Abstract
Object
The results of the International Subarachnoid Aneurysm Trial (ISAT) demonstrated lower rates of death and disability with endovascular treatment (coiling) than with open surgery (clipping) to secure the ruptured intracranial aneurysm. However, cost-effectiveness may not be favorable because of the greater need for follow-up cerebral angiograms and additional follow-up treatment with endovascular methods. In this study, the authors’ goal was to compare the cost-effectiveness of endovascular and neurosurgical treatments in patients with ruptured intracranial aneurysms who were eligible to undergo either type of treatment.
Methods
Clinical data (age, sex, frequency of retreatment, and rebleeding) and quality of life values were obtained from the ISAT. Total cost included those associated with disability, hospitalization, retreatment, and rebleeding. Cost estimates were derived from the Premier Perspective Comparative Database, data from long-term care in stroke patients, and relevant literature. Incremental cost-effectiveness ratios (ICERs) were estimated during a 1-year period. Parametric bootstrapping was used to determine the uncertainty of the estimates.
Results
The median estimated costs of endovascular and neurosurgical treatments (in US dollars) were $45,493 (95th percentile range $44,693–$46,365) and $41,769 (95th percentile range $41,094–$42,518), respectively. The overall quality-adjusted life years (QALY) in the endovascular group was 0.69, and for the neurosurgical group it was 0.64. The cost per QALY in the endovascular group was $65,424 (95th percentile range $64,178–$66,772), and in the neurosurgical group it was $64,824 (95th percentile range $63,679–$66,086). The median estimated ICER at 1 year for endovascular treatment versus neurosurgical treatment was $72,872 (95th percentile range $50,344–$98,335) per QALY gained. Given that most postprocedure angiograms and additional treatments occurred in the 1st year and the 1-year disability status is unlikely to change in the future, ICER for endovascular treatment will progressively decrease over time.
Conclusions
Using outcome and economic data obtained in the US at 1 year after the procedure, endovascular treatment is more costly but is associated with better outcomes than the neurosurgical alternative among patients with ruptured intracranial aneurysms who are eligible to undergo either procedure. With accrual of additional years with a better outcome status, the ICER for endovascular coiling would be expected to progressively decrease and eventually reverse.
Keywords: cost-effective analysis, endovascular coil placement, intracranial aneurysm, neurosurgical treatment, subarachnoid hemorrhage
Intracranial aneurysms affect 2% of the general population worldwide and are present in ~ 10 million people in the US.17 Until recently, the predominant technique for securing ruptured intracranial aneurysms was open operation (clipping). The advent and rapid technical evolution of endovascular treatments (coiling) together with supporting evidence have led to their widespread use.14 Using data from the National Inpatient Sample, we compared hospital costs in patients admitted with SAH in 1990–1991 with those admitted in 2000–2001.15 The mean hospital charges for SAH admissions increased significantly (from $37,400 to $65,900) over a 10-year period, and the in-hospital mortality rate declined from 29.4 to 26.5%. The incremental cost per survivor was $978,054, suggesting that the cost-effectiveness of new therapies may be steep.
In patients with ruptured intracranial aneurysms who were eligible to undergo either type of treatment, the ISAT showed lower rates of death and disability after endovascular treatment than after neurosurgical treatment.10 However, patients treated with coils more often underwent follow-up cerebral angiography and had higher rates of additional treatment, potentially influencing cost-benefit ratios for coiling versus clipping.2 The current study compares the cost-benefit of coiling and clipping at 1 posttreatment among patients with ruptured intracranial aneurysms eligible to be treated with either modality.
Methods
Data Sources
We obtained clinical data and quality of life values from the ISAT trial.2,10,11 Patients were eligible for the ISAT if they harbored a ruptured intracranial aneurysm that was judged by the neurosurgeon and the interventional neuroradiologist to be suitable for either technique (surgical or endovascular) on the basis of its angiographic anatomy. In addition the physicians had to agree that endovascular and surgical treatments were acceptable options for the patient.
Costs were derived from data on long-term care in stroke patients, relevant literature, and the Premier Perspective Comparative Database.4,7,8,12 The Premier database is the largest hospital-based comparative database in the US, providing detailed resource utilization data from ~ 38 million inpatient visits over the past 7 years from > 600 hospitals. This equates to 1 of every 6 inpatient discharges. Available data are demographic information (age, sex, and race/ethnicity), principal and secondary diagnoses, principal and secondary procedures, payor, length of hospital stay, and cost of care.12
Clinical Data
The following data were obtained from the ISAT: age, sex, severity of aneurysmal SAH, 1-year mortality, frequency of poor outcomes defined as death or dependency at 1 year, frequency of retreatment and rebleeding after the initial treatment, and number of cerebral angiograms.1,2,6,10,11
Cost Calculation
The mean net cost in each group was defined as the averaged total of the following costs: initial hospitalization, ongoing moderate and severe disability, cerebral angiography, retreatment, and rebleeding. The total final cost contained the cost of moderate to severe disability, including hospitalization, angiographic procedures, and the cost of retreatment and rebleeding associated with each treatment modality.
Cost of Acute Hospitalization
We determined the cost of hospitalization for aneurysmal SAH in the US in 2005–2006 using the Premier Perspective Comparative Database.12 We identified the patients with the primary discharge diagnosis of SAH using ICD-9-CM code 430. Furthermore, patients who underwent coil or clip placement were identified using ICD-9-CM procedure codes 39.51 and 39.52, respectively. Hospitalization costs were determined for patients with good neurological grade, following the same severity of ISAT enrolled patients (WFNS Grades I–III). Because the Premier database does not contain data pertaining to the initial Hunt and Hess scale or WFNS scale, we used the severity scale provided in the database. Therefore, we included patients with disease severity categorized as “mild” based on initial findings.
Cost of Moderate and Severe Disability
The cost of moderate to severe disability in this article represents the direct health system costs of continuing care after the initial hospitalization and the indirect cost of loss of productivity after acute stroke associated with moderate and severe disability per patient per year after acute hospitalization. The annual cost of medical care of moderate and severe disability was taken from data on long-term care in stroke patients, in which the average age was 50 and 65 years old, respectively, and the cost of long-term disability was adjusted for age.4,7 This health care cost is the median cost of stroke obtained from 1999 Medicare data for diagnosis-related group,16 which includes specific cerebrovascular disorders except transient ischemic attack. The cost of moderate to severe disability was adjusted for inflation for 2005–2006 using yearly inflation increments derived from the Consumer Price Index.
Cost of Cerebral Angiography
The direct cost of each cerebral angiogram was taken from nationally representative data (ICD-9-CM code 88.41) and previous studies.8,12
Cost of Additional Treatment
Because the vast majority of patients who underwent retreatment in the ISAT had not experienced aneurysm rupture, the cost of retreatment was extrapolated from the cost of treatment of an unruptured intracranial aneurysm. The cost of the treatment for unruptured aneurysm was extracted from the Premier Perspective Comparative Database.12 Patients were identified using ICD-9-CM primary diagnosis and procedure codes (437.3 for unruptured aneurysm; 39.51 for neurosurgical treatment; and 39.52 for endovascular treatment).
Cost of Rebleeding
The cost of rebleeding was calculated as the overall cost of treatment for any ruptured intracranial aneurysms by using the Premier database and combining the following ICD-9-CM codes: 430 for SAH, 39.51 for neurosurgical treatment, and 39.52 for endovascular treatment.
Treatment Effectiveness
The effectiveness of each treatment was measured in QALY. This measures the quality and the quantity of life lived as a means of quantifying the benefit of a medical intervention. The QALY is based on the number of years of life that would be added by the intervention.9 Each year in perfect health is assigned the value of 1.0 with progressively lower values assigned to increasing disabled statuses to 0 for death. If the extra years would not be lived in full health, for example, if one is disabled by a stroke, a value between 0 and 1 is given. The QALY was estimated at 1 year (healthy 1, mild disability 0.75, moderate and severe disability 0.39, and death 0) by the frequency distribution of the mRS for patients in each treatment group at 1 year according to the ISAT.10,11
Cost-Effectiveness Analysis
A cost-effectiveness analysis enumerates all additional resources consumed for an improvement in the effect associated with one health intervention over another. The result is summarized as an ICER. The ICER is a measure of the additional cost per unit of health gain.3 We calculated cost-effectiveness per QALY and ICER. The underlying calculation for the ICER comparing endovascular versus neurosurgical treatment in patients with SAH was as follows: ICER = (average costendovascular treatment − average costneurosurgical treatment)/(average effect endovascular treatment − average effect neurosurgical treatment). The average costs were estimated by multiplying each cost by the rates of clinical outcomes from ISAT findings.
To incorporate uncertainty into the cost-effectiveness analysis, we used Monte Carlo simulation of 10,000 replicates for clinical outcome rates and frequency distribution of the mRS using ISAT findings and fixed-cost parameters. We modeled the simulation for each clinical outcome rate and standard deviation equal to the standard error of the clinical outcome rate from ISAT results. The median and 95th percentile range are reported. In addition, we represented our results using the cost-effectiveness acceptability plane.3
Sensitivity Analysis
Sensitivity analysis was performed to explore uncertainty around the rate of retreatment in the endovascular group. A univariate sensitivity analysis of the retreatment rate of the endovascular group was done to estimate the ICER with the same rate of retreatment for the neurosurgical group.
Results
The ISAT randomized ~ 1000 patients in each group with the majority of them in good clinical condition (WFNS Grades I–III; Table 1). In the ISAT neurosurgical group (patients who underwent clip placement) outcomes at 1 year were 17.7% healthy, 51.4% mildly disabled, and 30.6% moderately to severely disabled or dead. In the endovascular group (patients who underwent coil placement) outcomes were 24.5% healthy, 52% mildly disabled, and 23.75% moderately to severely disabled or dead.10,11 The 1-year moderate to severe disability rate was 15.5% in the endovascular group and 21% in the neurosurgical group. Retreatment and rebleeding rates were 17.4 and 0.6% for the endovascular group and 3.8 and 0.3% for the neurosurgical group, respectively (Table 2). The mean number of cerebral angiograms in the endovascular group was 3.5, including the diagnostic cerebral angiogram prior to the intervention. In the neurosurgical group, the mean number of cerebral angiograms was 1.5, including pre- and postsurgical studies.1,6
TABLE 1.
Clinical characteristics of the patients treated in the ISAT*
| No. of Patients (%)* |
||
|---|---|---|
| Parameter | Endovascular Group | Neurosurgical Group |
| no. of patients | 1073 | 1070 |
| WFNS Grades I III | 1009 (94) | 1006 (94) |
| male sex | 400 (37) | 399 (37) |
| median age in yrs (range) | 52 (18–87) | 52 (18–84) |
Based on the studies by Mandelblatt, Molyneux (2002), and their colleagues.
TABLE 2.
Frequency of outcomes at 1 year in the patients treated in the ISAT used for the calculation of cost-effectiveness analysis
| No. of Patients (%)* |
|||
|---|---|---|---|
| Parameter | Endovascular Group | Neurosurgical Group | p Value |
| death at 1 yr9,10 | 86 (8) | 106 (9.9) | |
| death or dependency at | 252 (23.75) | 331 (30.6) | 0.0001 |
| 1 yr9,10 | |||
| mean no. of angiograms1,5 | 3.5 | 1.5 | <0.00001 |
| rate of retreatment2 | 187 (17.4) | 41 (3.8) | |
| rate of rebleeding2 | 6 (0.6) | 3 (0.3) | |
Unless stated otherwise.
The cost of the initial hospitalization for a low severity case of SAH treated by coiling was $35,143 (Table 3). The cost of moderate to severe disability was calculated at $21,645. The median cost adjusted by the rate of moderate to severe disability in the endovascular group was $3370 (95th percentile range $2858–$3930). The median cost of a cerebral angiogram was $2800, and the retreatment and rebleeding costs were $21,920 and $61,622, respectively. The median costs adjusted by the rates of retreatment and rebleeding in the endovascular group were $3809 (95th percentile range $3274–$4377) and $331 (95th percentile range $103–$774), respectively. The average 1-year total cost per patient with ruptured intracranial aneurysm treated by coiling over a period of 1 year was $45,493 (95th percentile range $44,639–$46,365).
TABLE 3.
Costs associated with each variable used for calculating the net cost of endovascular and neurosurgical treatment in patients with SAH*
| Variable | Source for Derivation of Data | Endovascular Group | Neurosurgical Group |
|---|---|---|---|
| cost of moderate to severe disability | Medicare costs on long-term care in stroke patients adjusted to inflation yr 2005–20064,6 | $21,645 ($3,370/$2,858–$3,930) | $21,645 ($4,445/$3,897–$5,036) |
| cost of hospitalization for a low severity case of ruptured intracranial aneurysm | Premier database using ICD-9-CM diagno- sis codes for SAH (430) & procedure (39.51 & 39.52)11 | $35,143 | $35,003 |
| cost of retreatment of unrup- tured intracranial aneurysm | Premier database using ICD-9-CM codes for unruptured intracranial aneurysms (437.3, 39.51, & 39.52)11 | $21,920 ($3,809/$3,274–$4,377) | $25,150 ($939/$639–$1,329) |
| cost of rebleeding | Premier database using ICD-9-CM codes for ruptured intracranial aneurysms (430, 39.51, & 39.52)11 | $61,622 ($331/$103–$774) | $58,426 ($137/$20–$459) |
| cost of cerebral angiogram | Premier database using ICD-9-CM code 88.417,11 | $800 ($2,800) | $800 ($1,200) |
| QALY | 0.69 (0.690–0.699) | 0.64 (0.639 0.649) | |
| total cost | $45,493 ($44,693–$46,365) | $41,769 ($41,094 –$42,518) | |
| cost per QALY | $65,424 ($64,178–$66,772) | $64,824 ($63,679–$66,086) |
Information in parentheses represents the cost adjusted to the incidence of that variable in the ISAT population/95th percentile ranges.
In the neurosurgical group the initial hospitalization for treatment of a low severity SAH case was $35,003. The rate-adjusted cost of moderate to severe disability was $4445 (95th percentile range $3897–$5036). The costs of retreatment and rebleeding were $25,150 and $58,426, respectively. The rate adjusted costs of retreat- ment and rebleeding were $939 (95th percentile range $639–$1329) and $137 (95th percentile range $20–$459). The estimated average 1-year total cost per patient with a ruptured intracranial aneurysm treated by clipping was $41,769 (95th percentile range $41,094–$42,518).
The overall QALY for coiling was 0.69 (95th percentile range 0.690–0.699) and for clipping was 0.64 (95th percentile 0.639–0.649). The cost per QALY for coiling was $65,424 (95th percentile range $64,178–$66,772) and for clipping was $64,824 (95th percentile range $63,679–$66,086) (Table 3). The mean estimated ICER for coiling versus clipping was $72,872 (95th percentile range $50,344–$98,335) per QALY gained.
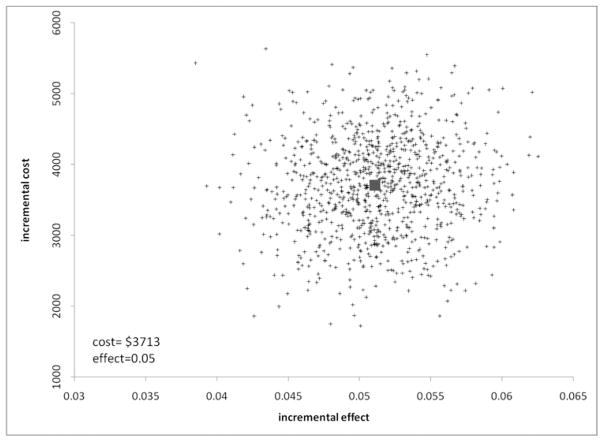
Figure 1 illustrates the uncertainty around the expected ICERs of coiling versus clipping. All replicates lie above zero on the cost axis, indicating a high degree of certainty that coiling is more costly than clipping. Similar results are true regarding the effectiveness of coiling over clipping.
Fig. 1.
Incremental cost-effectiveness plane scatterplot showing the estimated joint density of incremental costs and incremental effects of endovascular versus neurosurgical treatment.
Sensitivity Analysis
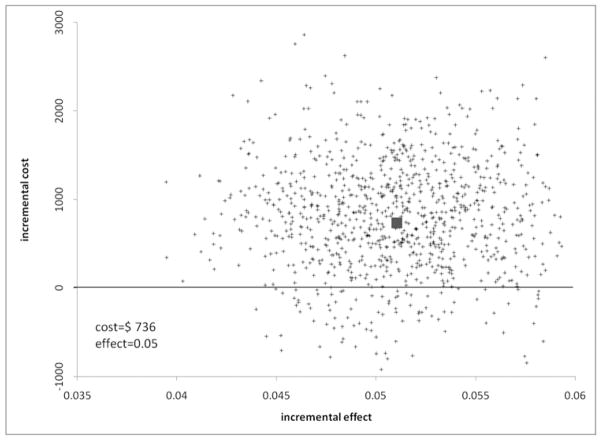
The high rate of retreatment in the endovascular group was most responsible for the higher cost associated with this treatment modality. If the rate of retreatment in the endovascular group is lowered to match the rate of retreatment of the neurosurgical group, the result is a lower and more beneficial ICER as shown in Table 4 and a more cost-effective treatment as shown in Fig. 2.
TABLE 4.
Costs and cost-effectiveness of univariate sensitivity analysis of the retreatment rate for endovascular intervention*
| Parameter | Endovascular | Neurosurgical | Difference |
|---|---|---|---|
| actual retreatment rate† | |||
| total cost | $45,493 ($44,693–$46,365) | $41,769 ($41,094–$42,518) | $3,715 ($2,617–$4,840) |
| cost per QALY | $65,424 ($64,178–$66,772) | 64,824 ($63,679–$66,086) | |
| ICER | $72,872 ($50,344–$98,335) | ||
| equivalent retreatment rate‡ | |||
| total cost | $42,506 ($41,851–$43,258) | $41,769 ($41,094–$42,518) | $743 ($251–$1,744) |
| cost per QALY | $61,128 ($60,121–62,288) | $64,824 ($63,679–$66,086) | |
| ICER | $14,594 ($–4,943 to $3,4562?) | ||
Values represent median with 95th percentiles in parentheses.
These values are based on the actual endovascular retreatment rate of 17.4.
These values are based on an endovascular retreatment rate equivalent to the neurosurgical retreatment rate of 3.8.
Fig. 2.
Incremental cost-effectiveness plane scatterplot showing the estimated joint density of incremental costs and incremental effects of endovascular versus neurosurgical treatment assuming the endovascular retreatment rate to be 3.8.
Discussion
The ISAT trial demonstrated that coiling results in better clinical outcomes (6.9% absolute risk reduction for death and dependency) in patients with ruptured intracranial aneurysms suitable for either coiling or clipping. In our analysis, the cost of coiling was higher than that for clipping. From other analyses, we anticipated that the reduced rate of disability in the patients treated endovascularly would offset the slightly higher cost of their acute hospitalization (A. I. Qureshi: Comparison between endovascular and surgical treatment for intracranial aneurysms: challenges for clinical trials. 29th International Stroke Conference San Diego, CA: February 5–7, 2004).
This analysis, however, found that the increased rates of follow-up cerebral angiograms and additional treatments in the endovascular group negated the savings when examined at 1 year. Most of these posttreatment costs are borne in the 1st year; hence comparative analysis at 1 year is most favorable for clipping. In the ISAT, the vast majority of the patients were retreated during the first 12 months after the original treatment. Retreatment was considered “late” if it happened within 18 months (median 4.2 months, range 1–18 months). Interestingly, aneurysm treatment did not cause significant additional morbidity. The risk of rebleeding in both groups was < 1% even after the 1st year.
Effectiveness measured in QALY favored coiling over clipping. Cost-utility analysis in which benefits of an intervention are quantified in terms of QALY has now become the standard type of cost-effectiveness analysis.16 Similarly, ICER is the accepted approach for comparing the value of 2 treatment approaches. An ICER lower than ~ $20,000 per additional QALY is exceptionally beneficial. An ICER between $20,000 and $40,000 per additional QALY is acceptable. An ICER between $60,000 and $100,000 per additional QALY is higher than most treatment alternatives, and ratios > $100,000 are not desirable. In our study, the mean estimated ICER for endovascular treatment versus neurosurgical treatment was $72,872 (95th percentile range $50,344–$98,335) per QALY gained. The analysis with 1-year data maximizes the cost related to coiling. On the other hand, available follow-up information suggests that the differences in patient outcome at 1 year are likely to continue year after year. Consequently, QALY differences will increase with time in favor of coiling. We hypothesize that the ICER associated with coiling will decrease with time and will reverse in years. This effect would be reached even sooner if the continuing yearly costs of disability were added on the side of clipping.
Certain limitations need to be considered prior to interpretation of this study. First, we used the ISAT data as the basis of comparison of effectiveness of coiling versus clipping in patients with ruptured intracranial aneurysms. The ISAT is the largest randomized trial we currently have that compares the effectiveness of endo- vascular and surgical treatment in patients with ruptured aneurysm. Although the ISAT is the best evidence that we have about treatment of ruptured intracranial aneurysms, most of the patients were enrolled outside the US. Harbaugh et al.5 pointed out that the results from ISAT might not be representative of practice patterns in the US, particularly because of differences in the degree of subspecialization of neurovascular surgeons in major centers. The better outcomes in patients undergoing endovascular procedures may be explained by a difference in practitioner experience and expertise. In the ISAT, patients with aneurysmal SAH were evaluated by a neurosurgeon and a neurointerventionist at each participating center. Eligibility for randomization was determined on the basis of subjective evaluations and the agreement of the physicians that endovascular and neurosurgical treatments were acceptable options. This may have introduced heterogeneity between centers because of subjective interpretations of the definition of eligibility.13 The recently presented Barrow Ruptured Aneurysm Trial, a single-center, randomized prospective trial of ruptured aneurysms in the US, provided new data about the broader applicability of the ISAT results (C. G. McDougall, unpublished data, 2008). Patients with aneurysmal SAH were randomized for endovascular and surgical treatment between March 2003 and January 2007. The primary outcome was an mRS score of 3–6 (dead or dependent) at 1 year or last available follow-up based on intention to treat. The preliminary results do not support superiority of surgical treatment over endovascular treatment, providing additional support to the assumptions used in our model.
Second, we used the data from the Premier database, which contains data that are nationally representative but lack specific details about certain clinical variables. We estimated the cost of hospitalization for good grade patients by including only those with low clinical severity determined by a scale particular to the database. We also assumed that the cost of retreatment of previously treated, ruptured aneurysms (in the absence of new re-bleeding) was similar to the hospitalization cost incurred for treating an unruptured intracranial aneurysm. These assumptions may not reflect the exact cost for such hospitalizations, but the bias is not based on the treatment modality used. The data are collected retrospectively and therefore are subject to ascertainment and documentation biases. However, it is unlikely that these biases are different based on the treatment modality used. The main difference in cost between the 2 modalities at 1 year was based on a differential rate of retreatment. The criteria for additional treatment of intracranial aneurysms in the ISAT study were not uniform, and therefore the rates of additional treatment may not be as high in practice as reported in the study.
Third, the evolution of technology particularly for endovascular treatment may make some of the assumptions in our model obsolete. This is a limitation of all cost-effectiveness studies that are evaluating technology in a rapid phase of evolution. Endovascular treatment is evolving at a much faster rate than neurosurgical treatment for intracranial aneurysms. The change in cost-effectiveness for endovascular treatment will be based on the ratio between increasing effectiveness and increasing cost. At this point, we cannot predict the exact magnitude of this ratio but we have performed sensitivity analysis to provide some estimate of the effect of variations in base- line assumptions used in the model.
Fourth, there may be unidentified -tween outcome data predominantly derived from studies in Europe and cost data derived from studies in the US. For example, the cost of treatment may be higher with either modality in the US, but the eventual beneficial effect on outcome of such expenditure will not be captured in our analysis. A similar study done in England by Wolstenholme et al.18 in which the authors used ISAT data and British costs showed no difference in the total 12-month cost of treatment between the 2 modalities. In another study of clinical and angiographic outcomes of patients enrolled in the ISAT in a North American center, there was no difference in cost in endovascular compared with traditional surgical clipping during hospitalization.6
Conclusions
Higher cost in the group treated with coils at 1 year was directly associated with the high incidence of retreatment of the originally treated intracranial aneurysm. From the perspective in the US, coiling results in higher cost at 1 year but is associated with better outcomes than clipping among patients with ruptured intracranial aneurysms that are suitable for either therapeutic modality. The accrual of years spent at better functional status among the coiled patients will reduce and ultimately reverse these 1-year results.
Acknowledgments
Disclosure
Dr. Lanzino has direct stock ownership in Johnson & Johnson. Dr. Suri is supported by National Institutes of Health (NIH) grant 5K12-RR023247-03.
Dr. Qureshi is supported in part by NIH grant RO-1-NS44976-01A2 and the American Heart Association’s Established Investigators Award 0840053N. Dr. Lakshminarayan is supported by an NINDS/NIH career development award (K23NS051377) and by grant U58DP000857 from the Centers for Disease Control.
Abbreviations used in this paper
- ICD-9-CM
International Classification of Diseases, 9th Revision, Clinical Modification
- ICER
incremental cost-effectiveness ratio
- ISAT
International Subarachnoid Aneurysm Trial
- mRS
modified Rankin Scale
- QALY
quality-adjusted life years
- SAH
subarachnoid hemorrhage
- WFNS
World Federation of Neurosurgical Societies
Footnotes
Disclaimer
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Bairstow P, Dodgson A, Linto J, Khangure M. Comparison of cost and outcome of endovascular and neurosurgical pro- cedures in the treatment of ruptured intracranial aneurysms. Australas Radiol. 2002;46:249–251. doi: 10.1046/j.1440-1673.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 2.Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RS, Sneade M, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT) Stroke. 2007;38:1538–1544. doi: 10.1161/STROKEAHA.106.466987. [DOI] [PubMed] [Google Scholar]
- 3.Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial -brillation. BMC Health Serv Res. 2006;6:52. doi: 10.1186/1472-6963-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gage BF, Cardinalli AB, Albers GW, Owens DK. Cost-effectiveness of warfarin and aspirin for prophylaxis of stroke in patients with nonvalvular atrial fibrillation. JAMA. 1995;274:1839–1845. [PubMed] [Google Scholar]
- 5.Harbaugh RE, Heros RC, Hadley MN. More on ISAT. Lancet discordance be. 2003;361:783–784. doi: 10.1016/S0140-6736(03)12641-4. [DOI] [PubMed] [Google Scholar]
- 6.Javadpour M, Jain H, Wallace MC, Willinsky RA, ter Brugge KG, Tymianski M. Analysis of cost related to clinical and angiographic outcomes of aneurysm patients enrolled in the international subarachnoid aneurysm trial in a North American setting. Neurosurgery. 2005;56:886–894. [PubMed] [Google Scholar]
- 7.Johnston SC, Gress DR, Kahn JG. Which unruptured cerebral aneurysms should be treated? A cost-utility analysis. Neurology. 1999;52:1806–1815. doi: 10.1212/wnl.52.9.1806. [DOI] [PubMed] [Google Scholar]
- 8.Kallmes DF, Kallmes MH, Lanzino G, Kassell NF, Jensen ME, Helm GA. Routine angiography after surgery for ruptured intracranial aneurysms: a cost versus benefit analysis. Neurosurgery. 1997;41:629–641. doi: 10.1097/00006123-199709000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Mandelblatt JS, Fryback DG, Weinstein MC, Russell LB, Gold MR. Assessing the effectiveness of health interventions for cost-effectiveness analysis. Panel on Cost-Effectiveness in Health and Medicine. J Gen Intern Med. 1997;12:551–558. doi: 10.1046/j.1525-1497.1997.07107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 11.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, re-bleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 12.Premier’s Perspective Comparative Database. Charlotte, NC; USA: 2006. [Accessed 6 August 2007]. ( http://www.premierinc.com) [Google Scholar]
- 13.Qureshi AI, Hutson AD, Harbaugh RE, Stieg PE, Hopkins LN. Methods and design considerations for randomized clinical trials evaluating surgical or endovascular treatments for cerebrovascular diseases. Neurosurgery. 2004;54:248–264. doi: 10.1227/01.neu.0000103446.26057.78. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi AI, Janardhan V, Hanel RA, Lanzino G. Comparisonof endovascular and surgical treatments for intracranial aneurysms: an evidence-based review. Lancet Neurol. 2007;6:816–825. doi: 10.1016/S1474-4422(07)70217-X. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi AI, Suri MF, Nasar A, Kirmani JF, Ezzeddine MA, Divani AA, et al. Changes in cost and outcome among US patients with stroke hospitalized in 1990 to 1991 and those hospitalized in 2000 to 2001. Stroke. 2007;38:2180–2184. doi: 10.1161/STROKEAHA.106.467506. [DOI] [PubMed] [Google Scholar]
- 16.Rasanen P, Roine E, Sintonen H, Semberg-Konttinen V, Ryynanen OP, Roine R. Use of quality-adjusted life years for the estimation of effectiveness of health care: a systematic literature review. Int J Technol Assess Health Care. 2006;22:235–241. doi: 10.1017/S0266462306051051. [DOI] [PubMed] [Google Scholar]
- 17.Wiebers DO, Whisnant JP, Huston J, III, Meissner I, Brown RD, Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- 18.Wolstenholme J, Rivero-Arias O, Gray A, Molyneux AJ, Kerr RS, Yarnold JA, et al. Treatment pathways, resource use, and costs of endovascular coiling versus surgical clipping after aSAH. Stroke. 2008;39:111–119. doi: 10.1161/STROKEAHA.107.482570. [DOI] [PubMed] [Google Scholar]