Abstract
Trypanosoma cruzi is the etiologic agent of Chagas disease, which affects millions of people in Latin America and has become a public health concern in the United States and areas of Europe. The possibility that kinase inhibitors represent novel anti-parasitic agents is currently being explored. However, fundamental understanding of the cell signaling networks requires the detailed analysis of the involved phosphorylated proteins. Here, we have performed a comprehensive mass spectrometry (MS)-based phosphorylation mapping of phosphoproteins from T. cruzi epimastigote forms. Our liquid chromatography (LC)-tandem MS (MS/MS, MS/MS/MS, and multistage activation) analysis has identified 237 phosphopeptides from 119 distinct proteins. Furthermore, 220 phosphorylation sites were unambiguously mapped: 148 on serine, 57 on threonine, and 8 on tyrosine. In addition, immunoprecipitation and Western blotting analysis confirmed the presence of at least seven tyrosine-phosphorylated proteins in T. cruzi. The identified phosphoproteins were subjected to Gene Ontology, InterPro, and BLAST analysis, and categorized based on their role in cell structure, motility, transportation, metabolism, pathogenesis, DNA/RNA/protein turnover, and signaling. Taken together our phosphoproteomic data provide new insights into the molecular mechanisms governed by protein kinases and phosphatases in T. cruzi. We discuss the potential roles of the identified phosphoproteins in parasite physiology and drug development.
Keywords: Cell Signaling, Chagas disease, Drug targets, Phosphoproteome, Trypanosoma cruzi
1 Introduction
Chagas disease or American trypanosomiasis is one of the most prevalent tropical illnesses with an estimated 11 million people infected and approximately 120 million people living in high risk areas. Chagas disease is a major public health problem in Latin America, where up to 50,000 people may die every year due to complications in the acute or chronic phases of the disease [1, 2]. In addition, due to the migration of chronically infected, asymptomatic people from endemic areas and lack of screening in blood banks, Chagas disease has become a public health concern in United States and certain areas of Europe, such as Catalonia [3, 4].
Chagas disease is caused by the protozoan parasite Trypanosoma cruzi, which is naturally transmitted by hematophagous Reduviidae insects, popular known as kissing bugs. The parasite, however, may also be transmitted congenitally or via blood transfusion or organ transplantation [1]. The T. cruzi life cycle comprises two stages in the insect vector and two stages in the human host. In the insect vector, epimastigote forms replicate in the midgut, whereas in the more distal portion of the gut, they are transformed into infective metacyclic trypomastigotes under nutritional stress. These metacyclic forms are expelled together with the insect’s excreta during a bloodmeal, and reach the host bloodstream through the bite wound or exposed ocular or oral mucosa. Inside the host, the parasite can infect different types of nucleated cells, where they immediately escape the parasitophorous vacuole and differentiate into amastigote forms, which reproduce freely in the cytoplasm by binary fission. After several divisions, amastigotes transform into trypomastigote forms, which are eventually released into the extracellular milieu and reach the bloodstream, where they can be taken up by the infect host cells or an insect vector, thus completing the natural life cycle [5].
The treatment of Chagas disease is currently restricted to two drugs, nifurtimox and benznidazole, which have limited efficacy and cause severe side effects [6, 7]. In addition, there is no human vaccine against T. cruzi [8, 9]. Therefore, there is a critical need to develop new therapeutic strategies for preventing or treating Chagas disease.
In line with the current interest in protein kinases as molecular targets for the treatment of a variety of diseases [10–13], the possibility that kinase inhibitors represent novel anti-parasitic agents is currently being explored [14]. Reversible protein phosphorylation is a key mechanism for the regulation of major biological processes including proliferation and differentiation. Approximately 2% of the T. cruzi genome encodes protein kinases, suggesting a major regulatory role in controlling parasite development and function [15]. A comparative study of the kinomes of trypanosomatids showed that T. brucei, T. cruzi, and Leishmania major have 176, 190, and 199 protein kinase genes, respectively [15, 16]. Of these kinases, approximately 12% are unique to trypanosomatids [15, 16]. Among the protein phosphatases, T. brucei, T. cruzi, and L. major have 78, 86, and 88 genes, respectively. About 40% of these phosphatase genes were atypical with no clear orthologs in other eukaryote genomes [17]. Taken all together, the significant differences between T. cruzi and host-cell protein kinases suggest that parasite specific inhibition can be achieved and, therefore, may represent a viable therapeutic approach to control Chagas disease.
Despite the importance of protein phosphorylation in many cellular processes, few studies have identified phosphorylation sites in trypanosomatid proteins. Recently, proteomic analysis of promastigote and amastigote forms of Leishmania donovani identified 73 phosphoproteins with diverse biological functions, however the specific phosphorylation sites (or phosphosites) were not identified [18]. Another proteomic study in L. donovani was able to identify 18 phosphosites from 16 distinct phosphopeptides [19]. However, to our knowledge the only phosphorylation site thus far determined in a T. cruzi protein is serine phosphorylation of the linker histone H1 [20]. In order to gain insight into the signaling networks that govern parasite function, we analyzed the phosphoproteome of T. cruzi at the non-infective, insect-derived epimastigote stage. We have chosen to initially perform the analysis of this stage, instead of the three other stages (i.e., metacyclic trypomastigote, amastigote, and trypomastigote), to better standardize the T. cruzi phosphoproteomic methodology, using a parasite form that can be obtained in large amounts in cell-free medium. Total epimastigote lysate was digested with trypsin, followed by the enrichment of phosphopeptides by strong-cation exchange (SCX) and immobilized metal-affinity chromatography (IMAC), and analysis by tandem LC-MS. Here, we show a comprehensive phosphorylation mapping study and discuss the potential roles of the identified phosphoproteins in parasite physiology and drug development.
2 Materials and Methods
2.1 Cell culture and protein extraction
T. cruzi epimastigotes (Y stain) were grown in liver infusion tryptose medium containing 10% fetal bovine serum at 28°C for 3–4 days [21]. The parasites were harvested and washed 3 times with phosphate-buffered saline (PBS), pH 7.4. Epimastigotes (2.4 × 1010) were lysed with 3 ml 8 M urea, 400 mM NH4HCO3, containing phosphatase inhibitor cocktail (Sigma-Aldrich), by vigorous vortexing for approximately 5 min. Disulfide bonds were reduced with 5 mM dithiothreitol for 15 min at 50°C, and the free thiol groups were alkylated with 10 mM iodoacetamide for 30 min at room temperature and protected from light. Protein content was measured by the Micro BCA assay (Pierce), according to the manufacturer’s protocol.
2.2 Trypsin Digestion
After BCA quantification, an equivalent of 10 mg total protein extract was diluted to 10 ml with 100 mM NH4HCO3 and digested with 100 μg sequencing-grade trypsin (Promega) for 24 h at 37°C. After the digestion, peptides were acidified by adding 100 μl formic acid (FA) and desalted in a C18 cartridge (DSC-18, Supelco, Sigma-Aldrich). The cartridge was activated with 4 ml methanol and equilibrated with 4 ml 0.05% trifluoroacetic acid (TFA). After loading and washing with 4 ml 0.05% TFA, the sample was eluted with 2 ml 80% ACN/0.05% TFA and dried in a vacuum centrifuge (Vacufuge, Eppendorf).
2.3 Strong Cation-Exchange (SCX) Chromatography
SCX fractionation was performed with 100 μl POROS HS 50 resin (Applied Biosystems) placed in a SPE support cartridge. After equilibrating the column with 25% ACN/0.5% FA (SCX buffer), the samples were loaded and eluted with 1 ml 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 150, 250, and 500 mM NaCl dissolved in SCX buffer. The fractions were dried in a vacuum centrifuge, dissolved in 200 μl 0.05% TFA, desalted in POROS R2 50 ziptips as described by Jurado et al. [22], and dried again.
2.4 Immobilized Metal-Affinity Chromatography (IMAC)
Fifty microliters of IMAC resin (PHOS Select Iron Affinity Gel, Sigma-Aldrich) was washed 2X with 450 μl 0.25 M acetic acid/30% ACN (IMAC buffer). Each fraction from SCX chromatography was redissolved in 100 μl IMAC buffer and incubated with the resin for 60 min with constant shaking. After the incubation, the resin was loaded onto ziptips, washed 5X with 100 μl IMAC buffer and eluted 2X with 100 μl 5% TFA/45% ACN. Samples were dried in a vacuum centrifuge prior to LC-MS analysis.
2.5 Liquid Chromatography-Mass Spectrometry (LC-MS)
Following IMAC purification each fraction was redissolved in 30 μl 0.05% TFA and 8 μl were used for each injection. The peptides were loaded in a C18 trap column (0.25 μl, Opti-Pak, Optimized Technologies) coupled to a nanoHPLC system (NanoLC-1DPlus, Eksigent). The separation was carried in a capillary reverse phase (RP) column (Acclaim, 3 μm C18, 75 μm × 25 cm, LC Packings, Dionex) in a gradient of 2–33.2% ACN/0.1% FA for 120 min. The eluted peptides were analyzed online in a linear ion trap mass spectrometer (LTQ XL/ETD, Thermo Fisher Scientific). The five most abundant ions were submitted to data-dependent collision-induced dissociation (CID) MS/MS, multistage activation (MSA), or MS/MS/MS fragmentation before being dynamically excluded for 2 min. The CID was set to 40% normalized collision energy. The MSA was set at −98.0, −49.0, and −32.6 Thompson (Th) relative to the precursor ion, and MS/MS/MS scans were triggered by the appearance of phosphate neutral loss (−98.0, −49.0, and −32.6 Th) in the MS2 scan event.
2.6 Bioinformatics analysis
MS/MS spectra were converted to DTA files using Bioworks (v3.3.1, Thermo Fisher Scientific) with the following criteria: peptide masses from 800 to 3500 Da, at least 15 ions, and a minimum of 10 counts. TurboSequest searches were done against the forward and reverse T. cruzi, bovine, human keratin, and porcine trypsin sequences (total 191,762 sequences, downloaded on March 17th, 2008, from GenBank). The parameters for database search were: fully trypsin digestion; up to 1 missed cleavage site; 2 Da for peptide mass tolerance; 1 Da for fragment mass tolerance; cysteine carbamidomethylation (+57 Da) as fixed modification; and methionine oxidation (+16 Da) and serine, tyrosine and threonine phosphorylation (+80 Da for MS/MS and −18 Da for MS/MS/MS) as variable modifications. The datasets were filtered with DCn ≥ 0.05, peptide probability ≤ 0.1, and Xcorr of 1.5, 2.2, and 2.7 (2.8 only for MS/MS/MS data) for singly-, doubly-, and triply-charged peptides, respectively. All phosphopeptide spectra were carefully examined for diagnostic b and y fragments to determine the exact modification site. For phosphorylation sites that could not be determined, the all possible modified amino acid residues were indicated by lower cap and the number of phosphate groups in parenthesis. The phosphorylation motifs were searched using Phosida phosphorylation site database (http://www.phosida.com/) [23]. All valid phosphoproteins were submitted to Gene Ontology (GO), Blast (e-value ≤ 1e-5) and InterPro annotations using Blast2go algorithm (February 11th, 2009, http://www.blast2go.de/) [24].
2.7 Immunoprecipitation and Western blot analysis
An equivalent of 1.2 × 109 epimastigote cells were solubilized in Triton lysis buffer (10 mM Tris-HCl (pH 7.6), 5 mM EDTA (pH 8.0), 50 mM NaCl, 30 mM Na4P2O7, 50 mM NaF, 1 mM Na3VO4, 1% Triton X-100) containing 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, 2 μg/ml leupeptin, 1 μg/ml pepstatin A, and clarified by centrifugation (16,000 × g, 10 min, 4°C). The supernatants were rotated with 10 μg of anti-phosphotyrosine monoclonal antibody (4G10, Upstate) for 2 h at 4 °C. The immune complex was captured by incubation with protein A-Sepharose beads (Rockland Immunochemicals), for 1 h at 4°C. The beads were then washed 3 times with cold lysis buffer and eluted by boiling in 2x SDS sample buffer (50 mM Tris-HCl (pH 6.8), 100 mM dithiothreitol, 2% SDS, 0.02% bromophenol blue, 10% glycerol, pH 6.8). Samples were resolved by 10% SDS-PAGE and transferred to PVDF membrane (Amersham Biosciences, GE Healthcare Life Sciences). After blocking with 1% BSA, the membrane was incubated overnight with anti-phosphotyrosine monoclonal antibody (4G10, Upstate), followed by 2 h incubation with horseradish peroxidase-conjugated goat anti-mouse IgG (H+L) (KPL), and visualized by using enhancedchemiluminescence reagent (ECL) and X-ray film (Phenix Research Products).
3 Results and Discussion
3.1 Phosphopeptide enrichment and phosphorylation site mapping
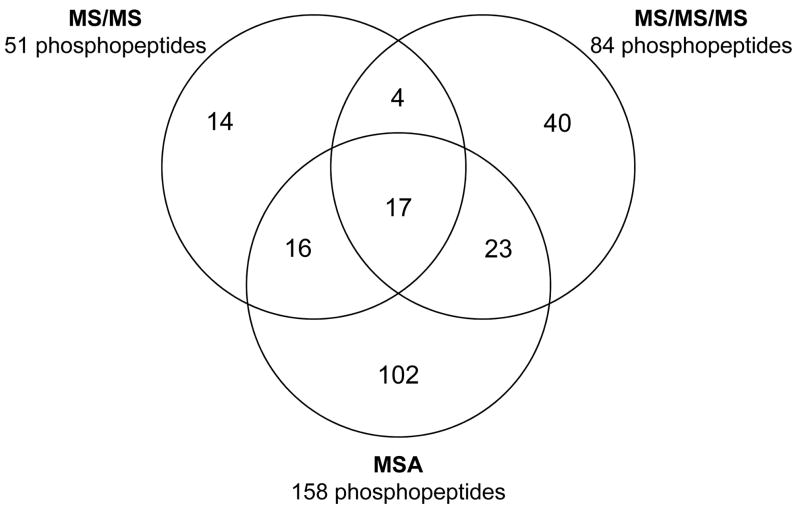
The identification of phosphoproteins can be limited due to their low abundance and the low stoichiometry of phosphorylation. In order to map the phosphorylation sites in T. cruzi proteins, whole epimastigote extracts were digested with trypsin, fractionated by SCX chromatography, and the resulting phosphopeptides enriched using IMAC. The enriched fractions were analyzed by LC-MS/MS, phosphate neutral loss-triggered LC-MS/MS/MS, or LC-MSA. It is well documented that MS/MS fragmentation of phosphopeptides results in a strong neutral loss of the phosphate group [25]. In many cases, the resulting spectra do not provide sufficient fragments to determine the phosphopeptide sequences. To circumvent this problem, neutral loss-triggered MS/MS/MS [26] and MSA (also known as pseudo-MS3) [27] were developed. To increase the phosphoproteome coverage, we applied all three dissociation strategies that resulted in the identification of similar numbers of non-phosphorylated peptides (Table 1). However, the MSA approach led to the identification of more phosphopeptides (n=160) as compared to the MS/MS (n=51) or MS/MS/MS (n=84) approach (Table 1, Supplementary Figures 1–3). In addition, only 17 peptides were sequenced using all three MSn acquisition regimens (Fig. 1), indicating that these regimens are complementary. It is noteworthy that the MS/MS/MS regimen led to an increased false-discovery rate (FDR), thus the Xcorr threshold had to be increased to validate the sequences. Two recent reports have compared the use of MS/MS, MS/MS/MS, and MSA for the analysis of phosphopeptides using high resolution mass spectrometers (Orbitrap-MS or FT-ICR-MS) for the first MS stage [28, 29]. On the one hand, Villen et al. showed no significant differences in the number of phosphopeptides identified and performance to locate the modification sites using all three methods of fragmentation [29]. On the other hand, Ulintz et al. reported that the MSA method outperformed the two other strategies [28]. The results shown herein indicate that indeed MSA had a superior performance compared to the other MSn strategies. These results suggest that the MSA-provided higher quality spectra [27] are more crucial for the analysis of phosphopeptides when lower resolution data is recorded in the first MS stage.
Table 1.
Statistics of phosphoproteome analysis of Trypanosoma cruzi epimastigote forms.
| Phosphoproteins |
||||||||
|---|---|---|---|---|---|---|---|---|
| MS approach | Non-phosphorylated proteins |
1P | 2P | 3P | 4P | >4P | Total phosphoproteins |
|
| MS/MS | 111 | 25 | 8 | 4 | 0 | 0 | 37 | |
| MS/MS/MS | 110 | 46 | 14 | 3 | 1 | 2 | 65 | |
| MSA | 80 | 60 | 22 | 11 | 5 | 1 | 99 | |
| Total | 156 | 74 | 30 | 18 | 5 | 4 | 131 | |
| T. cruzi | 139 | 72 | 24 | 16 | 3 | 4 | 119 | |
| Phosphopeptides | ||||||||
| Non-phosphorylated peptides | Phosphorylation sites | 1P | 2P | >2P | Total phosphopeptides | Reverse | FDR (%) | |
| MS/MS | 146 | 52 | 47 | 3 | 1 | 51 | 5 | 2.0 |
| MS/MS/MS | 124 | 65 | 75 | 5 | 4 | 84 | 9 | 4.1 |
| MSA | 96 | 162 | 146 | 9 | 5 | 160 | 9 | 3.4 |
| Total | 206 | 226 | 231 | 11 | 8 | 250 | 21 | 4.6 |
| T. cruzi | 196 | 221 | 227 | 5 | 5 | 237 | - | - |
| Distribution of T. cruzi phosphorylated sites |
||||||||
| Phosphoserine | Phosphothreonine | Phosphotyrosine | Ser or Thr | Ser or Tyr | ||||
| # of sites | 148 | 57 | 8 | 7 | 1 | |||
| % of Total | 65.5 | 25.2 | 3.5 | 3.1 | 0.4 | |||
Figure 1.
Venn diagram of T. cruzi phosphopeptides identified by MS/MS, MS/MS/MS, and MSA analyses.
In our analyses, 237 phosphopeptides were sequenced from 221 phosphorylation sites in 119 proteins from T. cruzi (Table 1, Supplementary Table 1). Of those 221 newly identified phosphorylation sites, 148 (65.5%) were on serine, 57 (25.2%) on threonine, and 8 (3.5%) on tyrosine (Table 1). Eight phosphorylation sites could not be assigned to specific amino acid residues, 7 on serine or threonine and 1 on serine or tyrosine (Table 1). From the identified phosphoproteins, approximately 40% had multiple phosphorylation sites (Table 1, Supplementary Table 1). The identification of these phosphoproteins further supports the notion that kinase-mediated signal transduction pathways are important in the regulation of parasite biological processes. Unlike metazoa and yeast, which utilize regulated transcription factors to direct the expression of certain genes, trypanosomatids indiscriminately transcribe most genes in large polycistronic units, thus emphasizing the critical role of posttranslational modifications (PTM) in the regulation of T. cruzi proteins.
3.2 Annotation of phosphorylation motifs
The specificity of protein kinases is determined by the amino acid residues adjacent to the phosphosite and is termed the consensus phosphorylation motif. To determine the potential kinase(s) responsible for modification of the identified phosphosites, the phosphorylation motifs were annotated using the Phosida search algorithm [15]. The most abundant motifs are those from CAMK2 (13.9%), CK1 (11.1%), PKA (8.71%), CK2 (7.7%), GSK3 (7.0%), ERK (5.9%), and CDK1 (4.2%) (Table 2). Fifty-seven (19.9%) phosphorylation sites did not match any known motif (Table 2), which may be due to kinases with undetermined specificity or atypical kinases that might only be present in trypanosomatids [15, 16]. As expected, there was a significant amount of redundancy among the identified motifs; therefore, more motifs (n=287) were present compared to phosphorylation sites (n=221). In these cases, it is probable that specificity is achieved by different spatial and temporal expression of the kinase(s). Intriguingly, a number of tyrosine-kinase phosphorylation motifs (ALK, SRC, and EGFR) were identified (Table 2). A key difference between host and parasite kinomes is the absence of receptor-linked and cytoplasmic tyrosine kinases in trypanosomatids [15]; however, the presence of protein-tyrosine phosphatases indicates that tyrosine phosphorylation is a key regulatory mechanism in T. cruzi [17]. It has been proposed that atypical tyrosine kinases such as Wee1 and dual-specificity kinases such as the DYRKs and CLKs, which are all present in the T. cruzi genome, are responsible for this activity [15].
Table 2.
Distribution of kinase specific motifs in T. cruzi.
| Phosphoproteome | All T. cruzi Sequences | |||
|---|---|---|---|---|
| Kinase motif | Number | % of total | Number | % of total |
| CAMK2 (R-X-X-S/T) | 40 | 13.94* | 170873 | 8.53 |
| CK1 (S-X-X-S/T, S/T-X-X-X-S) | 32 | 11.15* | 450808 | 22.49 |
| PKA (R-X-S/T, R-R/K-X-S/T) | 25 | 8.71 | 193369 | 9.65 |
| CK2 (S/T-X-X-E) | 22 | 7.67 | 183303 | 9.15 |
| GSK3 (S-X-X-X-S) | 20 | 6.97 | 134201 | 6.7 |
| ERK (V-X-S/T-P, P-X-S/T-P) | 17 | 5.92* | 23114 | 1.15 |
| CDK1 (S/T-P-K/R, S/T-P-X-K/R) | 12 | 4.18* | 30468 | 1.52 |
| NEK6 (L-X-X-S/T) | 10 | 3.48* | 250587 | 12.5 |
| CHK1 (M/I/L/V-X-R/K-X-X-S/T) | 10 | 3.48 | 81145 | 4.05 |
| PKD (L/V/I-X-R/K-X-X-S/T) | 9 | 3.14 | 57644 | 2.88 |
| Aurora (R/K-X-S/T-I/L/V) | 8 | 2.79 | 57021 | 2.85 |
| CDK2 (S/T-P-X-K/R) | 6 | 2.09 | 16244 | 0.81 |
| AKT (R-R/S/T-X-S/T-X-S/T, R-X-R-X-X-S/T) | 5 | 1.74 | 20994 | 1.05 |
| Aurora-A (R/K/N-R-X-S/T-M/L/V/I) | 4 | 1.39 | 6121 | 0.31 |
| EGFR (D/P/S/A/E/N-X-Y-V/L/D/E/I/N/P) | 3 | 1.05 | 69975 | 3.49 |
| ALK (Y-X-X-I/L/V/M) | 3 | 1.05* | 112032 | 5.59 |
| PLK1 (E/D-X-S/T-F/L/I/Y/W/V/M) | 2 | 0.70* | 91008 | 4.54 |
| SRC (E/D-X-X-Y-X-X-D/E/A/G/S/T) | 1 | 0.35 | 18509 | 0.92 |
| CHK1/2 (L-X-R-X-X-S/T) | 1 | 0.35 | 16716 | 0.83 |
| Unknown motif | 57 | 19.86 | - | - |
| Other motifs | - | - | 19945 | 1 |
| Total | 287 | 100 | 2004077 | 100 |
p < 0.01 by Fisher’s exact test.
To determine whether any motif was over- or under-represented, the distribution of motifs of epimastigote phosphoproteome was compared to that of the entire T. cruzi database. The CAMK2, ERK, and CDK1 motifs were significantly over-represented (p < 0.01 by Fisher’s exact test), whereas the CK1, NEK6, PLK1, and ALK motifs were under-represented (p < 0.01) (Table 2).
3.3 Functional categorization of identified phosphoproteins
Of 119 sequenced phosphoproteins, 68 (57%) were annotated in the GenBank database as hypothetical proteins, making it difficult to infer their biological function (Table 3). Therefore, we submitted all identified phosphoproteins to automated Blast, InterPro and Gene Ontology (GO) analysis (see Material and Methods for details). Since the majority of T. cruzi sequences were compiled in 2005 with the completion of the genome project [30], new entries in the GenBank database could help in the annotation of the identified sequences. Indeed, Blast analysis resulted in the annotation of 21 sequences previously described as hypothetical proteins (Table 3, Supplementary Table 2). In addition, InterPro analysis resulted in 74 entries, 25 of which were previously annotated as hypothetical proteins (Table 3, Supplementary Table 3). Finally, GO analysis generated 69 annotations, 22 of which were previously annotated as hypothetical proteins (Table 3, Supplementary Table 4). Taken together, Blast, InterPro and GO analyses, assigned 35 (51%) of the 68 hypothetical sequences to a predicted biological function (Table 3). The overall function of the identified phosphoproteins is discussed in more detail in the following subsections.
Table 3.
Functional categorization of identified phosphoproteins of Trypanosoma cruzi epimastigotes.
| Blast Analysis |
|||||||
|---|---|---|---|---|---|---|---|
| Hit # | Protein description | Accession # | P sites |
Sequence description | min. e-value | InterPro | Gene Ontology |
| Signal transduction | |||||||
| 1 | regulatory subunit of protein kinase a-like protein | EAN92816.1 | 5 | regulatory subunit of protein kinase a-like protein | 1.0E-0.0 | Cyclic nucleotide-binding, RmlC-like jelly roll fold | cAMP-dependent protein kinase complex |
| 2 | phosphoprotein phosphatase | EAN90617.1 | 2 | serine threonine protein | 1.0E-0.0 | Serine/threonine-specific protein phosphatase and bis(5-nucleosyl)-tetraphosphatase | hydrolase activity |
| 3 | rac serine-threonine kinase | EAN95526.1 | 2 | serine threonine protein kinase | 1.0E-0.0 | Zinc finger, FYVE-type | ATP binding, protein amino acid phosphorylation, protein serine/threonine kinase activity, zinc ion binding |
| 4 | hypothetical protein | EAN95573.1 | 2 | WD repeat domain 17 | 1.0E-0.0 | WD40/YVTN repeat-like | none |
| 5 | protein kinase-A catalytic subunit | AAL17691.2 | 1 | protein kinase a catalytic subunit | 1.0E-0.0 | Serine/threonine protein kinase | protein serine/threonine kinase activity |
| 6 | hypothetical protein | EAN96688.1 | 1 | hypothetical protein | 1.0E-0.0 | WD40 repeat-like | none |
| 7 | hypothetical protein | EAN97518.1 | 1 | hypothetical protein | 1.0E-0.0 | WD40/YVTN repeat-like | none |
| 8 | hypothetical protein | EAN88571.1 | 1 | WD-repeat proteinexpressed | 1.0E-0.0 | WD40/YVTN repeat-like | nucleotide binding |
| 9 | calmodulin | EAN86242.1 | 1 | calmodulin | 1.00E-80 | Calcium-binding EF-hand | calcium ion binding |
| 10 | protein kinase (GSK3 beta) | EAN98751.1 | 1 | protein kinase | 1.0E-0.0 | Serine/threonine protein kinase-related | ATP binding, protein amino acid phosphorylation, protein serine/threonine kinase activity |
| 11 | hypothetical protein | EAN99395.1 | 1 | EF-hand family protein | 1.0E-0.0 | Calcium-binding EF-hand | calcium ion binding |
| 12 | hypothetical protein | EAN93781.1 | 2 | hypothetical protein | 1.0E-0.0 | Forkhead-associated | none |
| 13 | hypothetical protein | EAN91302.1 | 2 | chromosome 6 open reading frame 224 | 1.0E-0.0 | Shikimate kinase, Adenylate kinase, YHS | shikimate kinase activity, ATP binding |
| 14 | hypothetical protein | EAN97116.1 | 1 | calmodulin-related protein | 1.00E-180 | none | calcium ion binding |
| 15 | hypothetical protein | EAN83100.1 | 1 | EF-hand domain | 1.0E-0.0 | Region of unknown function DM10, Protein of unknown function DUF1126 | calcium ion binding, flagellum, |
| 16 | hypothetical protein | EAN83235.1 | 1 | hypothetical protein | 1.0E-0.0 | C2 calcium-dependent membrane targeting | none |
| Cytoskeleton, flagellum and traffic proteins | |||||||
| 17 | paraflagellar rod component Par1b | AAC32103.1 | 3 | 69 kda paraflagellar rod protein | 1.0E-0.0 | Paraflagellar rod | calmodulin binding, microtubule-based flagellum |
| 18 | hypothetical protein | EAN99904.1 | 2 | hypothetical protein | 1.00E-134 | Major sperm protein, PapD-like | structural molecule activity |
| 19 | Beta tubulin 2.3 | AAL75957.1 | 1 | beta-tubulin | 1.0E-0.0 | Tubulin/FtsZ, GTPase | structural molecule activity |
| 20 | kinesin | EAN86063.1 | 1 | kinesin family member 14 | 1.0E-0.0 | Kinesin, motor region | microtubule motor activity, ATP binding, microtubule associated complex, microtubule-based movement |
| 21 | hypothetical protein | EAN84227.1 | 1 | dc2-related axonemal dynein intermediate chain | 1.0E-0.0 | none | none |
| 22 | dynein heavy chain | EAN97081.1 | 1 | dynein heavy chain | 1.0E-0.0 | ATPase associated with various cellular activities, AAA-5 | ATP binding, ATPase activity |
| 23 | clathrin coat assembly protein | EAN98227.1 | 1 | clathrin coat assembly protein | 1.0E-0.0 | ENTH/VHS, Epsin-like, N-terminal | phosphatidylinositol binding, clathrin coat, clathrin binding, clathrin cage assembly |
| 24 | hypothetical protein | EAN96083.1 | 1 | flagellar protein | 1.00E-154 | none | none |
| 25 | hypothetical protein | EAN88130.1 | 1 | Tctex1 domain | 1.00E-68 | Tctex-1 | flagellum, microtubule associated complex, microtubule-based process |
| 26 | hypothetical protein | EAN87422.1 | 1 | neurofilament heavy polypeptide (NF-H) | 1.0E-0.0 | none | structural molecule activity |
| Transporters | |||||||
| 27 | ABC transporter | EAN96763.1 | 6 | ABC transporter | 1.0E-0.0 | ABC-2 type transporter | ATP binding, ATPase activity |
| 28 | ABC transporter | EAN92058.1 | 3 | ABC transporter | 1.0E-0.0 | ABC transporter-like | ATP binding, ATPase activity |
| 29 | ABC transporter | EAN89676.1 | 2 | ABC transporter | 1.0E-0.0 | ABC-2 type transporter | ATP binding, ATPase activity |
| 30 | metal-ion transporter | EAN85275.1 | 1 | zinc transporter-like protein | 1.0E-0.0 | Cation efflux protein | cation transport, cation transmembrane transporter activity, membrane |
| 31 | chloride channel protein | EAN94356.1 | 1 | chloride channel 7 | 1.0E-0.0 | Cystathionine beta-synthase, Chloride channel, voltage gated | voltage-gated chloride channel activity, chloride transport, membrane |
| 32 | calcium channel protein | EAN97848.1 | 1 | calcium channel protein | 1.0E-0.0 | Ion transport | ion channel activity, ion transport, membrane |
| 33 | GPR1/FUN34/yaaH family | EAN82352.1 | 1 | GPR1/FUN34/yaaH family (Acetate transporter) | 3.44E-135 | GPR1/FUN34/yaaH | membrane |
| 34 | Na+-ATPase | BAC98847.1 | 1 | calcium motive p-type ATPase | 1.0E-0.0 | ATPase, P-type, K/Mg/Cd/Cu/Zn/Na/Ca/Na/H-transporter | ATP binding, membrane, cation transport, catalytic activity, metabolic process |
| DNA, RNA and protein turnover | |||||||
| 35 | GTP-binding elongation factor Tu family | EAN90873.1 | 4 | GTP binding protein 1 | 1.0E-0.0 | Translation elongation factor EF1A/initiation factor IF2gamma, C-terminal | GTPase activity, GTP binding |
| 36 | heterogeneous nuclear ribonucleoprotein H/F | EAN91678.1 | 4 | heterogeneous nuclear ribonucleoprotein h | 1.0E-0.0 | RNA recognition motif, RNP-1 | nucleic acid binding, nucleotide binding |
| 37 | mitochondrial DNA polymerase I protein D | EAN86491.1 | 3 | mitochondrial dna polymerase i protein | 1.00E-135 | DNA polymerase A | DNA binding, DNA-directed DNA polymerase activity, DNA replication |
| 38 | hypothetical protein | EAN95162.1 | 3 | hypothetical protein | 1.0E-0.0 | RNA recognition motif, RNP-1 | nucleic acid binding |
| 39 | eukaryotic translation initiation factor | EAN92887.1 | 2 | eukaryotic translation initiation factor | 1.0E-0.0 | Eukaryotic translation initiation factor 4E (eIF-4E) | RNA binding, translation initiation factor activity, cytoplasm, translational initiation |
| 40 | 25 kDa translation elongation factor 1-beta | EAN82455.1 | 1 | translation elongation factor 1-beta | 2.16E-79 | Translation elongation factor EF1B, beta and delta chains, guanine nucleotide | translation elongation factor activity |
| 41 | Tcc1a22.3 | AAL82703.1 | 1 | elongation factor 1-gamma | 1.0E-0.0 | Glutathione S-transferase, Thioredoxin fold | translation elongation, protein biosynthesis, |
| 42 | hypothetical protein | EAN84292.1 | 2 | hypothetical protein | 1.0E-0.0 | Signal recognition particle, SRP54 subunit, GTPase | GTP binding, SRP-dependent cotranslational protein targeting to membrane, 7S RNA binding, membrane |
| 43 | histone H2B | EAN83534.1 | 1 | histone H2B | 4.12E-58 | Histone H2B | nucleosome, DNA binding, nucleus, nucleosome assembly |
| 44 | hypothetical protein | EAN86654.1 | 1 | PAB1 binding protein | 1.0E-0.0 | Ataxin-2, N-terminal | mitochondrion, mRNA polyadenylation, cytoplasm, positive regulation of protein biosynthesis, polysome, nucleus |
| 45 | mitochondrial DNA polymerase I protein C | EAN88565.1 | 1 | mitochondrial dna polymerase i protein | 1.0E-0.0 | DNA-directed DNA polymerase, family A | DNA binding, DNA-directed DNA polymerase activity, DNA replication |
| 46 | ATP-dependent RNA helicase | EAN96171.1 | 1 | ATP-dependent rna helicase | 1.0E-0.0 | DNA/RNA helicase, DEAD/DEAH box type, N-terminal | nucleic acid binding, ATP binding, ATP-dependent helicase activity |
| 47 | tyrosyl or methionyl-tRNA synthetase | EAN96489.1 | 1 | tyrosyl-tRNA synthetase | 1.0–128 | Nucleic acid-binding, OB-fold | tRNA binding |
| 48 | glutamamyl carboxypeptidase | EAN85499.1 | 1 | acetylornithine deacetylase | 1.00E-152 | Peptidase M20, dimerisation | proteolysis, metallopeptidase activity, protein dimerization activity |
| 49 | calpain cysteine peptidase | EAN83138.1 | 1 | calpain-like cysteine peptidase | 1.0E-0.0 | Peptidase C2, calpain | calcium-dependent cysteine-type endopeptidase activity, intracellular, proteolysis |
| 50 | calpain-like cysteine peptidase | EAN88142.1 | 1 | calpain-like cysteine peptidase | 1.0E-0.0 | Peptidase C2, calpain | calcium-dependent cysteine-type endopeptidase activity, intracellular, proteolysis |
| 51 | calpain-like cysteine peptidase | EAN92790.1 | 1 | calpain-like cysteine peptidase | 1.00E-139 | Region of unknown function DUF1935 | proteolysis, calcium-dependent cysteine-type endopeptidase activity, intracellular |
| 52 | hypothetical protein | EAN99674.1 | 2 | hypothetical protein | 1.0E-0.0 | Zinc finger, CCCH-type | nucleic acid binding, zinc ion binding |
| 53 | hypothetical protein | EAN96152.1 | 3 | cysteine clan family c2 | 1.0E-0.0 | none | proteolysis, calcium-dependent cysteine-type endopeptidase activity, intracellular |
| 54 | nucleosome assembly protein | EAN95410.1 | 1 | nucleosome assembly protein | 1.00E-158 | Nucleosome assembly protein (NAP) | nucleus, nucleosome assembly |
| Metabolism | |||||||
| 55 | Tyrosine Aminotransferase | 1BW0 | 3 | tyrosine aminotransferase | 1.0E-0.0 | 1-aminocyclopropane-1-carboxylate synthase | tyrosine transaminase activity |
| 56 | ribose-phosphate pyrophosphokinase | EAN92422.1 | 3 | ribose-phosphate pyrophosphokinase | 1.0E-0.0 | Phosphoribosyl pyrophosphokinase | nucleoside metabolic process, magnesium ion binding, ribose phosphate diphosphokinase activity, nucleotide biosynthetic process |
| 57 | pyruvate dehydrogenase E1 alpha subunit | AAD11551.1 | 2 | pyruvate dehydrogenase e1 component alpha | 1.0E-0.0 | Pyruvate dehydrogenase (acetyl-transferring) E1 component, alpha subunit, | metabolic process |
| 58 | pyruvate phosphate dikinase 2 | AAG12986.1 | 1 | pyruvate phosphate dikinase | 1.0E-0.0 | Pyruvate/Phosphoenolpyruvate kinase, catalytic core | kinase activity |
| 59 | phosphoribosylpyrophosphate synthetase | EAN97393.1 | 1 | phosphoribosylpyrophosphate synthetase | 1.0E-0.0 | Phosphoribosyl pyrophosphokinase | nucleoside metabolic process, magnesium ion binding, ribose phosphate diphosphokinase activity, nucleotide biosynthetic process |
| 60 | ribose-phosphate pyrophosphokinase | EAN95566.1 | 1 | ribose-phosphate pyrophosphokinase | 1.0E-0.0 | Phosphoribosyl pyrophosphokinase | nucleoside metabolic process, magnesium ion binding, ribose phosphate diphosphokinase activity, nucleotide biosynthetic process |
| 61 | hypothetical protein | EAN95143.1 | 1 | hypothetical protein | 1.0E-0.0 | Methyltransferase type 11 | metabolic process, methyltransferase activity |
| 62 | hypothetical protein | EAN95748.1 | 1 | had superfamily | 1.0E-0.0 | HAD-superfamily hydrolase, subfamily IIA, CECR5 | metabolic process, hydrolase activity |
| Pathogenesis | |||||||
| 63 | trans-sialidase | EAN91292.1 | 3 | trans-sialidase | 1.0E-0.0 | Concanavalin A-like lectin/glucanase, subgroup | exo-alpha-sialidase activity, pathogenesis |
| 64 | trans-sialidase | EAN83746.1 | 2 | trans-sialidase | 1.0E-0.0 | Neuraminidase | sialidase activity, pathogenesis |
| 65 | trans-sialidase | EAN96241.1 | 2 | trans-sialidase | 1.0E-0.0 | Concanavalin A-like lectin/glucanase, subgroup | exo-alpha-sialidase activity, pathogenesis |
| 66 | trans-sialidase | EAN99282.1 | 1 | trans-sialidase | 1.0E-0.0 | Concanavalin A-like lectin/glucanase | exo-alpha-sialidase activity, pathogenesis |
| 67 | dispersed gene family protein 1 (DGF-1) | EAN86627.1 | 1 | dispersed gene family protein 1 (DGF-1) | 1.0E-0.0 | Parallel beta-helix repeat, Pectin lyase fold/virulence factor | none |
| Binding | |||||||
| 68 | hypothetical protein | EAN97824.1 | 3 | c11orf60 protein | 1.0E-0.0 | protein binding | cytoplasm, nucleus |
| 69 | hypothetical protein | EAO00106.1 | 3 | hypothetical protein | 1.0E-0.0 | none | protein binding |
| 70 | hypothetical protein | EAN92411.1 | 3 | p25-alpha domain-containing | 1.00E-74 | P25-alpha | none |
| 71 | hypothetical protein | EAN89957.1 | 3 | hypothetical protein | 1.0E-0.0 | Leucine-rich repeat | protein binding |
| 72 | hypothetical protein | EAN85866.1 | 1 | hypothetical protein | 1.0E-0.0 | Tetratricopeptide-like helical, Sel1-like, CS domain | binding |
| 73 | hypothetical protein | EAN88376.1 | 1 | hypothetical protein | 1.0E-0.0 | C2 calcium-dependent membrane targeting | none |
| 74 | hypothetical protein | EAN96978.1 | 1 | hypothetical protein | 1.0E-0.0 | none | nucleic acid binding, zinc ion binding |
| 75 | hypothetical protein | EAN92548.1 | 1 | T-complex-associated testis expressed 1 | 1.0E-0.0 | Leucine-rich repeat | protein binding |
| 76 | I/6 autoantigen | EAN91318.1 | 1 | I/6 autoantigen | 1.00E-83 | calcium ion binding | structural constituent of cytoskeleton, cytoplasm, calcium ion binding, cytoskeleton, microtubule |
| 77 | I/6 autoantigen | EAN94637.1 | 1 | I/6 autoantigen | 1.00E-82 | calcium ion binding | structural constituent of cytoskeleton, cytoplasm, calcium ion binding, cytoskeleton, microtubule |
| Other functions | |||||||
| 78 | retrotransposon hot spot (RHS) protein | EAN86249.1 | 2 | retrotransposon hot spotprotein | 1.0E-0.0 | SMAD/FHA domain | none |
| 79 | retrotransposon hot spot (RHS) protein | EAN99306.1 | 2 | retrotransposon hot spotprotein | 1.0E-0.0 | SMAD/FHA domain | none |
| 80 | retrotransposon hot spot (RHS) protein | EAN94997.1 | 1 | retrotransposon hot spotprotein | 1.0E-0.0 | SMAD/FHA domain | none |
| 81 | hypothetical protein | EAN88126.1 | 2 | isoform c | 1.0E-0.0 | CCR4-Not complex component, Not1 | muscle development, dendrite morphogenesis, sugar binding |
| 82 | hypothetical protein | EAN90373.1 | 2 | Mucoid inhibitor A | 1.0E-0.0 | Conserved hypothetical protein CHP02231 | none |
| 83 | hypothetical protein | EAN85046.1 | 1 | hypothetical multipass transmembrane protein | 1.0E-0.0 | none | none |
| 84 | peroxin 14 | EAN89658.1 | 1 | peroxin 14 | 1.0E-0.0 | Peroxisome membrane anchor protein Pex14p, N-terminal | peroxisome, membrane |
| 85 | hypothetical protein | EAN98546.1 | 1 | cg17097-isoform b | 1.0E-0.0 | none | none |
| 86 | hypothetical protein | EAN93909.1 | 1 | hypothetical multipass transmembrane protein | 1.0E-0.0 | none | none |
| Proteins without known function or domain | |||||||
| 87 | hypothetical protein | EAN90722.1 | 5 | hypothetical protein | 1.0E-0.0 | none | none |
| 88 | hypothetical protein | EAN94368.1 | 5 | hypothetical protein | 1.0E-0.0 | none | none |
| 89 | hypothetical protein | EAN88585.1 | 4 | hypothetical protein | 1.0E-0.0 | none | none |
| 90 | hypothetical protein | EAN91358.1 | 3 | hypothetical protein | 1.00E-49 | none | none |
| 91 | hypothetical protein | EAN91901.1 | 3 | hypothetical protein | 1.0E-0.0 | none | none |
| 92 | hypothetical protein | EAN94097.1 | 3 | hypothetical protein | 1.00E-105 | none | none |
| 93 | hypothetical protein | EAN94312.1 | 3 | hypothetical protein | 1.0E-0.0 | none | none |
| 94 | hypothetical protein | EAN83060.1 | 2 | hypothetical protein | 2.83E-114 | none | none |
| 95 | hypothetical protein | EAN85364.1 | 2 | hypothetical protein | 2.46E-121 | none | none |
| 96 | hypothetical protein | EAN93581.1 | 2 | hypothetical protein | 1.00E-159 | none | none |
| 97 | hypothetical protein | EAN95093.1 | 2 | hypothetical protein | 1.0E-0.0 | none | none |
| 98 | hypothetical protein | EAN96967.1 | 2 | hypothetical protein | 1.00E-124 | none | none |
| 99 | hypothetical protein | EAN99066.1 | 2 | hypothetical protein | 1.0E-0.0 | none | none |
| 100 | hypothetical protein | EAN99070.1 | 2 | hypothetical protein | 1.00E-178 | none | none |
| 101 | hypothetical protein | EAN86750.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 102 | hypothetical protein | EAN87778.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 103 | hypothetical protein | EAN88004.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 104 | hypothetical protein | EAN90456.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 105 | hypothetical protein | EAN93004.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 106 | hypothetical protein | EAN93058.1 | 1 | hypothetical protein | 1.00E-70 | none | none |
| 107 | hypothetical protein | EAN82852.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 108 | hypothetical protein | EAN94518.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 109 | hypothetical protein | EAN94661.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 110 | hypothetical protein | EAN94862.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 111 | hypothetical protein | EAN98665.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 112 | hypothetical protein | EAN99075.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 113 | hypothetical protein | EAN99719.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 114 | hypothetical protein | EAN99770.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 115 | hypothetical protein | EAN99804.1 | 1 | hypothetical protein | 1.00E-176 | none | none |
| 116 | hypothetical protein | EAN96262.1 | 1 | hypothetical protein | 1.60E-93 | none | none |
| 117 | hypothetical protein | EAN80998.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
| 118 | hypothetical protein | EAN86037.1 | 1 | hypothetical protein | 1.00E-67 | none | none |
| 119 | hypothetical protein | EAN91356.1 | 1 | hypothetical protein | 1.0E-0.0 | none | none |
3.3.1 Gene ontology analysis
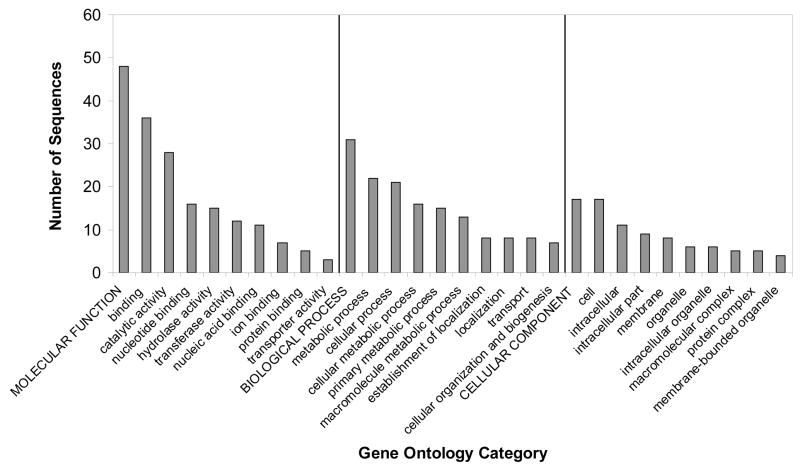
The overall functional distribution of the identified phosphoproteins, as determined by GO analysis (Supplementary Tables 4–5), is shown in Figure 2. The majority of proposed functions involved molecular interactions with proteins, nucleic acid, nucleotide, ions, and lipids. Enzymatic or catalytic activity and transport functions were also prevalent (Fig. 2, Supplementary Tables 4–5). The most abundant cellular components were intracellular, membrane bound, and localized in organelles (Fig. 2, Supplementary Tables 4–5). For the biological process category, a large proportion of the phosphoproteins were related to metabolism and other physiological processes. There were also proteins related to cell reproduction, development, differentiation and death, cell communication, response to stimulus, and locomotion (Fig. 2, Supplementary Tables 4–5). Overall, GO analysis further illustrates that protein phosphorylation is a significant regulatory mechanism governing a variety of T. cruzi biological functions.
Figure 2.
Gene ontology analysis. GO analysis was performed using Blast2GO algorithm. The representative categories for molecular function, cellular component, and biological process are shown in the graph. For the complete list, see Supplementary Tables 4 and 5.
3.3.2 Signaling transduction-related proteins
Signal transduction pathways are highly dynamic protein networks that integrate information from various stimuli. Sixteen of the identified proteins are proposed to be cell signaling modulators. Of these, six (37.5%) are classified as either protein kinases or phosphatases by Blast, InterPro, or GO analysis (Table 3), suggesting a number of signaling pathways are active. Indeed, calcium-binding proteins such as calmodulin and EF-hand domain-containing phosphoproteins were identified. Consistent with these observations, 13.9% of the phosphorylation motifs were characterized as CAMK2 consensus sites, indicating Ca2+ might play an important role in the regulation of many processes in T. cruzi physiology. Interestingly, a number of WD40 domain-containing phosphoproteins were identified. WD40 domain is an amino acid repeat with conserved tryptophan and aspartic acid residues [31]. This repeat has been shown to bind to phosphoserine/threonine and to act as a adaptor motif to anchor ubiquitin ligase, thus playing an important role in phosphorylation-dependent protein degradation pathways [32]. WD40 repeats are also present in several proteins from Chlamydomonas reinhardtii intraflagellar transport system [31], indicating that these proteins might also be involved in phosphorylation/ubiquitination-regulated cellular trafficking. Taken together, we have established a functional relationship between the proposed signaling molecules that provides unique insights into parasite biology with relevance to future drug development.
3.3.3 Cytoskeleton, flagellum, and trafficking proteins
Fifteen of the identified phosphoproteins were annotated as cytoskeleton, flagellum, and trafficking proteins (Table 3). In mammalian sperm, hyperactivated motility is mediated by Ca2+ [33]; however, it remains unclear whether the same phenomenon occurs in trypanosomatid flagellum. The presence of Ca2+ sensors in Trypanosoma sp. flagellum, such as calmodulin and flagellar calcium-binding protein (FCaBP), suggest that this phenomenon does occur in these protozoa [33–35]. We found that one paraflagellar rod protein (PAR1b) and one protein with orthologs in mammalian sperm are phosphorylated in a CAMK2 consensus motif, supporting the role of Ca2+-mediated protein phosphorylation in the regulation of motility in trypanosomes. In agreement with this data, tyrosine phosphorylation was recently shown to be enriched in T. brucei flagellum [36].
3.3.4 Transporters
We found 8 transporters in our analysis, 3 ABC, 1 acetate- and 4 ion-transporters. The ABC transporters are widely correlated with multidrug resistance from bacteria to humans [37]. The T. cruzi genome has 33 ABC transporter genes [38]. Two ABC-related transporters (tcpgp1 and tcpgp2) were characterized in T. cruzi, but their expression was not correlated with the efflux of nifurtimox or benznidazole [37]. Lara et al. proposed that the heme uptake by epimastigotes was dependent on ABC transporters [39]. More research is necessary to elucidate the ABC transporter functions in drug resistance, nutrient uptake, and metabolic secretion, and the role of phosphorylation in these processes.
3.3.5 DNA, RNA, and protein turnover
We have found two phosphoproteins with functions corresponding to nucleosome and chromatin assembly (i.e., histone H2B, nucleosome assembly protein). Marques Porto et al. showed by 32P-labeling the phosphorylation of T. cruzi histone H1 and H2B [40]. Although we have previously mapped the phosphorylation of histone H1 and now report herein the phosphorylation of histone H2B, the phospho-histone H1 was not detected in the current analysis. This could be because the phosphopeptide derived from histone H1 has an acetylated N-terminus and several miscleaved sites by trypsin digestion [20], thus they were not considered in current our analysis. Identification of heterogeneous nuclear ribonucleoprotein H/F, ATP-dependent RNA helicase, and pab1 binding protein (poly-A binding) suggests phosphorylation-mediated signal transduction pathways play a key role in T. cruzi RNA synthesis, processing, and degradation. Additionally, identification of protein synthesis modulators such as translation elongation factor and tRNA synthase as well as protein folding and processing modulators such as calpain indicates that phosphorylation may play a role in the regulation of these processes in T. cruzi.
3.3.6 Metabolism
Among the metabolic proteins, we identified enzymes involved in the late stages of the glycolytic pathway (i.e., pyruvate phosphate dikinase and pyruvate dehydrogenase E1 alpha subunit) (Table 3). Two phosphoproteins involved in nucleotide synthesis (i.e., ribose-phosphate pyrophosphokinase and phosphoribosylpyrophosphate synthetase) were also identified. These results suggest that these highly active metabolic pathways may be controlled at least in part by phosphorylation, thus feeding the machinery responsible for parasite cell growth and proliferation.
3.3.7 Pathogenesis
Although epimastigotes are noninfective forms, we found several proteins known to be related to pathogenesis of T. cruzi. For instance, five phosphoproteins belonging to two protein families (i.e., trans-sialidase (TS) and dispersed gene family (DGF)) were identified (Table 3). TS is a well known virulence factor [41] and comprises a protein superfamily (TS/gp85) encoded by about 1400 genes in T. cruzi genome [30]. Members of the TS/gp85 superfamily have the enzymatic activity to transfer sialic acid residues from the host to parasite glycoconjugates, which is related to protection against the host immune response [42]. Other members have been related to host cell recognition and invasion to protect the parasite from the host complement system [43]. DGF protein 1 is a multigene family that was recently shown to be expressed on the surface of bloodstream trypomastigotes [44]. DGF protein 1 was also reported to be N-glycosylated [45]. Here, we show that members of this family are also phosphorylated. The InterPro annotation suggests a pectin-lyase fold/virulence factor function, but its role in T. cruzi virulence yet remains to be elucidated (Table 3).
3.3.8 Proteins with other functions
A number of the phosphoproteins identified in our analysis seem to bind ions and other proteins; however, their exact functions are unknown. In addition, several of the identified phosphoproteins are from the retrotransposon hot spot (RHS) family. RHS is encoded by a large multigene family localized mainly in telomeric regions of trypanosomal chromosomes, and it is believed to be involved in gene duplication of multigene families, including virulence factors such as mucins and TS/gp85 glycoproteins [46, 47]. The finding that several members of RHS family are phosphorylated suggests that these gene products may be highly active, which accelerates gene duplication and evolution [46, 47]. This might explain the high divergence and differences in virulence between T. cruzi strains and phylogenetic lineages [47, 48].
3.4 Analysis of phosphotyrosine proteome
It has previously been estimated that 30% of all eukaryotic proteins contain covalently bound phosphate at any given time [49]. Furthermore, the proportion of pSer, pThr, and pTyr was recently reported in HeLa cells to be approximately 86.4%, 11.8%, and 1.8%, respectively [50]. The rarity of phosphorylation on tyrosine residues suggests there is a higher gain in signaling pathways because they are more tightly regulated. A genome-wise prediction of protein kinases of trypanosomatid parasites (T. cruzi, T. brucei, and Leishmania major) has shown that these protozoa lack typical tyrosine kinases; however, the presence of tyrosine phosphorylated proteins has been reported by a number of research groups [51, 52]. Das et al. showed that the major T. brucei tyrosine-phosphorylated protein is a nuclear RNA-binding protein (Nopp44/46) [51]. Recently, Nett et al. described the tyrosine phosphoproteome of T. brucei procyclic forms, where they mapped the tyrosine-phosphorylation sites of 34 proteins. Using anti-phosphotyrosine antibodies and immunofluorescence microscopy these authors showed that the tyrosine phosphorylated proteins were mostly localized at the basal body, flagellum, and nucleolus, indicating that tyrosine phosphorylation may play a central role in guiding signaling molecules to specific parasite locations. In T. cruzi, a 175-kDa protein is tyrosine-phosphorylated upon contact with the host cells, but the identity and the function of this protein during host cell invasion remains unknown [52].
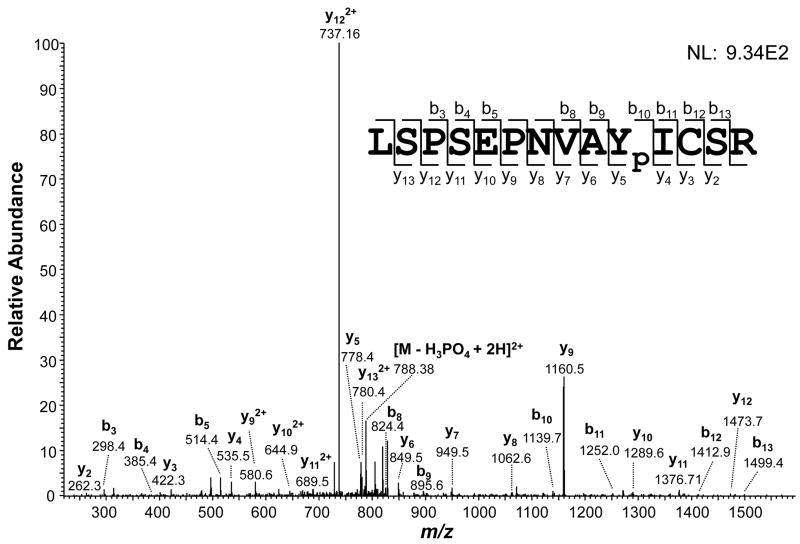
As described above, our MS/MS analysis identified 8 tyrosine phosphorylation sites from 6 distinct proteins: protein kinase (glycogen synthase kinase 3, GSK3); tyrosine aminotransferase; mitochondrial DNA polymerase I protein D; and three hypothetical proteins (EAN84292.1, EAN94368.1, and EAN95748.1) (Table 1 and Supplementary Table 1). The Figure 3A illustrates the identification of the tyrosine phosphorylated peptide LSPSEPNVAYpICSR from protein kinase GSK3. Initially, we observed an intense fragment corresponding to the neutral loss of phosphoric acid at m/z 788.38 ([M − H3PO4 + 2H]2+). After detailed analysis of remainder MS/MS fragments, we unambiguously mapped the phosphorylation to the Tyr10. Phosphorylation of Ser2, Ser4, or Ser13 was discarded based on the identification of fragment series b3–5 and b8–9 (for Ser2 and Ser4) and y2–4 (for Ser13). Furthermore, the presence of fragment series y5–10 corroborates the phosphorylation on Tyr10. Contrary to what has been reported for triple-quadrupole [53] and Q-TOF [54] mass spectrometers, intense neutral loss of the phosphate group from tyrosine phosphopeptides was previously observed by positive-ion mode MS/MS fragmentation in ion-trap instrument [25].
Figure 3.
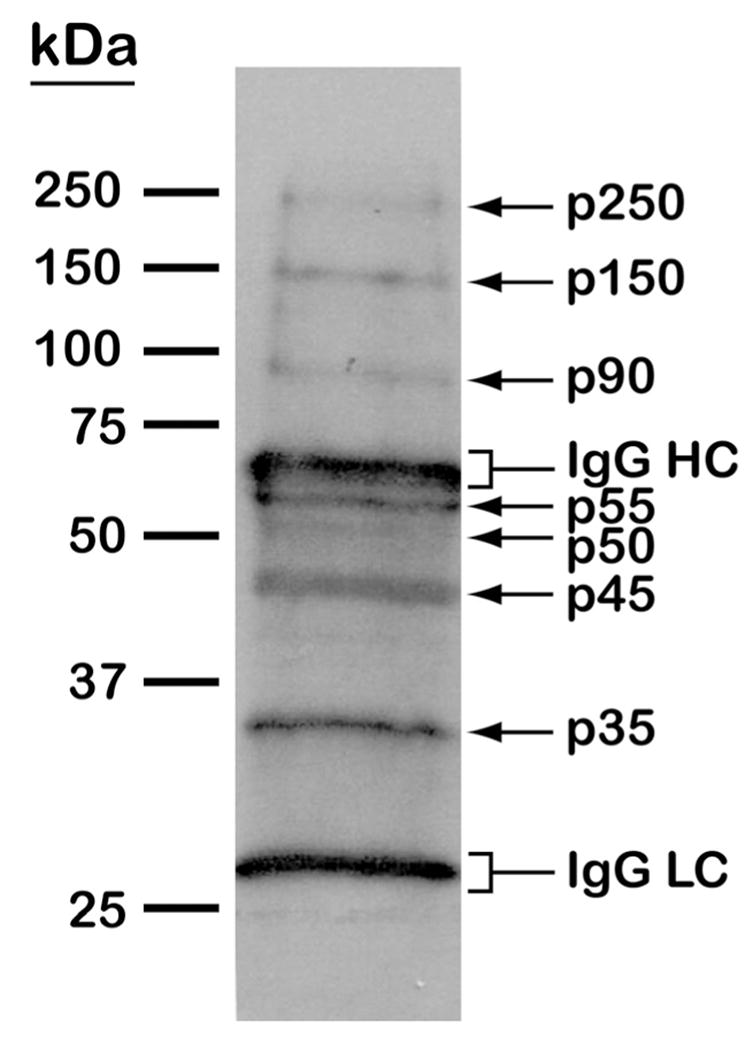
(A) MS/MS spectrum of the tyrosine-phosphorylated peptide LSPSEPNVAYpICSR from glycogen synthase kinase 3 (GSK3). Matched b and y fragments are indicated. (B) Western blotting analysis of tyrosine phosphorylated proteins in T. cruzi. Epimastigote extracts were subjected to anti-phosphotyrosine immunoprecipitation and separation by 10% SDS-PAGE. After blocking with BSA, the membrane was probed with the anti-phosphotyrosine antibody, followed by detection with horseradish peroxidase conjugated anti-mouse IgG and chemiluminescent reagent. Arrows denote T. cruzi tyrosine phosphorylated proteins. Brackets denote immunoglobulin G heavy (IgG HC) and light chains (IgG LC).
To further investigate tyrosine phosphorylation in T. cruzi, we performed an immunoprecipitation experiment using a monoclonal anti-phosphotyrosine antibody (see Material and Methods for details). Indeed, Western blotting analysis revealed 7 distinct tyrosine-phosphorylated proteins; p250, p150, p90, p55, p50, p45, and p35, which were assigned names according to their relative molecular masses (Fig. 3B). Interestingly, except for the p250, the predicted molecular masses of the tyrosine phosphorylated proteins identified by MS/MS seemed to closely correspond to those of the phosphoproteins detected by Western blotting: protein kinase (GSK3) (40.4 kDa), tyrosine aminotransferase (46.1 kDa), mitochondrial DNA polymerase I protein D (26.1 kDa), EAN84292.1 (103 kDa), EAN94368.1 (61.3 kDa), and EAN95748.1 (60 kDa). However, since other PTMs could alter the relative mobility of proteins on SDS-PAGE, the identity of the phosphorylated proteins observed on Western blot could not be inferred. Also, the low abundance of these proteins on the SDS-PAGE (as assessed by Coomassie blue staining) precluded their identification by LC-MS/MS.
The identification of GSK3 phosphorylation at Y187 suggests that this signaling pathway is important in T. cruzi biology. This enzyme is conserved throughout evolution; however, the parasite sequences are slightly truncated (353–355 residues) compared to the human, mouse, and Arabidopsis thaliana GSK orthologs (410–420 residues) (Supplementary Fig. 4). The amino acid sequence alignment also shows that T. cruzi GSK3 Y187 (arrow) is conserved in each of the included species (Supplementary Fig. 4). The corresponding tyrosine residue in human GSK3 (Y216) is in the “activation loop” of this kinase and its phosphorylation is critical for GSK3 catalytic activity [55, 56]. The significant proportion of GSK3 consensus phosphorylation sites (Table 2) further supports the presence of activated GSK3 in T. cruzi. Taken together, our results support the role of GSK3 in T. cruzi physiology and a selective inhibitor to this enzyme could potentially be used as an anti-parasitic agent. Indeed, GSK3 has been targeted for the development of selective drugs against Plasmodium ssp. and trypanosomatids [57, 58].
4 Concluding remarks
In this study, we have successfully mapped 237 phosphopeptides from 119 distinct proteins of epimastigote forms of T. cruzi. The results indicate that propagation of cell-signaling cascades by protein kinases and phosphatases play an important role in T. cruzi physiological processes, including cell motility, metabolism, ion transport, differentiation, and survival. We are currently analyzing the phosphoproteomes of other developmental stages of T. cruzi to expand the fundamental knowledge of the mechanisms regulating this medically relevant protozoan parasite. In addition to contributing to the understanding of the molecular aspects of T. cruzi biology, the information presented here will aid in the development of potential kinase-directed therapeutic strategies to treat Chagas disease.
Supplementary Material
Acknowledgments
This work was funded by grants from the National Institutes of Health (2S06GM008012-37 and 5G12RR008124). E.S.N. was partially supported by the George A. Krutilek memorial graduate scholarship from the Graduate School, UTEP. M.R.G. was supported by the Research Experience for Undergraduates (REU) Program/UTEP (National Science Foundation grant # DBI-0353887) and the University of Texas System Louis Stokes Alliance for Minority Participation (LSAMP) (NSF grant # HRD-0832951). T.J.P.S was supported by CAPES (Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior, Brazil). We thank the Biomolecule Analysis Core Analysis at the Border Biomedical Research Center/Biology/UTEP (NIH grant # 5G12RR008124), for the access to the LC-MS instruments.
Abbreviations
- BSA
bovine serum albumin
- CID
collision-induced dissociation
- ECL
enhanced chemiluminescent reagent
- EDTA
ethylenediaminetetraacetic acid
- FA
formic acid
- FCaBP
flagellar calcium-binding protein
- GO
gene ontology
- IMAC
immobilized metal-affinity chromatography
- LC-MS
liquid chromatography-mass spectrometry
- MS/MS
tandem mass spectrometry
- MS/MS/MS
dual-stage fragmentation
- MSA
multistage activation
- PAR
paraflagellar rod protein
- PBS
phosphate-buffered saline solution
- PVDF
polyvinylidene fluoride
- PTM
post-translational modification
- RP
reverse phase
- SCX
strong-cation exchange
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TFA
trifluoroacetic acid
- Th
Thompson
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
References
- 1.Barrett MP, Burchmore RJ, Stich A, Lazzari JO, et al. The trypanosomiases. Lancet. 2003;362:1469–1480. doi: 10.1016/S0140-6736(03)14694-6. [DOI] [PubMed] [Google Scholar]
- 2.Dias JC, Silveira AC, Schofield CJ. The impact of Chagas disease control in Latin America: a review. Mem Inst Oswaldo Cruz. 2002;97:603–612. doi: 10.1590/s0074-02762002000500002. [DOI] [PubMed] [Google Scholar]
- 3.Bern C, Montgomery SP, Herwaldt BL, Rassi A, Jr, et al. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA. 2007;298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- 4.Piron M, Verges M, Munoz J, Casamitjana N, et al. Seroprevalence of Trypanosoma cruzi infection in at-risk blood donors in Catalonia (Spain) Transfusion. 2008;48:1862–1868. doi: 10.1111/j.1537-2995.2008.01789.x. [DOI] [PubMed] [Google Scholar]
- 5.Tyler KM, Engman DM. The life cycle of Trypanosoma cruzi revisited. Int J Parasitol. 2001;31:472–481. doi: 10.1016/s0020-7519(01)00153-9. [DOI] [PubMed] [Google Scholar]
- 6.Urbina JA, Docampo R. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 2003;19:495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc Natl Acad Sci U S A. 2008;105:5022–5027. doi: 10.1073/pnas.0711014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumonteil E. DNA Vaccines against Protozoan Parasites: Advances and Challenges. J Biomed Biotechnol. 2007;2007:90520. doi: 10.1155/2007/90520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg N, Bhatia V. Current status and future prospects for a vaccine against American trypanosomiasis. Expert Rev Vaccines. 2005;4:867–880. doi: 10.1586/14760584.4.6.867. [DOI] [PubMed] [Google Scholar]
- 10.Arslan MA, Kutuk O, Basaga H. Protein kinases as drug targets in cancer. Curr Cancer Drug Targets. 2006;6:623–634. doi: 10.2174/156800906778742479. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 12.Force T, Kuida K, Namchuk M, Parang K, Kyriakis JM. Inhibitors of protein kinase signaling pathways: emerging therapies for cardiovascular disease. Circulation. 2004;109:1196–1205. doi: 10.1161/01.CIR.0000118538.21306.A9. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 14.Doerig C. Protein kinases as targets for anti-parasitic chemotherapy. Biochim Biophys Acta. 2004;1697:155–168. doi: 10.1016/j.bbapap.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 15.Parsons M, Worthey EA, Ward PN, Mottram JC. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics. 2005;6:127. doi: 10.1186/1471-2164-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naula C, Parsons M, Mottram JC. Protein kinases as drug targets in trypanosomes and Leishmania. Biochim Biophys Acta. 2005;1754:151–159. doi: 10.1016/j.bbapap.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brenchley R, Tariq H, McElhinney H, Szoor B, et al. The TriTryp phosphatome: analysis of the protein phosphatase catalytic domains. BMC Genomics. 2007;8:434. doi: 10.1186/1471-2164-8-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morales MA, Watanabe R, Laurent C, Lenormand P, et al. Phosphoproteomic analysis of Leishmania donovani pro- and amastigote stages. Proteomics. 2008;8:350–363. doi: 10.1002/pmic.200700697. [DOI] [PubMed] [Google Scholar]
- 19.Rosenzweig D, Smith D, Myler PJ, Olafson RW, Zilberstein D. Post-translational modification of cellular proteins during Leishmania donovani differentiation. Proteomics. 2008;8:1843–1850. doi: 10.1002/pmic.200701043. [DOI] [PubMed] [Google Scholar]
- 20.da Cunha JP, Nakayasu ES, Elias MC, Pimenta DC, et al. Trypanosoma cruzi histone H1 is phosphorylated in a typical cyclin dependent kinase site accordingly to the cell cycle. Mol Biochem Parasitol. 2005;140:75–86. doi: 10.1016/j.molbiopara.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Camargo EP. Growth and differentiation In Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev Inst Med Trop Sao Paulo. 1964;12:93–100. [PubMed] [Google Scholar]
- 22.Jurado JD, Rael ED, Lieb CS, Nakayasu E, et al. Complement inactivating proteins and intraspecies venom variation in Crotalus oreganus helleri. Toxicon. 2007;49:339–350. doi: 10.1016/j.toxicon.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Gnad F, Ren S, Cox J, Olsen JV, et al. PHOSIDA (phosphorylation site database): management, structural and evolutionary investigation, and prediction of phosphosites. Genome Biol. 2007;8:R250. doi: 10.1186/gb-2007-8-11-r250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotz S, Garcia-Gomez JM, Terol J, Williams TD, et al. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008;36:3420–3435. doi: 10.1093/nar/gkn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeGnore JP, Qin J. Fragmentation of phosphopeptides in an ion trap mass spectrometer. J Am Soc Mass Spectrom. 1998;9:1175–1188. doi: 10.1016/S1044-0305(98)00088-9. [DOI] [PubMed] [Google Scholar]
- 26.Beausoleil SA, Jedrychowski M, Schwartz D, Elias JE, et al. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc Natl Acad Sci U S A. 2004;101:12130–12135. doi: 10.1073/pnas.0404720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder MJ, Shabanowitz J, Schwartz JC, Hunt DF, Coon JJ. A neutral loss activation method for improved phosphopeptide sequence analysis by quadrupole ion trap mass spectrometry. Anal Chem. 2004;76:3590–3598. doi: 10.1021/ac0497104. [DOI] [PubMed] [Google Scholar]
- 28.Ulintz PJ, Yocum AK, Bodenmiller B, Aebersold R, et al. Comparison of MS(2)-Only, MSA, and MS(2)/MS(3) Methodologies for Phosphopeptide Identification. J Proteome Res. 2009;8:887–899. doi: 10.1021/pr800535h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villen J, Beausoleil SA, Gygi SP. Evaluation of the utility of neutral-loss-dependent MS3 strategies in large-scale phosphorylation analysis. Proteomics. 2008;8:4444–4452. doi: 10.1002/pmic.200800283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El-Sayed NM, Myler PJ, Bartholomeu DC, Nilsson D, et al. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science. 2005;309:409–415. doi: 10.1126/science.1112631. [DOI] [PubMed] [Google Scholar]
- 31.Cole DG. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic. 2003;4:435–442. doi: 10.1034/j.1600-0854.2003.t01-1-00103.x. [DOI] [PubMed] [Google Scholar]
- 32.Yaffe MB, Elia AE. Phosphoserine/threonine-binding domains. Curr Opin Cell Biol. 2001;13:131–138. doi: 10.1016/s0955-0674(00)00189-7. [DOI] [PubMed] [Google Scholar]
- 33.Hill KL. Biology and mechanism of trypanosome cell motility. Eukaryot Cell. 2003;2:200–208. doi: 10.1128/EC.2.2.200-208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broadhead R, Dawe HR, Farr H, Griffiths S, et al. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440:224–227. doi: 10.1038/nature04541. [DOI] [PubMed] [Google Scholar]
- 35.Maldonado RA, Mirzoeva S, Godsel LM, Lukas TJ, et al. Identification of calcium binding sites in the trypanosome flagellar calcium-acyl switch protein. Mol Biochem Parasitol. 1999;101:61–70. doi: 10.1016/s0166-6851(99)00055-9. [DOI] [PubMed] [Google Scholar]
- 36.Nett IR, Davidson L, Lamont D, Ferguson MA. Trypanosoma brucei: Identification and specific localization of tyrosine phosphorylated proteins. Eukaryot Cell. 2009 doi: 10.1128/EC.00366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones PM, George AM. Multidrug resistance in parasites: ABC transporters, P-glycoproteins and molecular modelling. Int J Parasitol. 2005;35:555–566. doi: 10.1016/j.ijpara.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Aguero F, Zheng W, Weatherly DB, Mendes P, Kissinger JC. TcruziDB: an integrated, post-genomics community resource for Trypanosoma cruzi. Nucleic Acids Res. 2006;34:D428–431. doi: 10.1093/nar/gkj108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lara FA, Sant’anna C, Lemos D, Laranja GA, et al. Heme requirement and intracellular trafficking in Trypanosoma cruzi epimastigotes. Biochem Biophys Res Commun. 2007;355:16–22. doi: 10.1016/j.bbrc.2006.12.238. [DOI] [PubMed] [Google Scholar]
- 40.Marques Porto R, Amino R, Elias MC, Faria M, Schenkman S. Histone H1 is phosphorylated in non-replicating and infective forms of Trypanosoma cruzi. Mol Biochem Parasitol. 2002;119:265–271. doi: 10.1016/s0166-6851(01)00430-3. [DOI] [PubMed] [Google Scholar]
- 41.Risso MG, Garbarino GB, Mocetti E, Campetella O, et al. Differential expression of a virulence factor, the trans-sialidase, by the main Trypanosoma cruzi phylogenetic lineages. J Infect Dis. 2004;189:2250–2259. doi: 10.1086/420831. [DOI] [PubMed] [Google Scholar]
- 42.Pereira-Chioccola VL, Acosta-Serrano A, Correia de Almeida I, Ferguson MA, et al. Mucin-like molecules form a negatively charged coat that protects Trypanosoma cruzi trypomastigotes from killing by human anti-alpha-galactosyl antibodies. J Cell Sci. 2000;113(Pt 7):1299–1307. doi: 10.1242/jcs.113.7.1299. [DOI] [PubMed] [Google Scholar]
- 43.Acosta-Serrano A, Hutchinson C, Nakayasu ES, Almeida IC, Carrington M. In: Trypanosomes: After the genome. Barry JD, Mottram JC, McCulloch R, Acosta-Serrano A, editors. Horizon Scientific Press; Norwich, UK: 2007. pp. 319–337. [Google Scholar]
- 44.Kawashita SY, da Silva CV, Mortara RA, Burleigh BA, Briones MRS. Homology, paralogy and function of DGF-1, a hihgly dispersed Trypanosoma cruzi specific gene family and its implications for information entropy if its encoded proteins. Mol Biochem Parasitol. 2009 doi: 10.1016/j.molbiopara.2008.12.010. Accepted. [DOI] [PubMed] [Google Scholar]
- 45.Atwood JA, 3rd, Minning T, Ludolf F, Nuccio A, et al. Glycoproteomics of Trypanosoma cruzi trypomastigotes using subcellular fractionation, lectin affinity, and stable isotope labeling. J Proteome Res. 2006;5:3376–3384. doi: 10.1021/pr060364b. [DOI] [PubMed] [Google Scholar]
- 46.Bringaud F, Biteau N, Melville SE, Hez S, et al. A new, expressed multigene family containing a hot spot for insertion of retroelements is associated with polymorphic subtelomeric regions of Trypanosoma brucei. Eukaryot Cell. 2002;1:137–151. doi: 10.1128/EC.1.1.137-151.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim D, Chiurillo MA, El-Sayed N, Jones K, et al. Telomere and subtelomere of Trypanosoma cruzi chromosomes are enriched in (pseudo)genes of retrotransposon hot spot and trans-sialidase-like gene families: the origins of T. cruzi telomeres. Gene. 2005;346:153–161. doi: 10.1016/j.gene.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Buscaglia CA, Di Noia JM. Trypanosoma cruzi clonal diversity and the epidemiology of Chagas’ disease. Microbes Infect. 2003;5:419–427. doi: 10.1016/s1286-4579(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 49.Venter JC, Adams MD, Myers EW, Li PW, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 50.Olsen JV, Blagoev B, Gnad F, Macek B, et al. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 51.Das A, Peterson GC, Kanner SB, Frevert U, Parsons M. A major tyrosine-phosphorylated protein of Trypanosoma brucei is a nucleolar RNA-binding protein. J Biol Chem. 1996;271:15675–15681. doi: 10.1074/jbc.271.26.15675. [DOI] [PubMed] [Google Scholar]
- 52.Favoreto S, Jr, Dorta ML, Yoshida N. Trypanosoma cruzi 175-kDa protein tyrosine phosphorylation is associated with host cell invasion. Exp Parasitol. 1998;89:188–194. doi: 10.1006/expr.1998.4285. [DOI] [PubMed] [Google Scholar]
- 53.Tholey A, Reed J, Lehmann WD. Electrospray tandem mass spectrometric studies of phosphopeptides and phosphopeptide analogues. J Mass Spectrom. 1999;34:117–123. doi: 10.1002/(SICI)1096-9888(199902)34:2<117::AID-JMS769>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 54.Steen H, Kuster B, Fernandez M, Pandey A, Mann M. Detection of tyrosine phosphorylated peptides by precursor ion scanning quadrupole TOF mass spectrometry in positive ion mode. Anal Chem. 2001;73:1440–1448. doi: 10.1021/ac001318c. [DOI] [PubMed] [Google Scholar]
- 55.Hughes K, Nikolakaki E, Plyte SE, Totty NF, Woodgett JR. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. Embo J. 1993;12:803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang QM, Fiol CJ, DePaoli-Roach AA, Roach PJ. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. J Biol Chem. 1994;269:14566–14574. [PubMed] [Google Scholar]
- 57.Droucheau E, Primot A, Thomas V, Mattei D, et al. Plasmodium falciparum glycogen synthase kinase-3: molecular model, expression, intracellular localisation and selective inhibitors. Biochim Biophys Acta. 2004;1697:181–196. doi: 10.1016/j.bbapap.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 58.Knockaert M, Wieking K, Schmitt S, Leost M, et al. Intracellular Targets of Paullones. Identification following affinity purification on immobilized inhibitor. J Biol Chem. 2002;277:25493–25501. doi: 10.1074/jbc.M202651200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.