Abstract
The Fischer 344 (F344) and Lewis (LEW) inbred rat strains react differently to morphine in a number of behavioral and physiological preparations, including the acquisition of aversions induced by this compound. The present experiment tested the ability of various compounds with relative selectivity at kappa, delta and mu receptor subtypes to assess the relative roles of these subtypes in mediating the differential aversive effects of morphine in the two strains. In the assessment of the role of the kappa receptor in morphine-induced aversions, animals in both strains were given access to saccharin followed by varying doses of the kappa agonist (−)−U50,488H (0.0, 0.28, 0.90 and 1.60 mg/kg). Although (−)−U50,488H induced aversions in both strains, no strain differences emerged. A separate subset of subjects was trained with the selective delta opioid agonist, SNC80 (0.0, 5.6, 10.0 and 18.0 mg/kg), and again although SNC80 induced aversions, there were no strain differences. Finally, a third subset of subjects was trained with heroin (0.0, 3.2, 5.6 and 10.0 mg/kg), a compound with activity at all three opiate receptor subtypes. Although heroin induced aversions in both strains, the aversions were significantly greater in the F344 strain, suggesting that differential activation of the mu opioid receptor likely mediates the reported strain differences in morphine-induced aversion learning. These data were discussed in terms of strain differences in opioid system functioning and the implications of such differences for other morphine-induced behavioral effects reported in F344 and LEW rats.
Keywords: F344, LEW, morphine, CTA, heroin, SNC80, (−)−U50, 488H, opioid
Introduction
Inbred rats and mice provide a useful tool in the study of the genetic mediation of many psychological disorders, including drug addiction (Crabbe, 2002; Cunningham et al., 2009). The F344 and LEW inbred rat strains reportedly differ on a variety of measures assessing the effects of drugs of abuse (Ambrosio et al., 1995; Davis et al., 2007; Kosten et al., 1994; 2007; Kruzich and Xi, 2006; Martin et al., 1999; 2003; Sanchez-Cardoso et al., 2007), suggesting that these strains could provide information regarding the underlying genetic differences in response to such drugs. For example, the F344 and LEW rats display differential patterns of drug self-administration for a variety of compounds (see Ambrosio et al., 1995; Kosten et al., 2007; Kruzich and Xi, 2006; Martin et al., 1999; 2003; Sanchez-Cardoso et al., 2007; for a review see Kosten and Ambrosio, 2002; Riley et al., 2009). Interestingly, these same strains also display differences in taste aversion learning induced by these self-administered compounds (Glowa et al., 1994; Gomez-Serrano et al., 2009; Grigson and Freet, 2000; Lancellotti et al., 2001; Pescatore et al., 2005; Roma et al., 2006; 2007; but see Davis and Riley, 2007; Kosten et al., 1994). Given that conditioned taste aversions (CTA) are thought to assess the aversive effects associated with drug administration and that these aversive drug effects may play a protective role in the development of substance abuse (Broadbent et al., 2002; Green and Grahame, 2008; Riley et al., 2009), understanding strain differences in aversion learning induced by self-administered compounds could provide valuable insights into the possible role genetic factors may play in the vulnerability to abuse drugs (Broadbent et al., 2002; Crabbe, 2002; Riley et al., 2009).
The drug to which the F344 and LEW rats display the most dramatic difference in taste aversion learning is morphine. Specifically, Lancellotti and her colleagues (2001) reported that when rats of both strains were given saccharin-morphine pairings during aversion conditioning, only the F344 rats acquired dose-dependent CTAs to the morphine-associated solution. No dose of morphine produced aversions in the LEW strain, even after repeated conditioning trials. Such differences between the two strains are not simply a function of differential learning ability in general (Herradon et al., 2008; Kearns et al., 2006; Martin et al., 2003) or aversion learning specifically (Davis and Riley, 2007; Foynes and Riley, 2004) and may instead reflect differences in the sensitivity to morphine's aversive effects. These differences in sensitivity may be important in understanding the differential self administration of morphine by the two strains in which the LEW strain displays a more rapid acquisition of morphine self administration (Ambrosio et al., 1995; Martin et al., 1999; 2003; Sanchez-Cardoso et al., 2007), i.e., the absence of aversive effects in the LEW strain may increase the overall acceptability of this drug, leading to increased drug intake.
Although the F344 and LEW strains differ significantly in the acquisition of morphine-induced aversions, the neurochemical basis for this difference remains unknown. Morphine binds to and is effective at all three opiate receptor subtypes (kappa, delta and mu; Fowler and Fraser, 1994; Self and Stein, 1992; see also Inturrissi et al., 1983; Oldendorf et al., 1972), and it is not clear which of the three mediates the differential aversive effects of morphine evident in the two strains. To address the role of various opiate receptor subtypes in aversions induced by morphine, the present series of experiments examined the ability of compounds with varying degrees of selectivity for kappa (U50,488H), delta (SNC80) and mu (heroin) receptors to induce aversions under conditions that support morphine-induced aversion learning.
Methods
Subjects and Apparatus
A total of 98 male F344 and 98 male LEW rats, weighing approximately 252 and 300 g, respectively, at the start of the experiment, served as subjects. Animals were housed in individual wire-mesh cages and maintained on a 12:12 h light/dark cycle and at an ambient temperature of 23°C for the duration of the experiment. Rat chow (Harlan Sprague-Dawley, Indianapolis, Indiana) was provided ad libitum. All fluids were presented in 50-ml Nalgene tubes affixed to the front of the cages. All procedures were in compliance with the US National Institutes of Health and National Research Council Guidelines (2003; 2006) and approved by the Institutional Animal Care and Use Committee at American University.
Drugs and Solutions
(−)−U50,488H hydrochloride (generously supplied by National Institute of Diabetes and Digestive and Kidney Diseases) was dissolved in saline (1 mg/ml). Given that the active isomer was used, doses common for the racemic mixture (and previously used in aversion learning, see Mucha and Herz, 1985) were halved. SNC80, prepared as a 2 mg/ml solution (generously supplied by National Institute of Diabetes and Digestive and Kidney Diseases), was dissolved in distilled water with a small amount (∼100μl) of HCl added to promote solubility. Heroin (generously supplied by the National Institute on Drug Abuse) was prepared as a 1 mg/ml solution in saline. All drug doses were expressed as the salt form and were administered subcutaneously (sc). Sodium saccharin (Sigma) was prepared as a 1g/l solution in tap water.
Procedure
Phase I: Habituation
After 232/3-h water deprivation, rats received 20-min access to water, beginning at 1000 h. This procedure was repeated daily until all rats were approaching and drinking from the tube within 2 s of its presentation. Once this criterion was reached, aversion conditioning began.
Phase II: CTA conditioning
Subjects were run in three different cohorts based on the specific compound on which they were trained, i.e., U50,488H, SNC80 or heroin. On Day 1 of this phase, animals received 20-min access to a novel saccharin solution during their daily fluid-access period. Immediately following saccharin access, rats within each strain and in each cohort were assigned to one of four groups such that consumption was comparable across groups.
1) U50,488H. Rats in this cohort were injected with vehicle (F344: n = 8; LEW: n = 8), 0.28 mg/kg (F344: n = 8; LEW: n = 8), 0.90 mg/kg (F344: n = 8; LEW: n = 8) or 1.60 mg/kg (F344: n = 8; LEW: n = 8) of (−)−U50,488H.
2) SNC80. Rats in this cohort were injected with vehicle (F344: n = 8; LEW: n = 8), 5.6 mg/kg (F344: n = 9; LEW: n = 8), 10.0 mg/kg (F344: n = 9; LEW: n = 8) or 18.0 mg/kg (F344: n = 7; LEW: n = 8) of SNC80.
3) Heroin. Rats in this cohort were injected with vehicle (F344: n = 8; LEW: n = 8), 3.2 mg/kg (F344: n = 8; LEW: n = 8), 5.6 mg/kg (F344: n = 9; LEW: n = 9) and 10.0 mg/kg (F344: n = 8; LEW: n = 9) of heroin.
On Days 2-4 of this phase, all animals received 20-min access to water during the fluid-access period. One conditioning day and three water-recovery days constituted a cycle, and five conditioning cycles were run. The fifth conditioning trial was treated as a one-bottle test of the aversion to saccharin.
Phase III: Two-bottle aversion test
On the day following the last water-recovery day of the fifth conditioning cycle, all animals were given both saccharin and water in a two-bottle test of the aversion to saccharin. Specifically, animals received two 50-ml Nalgene tubes, one tube containing saccharin and one containing water, during the 20-min fluid-access period. Total fluid consumed from both bottles was recorded.
Statistical Analysis
Individual analysis was performed for each compound. Water consumption during the habituation phase was analyzed using a two-factor (Day × Strain) ANOVA, with the repeated factor of Day, to determine if there were any strain differences in water consumption during this period. Independent-sample t-tests were used to examine differences between the strains on any specific day. Saccharin consumption on Trials 1-4 and on the one-bottle aversion test was analyzed using a three-way (Trial × Strain × Dose) ANOVA, with Trial as the repeating factor. When appropriate, Tukey-corrected post-hocs were used to examine specific group differences. The two-bottle aversion test data was analyzed using a two-factor (Strain × Dose) ANOVA to determine if there were any strain differences in percent of saccharin consumed or overall fluid consumption. Tukey-corrected post-hocs were employed where warranted.
Results
Kappa Agonism: U50,488H
Phase I: Habituation
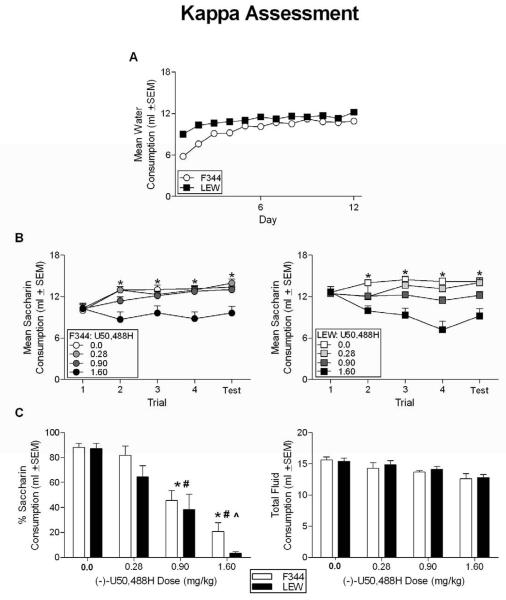
The 12 (Day) × 2 (Strain) repeated-measures ANOVA on water consumption revealed a significant effect of Strain [F(1, 62) = 53.662, p < 0.05] and Day [F(11, 682) = 68.056, p < 0.05] as well as a Day × Strain interaction [F(11, 682) = 9.072, p < 0.05]. Independent-sample t-tests revealed greater water consumption by the LEW animals on Days 1-6, 8, 10 and 12 (all p's < 0.05). On Days 7, 9 and 11, there were no strain differences in water consumption (all p's > 0.119) (see Figure 1, Panel A).
Figure 1.
(A) Mean water consumption during the habituation phase for all F344 and LEW animals prior to conditioning with various doses of (−)-U50,488H. LEW animals consumed more water than the F344 rats on all days during this phase, except for days 7, 9 and 11. (B) Mean saccharin consumption across conditioning trials for all F344 (left panel) and LEW (right panel) animals receiving different doses of (−)-U50,488H or vehicle. * indicates saccharin consumption of animals conditioned with vehicle, 0.28, and 0.90 mg/kg was significantly higher than that of animals conditioned with 1.60 mg/kg. (C) Percent saccharin consumption (left panel) and total fluid consumption (right panel) for all animals conditioned with varying doses of U50,488H or vehicle. * denotes significant difference from animals receiving vehicle. # denotes significantly less saccharin consumed compared to animals receiving 0.28 mg/kg. ^ denotes significantly less saccharin consumed compared to animals receiving 0.90 mg/kg
Phase II: CTA conditioning and one-bottle aversion test
The 5 (Trial) × 2 (Strain) × 2 (Dose) repeated-measures ANOVA on saccharin consumption revealed a significant effect of Dose [F(3,56) = 36.291, p < 0.05]; neither the effect of Strain nor the Strain × Dose interaction was significant (all p's ≥ 0.09). The Trial × Strain [F(4,224) = 8.103, p < 0.05] and Trial × Dose [F(12,224) = 7.351, p < 0.05] interactions were significant, and the main effect of Trial approached significance (p = 0.056). Tukey's-corrected post hoc tests revealed significant group differences. On the initial exposure to saccharin (Trial 1), LEW animals (regardless of dose) drank more saccharin on average than the F344 rats. On Trial 2, animals conditioned with the highest dose of (−)U50,488H decreased consumption relative to all other groups (all p's < 0.05). No other differences emerged on this trial. These same differences were apparent throughout the rest of the conditioning trials, i. e., only the highest dose of (−)U50,488H produced decreases in consumption compared to all other conditions (vehicle-injected subjects and those conditioned with 0.28 and 0.90 mg/kg; all p's < 0.05). Consumption by animals conditioned with 0.28 and 0.90 mg/kg never differed from that of vehicle animals. There were no differences between LEW and F344 subjects at any matched dose following the initial conditioning trial.
Phase III: Two-bottle aversion test
The 2 (Strain) × 4 (Dose) univariate ANOVA on the percent of saccharin consumption during the Two-Bottle Aversion Test revealed main effects of Strain [F(1, 63) = 4.325, p < .05] and Dose [F(3, 63) = 42.752, p < .05], but no significant Strain × Dose interaction (p = .605). Overall, the F344 animals (collapsed across Dose) displayed a higher percentage of saccharin consumption relative to the LEW animals (59% compared to 48%, respectively). The percent saccharin consumption by animals conditioned with the lowest dose (0.28 mg/kg) of (−)−U50,488H did not differ from those injected with vehicle, whereas there were significant decreases in percent of saccharin consumption (relative to vehicle-treated animals) in rats receiving 0.90 and 1.60 mg/kg (−)−U50,488H. Moreover, the percent saccharin consumption in subjects injected with 1.60 mg/kg was significantly lower than that of animals receiving 0.90 and 0.28 mg/kg (−)−U50,488H. Consumption at 0.90 mg/kg was significantly greater than that of the high dose group, but significantly lower than that of the lowest dose or vehicle. The 2 (Strain) × 4 (Dose) univariate ANOVA performed on the mean total fluid consumption during the two-bottle test revealed a significant effect of Dose [F(3, 63) = 8.106, p < .05], but not of Strain, and no significant Strain × Dose interaction (all p's > .599). Post-hocs revealed that total fluid consumption in animals injected with 0.90 and 1.60 mg/kg was significantly lower than those receiving vehicle. Moreover, consumption at the lowest dose (0.90 mg/kg) only differed from consumption at the highest dose (1.60 mg/kg) (See Figure 1, Panel C).
Delta Agonism: SNC80
Phase I: Habituation
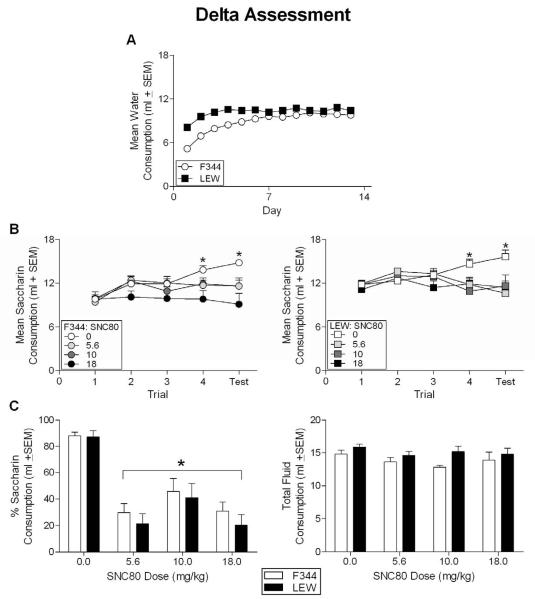
The 13 (Day) × 2 (Strain) repeated-measures ANOVA revealed a significant effect of Strain [F(1, 63) = 27.752, p < 0.05] and Day [F(12, 756) = 71.008, p < 0.05] as well as a significant Day × Strain interaction [F(12, 756) = 12.763, p < 0.05]. Independent-samples t-tests revealed that the LEW animals consumed more water on Days 1-6, 8-9 and 12 (all p's < 0.05). No strain differences were found on Days 7, 10-11 and 13 (all p's > 0.092) (see Figure 2, Panel A).
Figure 2.
(A) Mean water consumption during the habituation phase for all F344 and LEW animals prior to conditioning with various doses of SNC80. The LEW strain consumed more water on days 1-6, 8-9, and 12 compared to the F344 rats. Consumption was equivalent on all other days. (B) Mean saccharin consumption across conditioning trials for all F344 (left panel) and LEW (right panel) animals receiving varying doses of SNC80 or vehicle during aversion conditioning. * denotes saccharin consumption of animals conditioned with vehicle was significantly greater than consumption by all groups receiving SNC80. (C) Percent saccharin consumption (left panel) and total fluid consumption (right panel) for all animals conditioned with varying doses of SNC80 or vehicle. * denotes significant difference from vehicles (F344 and LEW).
Phase II: CTA conditioning and one-bottle aversion test
The 5 (Trial) × 2 (Strain) × 2 (Dose) repeated-measures ANOVA revealed a significant effect of Strain [F(1,57) = 10.82, p < 0.05], Dose [F(3,57) = 5.568. p < 0.05] and Trial [F(4,228) = 10.2, p < 0.05] as well as a significant Trial × Dose interaction [F(12,228) = 5.339, p < 0.05]. The Strain × Dose (p = 0.758) and Trial × Strain and Trial × Strain × Dose interactions were not significant (all p's > 0.347). Tukey-corrected post-hocs revealed significant group differences during acquisition. On Trials 1-3, no significant differences emerged (see Figure 2, Panel B). However, on Trial 4 and on the one-bottle aversion test, all doses of SNC80 (independent of strain) induced significant decreases in consumption relative to animals receiving vehicle. No differences emerged among the doses; all animals conditioned with SNC80 displayed similar decreases in saccharin consumption on Trial 4 and on the one-bottle aversion test (independent of dose and strain).
Phase III: Two-bottle aversion test
The 2 (Strain) × 4 (Dose) univariate ANOVA on the percent of saccharin consumption on the Two-Bottle Aversion Test revealed only a significant main effect of Dose [F(3, 64) = 28.035, p < .05]. The main effect of Strain and the Strain × Dose interaction were not significant. Post-hocs revealed that the percent of saccharin consumption on this test did not differ across the doses of SNC80, but that the percent saccharin consumption at each dose was significantly less than that of control animals. The 2 (Strain) × 4 (Dose) univariate ANOVA performed on total fluid consumption revealed a main effect of Strain [F(1, 64) = 8.063, p < .05], but no effect of Dose or a Strain × Dose interaction (all p's > .171). On average, the LEW animals consumed more fluid than the F344 rats. Average fluid consumption did not differ by dose (see Figure 2, Panel C).
Mu Agonism: Heroin
Phase I: Habituation
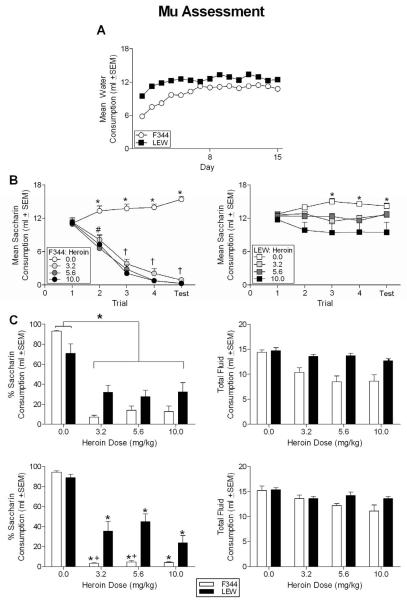
The 15 (Day) × 2 (Strain) repeated-measures ANOVA revealed a significant effect of Day [F(14, 910) = 76.906, p < 0.05] and Strain [F(1, 65) = 66.677, p < 0.05] as well as a significant Day × Strain interaction [F(14, 910) = 11.249, p < 0.05]. Independent-sample t-tests showed that LEW animals consumed more water on each day of the habituation phase (all p's < 0.05) except for Day 7, on which the strains did not differ (p = 0.148) (see Figure 3, Panel A).
Figure 3.
(A) Mean water consumption during the habituation phase for all F344 and LEW animals prior to aversion conditioning with heroin. LEW animals consumed more water on each day of this phase, except for Day 7 when the consumption by the strains was equivalent. Note: some SE bars fall within the symbol on graph. (B) Mean saccharin consumption across conditioning trials for all F344 (left panel) and LEW (right panel) animals receiving varying doses of heroin or vehicle during aversion conditioning. * denotes consumption by vehicle groups (F344 and LEW) significantly greater than all F344 animals receiving heroin. + indicates saccharin consumption by the vehicle groups (F344 and LEW) significantly greater than LEW animals conditioned with 10 mg/kg heroin. # indicates that the LEW animals conditioned with 3.2 and 5.6 mg/kg consumed significantly more saccharin than all F344 animals conditioned with heroin (3.2, 5.6, and 10 mg/kg). † indicates a significant strain difference in saccharin consumption with all LEW animals conditioned with heroin (3.2, 5.6, and 10 mg/kg) drinking more saccharin than all F344 animals conditioned with heroin. Note: some SE bars fall within the symbol on the graph. (C) Percent saccharin consumption (left panel) and total fluid consumption (right panel) for the first (top) and second (bottom) two-bottle aversion test for all animals conditioned with varying doses of heroin or vehicle. *denotes significant difference from vehicle groups. + denotes significant strain differences at a specific dose of heroin.
Phase II: CTA conditioning and one-bottle aversion test
The 5 (Trial) × 2 (Strain) × (Dose) repeated-measures ANOVA revealed significant effects of Strain [F(1,59) = 135.985, p < .05], Dose [F(3,59) = 40.351, p < 0.05] and Trial [F(4,236) = 57.601, p < 0.05] as well as a significant Strain × Dose [F(3,59) = 225.036, p < 0.05], Trial × Strain [F(4,236) = 48.964, p < 0.05], Trial × Dose [F(12,236) = 53.314, p < 0.05] and Trial × Strain × Dose [F(12,236) = 26.550, p < 0.05] interactions. On Trial 1, no differences were found in saccharin consumption between the different groups of F344 and LEW animals (see Figure 3, Panel B). On Trial 2, all groups of F344 rats receiving heroin (3.2, 5.6 and 10 mg/kg) displayed decreased saccharin consumption relative to F344 and LEW control groups (that displayed equivalent consumption throughout conditioning) and LEW animals conditioned with 3.2 and 5.6 mg/kg (all p's < 0.05). On this trial, LEW animals conditioned with 10 mg/kg heroin did not differ from any other group of animals (all p's > 0.05). On Trial 3, F344 animals conditioned with heroin did not differ from one another, but they did differ from both F344 and LEW rats receiving vehicle. All F344 rats receiving heroin also consumed significantly less saccharin than all LEW animals receiving heroin (all p's < 0.05). Only LEW animals receiving 10 mg/kg heroin differed from vehicle controls on this trial, but they did not differ from LEW animals receiving the other doses of heroin. These same animals (LEW 10 mg/kg) also drank significantly more saccharin than all F344 animals conditioned with heroin (all p's < 0.05). LEW animals receiving the two lower doses of heroin drank saccharin comparable to controls (all p's > 0.05). The differences that were evident on Trial 3 were also significant on Trial 4 and on the one-bottle aversion test. At no point during conditioning did LEW animals receiving 3.2 and 5.6 mg/kg heroin differ from controls of either strain.
Phase III: Two-bottle aversion test
The 2 (Strain) × 4 (Dose) univariate ANOVA performed on the percent of saccharin consumption revealed an effect of Dose [F(3, 66) = 43.383, p < .05] as well as a Strain × Dose interaction [F(3, 66) = 5.231, p < .05]. The main effect of Strain (p = .056) approached significance. Post-hocs revealed that percent saccharin consumption did not differ between the strains at any dose of heroin. All animals receiving heroin drank a lower percentage of saccharin on this test than control animals (all p's < .05), with the F344 and LEW control groups displaying a similar preference for saccharin. The 2 (Strain) × 4 (Dose) univariate ANOVA on total fluid consumption revealed effects of Strain [F(1, 66) =29.774, p , .05] and Dose [F(3, 66) = 8.608, p < .05] as well as a Strain × Dose interaction [F(3, 66) = 3.158, p < .05]. Post-hocs revealed that all F344 animals receiving heroin consumed less total fluid than controls, but did not differ in consumption across doses. The LEW animals across all conditioning doses, however, drank fluid comparable to that of controls. F344 and LEW animals receiving vehicle consumed similar amounts of fluid in the two-bottle test (see Figure 3, Panel C).
Given the decrease in overall fluid consumption in the F344 strain, animals were given a second two-bottle test following three water-recovery days. No injections were administered after the initial two-bottle test. The 2 (Strain) × 4 (Dose) univariate ANOVA was performed on the percent of saccharin consumed by all F344 and LEW animals during the second two-bottle test revealed significant main effects of Strain [F(1, 66) = 32.863, p < .05] and Dose [F(3, 66) = 88.938, p < .05] as well as a significant interaction of Strain × Dose [F(3, 66) = 6.907, p < .05]. Tukey-corrected post-hocs were used to examine specific group differences. Within the F344 strain, the percent of saccharin consumption did not differ between doses, but was significantly less than the F344 control group receiving vehicle during conditioning. A similar result was found with the LEW strain, i.e., the percent of saccharin consumption did not differ across the doses, but consumption at each dose of heroin was significantly lower than that of the LEW control group. The percent of saccharin consumption was similar for the F344 and LEW control groups. For animals injected with heroin, the F344 rats displayed a significantly lower percentage of saccharin consumption at the 3.2 and 5.6 mg/kg doses of heroin compared to LEW animals conditioned with these doses (all p's < .05). At the 10 mg/kg dose, the F344 and LEW animals did not differ in the percent of saccharin consumption.
Total fluid consumption on the second two-bottle test was analyzed using a 2 (Strain) × 4 (Dose) univariate ANOVA (see Figure 3C, right panel). Significant main effects of Strain [F(1, 66) = 6.429, p < .05] and Dose [F(3, 66) = 6.785, p < .05] were found; however, the Strain × Dose interaction was not significant. Post-hocs revealed that overall fluid consumption was significantly less than controls in animals conditioned with 5.6 and 10 mg/kg heroin. Moreover, the F344 strain displayed a significant decrease in overall fluid consumption relative to the LEW strain. Although there were significant differences in overall fluid consumption on the second two-bottle test, consumption by all animals receiving conditioning with heroin increased from the first two-bottle test. For example, F344 animals receiving 5.6 and 10 mg/kg heroin during conditioning consumed 8.5 and 8.6 ml on the first test and 12.2 and 11.1 ml on the second test, respectively.
Discussion
The present experiment examined the ability of various compounds with relative selectivity for the kappa (U50,488H), delta (SNC80) and mu (heroin) opiate receptor subtypes to induce taste aversions. The purpose of these assessments was to assess the possible role of these subtypes in the reported strain differences in aversions induced by morphine (see Lancellotti et al., 2001). As reported, although the kappa agonist (−)−U50,488H and the delta agonist SNC80 both induced aversions, there were no significant strain differences in the acquisition of these aversions. The only drug for which strain differences emerged was heroin. Given that heroin acts via all three receptor subtypes, including the MOR, these data implicate activation of the MOR (alone or in combination with the other subtypes; see below) in the strain differences seen with morphine-induced aversions.
The results of the study employing U50,488H demonstrate that equivalent taste aversions are induced by KOR agonism in the F344 and LEW rat strains. As noted, there was a significant Trial × Strain interaction during acquisition of the aversions induced by U50,488H. Specifically, the LEW animals (collapsed across dose) consumed more saccharin on Trials 1-3 and the F344 animals (collapsed across dose) consumed more saccharin on Trials 4 and on the one-bottle test. Given that this interaction was not affected by dose suggests that these differences were not a function of U50,488H, but simply a function of differences in consumption between the two strains. The significant Trial × Dose interaction supports the result that U50,488H had similar aversive effects in the F344 and LEW animals. As noted, only the highest dose of U50,488H caused a decrease in saccharin consumption during acquisition and on the one-bottle aversion test. Given that these effects were weak, it could be argued that higher doses would have had more robust effects and that these effects might have differed between the strains. However, clear dose-dependent decreases in saccharin consumption were evident in the two-bottle test demonstrating the behavioral activity of the doses employed. Given that the two-bottle aversion test is considered to be a more sensitive measure of aversion, it is unlikely that higher doses of U50,488H would have produced strain differences related to KOR agonism. Any differences emerging at higher doses would most likely be a consequence of non-specific binding at other opioid receptor sites. Taken together, the data from the first experiment suggest that there are no differences in the aversive effects of KOR activation between the two strains and that the strain differences reported in morphine aversion learning between the F344 and LEW rats are not a function of differential activation of the KOR only.
The analysis with the selective delta agonist, SNC80, did reveal a significant effect of Strain, i.e., a significant main-effect of Strain did emerge with the LEW animals (collapsed across Dose and Trial) consuming more saccharin than the F344 rats. However, since there was no interaction of Strain with any other factor (including Dose), these results suggest that the aversive effects of SNC80 did not differ between the two strains. The Trial × Dose interaction revealed differences between animals receiving vehicle and animals receiving SNC80 on Trial 4 and on the one-bottle test; the decreases in saccharin consumption were equivalent between the doses. It could again be suggested that the use of higher doses could have revealed strain differences in SNC80-induced aversions, given that the effects of the doses used in the present study were weak during acquisition and the one-bottle test. However, animals conditioned with SNC80 displayed at least a 50% decrease in saccharin consumption compared to vehicle-treated animals on the two-bottle aversion test. This effect demonstrates that the doses used in the present study had aversive effects that were evident in the more sensitive two-bottle test of the aversion. As with U50,488H, there do not appear to be any strain differences in the aversive effects of DOR agonism. Further, these data suggest that the strain differences previously reported for morphine in the F344 and LEW animals are unlikely due to differential activation of the DOR alone.
Given the lack of strain differences with compounds relatively selective for the KOR and DOR in aversion learning with the F344 and LEW rats, the final experiment examined the aversive effect of heroin in these strains. It was hypothesized that heroin would produce effects similar to that of morphine (more robust aversions in the F344 rats), given that this compound is a mu-preferring agonist with opioid activity similar to morphine. As described, stronger aversions were apparent in the F344 animals compared to LEW rats during acquisition and on the one-bottle and the two-bottle aversion tests. For example, the F344 rats acquired robust, dose-dependent, heroin-induced aversions during acquisition, whereas only the LEW animals conditioned with the highest dose of heroin displayed any decrease in saccharin consumption. These differences are very similar to those previously reported for morphine with the F344 animals displaying robust, dose-dependent aversions (see Lancellotti et al., 2001). In the two-bottle test, all animals conditioned with heroin displayed a significant decreased in the percentage of saccharin consumed compared to the vehicle-conditioned animals. However, on this test F344 rats conditioned with heroin had significantly less overall fluid than LEW subjects, making interpretation of the results of this two-bottle test difficult. A second two-bottle test was performed following three water recovery days (see Methods), and on this test total fluid consumption by all heroin conditioned animals increased. On this second two-bottle test, all animals receiving heroin decreased the percentage of saccharin consumed on this test as well. However, the F344 animals conditioned with 3.2 and 5.6 mg/kg drank a significantly lower percentage of saccharin than LEW animals receiving the same doses. F344 and LEW animals conditioned with 10 mg/kg heroin displayed similar decreases in percent saccharin consumption. The two-bottle test results parallel the one-bottle test results displaying stronger heroin-induced aversions in the F344 animals and support the position that the two strains differ in terms of the aversive effects of mu agonism. These differences in MOR activation could be an important mediator of the strain differences in morphine's aversive effects between these strains.
Taken together, these data demonstrating a lack of strain differences in aversion learning for the KOR and DOR agonists, with robust differences with the MOR-preferring agonist, suggest that the differences between the F344 and LEW rats in the aversive effects of morphine are most likely mediated by differential activity at the MOR. Interestingly, Liu and Grigson (2005) reported comparable aversions in F344 and LEW animals conditioned with DAMGO, a relatively specific MOR agonist. This work with DAMGO suggests that activation of the MOR alone does not differ between the F344 and LEW animals. Several possibilities exist that could explain the differences reported with morphine and heroin and the lack of strain differences reported with DAMGO. One possibility concerns the specific site of action of MOR-preferring agonists. For example, morphine's aversive effects are partially mediated by activation of peripheral opioid receptors (Bechara et al., 1987). If activation of peripheral opioid receptors is an important factor underlying morphine's aversive effects, it is possible that the F344 and LEW strains display differential activation of these receptors after morphine or heroin administration, given that both of these MOR-preferring compounds were administered systemically (Lancellotti et al., 2001 and the present study, respectively). Moreover, a difference in peripheral receptor activation would also account for the lack of strain differences in aversion learning associated with central activation of MORs induced by icv administered DAMGO (see Liu and Grigson, 2005), given that this route of administration would bypass any differences in peripheral MOR activation. In addition to the importance of peripheral opioid receptors in morphine's aversive effects, central opioid receptors have also been shown to be involved in these effects (Zito et al., 1988). It is possible that pharmacokinetic differences between the strains are responsible for these differences in aversion learning. For example, systemically administered drugs must cross the blood-brain barrier to have access to central MORs. If the speed at which systemically administered MOR-preferring compounds cross this barrier differs between the F344 and LEW rats, and central MOR activation is necessary for the expression of a MOR agonist-induced aversion, differences like those reported in morphine and heroin aversion learning, might be expected. Further, icv administration would again bypass this strain difference and would most likely lead to equivalent aversions between the strains. Although brain levels of systemically administered drugs were not measured in the present study, morphine brain levels reportedly are greater in the F344 rats 30 minutes post-injection (Gosnell and Krahn, 1993), even though strain differences in morphine blood levels have not been reported (Gosnell and Krahn, 1993; Davis et al., 2007).
A second possibility for the differences in the present report and those reported by Liu and Grigson (2005) is related to the relative selectivity of opiate activation. That is, heroin, unlike DAMGO, binds to all three opiate receptor subtypes, but demonstrates a higher preference for the MOR compared to the KOR and DOR, a binding profile similar to that of morphine. It is possible that the strain differences between the F344 and LEW rats in aversion learning with morphine and heroin are a consequence of a combination of opioid receptor binding, and this combination of binding/activation is what differs between the strains. If this is the case, isolating the MOR would be expected to produce no differences in aversion learning if activation of another receptor along with MOR activation is responsible for this difference. The idea that KOR and/or DOR activation is necessary in the production of morphine's effects has been reported previously for many morphine-induced behaviors, including analgesia and morphine-induced tolerance. For example, DOR antagonists can attenuate morphine-induced place preferences (Suzuki et al., 1994), decrease sensitization to morphine's rewarding effects (Shippenberg et al., 2009) and induce place aversions in morphine-dependent animals (Funada et al., 1996). Moreover, DORs are important in the formation of morphine tolerance and dependence (Fundytus et al., 1995; Zhu et al., 1999; see also Chefer and Shippenberg, 2009) and MORs are important for antinociception induced by DOR agonists, deltorphin II, DPDPE and SNC80 (Scherrer et al., 2004; Sora et al, 1999; see also Matthes et al., 1998). Further, morphine has been shown to produce thermal antinociception in MOR-knockout mice by activating spinal KORs (Yamada et al., 2006). Given the extensive interaction of the opioid receptors in response to morphine and other opioid drugs, it is possible that the strain differences evident with morphine and heroin are a consequence of some combination of MOR, DOR and/or KOR activity that differs between the strains. Furthermore, other neurotransmitter systems (e.g., dopaminergic, noradrenergic) reported to impact morphine aversion learning cannot be ruled out (see Grigson et al., 2000a; Koh and Bernstein, 2005; Mackey et al., 1986; Zito et al., 1988).
A better understanding of the differences in MOR agonist aversion learning between the F344 and LEW animals would provide much needed information about the opioid systems of both of these strains, possibly providing insights regarding strain differences in several other morphine-induced behaviors. Given the differences in morphine and other MOR agonist's antinociceptive effects between the F344 and LEW animals, it would be interesting to determine the effects of each of these agonists in aversion learning to more fully characterize the different behavioral profiles of these strains. Further, more work regarding the specific subtype of MOR in the F344 and LEW animals would prove beneficial to discussions of reported differences in aversion learning, self-administration and antinociception between these (and other) strains. For example, various strains of inbred mice displaying differing levels of specific MOR subtypes also display differing degrees of antinociception and cross-tolerance to various MOR agonists (Connelly et al., 1994; Narita et al., 2003; Sato et al., 1999; see Pasternak, 2001a,b; 2005 for reviews). Given that the LEW strain displayed decreases in saccharin consumption (relative to controls) in the heroin two-bottle test, but does not display any decrease associated with morphine administration supports the possibility that different subtypes of MORs could mediate the many different effects of various mu-preferring compounds. The possibility remains that the F344 and LEW rats differ in levels of these specific MOR subtypes, which could be the underlying biochemical mediation of their many behavioral differences to MOR agonist administration.
Acknowledgments
This work was supported by a grant from the Mellon Foundation to Anthony L. Riley, and a portion of this work was supported by the intramural research programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosio E, Goldberg SR, Elmer GI. Behavioral genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol. 1995;6:229–37. [PubMed] [Google Scholar]
- Bechara A, Zito KA, van der Kooy D. Peripheral receptors mediate the aversive conditioning effects of morphine in the rat. Pharmacol Biochem Behav. 1987;28:219–25. doi: 10.1016/0091-3057(87)90218-8. [DOI] [PubMed] [Google Scholar]
- Broadbent J, Muccino KJ, Cunningham CL. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav Neurosci. 2002;116:138–48. [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharm. 2009;34:887–98. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CD, Martinez RP, Schupsky JJ, Porreca F, Raffa RB. Etonitazene-induced antinociception in mu1 opioid receptor deficient CXBK mice: evidence for a role for mu2 receptors in supraspinal antinociception. Life Sci. 1994;54:PL369–74. doi: 10.1016/0024-3205(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Genetic contributions to addiction. Annu Rev Psychol. 2002;53:435–62. doi: 10.1146/annurev.psych.53.100901.135142. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Genetic influences on conditioned taste aversion. In: Reilly S, Schachtman TD, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. pp. 387–421. [Google Scholar]
- Davis CM, Riley AL. The effects of cocaine preexposure on cocaine-induced taste aversion learning in Fischer and Lewis rat strains. Pharmacol Biochem Behav. 2007;87:198–202. doi: 10.1016/j.pbb.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Davis CM, Roma PG, Dominguez JM, Riley AL. Morphine-induced place conditioning in Fischer and Lewis rats: acquisition and dose-response in a fully biased procedure. Pharmacol Biochem Behav. 2007;86:516–23. doi: 10.1016/j.pbb.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Fraser GL. Mu-, delta-, kappa-opioid receptors and their subtypes. A critical review with emphasis on radioligand binding experiments. Neurochem Int. 1994;24:401–26. doi: 10.1016/0197-0186(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Foynes MM, Riley AL. Lithium-chloride-induced conditioned taste aversions in the Lewis and Fischer 344 rat strains. Pharmacol Biochem Behav. 2004;79:303–8. doi: 10.1016/j.pbb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Funada M, Schutz CG, Shippenberg TS. Role of delta-opioid receptors in mediating the aversive stimulus effects of morphine withdrawal in the rat. Eur J Pharmacol. 1996;300:17–24. doi: 10.1016/0014-2999(95)00860-8. [DOI] [PubMed] [Google Scholar]
- Fundytus ME, Schiller PW, Shapiro M, Weltrowska G, Coderre TJ. Attenuation of morphine tolerance and dependence with the highly selective delta-opioid receptor antagonist TIPP[psi] Eur J Pharmacol. 1995;286:105–8. doi: 10.1016/0014-2999(95)00554-x. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Shaw AE, Riley AL. Cocaine-induced conditioned taste aversions: comparisons between effects in LEW/N and F344/N rat strains. Psychopharmacology (Berl) 1994;114:229–32. doi: 10.1007/BF02244841. [DOI] [PubMed] [Google Scholar]
- Gomez-Serrano MA, Kearns DN, Riley AL. The effects of light cycle phase on morphine-induced conditioned taste aversions in the Lewis, Fischer and Sprague-Dawley rat strains. Behav Brain Res. 2009;196:116–22. doi: 10.1016/j.bbr.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Krahn DD. Morphine-induced feeding: a comparison of the Lewis and Fischer 344 inbred rat strains. Pharmacol Biochem Behav. 1993;44:919–24. doi: 10.1016/0091-3057(93)90025-o. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is frees-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42:1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Freet CS. The suppressive effects of sucrose and cocaine, but not lithium chloride are greater in Lewis than in Fischer rats: evidence for the reward comparison hypothesis. Behav Neurosci. 2000;114:353–63. doi: 10.1037//0735-7044.114.2.353. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky P, Tanase D. Bilateral lesions of the gustatory thalamus disrupt morphine- but not lithium chloride-induced intake suppression in rats: evidence against the conditioned taste aversion hypothesis. Brain Res. 2000;858:327–37. doi: 10.1016/s0006-8993(00)01939-9. [DOI] [PubMed] [Google Scholar]
- Herradón G, Morales L, Gramage E, Alguacil LF. Comparative study of alpha2-adrenoceptors in Fischer 344 and Lewis rats. Evidence for clonidine-induced place aversion. Life Sci. 2008;82:1186–90. doi: 10.1016/j.lfs.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Inturrissi CE, Schultz M, Shin S, Umans JG, Angel L, Simon EJ. Evidence from opiate binding studies that heroin acts through its metabolites. Life Sci. 1983;33(Suppl 1):773–6. doi: 10.1016/0024-3205(83)90616-1. [DOI] [PubMed] [Google Scholar]
- Kearns DN, Gomez-Serrano MA, Weiss SJ, Riley AL. A comparison of Lewis and Fischer rat strains on autoshaping (sign-tracking), discrimination reversal learning and negative auto-maintenance. Behav Brain Res. 2006;169:193–200. doi: 10.1016/j.bbr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Koh MT, Bernstein IL. Mapping conditioned taste aversion associations using c-Fos reveals a dynamic role for insular cortex. Behav Neurosci. 2005;119:388–98. doi: 10.1037/0735-7044.119.2.388. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Ambrosio E. HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrinology. 2002;27:35–69. doi: 10.1016/s0306-4530(01)00035-x. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Chi S, Nestler EJ. Fischer and Lewis rat strains show differential cocaine effects in conditioned place preference and behavioral sensitization but not in locomotor activity or conditioned taste aversion. J Pharmacol Exp Ther. 1994;269:137–44. [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Haile CN. Strain differences in maintenance of cocaine self-administration and their relationship to novelty activity responses. Behav Neurosci. 2007;121:380–8. doi: 10.1037/0735-7044.121.2.380. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, Xi J. Differences in extinction responding and reinstatement of methamphetamine-seeking behavior between Fischer 344 and Lewis rats. Pharmacol Biochem Behav. 2006;83:391–5. doi: 10.1016/j.pbb.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Lancellotti D, Bayer BM, Glowa JR, Houghtling RA, Riley AL. Morphine-induced conditioned taste aversions in the LEW/N and F344/N rat strains. Pharmacol Biochem Behav. 2001;68:603–10. doi: 10.1016/s0091-3057(01)00461-0. [DOI] [PubMed] [Google Scholar]
- Liu C, Grigson PS. Mu opioid receptor agonist DAMGO-induce suppression of saccharin intake in Lewis and Fischer rats. Brain Res. 2005;1064:155–60. doi: 10.1016/j.brainres.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mackey WB, Keller J, van der Kooy D. Visceral cortex lesions block conditioned taste aversions induced by morphine. Pharmacol Biochem Behav. 1986;24:71–8. doi: 10.1016/0091-3057(86)90047-x. [DOI] [PubMed] [Google Scholar]
- Martín S, Lyupina Y, Crespo JA, González B, García-Lecumberri C, Ambrosio E. Genetic differences in NMDA and D1 receptor levels, and operant responding for food and morphine in Lewis and Fischer 344 rats. Brain Res. 2003;973:205–13. doi: 10.1016/s0006-8993(03)02482-x. [DOI] [PubMed] [Google Scholar]
- Martín S, Manzanares J, Corchero J, García-Lecumberri C, Crespo JA, Fuentes JA, Ambrosio E. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res. 1999;821:350–5. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- Matthes HWD, Smadja C, Valverde O, Vonesch JL, Foutz AS, Boudinot E, et al. Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-opioid receptor is maintained in mice lacking the mu-opioid receptor. J Neurosci. 1998;18:7285–95. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–80. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Narita M, Imai S, Ozaki S, Suzuki M, Narita M, Suzuki T. Reduced expression of a novel mu-opioid receptor (MOR) subtype MOR-1B in CXBK mice: implications of MOR-1B in the expression of MOR-mediated responses. Eur J Neurosci. 2003;18:3193–8. doi: 10.1111/j.1460-9568.2003.03052.x. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy; Washington DC: 2006. [Google Scholar]
- National Research Council . Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academy; Washington DC: 2003. [PubMed] [Google Scholar]
- Oldendorf WH, Hyman S, Braun L, Oldendorf SZ. Blood-brain barrier: penetration of morphine, codeine, heroin, and methadone after carotid injection. Science. 1972;178:984–6. doi: 10.1126/science.178.4064.984. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Insights into mu opioid pharmacology the role of mu opioid receptor subtypes. Life Sci. 2001a;68:2213–9. doi: 10.1016/s0024-3205(01)01008-6. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharmacol Sci. 2001b;22:67–70. doi: 10.1016/s0165-6147(00)01616-3. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Molecular biology of opioid analgesia. J Pain Symptom Manage. 2005;29:S2–9. doi: 10.1016/j.jpainsymman.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Pescatore KA, Glowa JR, Riley AL. Strain differences in the acquisition of nicotine-induced conditioned taste aversion. Pharmacol Biochem Behav. 2005;82:751–7. doi: 10.1016/j.pbb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Riley AL, Davis CM, Roma PG. Strain differences in taste aversion learning: implications for animal models of drug abuse. In: Reilly S, Schachtman TD, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford University Press; New York: 2009. pp. 226–61. [Google Scholar]
- Roma PG, Davis CM, Riley AL. Effects of cross-fostering on cocaine-induced conditioned taste aversions in Fischer and Lewis rats. Dev Psychobiol. 2007;49:172–9. doi: 10.1002/dev.20168. [DOI] [PubMed] [Google Scholar]
- Roma PG, Flint WW, Higley JD, Riley AL. Assessment of the aversive and rewarding effects of alcohol in the Fischer and Lewis rats. Psychopharmacology (Berl) 2006;189:187–99. doi: 10.1007/s00213-006-0553-6. [DOI] [PubMed] [Google Scholar]
- Sato T, Sakurada S, Takahashi N, Sakurada T, Tan-No K, Wako K, Kisara K. Contribution of spinal mu1-opioid receptors to morphine-induced antinociception. Eur J Pharmacol. 1999;369:183–7. doi: 10.1016/s0014-2999(99)00065-5. [DOI] [PubMed] [Google Scholar]
- Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: a parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci. 2004;19:2239–48. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- Self DW, Stein L. Receptor subtypes in opioid and stimulant reward. Pharmacol Toxicol. 1992;70:87–94. doi: 10.1111/j.1600-0773.1992.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Chefer VI, Thompson AC. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol Psychiatry. 2009;65:169–74. doi: 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Li XF, Funada M, Kinsey S, Uhl GR. Visceral chemical nociception in mice lacking mu-opioid receptors: effects of morphine, SNC80 and U-50,488. Eur J Pharmacol. 1999;366:R3–5. doi: 10.1016/s0014-2999(98)00933-9. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yoshiike M, Mizoguchi H, Kamei J, Misawa M, Nagase H. Blockade of δ-opioid receptors prevents morphine-induced place preference in mice. Jpn J Pharmacol. 1994;66:131–7. doi: 10.1254/jjp.66.131. [DOI] [PubMed] [Google Scholar]
- Yamada H, Shimoyama N, Sora I, Uhl GR, Fukuda Y, Moriya H, Shimoyama M. Morphine can produce analgesia via spinal kappa opioid receptors in the absence of mu opioid receptors. Brain Res. 2006;1083:61–9. doi: 10.1016/j.brainres.2006.01.095. [DOI] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pinter JE. Neuron. 1999;24:243–52. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- Zito KA, Bechara A, Greenwood C, van der Kooy D. The dopamine innervation of the visceral cortex mediates the aversive effects of opiates. Pharmacol Biochem Behav. 1988;30:693–9. doi: 10.1016/0091-3057(88)90086-x. [DOI] [PubMed] [Google Scholar]