Abstract
A variety of anatomic and functional neuroimaging findings are associated with Alzheimer's Disease (AD). One of the strongest imaging associations identified is between AD and hippocampal atrophy. The ∈4 allele of the apolipoprotein E (APOE) gene increases the risk of developing AD and lowers the mean age of onset of the disease. The purpose of this paper was to assess the association between hippocampal volume and APOE polymorphisms in elderly control subjects and patients with probable AD. We performed magnetic resonance imaging-based volume measurements of the hippocampus in 125 cognitively normal elderly controls and 62 patients with probable AD. APOE genotyping was performed using standard methods.
Hippocampal volumes were significantly smaller in AD cases than in control subjects (p <0.001). Hippocampal volumes did not differ significantly within either clinical group on the basis of APOE genotype. Both the ∈4 allele of APOE (p = 0.006) and hippocampal atrophy (p <0.001) were significantly but independently associated with AD.
Keywords: Alzheimer's Disease, Dementia, MRI, Quantitative MRI, Hippocampus
The ∈4 allele of the apolipoprotein E (APOE) gene confers an increased risk of developing AD and also lowers the mean age of onset in a dose dependent fashion while the ∈2 allele confers a protective effect [1–9]. The biologic basis for the effect of APOE ∈4 as a risk factor for developing AD is unknown at this point.
Several theories have been proposed, including that interaction between APOE ∈4 and amyloid β protein promotes development of senile plaques [2, 10, 11] that isoform-specific interaction exits between APOE ∈4 and τ protein in which ∈4 favors the formation of neurofibrillary tangles or ∈3 stabilizes τ, and that isoform-specific effects of ApoE exist for neuronal plasticity [13] and cholinergic function. [14]
The pathologic hallmarks of AD are senile plaques, neurofibrillary tangles, cell death or atrophy of selectively vulnerable neuronal populations, and decreased synapse density [15–18]. On a macroscopic level the primary abnormality associated with AD is cerebral atrophy which occurs earliest and with greatest severity in medial temporal lobe limbic areas. [15, 17, 19–23] Magnetic resonance imaging (MRI)-based volume measurements of the hippocampus are a sensitive marker of the medial temporal lobe pathology associated with AD even in its mildest form [24–32]. The above observations suggest two hypotheses concerning the relation between hippocampal formation volume and APOE genotype. First, we hypothesized that on average the hippocampal volumes of non-demented elderly individuals who were ∈4 carriers would be more atrophic than those of ∈4 non-carriers. This hypothesis was based on the assumption that given a large group of non-demented elderly subjects, some would have hippocampal atrophy due to preclinical AD and this was more likely in those who were ∈4 carriers than those who were ∈4 non-carriers. Second, we hypothesized that hippocampal volumes of AD patients who were ∈4 carriers would be similar to those who were not ∈4 carriers, provided that the duration of disease and severity of cognitive impairment was similar between the two groups of AD patients. This hypothesis was based several studies which have shown that the level cognitive impairment and its progression are not correlated with APOE genotype provided the duration of illness has been controlled for among AD patients with different APOE genotypes[33, 34, 35]. In turn, MRI determined hippocampal atrophy correlates closely with level of cognitive impairment in AD [24, 26, 29, 31, 36].
MATERIALS AND METHODS
Recruitment and Characterization of Subjects
Patients with AD and the cognitively normal control subjects for this study were recruited from the Alzheimer's Disease Center and Alzheimer's Disease Patient Registry at the Mayo Clinic[8, 37–39], which are prospective, longitudinal studies of aging and dementia. Informed consent was obtained for participation in the longitudinal studies which included clinical/cognitive assessment as well as MRI studies, and all studies were approved by the Mayo Institutional Review Board. The diagnosis of AD was made according to the NINCDS/ADRDA criteria [40] at a consensus conference attended by behavioral neurologists, neuropsychologists, a geriatrician, and nurses. Disease severity in AD cases was assessed by the Clinical Dementia Rating (CDR) scale; very mild - CDR 0.5; mild - CDR 1; moderate - CDR 2 [41]. An important distinction is made between establishing a diagnosis of AD and ranking its severity. The diagnosis of AD was established according to NINCDS/ADRDA criteria which emphasize a decline in cognitive performance over time as an important benchmark [40]. The CDR score was used as a staging instrument to rate disease severity at a specific point in time. It was therefore possible for cases to meet NINCDS/ADRDA criteria for AD and also be ranked as only very mildly demented (CDR 0.5). Cases were not excluded for the presence of ongoing medical problems such as diabetes, hypertension, or heart disease. All clinical diagnoses were established without knowledge of APOE genotype.
Control subjects were recruited from the same pool of patients coming to Mayo primary care physicians for a general medical examination and were evaluated in the same way as cases including review at a consensus conference. The criteria for cognitively normal controls were 1) no active neurological or psychiatric disorders, 2) not currently using psychoactive medications; and 3) like the cases, some had ongoing medical problems such as diabetes, hypertension, and heart disease, however the illnesses or their treatments did not interfere with cognitive function.
Apolipoprotein E Genotypes
DNA was extracted from peripheral blood leukocytes (Applied Biosystem 340A DNA Extractor, Applied Biosystems, Foster City, CA), and amplified by polymerase chain reaction (PCR) [6]. Each amplification reaction contained 250 ng of genomic DNA, 20 pmol of each primer, 200 μM of each deoxynucleoside triphosphate, 10% dimethyl sulfoxide, and 0.25 μL of Taq DNA polymerase in a final volume of 25 μL. Reaction conditions included denaturation for 30 seconds at 94°C, annealing for 30 seconds at 65°C, and extension for 30 seconds at 72°C, for 30 cycles (System 9600 Thermal Cycler, Perkin Elmer Cetus, Norwalk, Conn). The PCR products were digested with HhaI and the fragments were separated by electrophoresis on an 8% polyacrylamide nondenaturing gel. The gel was then treated with ethidium bromide for 30 minutes, and DNA fragments were visualized by ultraviolet illumination.
MR Image Acquisition
An MRI examination of the brain was performed within 4 months of the clinical assessment. For all AD cases in this study, the MRI was therefore performed with close temporal proximity to the initial diagnosis of AD. These MR studies were used in the diagnostic process only to exclude treatable causes of dementia. The volumetric data were not used to aid in the clinical diagnosis of AD.
All subjects were imaged at 1.5T using a standardized imaging protocol [42]. A T1-weighted sagittal set of images was used to measure total intracranial volume and for landmarking subsequent image acquisitions. Volume measurements of the hippocampus were derived from a T1-weighted 3D volumetric spoiled gradient echo sequence with 124 contiguous partitions, 1.6 mm slice thickness, a 22 × 16.5 cm field of view, 192 views, and 45° flip angle.
Image Processing
All image processing steps (including boundary tracing) in every subject were performed by the same trained research assistant who was blinded to all clinical information, including APOE genotype, in order to ensure that the volumetric data were generated in an unbiased fashion. Validation studies show the intra-rater, test-retest coefficient of variation of hippocampal volume measurements to be 1.9% with this method [43]. All anatomic tracing in every subject was reviewed later by CRJ who was also blinded to all clinical information and corrections were made at that time if necessary. This ensured rigorous quality control, as well as uniformity in the subjective aspects of image processing across all the participants in this study.
The borders of the hippocampi were manually traced sequentially on each slice from posterior to anterior [44]. The number of voxels in each was counted automatically using a summed region of interest function. These were multiplied by voxel volume to give a numeric value in mm3. In-plane hippocampal anatomic boundaries were defined to include the CA1-CA4 sectors of the hippocampus proper, the dentate gyrus, and subiculum [26, 43–47]. The inferior border of the hippocampus was demarcated by the high intrinsic contrast on T1-weighted images between the white matter of the parahippocampal gyrus and the gray matter of the subiculum. Contrast between the hippocampal and cerebrospinal fluid in the temporal horn, choroidal fissure, and uncal cistern were used to identify the remaining inplane boundaries of the hippocampus. Segmentation of the hippocampal head from the overlying amygdala was facilitated by identifying the alveus which provides a thin high intensity line demarcating the undulating superior surface of the head of the hippocampus. Identification of these anatomic landmarks allowed us to include the full anterior extent of the hippocampus in the measured volumes. The posterior boundary of the hippocampus was determined by the oblique coronal anatomic section on which the crura of the fornices were identified in full profile [48]. Thus, essentially the entire hippocampus was included in these measurements.
Statistical Methods
Individual hippocampal volumes were normalized for intersubject variation in head size by dividing structure volume (in mm3) by the total intracranial volume (TIV in cm3) of that particular subject [26, 46]. Volumes were converted to normal deviates, referred to as W scores. Specifically, for each subject we obtained the age and gender specific normal percentile based on the results in [42]. Percentile values were converted to normal deviates using the inverse of the standard normal distribution (a percentile value of 95 corresponding to a W score of 1.645, for example) [49]. Hippocampal symmetry was assessed by step-wise regression with the normalized (by TIV) right-left hippocampal volume difference as the dependent variable and with independent variables of age, gender, APOE genotype, and clinical group. Analyses of APOE were performed on the basis of individual genotypes, and also by grouping ∈4 carriers into an ∈4+ group (∈3/4 and ∈4/4), and ∈4 non-carriers into an ∈4- group (∈2/3 and ∈3/3). Participants with the ∈2/4 genotype were treated separately.
Comparisons between two groups with respect to quantitative variables were performed using two sample t-tests. Correlations between cardinal quantitative variables were performed using product moment correlations. Spearman rank correlations were used if either variable was ordinal. Chi-square tests were used for testing association between dichotomous variables.
Stepwise logistic regression was used to assess the relationship between disease status and the following independent variables: age, hippocampal W value, and APOE. Stepping down, we first considered a main effects model, using P<0.05 as the criteria for remaining in the model. We then considered nonlinear terms and interaction terms for significant main effects. A similar stepwise approach was used to assess asymmetry, in which the dependent variable was the difference between left and right volumes. The independent variables were age, gender, APOE, and disease status.
RESULTS
One-hundred-eighty-seven subjects are included in this report, ie, 125 controls and 62 AD cases (Table 1). Ninety-two of the controls and 51 of the cases were women. None of the female patients or controls were undergoing estrogen replacement therapy. No significant differences in mean age or educational attainment were present between ∈4+ controls, ∈4− controls, ∈4+ AD cases, or ∈4− AD cases. Dementia rating scale [50] and Mini-Mental State Exam (MMSE) [51] scores were significantly lower in AD cases than control subjects, but did not differ on the basis of APOE genotype within either clinical group. The CDR scores and MMSE scores did not differ significantly between the ∈4− and ∈4+ AD cases. The duration of clinical disease did not differ significantly between ∈4+ and ∈4− AD cases.
Table 1.
Demographic Data by Diagnosis Group and Genotype
| Controls (N = 125) | AD cases (N = 62) | |||
|---|---|---|---|---|
| Variable | ∈4− (N = 95) | ∈4+ (N = 30) | ∈4− (N = 26)+ | ∈4+ (N = 36)++ |
| Age | 78.77 ± 6.98 | 80.13 ± 5.86 | 75.42 ± 9.25 | 75.47 ± 6.56 |
| Education | 13.42 ± 3.05 | 13.37 ± 2.68 | 12.92 ± 2.50* | 13.34 ± 3.01* |
| MMSE | 28.67 ± 1.29 | 28.30 ± 1.12 | 19.96 ± 5.73** | 19.57 ± 5.62* |
| DRS | 135.28 ± 7.05* | 134.47 ± 6.68 | 103.50 ± 19.57** | 104.46 ± 18.43* |
one with missing data
two with missing data
6 with CDR = 0.5, 15 with CDR = 1.0, 5 with CDR = 2.0
13 with CDR = 0.5, 18 with CDR = 1.0, 5 with CDR = 2.0
Hippocampal W values by diagnosis group and genotype are found in Table 2. The proportion of AD cases who were ∈4+ (53%) was significantly greater than the proportion of control subjects who were ∈4+ (22%) (p <0.001). Genotypes are arranged in Table 2 from left to right in order of increasing risk for AD. Two control subjects and 3 AD cases had the ∈2/4 genotype. These subjects were segregated to the left hand column of Table 2 as the risk of AD conferred by ∈2/4 is uncertain. In control subjects, a trend toward decreasing hippocampal W score (more atrophic hippocampi relative to controls) was present with genotypes associated with greater risk of AD. However this trend did not reach statistical significance (p = 0.165, r =−0.126). When the individual genotypes were combined into ∈4+ and ∈4− groups, a trend toward smaller hippocampal W values in the ∈4+ compared to the ∈4− groups was present both for control subjects and for AD cases, but this trend did not reach significance in either clinical group. Among controls, mean hippocampal W values were −0.15 (95% C.I. −0.51,0.22) for ∈4+ subjects and 0.06 (95% C.I. −0.15,0.27) for ∈4− subjects (p = 0.320). Among cases, mean hippocampal W values were −2.06 (95% C.I. −2.56,−1.78) for ∈4+ subjects and −1.93 (95% C.I. −2.35,−1.52) for ∈4− subjects (p = 0.679). Hippocampal W values were significantly smaller in both ∈4+ and ∈4− AD cases (−2.19 and −1.93 respectively) when compared to ∈4+ or ∈4− control subjects (−0.15 and 0.06 respectively; p <0.001).
Table 2.
Hippocampal W Values by Diagnosis Group and Genotype
| Group | Genotype | ||||||
|---|---|---|---|---|---|---|---|
| ∈2/4 | ∈2/3 | ∈3/3 | ∈3/4 | ∈4/4 | ∈4− (∈2/3 + ∈3/3) | ∈4+ (∈3/4 + ∈4/4) | |
| Controls (n = 125) | −0.21 (2) | 0.27 (20) | 0.00 (75) | −0.13 (27) | −0.71 (1) | −0.06 (95) | −0.15 (28) |
| AD (n = 62) | −0.63 (3) | −1.93 (2) | −1.93 (24) | −2.20 (23) | −2.15 (10) | −1.93 (26) | −2.19 (33) |
Values in the table represent the mean hippocampal W score for each group. The number of subjects in each group is in parentheses.
No significant differences in hippocampal symmetry were present among groups (AD cases vs controls, or ∈4+ vs ∈4− ) or in stepwise regression analysis including age, gender, APOE genotype and clinical group in the model. Within both the case and control groups, no association between hippocampal W value and age was observed.
A logistic regression model was constructed (Table 3) with clinical status (case vs control) as the dependent variable. Age and hippocampal W value were modeled as continuous variables, and ∈4 was modeled as a dichotomous variable—present (3/4 or 4/4) or absent (2/3 or 3/3). An ∈4+ positive individual was approximately 3.5 times more likely to have AD than an ∈4− negative person. Each 1 unit increase in the hippocampal W score reduced the odds of disease by approximately 80%. Both the ∈4 allele of APOE (p=0.006) as well as the hippocampal W score (p < 0.001) were significantly and independently related to clinical status. There was no statistically significant interaction between hippocampal W scores and APOE genotype.
Table 3.
Logistic Regression Model
| Variable | Odds Ratio | 95% C.I. | P |
|---|---|---|---|
| Hippocampal W value | 0.196 | (0.121, 0.317) | <.001 |
| APOE | 3.581 | (1.411, 9.091) | .006 |
DISCUSSION
In accordance with most volumetric anatomic imaging studies, the hippocampi of AD cases were significantly more atrophic than those of control subjects although overlap between groups was present [24–32] (Figure). The method of analysis we employed corrects for the potentially confounding effects of age, gender, and head size when comparing hippocampal volume among individuals [42, 46]. The observed difference in hippocampal W scores between AD cases and control subjects was therefore interpreted as a direct effect of the pathology of AD [15, 17, 23].
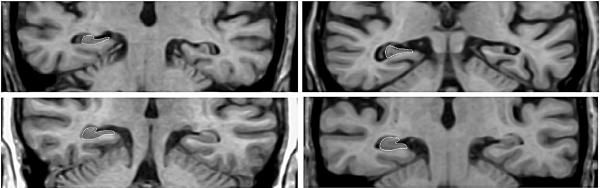
Figure 1. Relationships between hippocampal volume, clinical group, and APOE genotype.
This four panel collage illustrates a selected imaging slice through the body of the hippocampi in four different subjects. All four were women. The top left panel is a 79-year-old ∈4+ AD patient with normalized hippocampal volumes of 2.26. Top right is a 77-year-old e4− AD patient with normalized hippocampal volumes of 2.42. Bottom left is an 80-year-old ∈4+ control with normalized hippocampal volumes of 4.23. Bottom right is an 80-year-old ∈4− control with normalized hippocampal volumes of 4.36. The right hippocampus is traced as it would be for quantitation in each subject. The figure illustrates significant hippocampal atrophy in AD subjects compared to controls, but no difference in hippocampal volume between ∈4+ and ∈4− members of either clinical group. The hippocampal volumes are normalized by dividing the sum of the right plus left hippocampal volume in mm3 by total intracranial volume in cm3.
Among control subjects those who were ∈4+ positive tended to have slightly smaller hippocampi than those who were ∈4− negative. This same was true among AD cases; however this difference between ∈4+ positive and ∈4− negative subjects was not significant for either clinical group (Figure). The absence of any significant association between APOE genotype and hippocampal W score within either clinical group was confirmed by a logistic regression analysis in which no significant interaction between hippocampal W score and APOE genotype was found (Table 3).
APOE and Hippocampal Volume in Controls
In each person who develops AD, progressive accumulation of degenerative pathologic changes is ongoing for years, perhaps decades, prior to manifestation of the definitive clinical syndrome. Among the 125 non-demented elderly control subjects in our study it is almost certain that some will develop AD at some point in the future. It therefore follows that some of these non-demented control subjects had subclinical accumulation of AD pathology at the time of the MR scan, and that this was more likely in those who were ∈4+ positive than those who were ∈4− negative. For this reason the absence of a significant difference in hippocampal volumes between ∈4+ positive and ∈4− negative controls in our study was unexpected. Plassman and colleagues [52] did find smaller hippocampal volumes in ∈4+ positive than in ∈4− negative twins who were cognitively normal but discordant for APOE genotype. One plausible explanation for the discrepancy between our findings and those of Plassman and colleagues [52] is the different demographics of the non-demented subjects in the two studies. Our controls were on average a decade and a half older than the non-demented subjects reported by Plassman and colleagues [52]. It is widely accepted that intersubject anatomic variability increases with advancing age particularly in older subjects. The effect of age related intersubject anatomic variability was therefore probably substantially greater in our normal group than that of Plassman and colleagues [52]. It is possible that an ∈4 effect on hippocampal volume was present in our group of 125 control subjects but was small enough to have been overwhelmed by the much greater effect of intersubject anatomic variability. Another contributing factor to the discrepancy between our results and those of Plassman and colleagues [52] was their use of twin pairs, which would act to reduce the intersubject anatomic variability due to genetic control over brain morphology.
APOE and Hippocampal Volume in AD patients
The second main finding of our study was the absence of a significant difference in the hippocampal W scores among AD patients of different APOE genotypes. We anticipated this result based on the following two lines of reasoning: 1) the relationship between AD pathology, APOE genotype, and hippocampal atrophy; 2) the relationship between cognitive decline, APOE genotype, and hippocampal atrophy. MRI volumetric measurements of hippocampal atrophy are related to the severity of AD pathology in the medial temporal lobe. Of the two primary pathologic features of AD, senile plaques and neurofibrillary change, neurofibrillary change occurs earliest and with greatest severity in the medial temporal lobe limbic areas in a highly stereotypical fashion, whereas senile plaques are distributed in limbic cortex and association neocortex with no apparent preference toward the medial temporal lobe [15, 19, 21, 22]. MRI measures of hippocampal atrophy might therefore be expected to track more closely with neurofibrillary pathology than with senile plaques, and Gomez-Isla et al [33] have shown that APOE ∈4 is correlated with the plaque burden but not with the number of neurofibrillary tangles provided that differences in the duration of disease between different APOE genotype groups were controlled for. The degree of cognitive impairment in AD correlates with neurofibrillary tangles but not with the senile plaque burden [53]. MRI measurements of hippocampal atrophy have been shown to track closely with cognitive measures of memory performance that are critically dependent on the medial temporal lobe limbic areas. [24, 29, 31, 36, 42] Although the relationship between cognitive decline and APOE genotype is controversial [54, 55], several studies have shown that once a person has clinically evident AD the APOE genotype does not correlate with the rate of clinical progression of the disease nor with dementia severity [33–35]. By the above reasoning therefore hippocampal atrophy should best correlate with those features of AD which are tightly linked with medial temporal lobe dysfunction—neurofibrillary tangles and the cognitive disturbance—neither of which are closely correlated with APOE ∈4 genotype [19, 33, 34, 53].
Our results in AD patients are in accord with those reported by Yamaguchi and collaborators [56], who found that the annual change in the interuncal distance (linear distance between the unci of the temporal lobes at the level of the suprasellar cistern) was greater for AD cases than controls, but not different among AD patients of different APOE genotypes. Our results differ, however, from those reported by Lehtovirta [57, 58], who found smaller right, (but not left) hippocampal and amygdala volumes in AD patients who were ∈4/4 than those who were ∈3/4, ∈3/3, or ∈3/2. A difference between our AD patients and those described by Lehtovirta and colleagues [57, 58] is that the mean age of both the ∈4+ positive and ∈4− negative AD patients in our study was 75 years, where as the mean age of AD subjects in each of the APOE genotype groups was <70 years old in the studies of Lehtovirta and colleagues [57, 58]. One explanation for the discrepancy between our findings in AD patients and those of Lehtovirta and colleagues [57, 58] rests with the notion that the effect of APOE ∈4 as a risk factor for AD may be age-dependent. Although this concept is controversial [5, 59], both Rebecket and collaborators [60] and Blacker and co-workers [9] found that the prevalence of APOE ∈4 was greatest in AD patients with onset of the disease under age 70, and that it decreases with advancing age in AD patients thereafter. Growden and associates [34] have suggested that “the influence of APOE ∈4 either preceeds or occurs at an early point in the AD disease process”. Data from the patient registry from which our study patients were derived [35] suggests much the same, with declining prevalence of the ∈4 allele in AD patients after the age of 75 years. If the association between APOE ∈4 and AD risk is age dependent then the most pronounced difference in hippocampal volume among patients with different APOE genotypes would be expected in younger AD patients, which is what Lehtovirta and colleagues [57, 58] found. In contrast, in a group of older AD patients such as ours, one could hypothesize that the association between APOE∈4 and hippocampal volume had already passed its peak and was waning.
A second difference between our study and those of Lehtovirta and colleagues [57, 58], was that they found significant differences between ∈4/4 AD patients and AD patients of other genotypes for the right (but not the left) hippocampus and amygdala. In contrast, we found no association between hippocampal asymmetry and APOE ∈4/4 in AD cases. We are not aware of any pathologic literature which suggests selective vulnerability of the right medial temporal regions in AD and can only suggest the possibility that this finding of Lehtovirta and colleagues [57, 58] may be due to small sample sizes of ∈4/4 subjects (n=4 [58] and n=5 [57]).
Clinical Utility of Hippocampal Volume Measurements
Consistent with prior studies, the proportion of subjects who carry the ∈4 allele was substantially greater in AD cases (53 %) than control subjects (22 %) indicating the power of this genetic marker as a risk factor for developing AD [3–9]. However, half of the AD cases in our study were ∈4− negative, and in these individuals APOE genotype would have failed to identify the risk of AD prior to its clinical onset. From the perspective of the ability of MR-based hippocampal volume measurements to predict which individuals are at risk for subsequently developing AD, the important finding in this study was that on average the hippocampi of AD cases were significantly atrophic regardless of APOE genotype.
The data presented here are consistent with the notion that hippocampal atrophy is a marker of the presence of AD while the ∈4 allele is a marker of the risk for developing AD. However, several studies have shown that the hippocampal atrophy associated with AD can be identified in the mildest forms of the disease, indicating that this neuroimaging marker is a sensitive indicator of the pathology of AD [25, 30, 31, 42]. As indicated by the distribution of CDR scores in Table 1, the majority of the AD cases in this study were only mildly demented. A critical question therefore is whether hippocampal atrophy which precedes the onset of the earliest clinical symptoms in AD can be detected reliably by MRI volumetry. If this were true, then MRI-based hippocampal volumetry may represent a method that can aid in identifying asymptomatic individuals who are at risk for developing AD [61, 62]. An equally important issue is the interrelation between hippocampal atrophy and APOE genotype as predictors of the risk of developing AD. The independent association of both ∈4 and hippocampal atrophy with clinical AD demonstrated here provides a rationale for further study focused on the question: Can accurate prediction models be developed that incorporate both neuroimaging variables, and genetic variables as independent markers of the risk of developing AD?
In addition to pre-symptomatic diagnosis, a second potentially useful clinical role for hippocampal volume measurements may be in clarifying the cause of dementia of clinically affected individuals. The presence of ∈4 in an elderly demented individual increases confidence in the clinical diagnosis of AD as opposed to other causes of dementia. [63–66]. The presence of significant hippocampal atrophy may similarly increase confidence in the clinical diagnosis of AD, especially in demented ∈4− negative individuals in whom ApoE genotyping does not aid greatly with the differential diagnosis. However, confirmation of this possibility will require studies which correlate premortem imaging findings with pathologically proven diagnoses.
Acknowledgments
Brenda Maxwell - Typing
Ruth Cha - Statistical Analysis
Supported by NIH-NIA-AG11378; AG-08031; AG-06786; NINDS-NS29059; The DANA Foundation; The Alzheimer's Association
Abbreviations
- MRI
Magnetic Resonance Imaging
- TIV
Total Intracranial Volume
- PHG
Parahippocampal Gyrus
- MTL
Medial Temporal Lobe
- AD
Alzheimer's Disease
References
- 1.Corder EH, Saunders AM, Strittmatter WJ. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 2.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high acidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer's disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corder EH, Saunders AM, Risch NJ, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer's disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 4.Kuussisto J, Koivisto K, Kervinen K, et al. Association of apolipoprotein E phenotypes with late onset Alzheimer's disease: population based study. BMJ. 1994;309:636–638. doi: 10.1136/bmj.309.6955.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayeux R, Stern Y, Ottman R, et al. The apolipoprotein E4 allele in patients with Alzheimer's disease. Ann Neurol. 1993;34:752–754. doi: 10.1002/ana.410340527. [DOI] [PubMed] [Google Scholar]
- 6.Tsai MS, Tangalos EG, Petersen RC, et al. Apolipoprotein E: risk factor for Alzheimer's disease. Am J Hum Genet. 1994;54:643–649. [PMC free article] [PubMed] [Google Scholar]
- 7.Roses AD. A model for susceptibility polymorphisms for complex disease: apolipoprotein E and Alzheimer disease. Neurogenetics. 1997;1:3–11. doi: 10.1007/s100480050001. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 9.Blacker D, Haines JL, Rodes L, et al. ApoE-4 and age at onset of Alzheimer's disease: The NIMH Genetics Initiative. Neurology. 1997;48:139–147. doi: 10.1212/wnl.48.1.139. [DOI] [PubMed] [Google Scholar]
- 10.Strittmatter WJ, Weisgraber KH, Goedert M, et al. Microtubule instability and paired helical filament formation in the Alzheimer disease brain are related to apolipoprotein E genotype. Exp Neurol. 1994;125:163–171. doi: 10.1006/exnr.1994.1019. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Z, Smith JD, Greengard P, et al. Alzheimer amyloid-Beta peptide forms denaturant-resistant complex with type ∈3 but not type ∈4 isoform of native apolipoprotein E. Mol Med. 1996;2:175–180. [PMC free article] [PubMed] [Google Scholar]
- 12.Strittmatter WJ, Weisgraber KH, Huang DY, et al. Binding of human apolipoprotein E to synthetic amyloid beta-peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci. 1994;17:525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 14.Poirier J, Delisle M-C, Quirion R, et al. Apolipoprotein ∈4 allele as a predictor of cholinergic deficits and treatment outcome in Alzheimer disease. Proc Natl Acad Sci USA. 1995;92:12260–12264. doi: 10.1073/pnas.92.26.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- 16.Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch Neurol. 1985;42:1097–1104. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson BE, Blessed G, Roth M. Observations on the brains of demented old people. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 18.Terry RD, Peck A, DeTeresa R, et al. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981;10:184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- 19.Arnold SE, Hyman BT, Flory J, et al. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cerebr Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 20.Brun A, Englund E. Regional pattern of degeneration in Alzheimer's disease: neuronal loss and histopathological grading. Histopathology. 1981;5:549–564. doi: 10.1111/j.1365-2559.1981.tb01818.x. [DOI] [PubMed] [Google Scholar]
- 21.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 22.Hyman BT, Gomez-Isla T. Alzheimer's disease is a laminar, regional, and neural system specific disease, not a global brain disease. Neurobiol Aging. 1994;15:353–354. doi: 10.1016/0197-4580(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 23.Hooper MW, Vogel FS. The limbic system in Alzheimer's disease: a neuropathologic investigation. Am J Pathol. 1976;85:1–19. [PMC free article] [PubMed] [Google Scholar]
- 24.Convit A, de Leon MJ, Golomb J, et al. Hippocampal atrophy in early Alzheimer's disease: anatomic specificity and validation. Psychiatr Q. 1993;64:371–387. doi: 10.1007/BF01064929. [DOI] [PubMed] [Google Scholar]
- 25.Convit A, de Leon MH, Tarshish C, et al. Hippocampal volume losses in minimally impaired elderly. Lancet. 1995;345:266. doi: 10.1016/s0140-6736(95)90265-1. [DOI] [PubMed] [Google Scholar]
- 26.Jack CR, Jr, Petersen RC, O'Brien PC, et al. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 27.Kesslak JP, Nalcioglu O, Cotman CW. Quantification of magnetic resonance scans for hippocampal and parahippocampal atrophy in Alzheimer's disease. Neurology. 1991;41:51–54. doi: 10.1212/wnl.41.1.51. [DOI] [PubMed] [Google Scholar]
- 28.Killiany RJ, Moss MB, Albert MS, et al. Temporal lobe regions on magnetic resonance imaging identify patients with early Alzheimer's disease. Arch Neurol. 1993;50:949–954. doi: 10.1001/archneur.1993.00540090052010. [DOI] [PubMed] [Google Scholar]
- 29.Laakso MP, Soininen H, Partanen K, et al. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer's disease: correlation with memory functions. J Neural Transm. 1995;9:73–86. doi: 10.1007/BF02252964. [DOI] [PubMed] [Google Scholar]
- 30.Lehericy S, Baulac M, Chiras J, et al. Amygdalohippocampal MR volume measurements in the early stages of Alzheimer disease. AJNR. 1994;15:927–937. [PMC free article] [PubMed] [Google Scholar]
- 31.Soininen HS, Partanen K, Pitkanen A, et al. Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology. 1994;44:1660–1668. doi: 10.1212/wnl.44.9.1660. [DOI] [PubMed] [Google Scholar]
- 32.Pearlson G, Harris GJ, Powers RE, et al. Quantitative changes in mesial temporal volume, regional cerebral blood flow, and cognition in Alzheimer's disease. Arch Gen Psychiatry. 1992;49:402–408. doi: 10.1001/archpsyc.1992.01820050066012. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Isla T, West HL, Rebeck GW, et al. Clinical and pathological correlates of apolipoprotein E ∈4 in Alzheimer's disease. Ann Neurol. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- 34.Growdon J, Locasio J, Corkin S, et al. Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer's disease. Neurology. 1996;46:A419. doi: 10.1212/wnl.47.2.444. Abstract. [DOI] [PubMed] [Google Scholar]
- 35.Waring S, Rocca W, Smith G, et al. Apolipoprotein E and rare of clinical progression in Alzheimer's disease. Neurology. 1996;46:A348. Abstract. [Google Scholar]
- 36.de Leon MJ, George AE, Golomb J, et al. Frequency of hippocampal formation atrophy in normal aging and Alzheimer's disease. Neurobiol Aging. 1997;18:1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- 37.Petersen RC, Kokmen E, Tangalos EG, et al. Mayo Clinic Alzheimer's Disease Patient Registry. Aging. 1990;2:408–415. doi: 10.1007/BF03323961. [DOI] [PubMed] [Google Scholar]
- 38.Petersen RC, Smith G, Kokmen E, et al. Memory function in normal aging. Neurology. 1992;42:396–401. doi: 10.1212/wnl.42.2.396. [DOI] [PubMed] [Google Scholar]
- 39.Petersen RC, Smith GE, Ivnik RJ, et al. Memory function in very early Alzheimer's disease. Neurology. 1994;44:867–872. doi: 10.1212/wnl.44.5.867. [DOI] [PubMed] [Google Scholar]
- 40.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 41.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 42.Jack CR, Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jack CR, Jr, Bentley M, Twomey CK, Zinsmeister AR. MR-based volume measurements of the hippocampal formation and anterior temporal lobe: validation studies. Radiology. 1990;176:205–209. doi: 10.1148/radiology.176.1.2353093. [DOI] [PubMed] [Google Scholar]
- 44.Jack CR., Jr MRI-based hippocampal volume measurements in epilepsy. Epilepsia. 1994;35(suppl 6):S21–S29. doi: 10.1111/j.1528-1157.1994.tb05986.x. [DOI] [PubMed] [Google Scholar]
- 45.Jack CR, Jr, Sharbrough FW, Twomey CK, et al. Temporal lobe seizures: lateralization with MR volume measurements of hippocampal formation. Radiology. 1990;175:423–429. doi: 10.1148/radiology.175.2.2183282. [DOI] [PubMed] [Google Scholar]
- 46.Jack CR, Jr, Twomey CK, Zinsmeister AR, et al. Anterior temporal lobes and hippocampal formations: normative volumetric measurements with MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- 47.Duvernoy HM. An atlas of applied anatomy. JF Bergmann; Munich: 1988. The human hippocampus; pp. 77–91. [Google Scholar]
- 48.Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- 49.O'Brien PC, Dyck PJ. Procedures for setting normal values. Neurology. 1995;45:17–23. doi: 10.1212/wnl.45.1.17. [DOI] [PubMed] [Google Scholar]
- 50.Mattis S. Mental status examination for organic mental syndromes in the elderly patient. In: Bellak KT, editor. Geriatric psychiatry. Grune and Stratton; New York: 1976. [Google Scholar]
- 51.Folstein MF, Folstein SE, McHugh PR. "Mini Mental State": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 52.Plassman BL, Welsh-Bohmer KA, Bigler ED, et al. Apolipoprotein E4 allele and hippocampal volume in twins with normal cognition. Neurology. 1997;48:985–989. doi: 10.1212/wnl.48.4.985. [DOI] [PubMed] [Google Scholar]
- 53.Arriagada PV, Growdow JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but no senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 54.Feskens EJM, Havekes LM, Kalmijn S, et al. Apolipoprotein ∈4 allele and cognitive decline in elderly men. BMJ. 1994;309:1202–1206. doi: 10.1136/bmj.309.6963.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henderson A, Easteal S, Jorm A, et al. Apolipoprotein E allele 4, dementia, and cognitive decline in a population sample. Lancet. 1995;346:1387–1390. doi: 10.1016/s0140-6736(95)92405-1. [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi S, Nakagawa T, Arai H, et al. Temporal progression of hippocampal atrophy and apolipoprotein E gene in Alzheimer's disease. J Am Geriatr Soc. 1996;44:216–217. doi: 10.1111/j.1532-5415.1996.tb02451.x. [DOI] [PubMed] [Google Scholar]
- 57.Lehtovirta M, Soininen H, Laakso MP, et al. SPECT and MRI analysis in Alzheimer's disease: relation to apolipoprotein E ∈4 allele. J Neurol Neurosurg Psychiatry. 1996;60:644–649. doi: 10.1136/jnnp.60.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehtovirta M, Laakso MP, Soininen H, et al. Volumes of hippocampus, amygdala and frontal lobe in Alzheimer's patients with different apolipoprotein E genotypes. Neuroscience. 1995;67:65–72. doi: 10.1016/0306-4522(95)00014-a. [DOI] [PubMed] [Google Scholar]
- 59.Evans DA, Beckett LA, Field TS, et al. Apolipoprotein E ∈4 and incidence of Alzheimer disease in a community population of older persons. JAMA. 1997;277:822–824. [PubMed] [Google Scholar]
- 60.Rebeck GW, Reiter JS, Strickland DK, et al. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 61.de Leon MJ, Golomb J, George AE, et al. The radiologic prediction of Alzheimer disease: the atrophic hippocampal formation. AJNR. 1993;14:897–906. [PMC free article] [PubMed] [Google Scholar]
- 62.Kaye JA, Swihart T, Howieson D, Dame A. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 63.Kakulas BA, Wilton SD, Fabian VA, et al. Apolipoprotein-E genotyping in the diagnosis of Alzheimer's disease in autopsy confirmed series. Lancet. 1996;348:483. doi: 10.1016/s0140-6736(05)64588-6. Letter. [DOI] [PubMed] [Google Scholar]
- 64.Relkin NR, Tanzi R, Breitner J, et al. Apolipoprotein E genotyping in Alzheimer's disease. Lancet. 1996;347:1091–1095. [Google Scholar]
- 65.Roses AD. Apolipoprotein E genotype: utility in clinical practice in Alzheimer's disease. J Am Gerontol Soc. 1996;44:1–3. doi: 10.1111/j.1532-5415.1996.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 66.Saunders AM, Hulette C, Welsch KA, et al. Specificity, sensitivity and predictive value of apolipoprotein E genotyping for sporadic Alzheimer disease. Lancet. 1996;348:90–93. doi: 10.1016/s0140-6736(96)01251-2. [DOI] [PubMed] [Google Scholar]
- 67.Hyman BT. Apolipoprotein E genotype: utility in clinical practice in Alzheimer's disease. J Am Gerontol Soc. 1996;44:1469–1471. doi: 10.1111/j.1532-5415.1996.tb04073.x. [DOI] [PubMed] [Google Scholar]