Abstract
The synthetic oleanolic triterpenoid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Imidazolide or CDDO-Im) is an extremely potent activator of Nrf2 signaling. In cells undergoing adipogenesis, CDDO-Im prevents lipid accumulation in an Nrf2-dependent manner. However, in vivo evidence for effects of CDDO-Im on obesity is lacking. The goals of these studies were to determine if CDDO-Im can prevent high-fat diet-induced obesogenesis in the mouse, and to elucidate the molecular target of drug action. Wild-type and Nrf2-disrupted C57BL/6J female mice were dosed 3 times per week with 30 μmol/kg CDDO-Im or vehicle by oral gavage, during 95 days of access to a control diet or a high-fat diet. Body weights, organ weights, hepatic fat accumulation and gene expression were measured. Treatment with CDDO-Im effectively prevented high-fat diet-induced increases in body weight, adipose mass, and hepatic lipid accumulation in wild-type mice but not in Nrf2-disrupted mice. Wild-type mice on a high-fat diet and treated with CDDO-Im exhibited higher oxygen consumption and energy expenditure than vehicle-treated mice, while food intake was lower in CDDO-Im-treated than vehicle-treated mice. Levels of gene transcripts for fatty acid synthesis enzymes were downregulated after CDDO-Im treatment in the liver of wild-type mice. This inhibitory effect of CDDO-Im on lipogenic gene expression was significantly reduced in Nrf2-disrupted mice. The results indicate that CDDO-Im is an exceedingly potent agent for preventing obesity, and identify the Nrf2 pathway as a novel target for management of obesogenesis.
Keywords: Nrf2, Diet-induced obesity, Fatty acid synthase, Triterpenoid
1. Introduction
There has been a striking increase in the incidence of obesity worldwide over the past several decades. In 2005–2006, a third of adults in the USA were defined as obese (Centers for Disease Control and Prevention). Interest in obesity has expanded over the past decade because obesity is associated with increased morbidity and mortality (Rosen and MacDougald, 2006). Obesity is linked with type 2 diabetes, cardiovascular disease, osteoarthritis, as well as certain type of cancers (Rosen, 2005; Ginter and Simko, 2008; Cerulli et al., 2007). A wide range of pharmacologic approaches for the management of obesity such as appetite suppressants and lipase inhibitors have been evaluated (Cerulli et al., 2007), but their efficacy is often restricted to a weight reduction of 5–10% (Cerulli et al., 2007; Weigle, 2003), necessitating development of alternative options.
The synthetic triterpenoid analog of oleanolic acid 1-[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Im) is a potent inducer of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) signaling both in vitro and in vivo (Yates et al., 2007). Nrf2 is a member of the Cap’n’Collar subfamily of basic leucine zipper transcription factors that regulate expression of antioxidant and cytoprotective genes. Nrf2-disrupted mice do not exhibit any remarkable phenotype in the absence of exogenous stress, but are more sensitive to toxic challenges such as exposure to carcinogens, allergens and other inflammatory factors (Kensler et al., 2007). Therefore, studies using Nrf2-disrupted mice and/or CDDO-Im have been focused on its protective effects against electrophilic and oxidative stresses. However, previous studies have indicated that CDDO-Im inhibits accumulation of lipid droplets in an Nrf2-dependent manner in an adipocyte differentiation model in mouse embryonic fibroblasts (Shin et al., 2007), and CDDO-Im downregulates expression of fatty acid synthase (encoded by Fasn) in a liposarcoma cell line (Hughes et al., 2008). Moreover, recent expression analyses in our lab indicate that hepatic genes involved in fat and carbohydrate metabolism are major targets of CDDO-Im and Keap1, a cytoplasmic protein that binds Nrf2, thereby preventing its nuclear translocation and nuclear accumulation (Yates et al., 2009).
Oral administrations of CDDO-Im have been shown to ameliorate chronic diseases such as carcinogenesis and emphysema in rodent models (Yates et al., 2006, Sussan et al., 2009). However, the effects of this pharmacologic agent on obesogenesis in vivo remain unknown. We evaluated the potential for CDDO-Im to function as an anti-obesity drug in mice fed a high-fat diet. In order to determine whether and how CDDO-Im regulates whole body energy balance, and to identify molecular target responsible for drug actions on obesogenesis, effects of CDDO-Im treatment were examined on multiple parameters associated with obesity in wild-type and Nrf2-disrupted mice.
2. Materials and methods
2.1. Experimental animals
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee. Wild-type C57BL/6J mice were obtained from The Jackson Laboratories (Bar Harbor, ME). Nrf2-disrupted mice on a C57BL/6J background were generated as described previously (Itoh et al., 2004). Female mice (6–7 week-old) were fed a control diet (10 kcal% fat, Research Diets, New Brunswick, NJ) or a high-fat diet (60 kcal% fat, Research Diets) for 21 days or 95 days. Mice were dosed by oral gavage with vehicle (10% DMSO, 10% Cremophor-EL, and PBS) or CDDO-Im (30 μmol/kg body weight, obtained from Reata Pharmaceuticals, Dallas, Tx) 3 times per week throughout the feeding period (Monday, Wednesday, Friday). Organ weights, blood chemistry, and gene expression changes were determined 2 days after the last dose of CDDO-Im.
2.2. Indirect calorimetry
Wild-type mice were fed a high-fat diet for 82 days. Mice were dosed by oral gavage with vehicle or CDDO-Im (30 μmol/kg body weight) 3 times per week. Indirect calorimetry studies were conducted in an open-flow indirect calorimeter (Oxymax Equal Flow System; Columbus Instruments, Columbus, OH) at the Center for Metabolism and Obesity Research (Johns Hopkins University). Calorimetry, daily body weight, and daily food intake data were acquired during a 6-day acclimation period, followed by 4 days of experimental period. Oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured for each chamber every 17 minutes. The respiratory exchange ratio (RER = VCO2/VO2) was calculated by Oxymax software (v. 5.9). Energy expenditure was calculated as EE = VO2 × (3.815 + (1.232 × RER)) (Lusk, 1928), and normalized for subject body mass.
2.3. Blood chemistry
Blood samples were taken from the heart of sacrificed mice (unfasted) at the beginning of the light cycle at day 95, and allowed to clot at room temperature for 30 min. Serum samples were collected by centrifugation at 10 000 × g for 1 min at 4°C. Serum triglycerides, cholesterol, high-density lipoprotein (HDL)-cholesterol and low-density lipoprotein (LDL)-cholesterol levels were determined using a Vet ACE® chemistry system (Alfa Wassermann, West Caldwell, NJ) at the Johns Hopkins University Phenotyping Core.
2.4. Isolation of RNA and RT-PCR
RNA from liver and periuterine white adipose tissue was purified using the PerfectPure RNA Tissue Kit (5 PRIME, Gaithersburg, MD). Real-time PCR was performed on a My-IQ real-time PCR instrument (Bio-Rad Laboratories, Hercules, CA) using SYBR GREEN PCR Master Mix (Applied Biosystems, Warnington, UK). The PCR efficiency was determined as previously described (Pfaffl, 2001). Glyceraldehyde 3-phosphate dehydrogenase was used as normalizing controls. Primer sequences are shown in the Supplementary Table 1.
2.5. Fatty acid synthase assay
Fatty acid synthase activity was assayed as described previously (Pope and Rooney, 1987). Livers were homogenized in ice-cold 330 mM sucrose/l0 mM Tris-HCl/l mM EDTA (pH 7.4) for 1 min. The homogenate was centrifuged at 10 000 × g for 15 min followed by 105 000 × g for 1 h. The assay mixture contained 200 mM potassium phosphate (pH 7.0), 0.85 mM EDTA, 0.2 mM NADPH, 0.03 mM acetyl-CoA, 0.1 mM malonyl-CoA and supernatant fraction (100 μg of protein) in a total volume of 0.5 ml. The amount of NADPH oxidized was measured at 340 nm at 37 °C.
2.6. Histology
Formalin-fixed frozen sections of livers were stained with 0.5% Oil Red O solution in propylene glycol for 1 h, and counterstained with hematoxylin for 30 sec. For each slide, 6 random fields were captured with an Olympus BX60 microscope (Olympus America, Center Valley, PA) and the area stained with Oil Red O was measured by Image-Pro Plus software (Media Cybernetics, Bethesda, MD).
2.7. Statistical analysis
Data represent mean ± S.E.M. Statistical significance was determined by Student’s t test or by repeated measures analysis of variance followed by Student-Newman-Keuls test as appropriate. P values of ≤0.05 are considered significant.
3. Results
3.1. CDDO-Im prevents body weight gain following a high-fat diet in an Nrf2-dependent manner
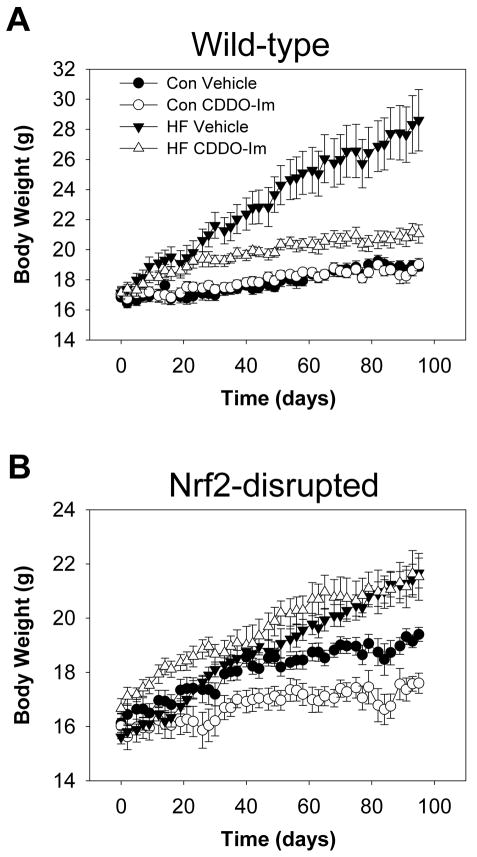
Previous studies indicated that CDDO-Im inhibits fat accumulation in cell culture models (Shin et al., 2007; Hughes et al., 2008). In order to determine effects of CDDO-Im on body weight gain, wild-type mice fed a control diet or a high-fat diet were dosed chronically with vehicle or 30 μmol/kg CDDO-Im (Fig. 1A). Our previous study indicated that 30 μmol/kg CDDO-Im was optimal to induce the Nrf2-target gene NAD(P)H: quinone acceptor oxidoreductase 1 in mouse liver (Yates et al., 2007). A long-term study, in which mice on a standard diet were fed a higher dose of CDDO-Im for 6 months, indicated no significant effect on weight gain (Sussan et al., 2009). In the present study, CDDO-Im effectively prevented weight gain only in high-fat diet-fed but not in control diet-fed wild-type mice, without any evidence of gross toxicity (Fig. 1A). In order to determine whether this inhibitory effect of CDDO-Im was Nrf2-dependent, the same experiment was repeated using Nrf2-disrupted mice. Although there was no sign of gross toxicity such as lethargy or ruffled fur, CDDO-Im had a slight inhibitory effect on rate of weight gain in Nrf2-disrupted mice in contrast to the wild-type mice. Despite this effect in control mice, the inhibitory effect of CDDO-Im on weight gain following a high-fat diet was completely lost in Nrf2-disrupted mice (Fig. 1B), suggesting that CDDO-Im prevents obesogenesis in an Nrf2-dependent manner.
Fig. 1.
Effects of CDDO-Im on body weight gain of (A) wild-type mice and (B) Nrf2-disrupted mice. Mice fed a control diet (Con) or a high-fat diet (HF) for 95 days were dosed with 30 μmol/kg body weight CDDO-Im or vehicle by gavage 3 times per week throughout the feeding period with both diets. Body weights were monitored before each treatment, 3 times per week. Data represent mean ± S.E.M., n = 8–15 per group.
Inhibition of body weight gain was associated with a dramatic reduction of periuterine and mesenteric white adipose tissues in high-fat diet-fed animals following treatment with CDDO-Im in wild-type but not in Nrf2-disrupted mice (Table 1). Liver weights of wild-type mice were increased slightly following CDDO-Im treatment (Table 1), a response seen with other chemical classes of activators of the Keap1-Nrf2 pathway. Pretreatment with CDDO-Im did not prevent high-fat diet-induced elevation of levels of total cholesterol, low-density lipoprotein (LDL)-cholesterol, and high-density lipoprotein (HDL)-cholesterol, in either genotype (Table 2). Conversely, CDDO-Im prevented high-fat diet-induced elevation of serum triglyceride levels in wild-type mice, but triglyceride levels were increased in CDDO-Im-treated Nrf2-disrupted mice (Table 2). We were not able to detect statistically significant increases in levels of triglyceride and LDL-cholesterol in Nrf2-disrupted mice following a high-fat diet. This likely due to the fact that rate of body weight increment following a high-fat diet was slower in vehicle-treated Nrf2-disrupted mice than in wild-type mice (0.063 g/day and 0.119 g/day, respectively).
Table 1.
Organ weights (mg) of control diet and high-fat diet-fed mice
| Control diet | High-fat diet | |||
|---|---|---|---|---|
| Vehicle | CDDO-Im | Vehicle | CDDO-Im | |
|
Wild-type | ||||
| Periuterine white adipose tissue | 443 ± 25 (2.3) | 400 ± 27 (2.1) | 2164 ± 424b (7.0) | 713 ± 121ab (3.3) |
| Mesenteric white adipose tissue | 131 ± 17 (0.7) | 122 ± 18 (0.6) | 422 ± 107b (1.4) | 155 ± 8a (0.7) |
| Pancreas | 167 ± 13 (0.9) | 149 ± 6 (0.8) | 242 ± 14b (0.9) | 176 ± 10a (0.8) |
| Liver | 860 ± 22 (4.6) | 1107 ± 45a (5.8) | 966 ± 61 (3.4) | 1065 ± 61 (5.1) |
| Kidney | 127 ± 3 (0.7) | 134 ± 5 (0.7) | 148 ± 8b (0.5) | 142 ± 4 (0.7) |
|
Nrf2-distupted | ||||
| Periuterine white adipose tissue | 464 ± 34 (2.4) | 385 ± 65 (2.2) | 834 ± 100b (3.7) | 827 ± 160b (3.6) |
| Mesenteric white adipose tissue | 107 ± 10 (0.6) | 139 ± 25 (0.8) | 199 ± 26b (0.9) | 221 ± 39 (1.0) |
| Pancreas | 213 ± 10 (1.1) | 182 ± 13 (1.0) | 238 ± 12 (1.1) | 203 ± 14 (0.9) |
| Liver | 760 ± 34 (3.8) | 709 ± 43 (4.1) | 777 ± 25 (3.6) | 803 ± 29 (3.7) |
| Kidney | 122 ± 5 (0.6) | 115 ± 6 (0.7) | 138 ± 7 (0.6) | 128 ± 3 (0.6) |
Organ weights as % of total body weight are shown in the parenthesis. Data represent mean ± S.E.M., n = 9–15.
P < 0.05, CDDO-Im vs. vehicle.
P < 0.05, high-fat diet vs. control diet.
Table 2.
Metabolic parameters of control diet and high-fat diet-fed mice
| Control diet | High-fat diet | |||
|---|---|---|---|---|
| Vehicle | CDDO-Im | Vehicle | CDDO-Im | |
|
Wild-type | ||||
| Triglyceride (mg/dl) | 83 ± 9 | 84 ± 9 | 123 ± 8b | 115 ± 27 |
| Cholesterol (mg/dl) | 74 ± 5 | 95 ± 4a | 132 ± 8b | 151 ± 8b |
| LDL-cholesterol (mg/dl) | 28 ± 2 | 34 ± 3 | 45 ± 4b | 55 ± 7b |
| HDL-cholesterol (mg/dl) | 29 ± 4 | 44 ± 3a | 63 ± 3b | 74 ± 4b |
|
Nrf2-distupted | ||||
| Triglyceride (mg/dl) | 102 ± 9 | 101 ± 10 | 95 ± 7 | 132 ± 10ab |
| Cholesterol (mg/dl) | 84 ± 6 | 85 ± 10 | 104 ± 7b | 116 ± 8b |
| LDL-cholesterol (mg/dl) | 31 ± 3 | 30 ± 5 | 37 ± 3 | 36 ± 4 |
| HDL-cholesterol (mg/dl) | 33 ± 3 | 35 ± 5 | 49 ± 4b | 54 ± 4b |
Data represent mean ± S.E.M., n = 7–10.
P < 0.05, CDDO-Im vs. vehicle.
P < 0.05, high-fat diet vs. control diet.
In summary, our data indicate that CDDO-Im prevents increases in body weight, adipose mass and triglyceride following a high-fat diet through Nrf2 signaling.
3.2. Energy expenditure of CDDO-Im-treated mice is higher compared to vehicle-treated mice
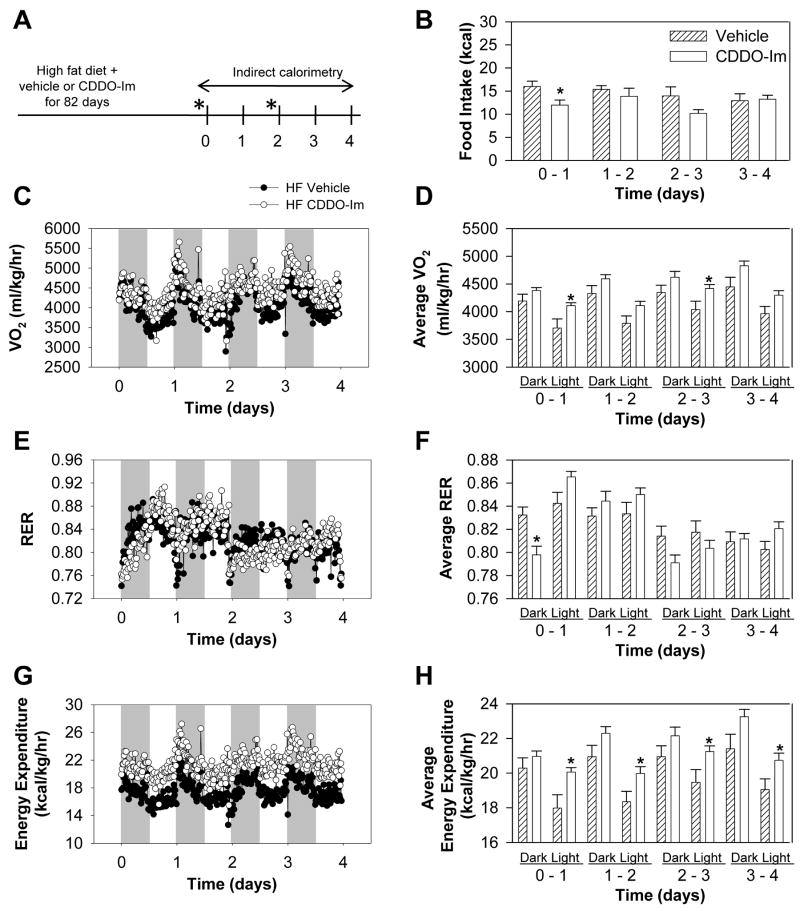
Both increased energy intake and a reduced metabolic rate contribute to the weight gain observed from ingesting a high-fat diet (Winzell and Ahrén 2004). Therefore, for accurate measurement of energy intake and energy expenditure, indirect calorimetry was performed in wild-type mice following 82 days of the high-fat diet in combination with CDDO-Im or vehicle treatment (Fig. 2A). Oxygen consumption and energy expenditure were increased following CDDO-Im treatment (Fig. 2C, 2D, 2G, 2H). Average oxygen consumption values were increased following CDDO-Im treatment by 4.5, 6.1, 6.4, 8.6% and 10.9, 8.4, 9.5, 8.4% at days 0, 1, 2, 3 during each dark and light cycle, respectively (Fig. 2D). Average energy expenditure was increased following CDDO-Im treatment by 3.3, 6.5, 5.8, 8.5% and 11.5, 8.9, 9.1, 8.9% at days 0, 1, 2, 3 during each dark and light cycle, respectively (Fig. 2H). These data suggest that the effects of CDDO-Im on oxygen consumption and energy expenditure were more prominent during light cycles than dark cycles of each day. CDDO-Im produced transient decreases in respiratory exchange ratio during the first day after dosing by gavage at days 0 and 2 of the indirect calorimetry measurements, indicating increased fat oxidation, but there was a rebound effect (Fig. 2E, 2F). CDDO-Im also produced transient hypophagia that was significant (day 0) or nearly so (day 2) on dosing days (Fig. 2B). Overall, the data indicate that repeated dosing of CDDO-Im results in a chronic increase in energy expenditure and an acute increase in fat oxidation and decrease in food intake.
Fig. 2.
Indirect calorimetry and food intake analysis in wild-type mice fed a high-fat diet. (A) Protocol for indirect calorimetry. High-fat diet-fed mice were treated with 30 μmol/kg body weight CDDO-Im or vehicle for 82 days and indirect calorimetry was performed thereafter from days 0 to 4. Mice were dosed with CDDO-Im on days 0 and 2 (*). (B–H) All data represent mean ± S.E.M., n = 8 per group. * P < 0.05, CDDO-Im vs. vehicle. (B) Food intake (C) Oxygen consumption (VO2) (D) Average oxygen consumption (E) Respiratory exchange ratio (RER) (F) Average respiratory exchange ratio (G) Energy expenditure normalized to body weight (H) Average energy expenditure normalized to body weight.
3.3. CDDO-Im prevents fat accumulation in liver in an Nrf2-dependent manner
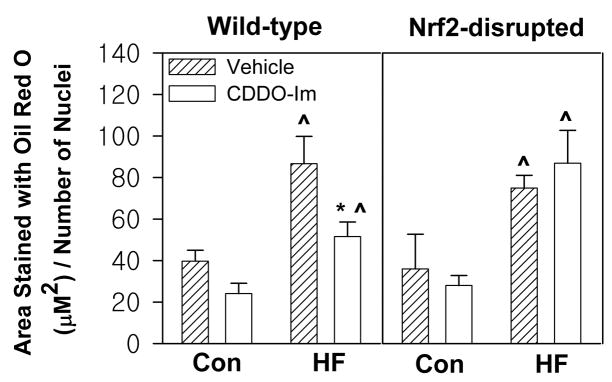
Non-alcoholic fatty liver disease is the most common cause of chronic liver disease and prevails in approximately 20% of the US population (Qureshi and Abrams, 2007). In order to determine the effect of CDDO-Im treatment on hepatic lipid homeostasis, lipid content of the liver of control diet-fed or high-fat diet-fed mice treated with CDDO-Im or vehicle was examined by Oil Red O staining. CDDO-Im treatment reduced fat accumulation in the liver by 40% in wild-type animals on a high-fat diet but not in Nrf2-disrupted mice (Fig. 3). These data suggest that CDDO-Im regulates hepatic lipid homeostasis in an Nrf2-dependent manner, and that this pharmacologic agent may be used for prevention of fatty liver due to obesity.
Fig. 3.
Effects of CDDO-Im on hepatic lipid accumulation in wild-type mice and Nrf2-disrupted mice. Liver sections were stained with Oil Red O, and surface area of stained lipid droplets was normalized by number of hepatocyte nuclei per field. All data represent mean ± S.E.M., n = 5–8 per group. * P < 0.05, CDDO-Im vs. vehicle. ^ P < 0.05, high-fat diet vs. control diet.
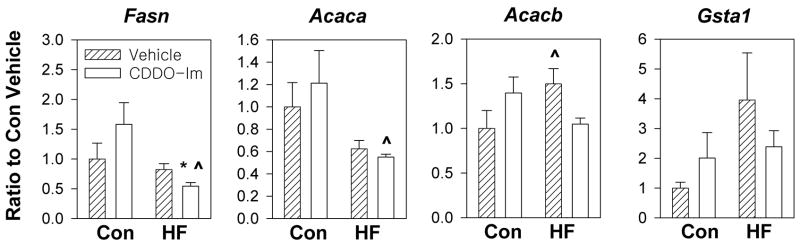
3.4. CDDO-Im regulates expression of fatty acid synthesis and oxidation enzymes in liver
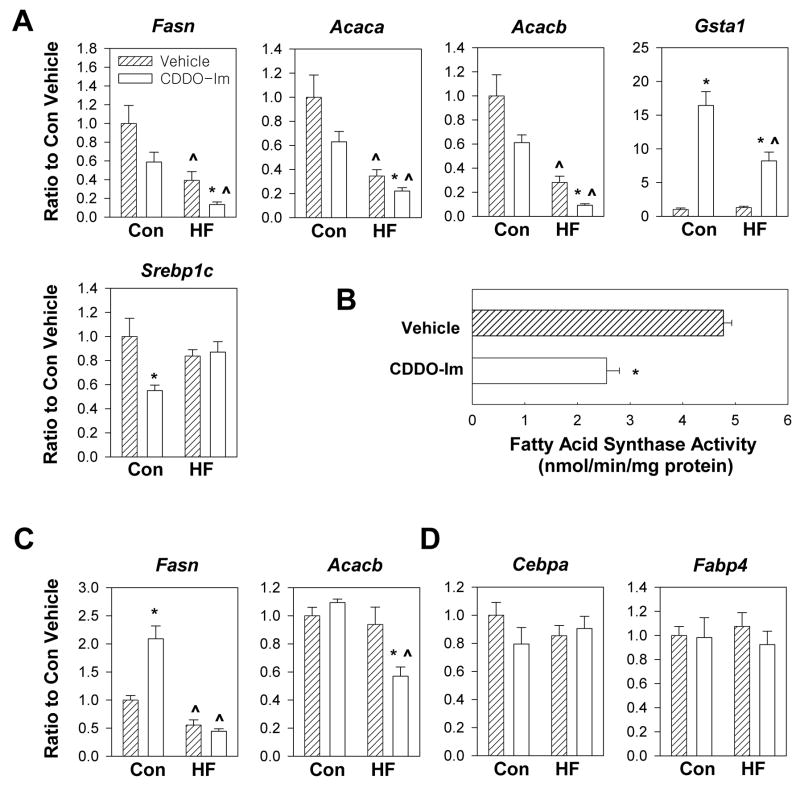
In order to determine how CDDO-Im regulates body weight gain, quantitative real-time PCR was used to study hepatic gene expression of wild-type mice. Fatty acid synthase and acetyl-CoA carboxylase are key enzymes involved in the biosynthesis of fatty acids. Acetyl-CoA carboxylase catalyzes the conversion of acetyl-CoA to malonyl-CoA, and fatty acid synthase uses malonyl-CoA and acetyl-CoA to synthesize long-chain fatty acids. In addition to its role as a substrate, malonyl-CoA plays a central role in the control of fat oxidation through an inhibition of carnitine palmitoyl transferase I, the rate-limiting step in fatty acid uptake into mitochondria (Wolfgang and Lane MD 2006). While acetyl-CoA carboxylase 1 (encoded by Acaca) provides malonyl-CoA to fatty acid synthase in the cytoplasm, acetyl-CoA carboxylase 2 (encoded by Acacb) inhibits beta oxidation by producing malonyl-CoA at the mitochondrial membrane. Levels of mRNA for Fasn was downregulated by CDDO-Im by 41% (P = 0.09) and 66% (P = 0.02) in control diet and high-fat diet-fed mice, respectively (Fig. 4A). The specific activity of fatty acid synthase was also decreased by 47% (P = 0.0003) following CDDO-Im treatment in mice fed a high-fat diet (Fig. 4B). CDDO-Im treatment decreased mRNA expression for Acaca by 37% (P = 0.10) and 36% (P = 0.05) in control diet and high-fat diet-fed mice, respectively (Fig. 4A). CDDO-Im treatment downregulated mRNA expression of Acacb by 39% (P = 0.06) and 68% (P = 0.003) in control diet and high-fat diet-fed mice, respectively (Fig. 4A). Sterol regulatory element-binding protein-1c (encoded by Srebp1c), a transcription factor that enhances expression of fatty acid synthase and acetyl-CoA carboxylase (Yang et al., 2001), was downregulated by 45% (P = 0.02) in control diet fed-mice but not in high-fat diet-fed mice (Fig. 4A), implying that CDDO-Im regulates expression of these genes through alternative mechanisms. Gluthathione S-transferase alpha-1 (encoded by Gsta1) is a well characterized target gene of CDDO-Im that is regulated through the Keap1-Nrf2 signaling pathway (Shin et al., 2007). CDDO-Im increased the level of Gsta1 transcripts in livers of control diet-fed mice by more than 16-fold. This response is substantially blunted in mice fed a high-fat diet. High-fat diets are known to downregulate the expression of hepatic genes encoding glutathione S-transferases (Toye et al., 2007). Overall, CDDO-Im downregulated the expression of enzymes involved in fatty acid synthesis, and its inhibitory effects on Fasn and Acacb mRNA expression were greater than 60% in high-fat diet-fed mice.
Fig. 4.
Effects of CDDO-Im on gene expression and fatty acid synthase activity in wild-type mice fed a control diet or a high-fat diet for 95 days (A, B, D) or 21 days (C). (A) Analysis of hepatic gene expression using quantitative real-time PCR following 95 days of treatment. Data represent mean ± S.E.M., n = 6–8 per group. * P ≤ 0.05, CDDO-Im vs. vehicle. ^ P < 0.05, high-fat diet vs. control diet. (B) Specific activity of fatty acid synthase in the liver of mice fed a high-fat diet for 95 days (nmol NADPH oxidized per min per mg protein). Data represent mean ± S.E.M., n = 4 per group. * P < 0.05, CDDO-Im vs. vehicle. (C) Analysis of hepatic gene expression using quantitative RT-PCR following 21 days of treatment. Data represent mean ± S.E.M., n = 6 per group. * P < 0.05, CDDO-Im vs. vehicle. ^ P < 0.05, high-fat diet vs. control diet. (D) Analysis of gene expression in white adipose tissue using quantitative RT-PCR following 95 days of treatment. Data represent mean ± S.E.M., n = 6 per group. * P < 0.05, CDDO-Im vs. vehicle. ^ P < 0.05, high-fat diet vs. control diet.
In order to further investigate the mechanisms underlying the inhibitory effect of CDDO-Im against body weight gain, mice were fed a high-fat diet or a control diet for 3 weeks and co-treated with vehicle and CDDO-Im, and gene expression in the liver was analyzed. No statistically significant differences were detected in this short time-frame between vehicle or CDDO-Im-treated mice fed a high-fat diet in terms of body weight, periuterine and mesenteric adipose tissue mass and serum triglyceride level (data not shown). The mRNA levels of Fasn were not affected by CDDO-Im treatment in mice fed a high-fat diet (Fig. 4C), although CDDO-Im downregulated mRNA levels of Acacb (39.3 %). There results suggest that sustained dosing of CDDO-Im is required to induce changes in hepatic expression of lipogenic enzymes in the liver of mice fed a high-fat diet.
3.5. CDDO-Im does not affect expression of genes involved in lipogenesis and adipocyte differentiation in white adipose tissue
In addition to the liver, white adipose tissue is another key organ that regulates lipogenesis. Therefore, expression of genes involved in fatty acid metabolism was analyzed in periuterine white adipose tissue. Gsta1 expression was induced 28-fold in high-fat diet-fed mice following CDDO-Im treatment (data not shown) indicating that CDDO-Im distributes to adipose tissues. However, although levels of Fasn and Acacb transcripts were lower in high-fat diet-fed mice than in control diet-fed mice, none of these genes were downregulated following CDDO-Im treatment in white adipose tissue in contrast to the liver (data not shown).
Our previous report demonstrated that Nrf2 inhibits adipocyte differentiation in mouse embryonic fibroblasts, and that this inhibitory effect is associated with modulation of expression of terminal markers of differentiation such as CCAAT/enhancer-binding protein alpha (encoded by Cebpa) and fatty acid binding protein (encoded by Fabp4) (Shin et al. 2007). In order to evaluate the effects of Nrf2 genotype on adipocyte differentiation, mRNA levels of Cebpa and Fabp4 were analyzed in white adipose tissue of Nrf2-disrupted mice and wild-type mice fed a control diet or a high-fat diet for 13 weeks. We were not able to detect an increase in the expression of differentiation markers following a high-fat diet in white adipose tissue in either genotype, and no differences in the mRNA levels of these markers between Nrf2+/+ and Nrf2−/− mice were detected (data not shown). Consistent with these results, mRNA levels of Cebpa and Fabp4 were not affected by CDDO-Im treatment (Fig. 4D). Overall, our data indicate that CDDO-Im modulates gene expression in liver rather than in white adipose tissue.
3.6. Inhibitory effects of CDDO-Im on expression of fatty acid synthesis and oxidation enzymes is suppressed in the liver of Nrf2-disrupted mice
Gene expression in the liver of Nrf2-disrupted mice fed a control diet or a high-fat diet for 95 days dosed with CDDO-Im or vehicle was analyzed to determine whether CDDO-Im regulates expression of lipogenic genes in an Nrf2-dependent manner. Levels of mRNA for Fasn was downregulated by CDDO-Im by 33.8% (P = 0.04) in high-fat diet-fed mice but not in control diet-fed mice (Fig. 5). Inhibitory effects of CDDO-Im on expression of Acaca and Acacb and positive effects on expression of Gsta1 were completely abrogated in Nrf2-disrupted mice fed a high-fat diet and a control diet. Decreases in levels of Fasn, Acaca, Acacb and Gsta1 following a high-fat diet were also substantially suppressed in Nrf2-disrupted mice. These results indicate that CDDO-Im regulates transcription of Fasn, Acaca and Acacb through Nrf2 signaling.
Fig. 5.
Effects of CDDO-Im on hepatic gene expression in Nrf2-disrupted mice. Data represent mean ± S.E.M., n = 6 per group. * P < 0.05, CDDO-Im vs. vehicle. ^ P < 0.05, high-fat diet vs. control diet.
4. Discussion
Prevalence of obesity has markedly increased in many parts of the world (Ginter and Simko, 2008). According to the Centers for Disease Control and Prevention, health consequences of obesity include coronary heart disease, type 2 diabetes, cancers, liver and gallbladder disease, highlighting the importance of prevention of obesity. An inhibitory effect of CDDO-Im on lipid synthesis has been demonstrated in vitro (Shin et al., 2007; Hughes et al., 2008), but its effect on obesogenesis in vivo remains unknown. Herein, we investigated the effect of CDDO-Im on obesity in mice chronically fed a high-fat diet and observed that CDDO-Im selectively prevents body weight gain in high-fat-fed mice but not in control diet-fed mice.
To investigate the mechanism underlying the effects of CDDO-Im on body weight gain, a number of parameters were addressed. First, analysis of blood chemistry indicated that CDDO-Im prevented diet-induced increases in serum triglycerides in wild-type mice. However, CDDO-Im upregulates serum cholesterol levels and HDL-cholesterol levels, especially in wild-type mice fed a control diet. This effect might be partly explained by the fact that CDDO-Im affects multiple genes involved in biliary cholesterol excretion (Yates et al., 2009). In addition to blood chemistry, quantitative real-time PCR analyses were used to evaluate effects of CDDO-Im on expression of hepatic genes. Transcript levels of Fasn, Acaca, and Acacb were dramatically downregulated in liver following chronic CDDO-Im treatment. These data were consistent with our observation that CDDO-Im prevents lipid accumulation in liver. While the CDDO-Im-induced changes in Fasn transcript levels in the liver of control diet-fed mice were not statistically significant, Fasn mRNA was decreased by 66% in obese mice following CDDO-Im treatment. These differing effects of CDDO-Im on Fasn expression in high-fat diet-fed mice and control diet-fed mice may partly explain the selective effects of CDDO-Im on body weight gain in the high-fat-fed mice. However, mRNA expression of other genes involved in fat oxidation and energy expenditure such as peroxisome proliferator-activated receptor alpha, peroxisome proliferator-activated receptor gamma coactivator-1 alpha, acyl-CoA oxidase 1, and carnitine palmitoyl transferase I alpha (the liver isoform of carnitine palmitoyl transferase I) were not affected by CDDO-Im in the liver of mice fed a high-fat diet (data not shown). We also investigated effects of CDDO-Im on gene expression in other organs involved in fat synthesis and oxidation, i.e white adipose tissue and skeletal muscle. CDDO-Im did not downregulate levels of Fasn, Acaca, and Acacb in white adipose tissue (data not shown), implying involvement of hepatic-specific transcriptional regulation. In addition, CDDO-Im did not alter expression of genes involved in fatty acid oxidation in skeletal muscle (data not shown). Since our data indicate that CDDO-Im inhibits food intake and increases fat oxidation and energy expenditure, it remains to be determined whether and how inhibition of hepatic lipogenic enzymes by CDDO-Im contributes to alterations in systemic energy balance.
To investigate the mechanism of CDDO-Im at an earlier time-point before its anti-obesogenic effects is evident, gene expression in mice fed a control diet or a high-fat diet for 21 days has been analyzed. Transcript levels of Fasn and Acacb were increased or unchanged following CDDO-Im-treatment in mice fed a low fat diet (Fig. 4C). CDDO-Im did not affect expression of Fasn in the liver of mice-fed a high-fat diet for 21 days, and the extent of inhibition of Acacb by CDDO-Im in the liver of mice fed a high-fat diet was diminished in mice treated for 21 days compared to mice treated for 95 days (39% and 68%, respectively). A previous report from our lab indicates that mRNA expression of fatty acid synthase was downregulated in mouse liver following a single dose of CDDO-Im (Yates et al., 2009). Consistent with our finding, Tanaka et al. (2008) has reported that hepatic mRNA levels of Fasn and Acaca are higher in Nrf2-disrupted mice than in wild-type mice following 4 weeks of high-fat diet. One possible explanation for this lack of inhibition of Fasn expression after 21days of treatment is that mice become transiently resistant to CDDO-Im following repeated dosing during 21 days, but eventually respond to the inhibitory effect of CDDO-Im on obesogenesis following sustained dosing.
In the present study, we hypothesized that CDDO-Im will downregulate expression of markers of adipocyte differentiation in white adipose tissue following a high-fat diet, based on the observation that Nrf2 inhibits adipocyte differentiation in mouse embryonic fibroblasts (Shin et al., 2007). Therefore, we evaluated effects of Nrf2 genotype and CDDO-Im on body weight gain in mice chronically fed a high-fat diet. However, we were not able to detect effects of diet or CDDO-Im on expression of Cebpa and Fabp4. A prolonged feeding period will be required to detect changes of transcript levels in white adipose tissue where expression levels of markers of adipogenesis are relatively abundant, and to detect effects of Nrf2 genotype on adipogenesis.
Based on the fact that CDDO-Im is known to target Nrf2 signaling (Dinkova-Kostova et al., 2005; Yates et al., 2006), we evaluated effects of CDDO-Im on obesogenesis in Nrf2-disrupted mice. However, studies using Nrf2-disrupted mice are confounded because the rate of body weight increment following a high-fat diet was substantially slower in vehicle-treated Nrf2-disrupted mice than in wild-type mice, and weights of periuterine and mesenteric white adipose tissues as % of total body weight were smaller in Nrf2-disrupted mice than in wild-type mice. There were no statistically significant differences in adipose mass between two genotypes fed a control diet treated along with dosing with the vehicle. However, initial body weights of Nrf2-disrupted mice are smaller than wild type mice (16.0 g and 16.9 g, respectively). These results suggest that while activation of Nrf2 signaling by CDDO-Im negatively affects accumulation of fat, constitutive levels of Nrf2 may contribute to accumulation of fat following a high-fat diet. Furthermore, rate of body weight increment following a control diet was slightly slower in CDDO-Im-treated Nrf2-disrupted mice than in vehicle-treated mice. Despite its potential toxicity in Nrf2-disrupted mice, CDDO-Im did not prevent weight gain following a high-fat diet in Nrf2-disrupted mice, and the inhibitory effects of CDDO-Im on triglyceride levels, hepatic lipid accumulation and expression of lipogenic genes were significantly suppressed in the Nrf2-disrupted mice.
In summary, our data indicate that CDDO-Im effectively prevents body weight gain following a high-fat diet, and that Nrf2 can be an attractive target for management of obesogenesis. Further studies will be required to elucidate mechanisms underlying molecular regulation of systemic energy balance and transcription of lipogenic enzymes by triterpenoids and other classes of activators of Nrf2 signaling.
Supplementary Material
Acknowledgments
This work was supported by NIH grants CA094076 (TWK) and CA78814 (MBS), and by a grant from Reata Pharmaceuticals (MBS). Soona Shin is recipient of a Samsung Scholarship (Samsung Foundation of Culture). This forms part of the dissertation of S.S. at the Johns Hopkins University. We thank Brian H. Schofield, Nadine Forbes, and Patricia Egner for assistance with histology, the lipid panel, and fatty acid synthase assay, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Centers for Disease Control and Prevention. ( http://www.cdc.gov/nccdphp/dnpa/obesity/index.htm)
- Cerulli J, Lomaestro BM, Malone M. Update on the pharmacotherapy of obesity. Ann Pharmacother. 2007;41:1505–1517. doi: 10.1345/aph.140066. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Liby KT, Stephenson KK, Holtzclaw WD, Gao X, Suh N, Williams C, Risingsong R, Honda T, Gribble GW, Sporn MB, Talalay P. Extremely potent triterpenoid inducers of the phase 2 response: correlations of protection against oxidant and inflammatory stress. Proc Natl Acad Sci U S A. 2005;102:4584–4569. doi: 10.1073/pnas.0500815102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginter E, Simko V. Adult obesity at the beginning of the 21st century: epidemiology, pathophysiology and health risk. Bratisl Lek Listy. 2008;109:224–230. [PubMed] [Google Scholar]
- Hughes DT, Martel PM, Kinlaw WB, Eisenberg BL. The synthetic triterpenoid CDDO-Im inhibits fatty acid synthase expression and has antiproliferative and proapoptotic effects in human liposarcoma cells. Cancer Invest. 2008;26:118–127. doi: 10.1080/07357900701522612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K, Itoh K, Kumagai Y, Oyasu R, Hattori K, Kawai K, Shimazui T, Akaza H, Yamamoto M. Nrf2 is essential for the chemopreventive ef cacy of oltipraz against urinary bladder carcinogenesis. Cancer Res. 2004;64:6424–6431. doi: 10.1158/0008-5472.CAN-04-1906. [DOI] [PubMed] [Google Scholar]
- Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- Lusk G. The elements of the science of nutrition. 4. WB Saunders; Philadelphia, PA: 1928. [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope TS, Rooney SA. Effects of glucocorticoid and thyroid hormones on regulatory enzymes of fatty acid synthesis and glycogen metabolism in developing fetal rat lung. Biochim Biophys Acta. 1987;918:141–148. doi: 10.1016/0005-2760(87)90189-5. [DOI] [PubMed] [Google Scholar]
- Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver. World J Gastroenterol. 2007;13:3540–3553. doi: 10.3748/wjg.v13.i26.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED. The transcriptional basis of adipocyte development. Prostaglandins Leukot Essent Fatty Acids. 2005;73:31–34. doi: 10.1016/j.plefa.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW. NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis. Mol Cell Biol. 2007;27:7188–7197. doi: 10.1128/MCB.00915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussan TE, Rangasamy T, Blake DJ, Malhotra D, El-Haddad H, Bedja D, Yates MS, Kombairaju P, Yamamoto M, Liby KT, Sporn MB, Gabrielson KL, Champion HC, Tuder RM, Kensler TW, Biswal S. Targeting Nrf2 with the triterpenoid CDDO-imidazolide attenuates cigarette smoke-induced emphysema and cardiac dysfunction in mice. Proc Natl Acad Sci U S A. 2009;106:250–255. doi: 10.1073/pnas.0804333106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- Toye AA, Dumas ME, Blancher C, Rothwell AR, Fearnside JF, Wilder SP, Bihoreau MT, Cloarec O, Azzouzi I, Young S, Barton RH, Holmes E, McCarthy MI, Tatoud R, Nicholson JK, Scott J, Gauguier D. Subtle metabolic and liver gene transcriptional changes underlie diet-induced fatty liver susceptibility in insulin-resistant mice. Diabetologia. 2007;50:1867–1879. doi: 10.1007/s00125-007-0738-5. [DOI] [PubMed] [Google Scholar]
- Weigle DS. Pharmacological therapy of obesity: past, present, and future. J Clin Endocrinol Metab. 2003;88:2462–2469. doi: 10.1210/jc.2003-030151. [DOI] [PubMed] [Google Scholar]
- Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53 (Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- Wolfgang MJ, Lane MD. The role of hypothalamic malonyl-CoA in energy homeostasis. J Biol Chem. 2006;281:37265–37269. doi: 10.1074/jbc.R600016200. [DOI] [PubMed] [Google Scholar]
- Yang J, Goldstein JL, Hammer RE, Moon YA, Brown MS, Horton JD. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc Natl Acad Sci U S A. 2001;98:13607–13612. doi: 10.1073/pnas.201524598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates MS, Kwak MK, Egner PA, Groopman JD, Bodreddigari S, Sutter TR, Baumgartner KJ, Roebuck BD, Liby KT, Yore MM, Honda T, Gribble GW, Sporn MB, Kensler TW. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 2006;66:2488–2494. doi: 10.1158/0008-5472.CAN-05-3823. [DOI] [PubMed] [Google Scholar]
- Yates MS, Tauchi M, Katsuoka F, Flanders KC, Liby KT, Honda T, Gribble GW, Johnson DA, Johnson JA, Burton NC, Guilarte TR, Yamamoto M, Sporn MB, Kensler TW. Pharmacodynamic characterization of chemopreventive triterpenoids as exceptionally potent inducers of Nrf2-regulated genes. Mol Cancer Ther. 2007;6:154–162. doi: 10.1158/1535-7163.MCT-06-0516. [DOI] [PubMed] [Google Scholar]
- Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams CR, Liby KT, Sporn MB, Sutter TR, Kensler TW. Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice. Carcinogenesis. 2009;30:1024–1031. doi: 10.1093/carcin/bgp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.