Abstract
Differentiation of serious bacterial infection (SBI) from self-limiting viral illness in febrile infants younger than three months is a significant challenge for clinicians. We aimed to assess the risk factors for SBI in febrile infants. Data were obtained from 221 infants younger than three months who visited a single community referral hospital for fever and underwent a complete sepsis workup between August 2003 and July 2006. The causes of fever were febrile illness without a documented cause (FISDC, 65%), urinary tract infection (UTI, 12%), aseptic meningitis (12%), bacteremia (4%), bacterial meningitis (2%). Cerebrospinal fluid enterovirus polymerase chain reaction was positive in 28% of FISDC and 48% of aseptic meningitis cases. When UTI was excluded, the risk factors for SBI were 1) C-reactive protein (CRP) level of ≥1.87 mg/dL and 2) fevers of ≥38.9℃. The specificity and negative predictive values of risk factors 1) and 2) for the diagnosis of SBI were 94% and 95%, respectively. We concluded that enteroviral infection may be a major cause of febrile episodes in infants younger than three months. If UTI could be excluded, the presence of CRP levels ≥1.87 mg/dL and fevers of ≥38.9℃ can be used as criteria to rule out SBI in these infants.
Keywords: C-reactive Protein, Enterovirus Infections, Fever, Neonatal Sepsis, Serious Bacterial Infection
INTRODUCTION
Fever is the most common chief symptom in infants younger than three months visiting emergency departments or outpatient clinics. The causes of fever in these infants vary from mild viral infections (which are usually self-limiting) to serious bacterial infections (SBIs) (such as urinary tract infections [UTIs], bacteremia, and bacterial meningitis) which are progressive and lead to permanent neurological sequelae or death without proper antibiotic treatment (1-3). The incidence of SBI in febrile infants younger than three months old has been reported from 7% to 18% (4-7). However, the symptoms and signs of febrile infants who are suffering from SBIs are usually nonspecific and not discernable from mild viral infections, especially in the early phase of SBI (6, 7). Moreover, the definite diagnosis of SBI is usually made on the basis of culture studies, which are typically unavailable at the time of initial evaluation. Therefore, it is not easy to decide which infants require hospitalization and antibiotic therapy. Consequently, there have been efforts to classify febrile infants by level of risk to indicate which cases might require more aggressive management (8-11). These efforts resulted in the development of scales or criteria to determine which infants are at higher risk. Although these scales or criteria have served as reliable guidelines in the management of febrile infants, they are imperfect tools, in terms of their applicability to all geographic regions and clinical situations. Therefore, it may be necessary to develop individual criteria to identify high-risk febrile infants based on epidemiology and clinical setting for each health care facility.
The object of our study was to develop clinical criteria to help guide clinicians in distinguishing high-risk vs. low-risk febrile infants for SBI among infants younger than three months old in a community referral hospital.
MATERIALS AND METHODS
Subjects
There were 221 study subjects. They represent all infant patients younger than three months old who visited the emergency department or outpatient clinic of Seoul National University Bundang Hospital for fevers (≥38℃ at axilla) of unknown focus and who were directly hospitalized on the suspicion of neonatal sepsis between August, 2003 and July, 2006. Febrile infants who had an evident comorbid condition such as pneumonia, bronchiolitis, gastroenteritis, and arthritis were excluded. However, febrile infants with mild upper respiratory tract infection (URI) symptoms such as runny nose, nasal obstruction, cough, and mild gastrointestinal (GI) symptoms (such as regurgitation and loose stool) were not excluded, because the presence of such conditions was not sufficient to rule out neonatal sepsis. Preterm infants who had fevers in the neonatal intensive care unit and term infants who had fevers within the first 72 hr after birth (and thus were suspected to be affected with early-onset neonatal sepsis) were also excluded. All infants underwent a complete sepsis workup, the indication of which was an ill-looking appearance or a fever of ≥39℃ (2, 3). Body temperature was measured at the axilla, and the highest body temperature documented during the stay in the emergency department or outpatient clinic was recorded. The institutional review board approved this study. Informed consent was obtained from parents before inclusion in the study.
Methods
Complete sepsis workup included blood and urine cultures, complete blood cell count, C-reactive protein (CRP), urinalysis with microscopic examination of urinary sediment, chest radiography, and lumbar puncture. Cerebrospinal fluid (CSF) samples from lumbar puncture were used for cell counts, chemistry including glucose and protein, bacterial and fungal cultures, viral cultures for Herpes simplex virus and Enterovirus and polymerase chain reaction (PCR) for Herpes simplex virus and Enterovirus. For Enterovirus PCR, real-time nested reverse-transcription PCR was used. For any URI symptoms, viral cultures for influenza virus, parainfluenza virus, and respiratory syncytial virus were performed with nasal aspirate. For any GI symptoms, latex agglutination tests for rotavirus and adenovirus were done. Febrile illness without a documented cause (FISDC) was applied when the fever subsided spontaneously within five days after admission and the febrile infant left the hospital without any complications or sequelae. Aseptic meningitis was defined as CSF pleocytosis with a negative CSF culture, whereas bacterial meningitis was defined as CSF pleocytosis with a positive CSF culture. Bacteremia was defined as a positive blood culture with or without sepsis syndrome. Urinary tract infection (UTI) was defined as culture-positive (≥105 CFU/HPF) urine obtained by a urine bag with a concurrent marked pyuria (WBC ≥50/HPF).
Receiver-operating characteristic (ROC) curves were constructed to select the cut-off values of CRP and peripheral blood WBC counts suggesting SBI. Independent significance of risk factors was evaluated using multivariate logistic regression analysis.
RESULTS
Demographic variables
The mean gestational age and birth weight of the febrile infants were 38+6±1+6 weeks and 3,263±490 g, respectively. The mean age at visit was 43±25 days old (34%, ≤30 days old; 39%, 31-60 days old; 27%, 61-90 days old). Male infants were 1.7 fold more common than female infants. A total of 65% of the infants were born through vaginal delivery, whereas 35% were delivered through a Caesarean section.
Final diagnosis of febrile infants
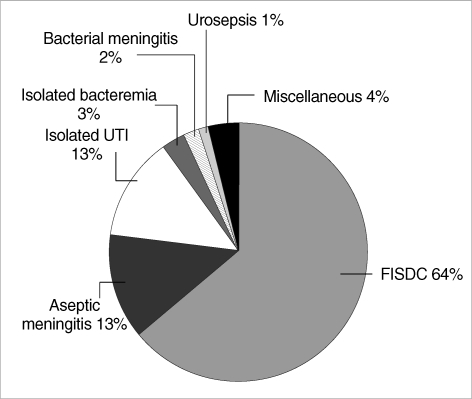
FISDC was the most common final diagnosis of the febrile infants and accounted for 63% (n=142) of all febrile infants. Aseptic meningitis and isolated UTI were the next common diagnoses, accounting for 13% (n=28) of cases. Isolated bacteremia accounted for 3% (n=6), bacterial meningitis for 2% (n=4), UTI with concurrent bacteremia for 1% (n=3), and miscellaneous for 5% (n=10) of the febrile infants (Fig. 1).
Fig. 1.
Final diagnosis of febrile infants younger than three months old.
FISDC, febrile illness without a documented cause; UTI, urinary tract infection.
Among thirteen infants with bacteremia, 54% (n=7) were caused by group B Streptococcus (GBS) and 23% (n=3) by Escherichia coli. Other bacterial etiologies were Streptococcus pneumoniae, Staphylococcus aureus and Streptococcus pyogenes. Among the seven infants with GBS bacteremia, four infants had concurrent meningitis, one infant had concurrent septic arthritis, and only two infants had isolated bacteremia. In cases of E. coli bacteremia, all had concurrent UTIs, but none had meningitis. All cases of bacterial meningitis were caused by GBS, which was also cultured in the blood. In cases of UTI, E. coli was cultured in 81% (n=25) and K. pneumoniae in 19% (n=6) of cases.
The miscellaneous causes of fever were rotaviral gastroenteritis (n=1), Kawasaki disease (n=2), pneumonia (n=2), acute otitis media (n=1), influenza virus infection (n=1), and respiratory syncytial virus bronchiolitis (n=1). These infants demonstrated no definite fever focus during the initial evaluation process and underwent complete sepsis work up.
Seasonal trends
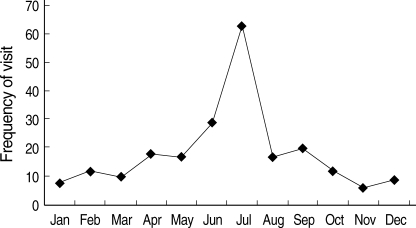
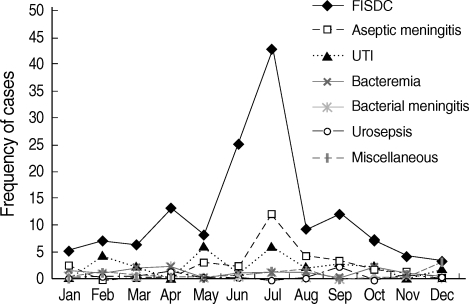
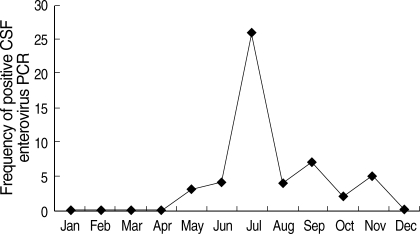
The frequency of febrile infants younger than three months old displayed definite seasonal trending. Nearly half of the visits of febrile infants were during the months of June (13%) and July (29%) (Fig. 2). Approximately 48% of the FISDC cases also occurred in June (18%) and July (30%). Most cases (86%) of aseptic meningitis occurred between May and September, with peak prevalence in July (43%) (Fig. 3). Moreover, 84% of positive CSF enterovirus PCR cases occurred between June and September, with peak prevalence in July (43%) (Fig. 4). CSF enterovirus PCR was positive in 28% of FISDC cases and 48% of aseptic meningitis cases. FISDC and aseptic meningitis accounted for 77% of all febrile episodes. Most of the enterovirus-positive cases were detected by PCR (n=52) rather than by culture (n=8). Six CSF specimens were positive for both enterovirus PCR and culture. There was not a documented epidemic of enterovirus during the study period.
Fig. 2.
Monthly occurrence of visits of febrile infants younger than three months old.
Fig. 3.
Monthly occurrence of specific diagnoses of febrile infants younger than three months old.
FISDC, febrile illness without a documented cause; UTI, urinary tract infection.
Fig. 4.
Monthly occurrence of positive cerebrospinal fluid enterovirus polymerase chain reaction.
CSF, cerebrospinal fluid; PCR, polymerase chain reaction.
Risk factors and diagnostic indices for serious bacterial infection
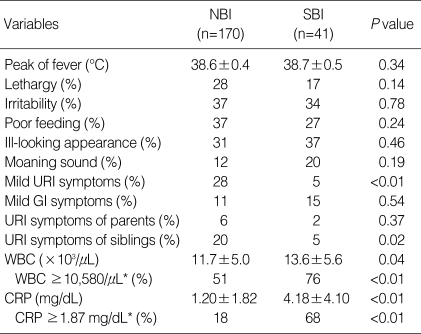
FISDC and aseptic meningitis could be categorized as non-bacterial infections (NBIs) and UTIs, bacterial meningitis and bacteremia could be categorized as SBIs. Among clinical manifestations, the absence of mild URI symptoms in the patient and the absence of URI symptoms in the patient's siblings were the risk factors that differentiated SBI from NBI. Among the laboratory findings, WBC counts ≥10,580/µL and CRP levels ≥1.87 mg/dL were risk factors that could differentiate SBI from NBI (Table 1). These risk factors were further assessed using logistic regression analysis. Resultant independent risk factors suggesting SBI rather than NBI were as follows: 1) CRP levels ≥1.87 mg/dL (odds ratio [OR] 10.9, 95% confidence interval [CI] 4.8-24.6), 2) lack of mild URI symptoms in the patient (OR 6.8, 95% CI 1.5-31.8), and 3) no URI symptoms in the patient's siblings (OR 4.7, 95% CI 1.0-23.1). Combined criteria were determined to be 1) and 2) and 3) having an OR of 14.1 and 95% CI of 6.3-31.4. The sensitivity, specificity, positive predictive value, and negative predictive values of these combined criteria for SBI were 61% (25/41), 90% (153/170), 60% (25/42), and 91% (153/169), respectively. This means that if a febrile infant does not satisfy all of these criteria, the probability of his or her not having SBI is 91%.
Table 1.
Clinical characteristics and laboratory findings for nonbacterial and serious bacterial infections
Values are presented as mean±SD.
*Cut-off values were selected by using receiver-operating characteristic curves.
NBI, non-bacterial infection; SBI, serious bacterial infection; URI, upper respiratory tract infection; GI, gastrointestinal; WBC, white blood cell; CRP, C-reactive protein.
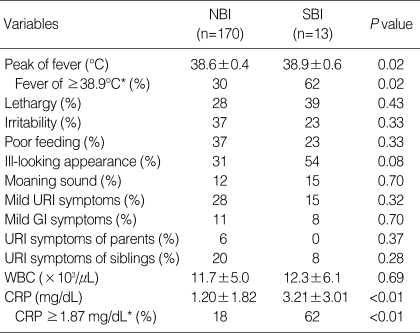
After excluding UTI, which could be indicated early in the initial evaluation process through the presence of marked pyuria, the same analytic procedure was performed to search for the independent risk factors for SBI, excepting UTI. Resultant independent risk factors were as follows: 1) CRP levels ≥1.87 mg/dL (OR 6.6, 95% CI 2.0-21.9) and 2) fevers of ≥38.9℃ (OR 3.3, 95% CI 1.0-11.1) (Table 2). Combined criteria were 1) and 2) with an OR of 9.0 and 95% CI of 2.5-32.3. The sensitivity, specificity, positive predictive value, and negative predictive values of these combined criteria were 38% (5/13), 94% (159/170), 31% (5/16), and 95% (159/167), respectively. This means that if a febrile infant is not affected with UTI and does not satisfy both of these criteria, the probability of his or her not having SBI is 95%.
Table 2.
Clinical characteristics and laboratory findings for non-bacterial infection and serious bacterial infection after excluding isolated urinary tract infection cases
Values are presented as mean±SD.
*Cut-off values were selected by using receiver-operating characteristic curves.
NBI, non-bacterial infection; SBI, serious bacterial infection; URI, upper respiratory tract infection; GI, gastrointestinal; WBC, white blood cell; CRP, C-reactive protein.
DISCUSSION
It is always not easy for physicians to discriminate SBI requiring prompt and proper antibiotic treatment from other self-limiting viral illnesses. To date, according to the conservative guidelines, most physicians hospitalized all febrile infants younger than three months old and initiated empirical antibiotic therapy. However, these unreserved admission policies brought up problems such as increased medical and socioeconomic costs, waste of community medical resources, and injudicious exposure of young infants to antibiotics (11). Since then, many studies have attempted to develop a stratification system which could guide physicians to distinguish SBI from self-limiting viral illness (8-11). As a result, several stratification systems have been developed. However, they could not be applicable in all situations due to differences in epidemiology and clinical settings. Therefore, it is advisable to develop a suitable stratification system based on the epidemiology and clinical setting of each individual health care center.
Although the patients we enrolled were febrile infants with ill-looking appearances or high fevers, we tried to find some indicators which could guide us in differentiating SBI from self-limiting viral illness in clinical manifestations and laboratory findings. The combined criteria are 1) CRP levels ≥1.87 mg/dL, 2) no URI symptoms, and 3) no URI symptoms in the patient's siblings. This combined ctiteria have an OR of 14.1, specificity of 90%, and a negative predictive value of 91% for SBI. Furthermore, in the emergency department or outpatient clinic setting, physicians can easily and promptly screen UTI by urinalysis. Thus, we searched for risk factors for SBI after excluding isolated UTI cases. Resultant combined criteria are 1) CRP levels ≥1.87 mg/dL and 2) fevers of ≥38.9℃ have an OR of 9.0, specificity of 94%, and negative predictive value of 95% for SBIs, except for UTI. This implies that, in the context of the emergency department or outpatient clinic setting, if we could exclude febrile infants whose urinalysis demonstrates marked pyuria, the remaining febrile infants who do not satisfy both CRP level of ≥1.87 mg/dL and fever of ≥38.9℃ could be considered to be in the low-risk group and are unlikely to have an SBI, with a probability of 95%. This low-risk group of infants may be discharged from emergency department or outpatient clinic without hospitalization. However, a remaining probability of 5% and the fact that these criteria have not been validated yet necessitates a meticulous follow-up plan for discharged febrile infants. This follow-up plan should include a reliable caretaker, detailed directions for revisiting the hospital, and easy accessibility to the hospital. If these conditions cannot be satisfied, it would be safer to hospitalize the febrile infants.
There was an interesting feature in monthly prevalence of febrile infants (Fig. 2). Nearly half of the visits of febrile infants were concentrated in June (13%) and July (29%). Approximately 48% of FISDC occurred in June (18%) and July (30%). Aseptic meningitis also had similar seasonal incidence, occurring between May to September, with the most cases occurring in July (43%) and August (14%). Moreover, positive CSF enterovirus PCRs occurred mostly from June to September, with a peak in July (43%). In terms of specific diagnosis, CSF enterovirus PCR was positive in 28% of FISDC cases and 48% of aseptic meningitis cases. These two morbidities accounted for 77% of all febrile episodes in our subject infants. The shared peak season for the visits of febrile infants, FISDC, aseptic meningitis and positive CSF enterovirus PCR and the high frequency of positive CSF enterovirus PCR in FISDC and aseptic meningitis cases suggest that enteroviral infection may be a major cause of febrile episodes in these infants and that FISDC and aseptic meningitis may be parts of a continuum of enteroviral infection rather than separate diseases. Enteroviral infection is common in early infancy and most cases are asymptomatic or self-limiting (12). In a review of 338 enteroviral infections in early infancy, 9% were classified as mild nonspecific febrile illness (13). However, enteroviral infection may manifest as a sepsis-like illness which is characterized by fever, poor feeding, abdominal distention, irritability, rash, lethargy, and hypotonia (14). In our study, many febrile infants who presented with an ill-looking appearance or high fever and were suspected to be affected with bacterial sepsis were in fact revealed to be free of SBI and instead had evidence of enteroviral infection. In this context, it may be said that some culture-negative neonatal sepsis cases might have been attributed to neonatal enteroviral infection. Therefore, it may be necessary to search for evidence of enteroviral infection in the evaluation process of febrile infants. In our study, we used both PCR and culture methods for the detection of enterovirus in CSF. Most of the enterovirus positive cases were detected by PCR rather than by culture. The superiority of CSF enterovirus PCR to traditional culture methods in the diagnosis of neonatal enteroviral infection has already been described by many researchers (15-18).
In conclusion, enteroviral infection may be a major cause of febrile episodes in infants younger than three months. If UTI is excluded, the presence of a CRP level ≥1.87 mg/dL and a fever of ≥38.9℃ can be used as criteria to rule out SBI in febrile infants of this age. By using these criteria, unnecessary hospitalization and exposure to antibiotics may be reduced. However, a meticulous follow-up plan for discharged febrile infants must be provided to minimize the risk of not treating SBI.
References
- 1.Nelson DS, Walsh K, Fleisher GR. Spectrum and frequency of pediatric illness presenting to a general community hospital emergency department. Pediatrics. 1992;90:5–10. [PubMed] [Google Scholar]
- 2.Baraff LJ, Bass JW, Fleisher GR, Klein JO, McCracken GH, Jr, Powell KR, Schriger DL. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med. 1993;22:1198–1210. doi: 10.1016/s0196-0644(05)80991-6. [DOI] [PubMed] [Google Scholar]
- 3.Luszczak M. Evaluation and management of infants and young children with fever. Am Fam Physician. 2001;64:1219–1226. [PubMed] [Google Scholar]
- 4.Levine DA, Platt SL, Dayan PS, Macias CG, Zorc JJ, Krief W, Schor J, Bank D, Fefferman N, Shaw KN, Kuppermann N. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infections. Pediatrics. 2004;113:1728–1734. doi: 10.1542/peds.113.6.1728. [DOI] [PubMed] [Google Scholar]
- 5.Pantell RH, Newman TB, Bernzweig J, Bergman DA, Takayama JI, Segal M, Finch SA, Wasserman RC. Management and outcomes of care of fever in early infancy. JAMA. 2004;291:1203–1212. doi: 10.1001/jama.291.10.1203. [DOI] [PubMed] [Google Scholar]
- 6.Bachur RG, Harper MB. Predictive model for serious bacterial infections among infants younger than 3 months of age. Pediatrics. 2001;108:311–316. doi: 10.1542/peds.108.2.311. [DOI] [PubMed] [Google Scholar]
- 7.Trautner BW, Caviness AC, Gerlacher GR, Demmler G, Macias CG. Prospective evaluation of the risk of serious bacterial infection in children who present to the emergency department with hyperpyrexia (temperature of 106°F or higher) Pediatrics. 2006;118:34–40. doi: 10.1542/peds.2005-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy PL, Sharpe MR, Spiesel SZ, Dolan TF, Forsyth BW, De-Witt TG, Fink HD, Baron MA, Cicchetti DV. Observation scales to identify serious illness in febrile children. Pediatrics. 1982;70:802–809. [PubMed] [Google Scholar]
- 9.Dagan R, Sofer S, Phillip M, Shachak E. Ambulatory care of febrile infants younger than 2 months of age classified as being at low risk for having serious bacterial infections. J Pediatr. 1988;112:335–360. doi: 10.1016/s0022-3476(88)80312-3. [DOI] [PubMed] [Google Scholar]
- 10.Baskin MN, O'Rourke EJ, Fleisher GR. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatr. 1992;120:22–27. doi: 10.1016/s0022-3476(05)80591-8. [DOI] [PubMed] [Google Scholar]
- 11.Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329:1437–1441. doi: 10.1056/NEJM199311113292001. [DOI] [PubMed] [Google Scholar]
- 12.Melnick JL. Enterovirus. In: Evans AS, editor. Viral infections of humans-epidemiology and control. 3rd edn. New York: Plenum; 1989. pp. 191–203. [Google Scholar]
- 13.Morens DM. Enteroviral disease in early infancy. J Pediatr. 1978;92:374–377. doi: 10.1016/s0022-3476(78)80422-3. [DOI] [PubMed] [Google Scholar]
- 14.Cherry JD. Enteroviruses. In: Remington JS, Klein JO, editors. Infectious diseases of the fetus and newborn infant. 5th edn. Philadelphia: W.B. Saunders Company; 2001. pp. 477–518. [Google Scholar]
- 15.Furione M, Zavattoni M, Gatti M, Percivalle E, Fioroni N, Gerna G. Rapid detection of enteroviral RNA in cerebrospinal fluid (CSF) from patients with aseptic meningitis by reverse transcription-nested polymerase chain reaction. New Microbiol. 1998;21:343–351. [PubMed] [Google Scholar]
- 16.Palacios Poggio G, Cisterna D, Freire MC, Cello J. RT-nested PCR for the detection of enterovirus in biological samples from patients with suspected enteroviral infections. Rev Argent Microbiol. 2000;32:165–172. [PubMed] [Google Scholar]
- 17.Glimaker M, Johansson B, Olcen P, Ehrnst A, Forsgren M. Detection of enteroviral RNA by polymerase chain reaction in cerebrospinal fluid from patients with aseptic meningitis. Scand J Infect Dis. 1993;25:547–557. doi: 10.3109/00365549309008542. [DOI] [PubMed] [Google Scholar]
- 18.Thoren A, Widell A. PCR for the diagnosis of enteroviral meningitis. Scand J Infect Dis. 1994;26:249–254. doi: 10.3109/00365549409011792. [DOI] [PubMed] [Google Scholar]