Abstract
We designed a randomized, double-blinded study to determine the efficacy and safety of 0.5 mg/kg intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. Patients were randomly allocated into two groups: ephedrine group (n=21) and control group (n=21). Intravenous preload of 15 mL/kg lactated Ringer's solution was given. Shortly after the spinal injection, ephedrine 0.5 mg/kg or saline was injected intravenous for 60 sec. The mean of highest and lowest heart rate in the ephedrine group was higher than those of control group (P<0.05). There were significant lower incidences of hypotension and nausea and vomiting in the ephedrine group compared with the control group (8 [38.1%] vs. 18 [85.7%]); (4 [19%] vs. 12 [57.1%], respectively) (P<0.05). The first rescue ephedrine time in the ephedrine group was significantly longer (14.9±7.1 min vs. 7.9±5.4 min) than that of the control group (P<0.05). Neonatal outcome were similar between the study groups. These findings suggest, the prophylactic bolus dose of 0.5 mg/kg intravenous ephedrine given at the time of intrathecal block after a crystalloid fluid preload, plus rescue boluses reduce the incidence of hypotension.
Keywords: Anesthesia, Spinal; Cesarean Section; Ephedrine; Hypotension
INTRODUCTION
Spinal anesthesia provides a fast, profound, and symmetrical sensory and motor block of high quality in patients undergoing cesarean delivery (1, 2). The most common serious adverse effect of spinal anesthesia for cesarean delivery is hypotension, with a reported incidence greater than 80% (3).
A number of strategies for preventing hypotension have been investigated, because it may have detrimental maternal and neonatal effects. The use of lateral uterine displacement is routine procedure to prevent hypotension (4). Other strategies have included the use of intravenous fluid preload, gravity (Trendelenburg or leg rising), compression devices on the legs, and prophylactic vasopressors (1). However, no methods have proved satisfactory. Ephedrine is the most commonly used drug among the vasopressors.
The prophylactic administration of ephedrine by the intramuscular route is very controversial because its systemic absorption and peak effect are difficult to predict, thus, possibly resulting in rebound hypertension (5). The intravenous route may be more effective and controllable, although large doses are used; the incidence of hypotension was still high in some studies (6, 7).
Intravenous ephedrine given immediately after the induction of spinal anesthesia has been described (7, 8). Doses of 10-20-30 mg or 0.25 mg/kg were not effective in eliminating hypotension completely (7-10). Therefore, we designed a randomized, double-blinded study to determine efficacy and safety of 0.5 mg/kg intravenous ephedrine for preventing hypotension during spinal anesthesia for cesarean delivery.
MATERIALS AND METHODS
During the study period, 52 consecutive patients were identified suitable for the study. They were women, ASA status I or II, undergoing elective cesarean section under spinal anesthesia. Three had to be excluded because of hypertension, two refused to participate, and one was missed because of high workload. Written informed consent was obtained from each subject, and the study protocol was approved by the Human Ethics Committee of our medical school. Patients with preexisting or pregnancy-induced hypertension, known cardiovascular or cerebrovascular disease, abnormal cardiotocography (CTG) tracing, or contraindications to spinal anesthesia were excluded. Randomization was based on a computer-generated code that was prepared at a remote site and sealed in opaque, sequentially numbered envelopes. The patients were randomly divided into 2 groups: ephedrine group (n=23) and control group (n=23) after spinal anesthesia.
None of patients was premedicated. On arrival in the operation room, baseline measurements of systolic arterial pressure (SAP) and heart rate (HR) were calculated with a Criticare System 1100 monitor (Criticare System Inc., Waukesha, WI, U.S.A.) as the mean of three successive measurements, 1 min apart and in the modified supine position with at least 15° of left lateral tilt. 18-gauge intravenous cannula was sited in the non-dominant hand and intravenous preload of 15 mL/kg lactated Ringer's solution was given, within 15 min, after which the intravenous infusion was slowed to the minimum rate required to maintain vein patency.
Spinal anesthesia was administered with the patient in the right lateral position. After skin infiltration with lidocaine, a 25-gauge Whitacre needle was inserted at the L2-3 or L3-4 vertebral interspace and hyperbaric 0.5% bupivacaine 2 mL with fentanyl 10 µg was injected intrathecally. The patient was then immediately turned supine with left lateral tilt. Oxygen 4 L/min was given by nasal cannula until delivery.
Shortly after the spinal injection, ephedrine 0.5 mg/kg in the ephedrine group or saline in the control group was injected intravenous for 60 sec. All study medications were administered by an anesthetist not be involved in the care of the patient or collection of data. A second anesthetist, blind to identity of the study medication, managed the patient. The study period started at the time of intrathecal injection and ended when the umbilical cord was clamped. Systolic arterial pressure and heart rate were recorded at 1-min intervals. Fetal heart rate was monitored by using CTG continuously until delivery. The baseline SAP and HR, lowest and highest SAP and HR, nausea, vomiting, dizziness, and chest symptoms were recorded every minute. The first rescue ephedrine time, total doses of rescue ephedrine, and total dose of used ephedrine were also recorded. Upper sensory level of anesthesia was measured by assessing loss of pinprick discrimination at 10 min. All blocks extended to T5 or above, before surgery was allowed to start.
Hypotension was defined as 20% decrease in SAP from baseline. Hypertension was defined as 20% increase in SAP from baseline. Maternal bradycardia was defined as heart rate <60 beats/min and treated immediately by using intravenous atropine 0.5 mg. Tachycardia was defined as heart rate >120 beats/min. Hypotension was treated immediately by using rescue intravenous ephedrine 5 mg every minute until SAP returned to normal values (>80% of baseline value).
After delivery, Apgar scores were assessed at 1 and 5 min by the attending pediatrician. Arterial blood samples were taken from umbilical cord for blood-gas analysis within 2 min. All patients received oxytocin 20 units/L in crystalloid after delivery.
Prospective power analysis showed that a sample size of 20 patients per study group would have 80% power at the 5% significance level to detect a difference of 50% in the incidence of hypotension in the study group compared with control, assuming a baseline incidence of 80%, as reported in a published study of a similar patient group (10).
Data were presented as mean±standard deviation, median (range), or percentage, as appropriate. Statistical analyses were performed Statistica 7.0. Software (Statsoft, Inc., Tulsa, AR, U.S.A.). Demographic parameters, delivery time, first rescue ephedrine time, umbilical arterial pH, SAP, and HR were compared with t-test. Changes over time in SAP and HR between and within the study groups, comparing values at each time point, were analyzed with repeated measures ANOVA followed by a post hoc Bonferroni test to identify significant differences. Doses of total rescue and used ephedrine, Apgar scores, and upper sensory level were compared with Mann-Withney U test. Hypotension, hypertension, tachycardia, bradycardia and nausea and vomiting of the study groups were compared with χ2 or Fisher's exact test, as appropriate. A P value of <0.05 was considered significant.
RESULTS
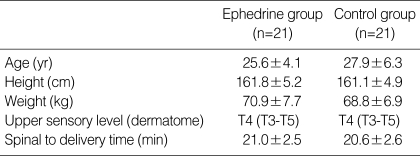
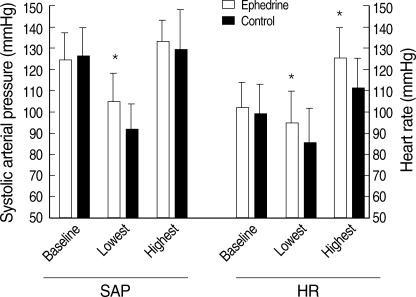
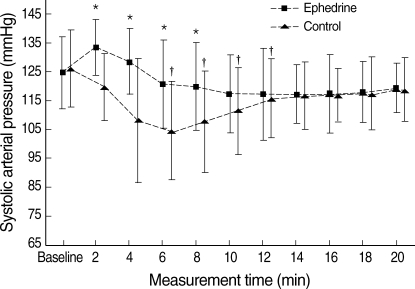
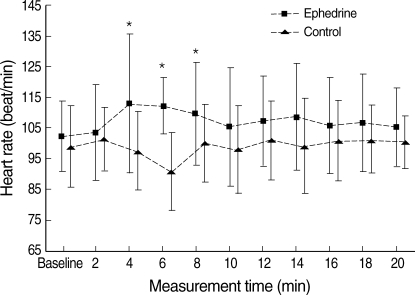
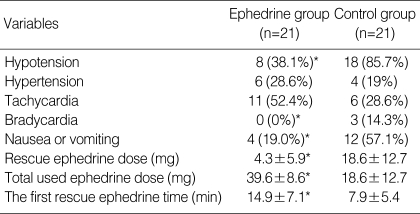
Of 46 patients randomized, two in the both groups (n=21) had to be excluded from data analysis because of the protocol violations. In each study group, 21 patients completed the study protocol. There was no difference between the study groups with regard to the age, weight, height, and delivery time (P>0.05) (Table 1). All patients had adequate surgical anesthesia. The median upper sensory level 10 min after the intrathecal injection was T4 (T3-T5) for all the study groups. There was no significant difference in the SAP and HR vaues at baseline between the study groups (P>0.05). The mean highest and lowest HR in the ephedrine group was higher than those of control group (P<0.05). There were significant differences in mean lowest SAP between the study groups (P<0.05). The mean highest SAP in the ephedrine group was higher than that of control group, but this difference was not significant (Fig. 1). From 2 to 8 min, the mean SAPs in the control group were significantly lower than those of the ephedrine group (P<0.05) (Fig. 2). From 6 to 12 min, significant decreases of the mean SAP in the control group were observed as compared with baseline (P<0.05) (Fig. 2). From 4 to 8 min, the mean HR in the control group was significantly lower than those of the ephedrine group (P<0.05) (Fig. 3). The incidences of hypotension, hypertension, tachycardia, bradycardia, nausea or vomiting, the total doses rescue and used ephedrine, and the first rescue ephedrine time are summarized in Table 2. There was significant lower incidences of hypotension in the ephedrine group compared with the control group (8 [38.1%] vs. 18 [85.7%]) (P<0.05). There were significant lower incidences of nausea and vomiting in the ephedrine group compared with the control group (4 [19%] vs. 12 [57.1%]) (P<0.05). There was no difference in the ratio of hypertension between the study groups (P>0.05). The ratio of bradycardia in the control group was significantly higher than that of the ephedrine group (14.3% vs. 0%; P<0.05). There were significant decrease total doses of rescue ephedrine required in the ephedrine group (P<0.05) (Table 2). Total doses of used ephedrine in the ephedrine group were significant higher than that of control group. The first rescue ephedrine time in the ephedrine group was significantly longer (14.9±7.1 min vs. 7.9±5.4 min) than that of the control group (P<0.05) (Table 2).
Table 1.
Patient characteristics
Values are mean±SD or median (min-max).
Fig. 1.
Baseline, the lowest, and highest systolic arterial pressure (SAP) and heart rate (HR) of the study groups.
Values are mean±SD.
*P<0.05 vs. control group.
Fig. 2.
Systolic arterial pressure of the ephedrine and control groups.
Values are mean±SD.
*P<0.05 vs. control group; †P<0.05 vs. baseline.
Fig. 3.
Heart rate of the ephedrine and control groups.
Values are mean±SD.
*P<0.05 vs. control group.
Table 2.
Hemodynamic data
Values are number of patients (%) or mea±SD.
*P<0.05 vs. control group.
No abnormal CTG tracing was observed until delivery. Analysis of neonatal data showed no differences between the study groups. No Apgar scores were below 7 at 1 min or 5 min. Umbilical arterial pH were similar between the study groups (P>0.05). There was no pH <7.2 in the both groups (Table 3).
Table 3.
Neonatal data
Values are mean±SD or median (min-max).
DISCUSSION
This is the first report to our knowledge to investigate the effect of intravenous ephedrine given according to maternal weight dose of 0.5 mg/kg after the induction of spinal anesthesia for cesarean section to prevent hypotension related to spinal anesthesia. Our findings demonstrated that prophylactic intravenous ephedrine during spinal anesthesia for cesarean section can prevent hypotension without significant maternal tachycardia or hypertension, and also it increases the first rescue ephedrine time and decreases the ratio of nausea and vomiting. Umbilical arterial pH and Apgar scores were not influenced by hypotension or ephedrine medication.
The incidence of hypotension during spinal anesthesia for cesarean section is reported to be as high as 80%, despite fluid preload, lateral uterine displacement and use of vasopressor agents (11). In the anesthesia practice, prevention and management of hypotension related to spinal anesthesia remains a difficult problem and there was no consensus on its optimal management.
Phenylephrine, α1-adrenergic agonist whose action would be expected to counteract the decrease in systemic vascular resistance induced by spinal anesthesia (12). Phenylephrine can be used for the prevention and treatment of maternal hypotension (13-15) but a reduction of fetal oxygenation due to uterine vasoconstriction has been observed in animals (16). It may cause maternal bradycardia (14, 17). Loughrey et al. (18) compared intravenous bolus of ephedrine and phenylephrine combination with ephedrine alone. They found the combination of ephedrine and phenylephrine given as an intravenous bolus was not superior regarding to the incidence of hypotension, maternal side effects, or umbilical blood gases when administered as a prophylactic bolus followed by rescue boluses and compared to ephedrine alone.
Ephedrine, an indirectly acting sympathomimetic amine, is probably the vasopressor of choice in obstetric anesthesia. Although ephedrine has mixed α- and β-adrenoreceptor activity, it maintains arterial pressure mainly by increases in cardiac output (CO) and heart rate as a result of its predominant activity on β1-adrenoreceptors (19). Variable intravenous infusions of ephedrine appear to be successfu1 (14, 20-22). Kee et al. (10) investigated the efficacy and optimum dose of intravenous ephedrine for prevention of hypotension during spinal anesthesia for cesarean delivery. They compared the effect of ephedrine 10, 20, or 30 mg intravenous for the prevention of hypotension. They found that a bolus dose of 30 mg intravenous ephedrine was required to reduce the incidence of hypotension during spinal anesthesia for cesarean delivery. They concluded that although the incidence of hypotension was reduced to 35% in the patients who received ephedrine 30 mg compared with the control rate of 95%, this was at the expense of an increased incidence of hypertension, which occurred in 45% of the patients. They suggested that 30-mg intravenous ephedrine may not be suitable in some patients such as those with cardiovascular or cerebrovascular disease. Compared with the study of Kee et al. (10), the incidence of reactive hypertension is lower in our study (45% vs. 28.6%). Duration of ephedrine administration in the study of Kee et al. was 30 sec, however, in our study; it was 60 sec. Decreased ratio of reactive hypertension in the ephedrine group in our study may result from the longer duration of ephedrine administration. Particularly if sympathetic block level is low, reactive hypertension may be a problem. In the ephedrine and control groups, upper sensory level was T4 (T3-T5), however, it was T4 (C2-T7) in the study of Kee et al. (10), and the range of sensorial block was wide compared to our study. Increased sympathetic activity might be related to compensatory stimulation of thoracic sympathetic nerves, including the fibers supplying the heart (T1-T4) in the patients undergoing spinal anesthesia (23). Such event also was reported in low spinal anesthesia and epidural blocks in which sympathetic block does not reach the T4 level (24). The ratio of reactive hypertension was similar the patients given intravenous ephedrine and saline (28.6% vs. 19%). In the control group, cause of reactive hypertension may result from the administration of higher doses of rescue ephedrine.
Lee et al. (9) reviewed available studies to determine the dose-response characteristics of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. They reported that, significant dose-response relationships were found for hypotension, hypertension and umbilical arterial pH. They suggested that, the use of larger doses of ephedrine (>14 mg) does not completely eliminate hypotension but causes reactive hypertension and a minor decrease in umbilical arterial pH. They found no evidence of a dose-response relationship for nausea or vomiting, fetal acidosis, or Apgar scores. Both ratio of hypotension and nausea and vomiting decreased with ephedrine dose used in this study.
Some studies found significantly higher umbilical arterial pH when using prophylactic ephedrine (7). Thus, it seems that ephedrine must be used during cesarean section to avoid spinal hypotension, which remains a major determinant of fetal acidemia (10, 25). Ephedrine has been shown to cross the placenta and to affect the fetal and neonatal heart rate (26) due to β-adrenoreceptor activity. A greater proportion of low umbilical artery pH has been observed with ephedrine than phenylephrine (12). Previous studies have shown that the use of ephedrine to prevent or treat hypotension associated with spinal and epidural anesthesia for cesarean delivery may not correct fetal acidosis and may even increase it, especially if hypotension still occurs (5, 22, 27). Kee et al. (10) found that umbilical blood pH values were lower in patients who had hypotension compared with patients who did not, whereas hypertension was not associated with adverse effects. Although they did not measure uteroplacental flow, their results suggest that, within the range of doses used in their study (10, 20, or 30 mg), the potential vasoconstrictive effects of ephedrine may have a less detrimental effect on uteroplacental blood flow than the effects of hypotension. Eisler et al. (28) demonstrated that fetal catecholamine stimulation before delivery might be beneficial. They suggested that when a β-adrenergic agonist was administered before elective cesarean section, lower respiratory morbidity, and better lung function and reduced risk of hypoglycaemia in the newborn infant were found. In our study, lowest SAP was maintained better in patients who received intravenous ephedrine compared with the control groups. We found no significant difference in neither Apgar scores nor umbilical arterial blood gases data between the study groups, despite a difference in the incidence of hypotension, probably reflecting the early recognition and restoration of hypotension with rescue ephedrine.
Although mean highest HR in the ephedrine group was higher, we found no difference in ratio of tachycardia between the study groups. This could be explained by both the effect of "rescue" ephedrine and baroreceptor-mediated reflex increases in heart rate in patients who became hypotensive. In addition, atropine was applied for bradycardia in the control group.
These findings suggest, the prophylactic bolus dose of 0.5 mg/kg intravenous ephedrine given at the time of intrathecal block after a crystalloid fluid preload, plus rescue boluses reduce the incidence of hypotension. It has not been shown to eliminate the need to treat maternal hypotension during spinal anesthesia for elective cesarean delivery compared to intravenous rescue boluses alone.
References
- 1.Park GE, Hauch MA, Curlin F, Datta S, Bader AM. The effects of varying volumes of crystalloid administration before cesarean delivery on maternal hemodynamics and colloid osmotic pressure. Anesth Analg. 1996;83:299–303. doi: 10.1097/00000539-199608000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Cheun JK, Kim AR. Intrathecal meperidine as the sole agent for cesarean section. J Korean Med Sci. 1989;4:135–138. doi: 10.3346/jkms.1989.4.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rout CC, Rocke DA, Levin J, Gouws E, Reddy D. A reevaluation of the role of crystalloid preload in the prevention of hypotension associated with spinal anesthesia for elective cesarean section. Anesthesiology. 1993;79:262–269. doi: 10.1097/00000542-199308000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Clark SL, Cotton DB, Pivarnik JM, Lee W, Hankins GD, Benedetti TJ, Phelan JP. Position change and central hemodynamic profile during normal third-trimester pregnancy and post partum. Am J Obstet Gynecol. 1991;164:883–887. doi: 10.1016/s0002-9378(11)90534-1. [DOI] [PubMed] [Google Scholar]
- 5.Webb AA, Shipton EA. Re-evaluation of i.m. ephedrine as prophylaxis against hypotension associated with spinal anaesthesia for Caesarean section. Can J Anaesth. 1998;45:367–369. doi: 10.1007/BF03012030. [DOI] [PubMed] [Google Scholar]
- 6.Husaini SW, Russell IF. Volume preload: lack of effect in the prevention of spinal-induced hypotension at caesarean section. Int J Obstet Anesth. 1998;7:76–81. doi: 10.1016/s0959-289x(98)90001-2. [DOI] [PubMed] [Google Scholar]
- 7.Chan WS, Irwin MG, Tong WN, Lam YH. Prevention of hypotension during spinal anaesthesia for caesarean section: ephedrine infusion versus fluid preload. Anaesthesia. 1997;52:908–913. doi: 10.1111/j.1365-2044.1997.190-az0323.x. [DOI] [PubMed] [Google Scholar]
- 8.King SW, Rosen MA. Prophylactic ephedrine and hypotension associated with spinal anesthesia for cesarean delivery. Int J Obstet Anesth. 1998;7:18–22. doi: 10.1016/s0959-289x(98)80023-x. [DOI] [PubMed] [Google Scholar]
- 9.Lee A, Ngan Kee WD, Gin T. A dose-response meta-analysis of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for elective cesarean delivery. Anesth Analg. 2004;98:483–490. doi: 10.1213/01.ANE.0000096183.49619.FC. [DOI] [PubMed] [Google Scholar]
- 10.Ngan Kee WD, Khaw KS, Lee BB, Lau TK, Gin T. A dose-response study of prophylactic intravenous ephedrine for the prevention of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2000;90:1390–1395. doi: 10.1097/00000539-200006000-00024. [DOI] [PubMed] [Google Scholar]
- 11.Rout CC, Rocke DA. Prevention of hypotension following spinal anesthesia for cesarean section. Int Anesthesiol Clin. 1994;32:117–135. [PubMed] [Google Scholar]
- 12.Thomas DG, Robson SC, Redfern N, Hughes D, Boys RJ. Randomized trial of bolus phenylephrine or ephedrine for maintenance of arterial pressure infusion during spinal anaesthesia for Caesarean section. Br J Anaesth. 1996;76:61–65. doi: 10.1093/bja/76.1.61. [DOI] [PubMed] [Google Scholar]
- 13.LaPorta RF, Arthur GR, Datta S. Phenylephrine in treating maternal hypotension due to spinal anaesthesia for caesarean delivery: effects on neonatal catecholamine concentrations, acid base status and Apgar scores. Acta Anaesthesiol Scand. 1995;39:901–905. doi: 10.1111/j.1399-6576.1995.tb04195.x. [DOI] [PubMed] [Google Scholar]
- 14.Hall PA, Bennett A, Wilkes MP, Lewis M. Spinal anaesthesia for caesarean section: comparison of infusions of phenylephrine and ephedrine. Br J Anaesth. 1994;73:471–474. doi: 10.1093/bja/73.4.471. [DOI] [PubMed] [Google Scholar]
- 15.Desalu I, Kushimo OT. Is ephedrine infusion more effective at preventing hypotension than traditional prehydration during spinal anaesthesia for caesarean section in African parturients? Int J Obstet Anesth. 2005;14:294–299. doi: 10.1016/j.ijoa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Greiss FC, Crandell DL. Therapy for hypotension induced by spinal anesthesia during pregnancy: observations on gravid ewes. JAMA. 1965;191:793–796. doi: 10.1001/jama.1965.03080100011002. [DOI] [PubMed] [Google Scholar]
- 17.Lee A, Ngan Kee WD, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2002;94:920–926. doi: 10.1097/00000539-200204000-00028. [DOI] [PubMed] [Google Scholar]
- 18.Loughrey JP, Yao N, Datta S, Segal S, Pian-Smith M, Tsen LC. Hemodynamic effects of spinal anesthesia and simultaneous intravenous bolus of combined phenylephrine and ephedrine versus ephedrine for cesarean delivery. Int J Obstet Anesth. 2005;14:43–47. doi: 10.1016/j.ijoa.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Critchley LA, Stuart JC, Conway F, Short TG. Hypotension during subarachnoid anaesthesia: haemodynamic effects of ephedrine. Br J Anaesth. 1995;74:373–378. doi: 10.1093/bja/74.4.373. [DOI] [PubMed] [Google Scholar]
- 20.Jackson R, Reid JA, Thorburn J. Volume preloading is not essential to prevent spinal-induced hypotension at caesarean section. Br J Anaesth. 1995;75:262–265. doi: 10.1093/bja/75.3.262. [DOI] [PubMed] [Google Scholar]
- 21.Kang YG, Abouleish E, Caritis S. Prophylactic intravenous ephedrine infusion during spinal anesthesia for cesarean section. Anesth Analg. 1982;61:839–842. [PubMed] [Google Scholar]
- 22.Lee A, Ngan Kee WD, Gin T. Prophylactic ephedrine prevents hypotension during spinal anesthesia for cesarean delivery but does not improve neonatal outcome: a quantitative systematic review. Can J Anaesth. 2002;49:588–599. doi: 10.1007/BF03017387. [DOI] [PubMed] [Google Scholar]
- 23.Owczuk R, Sawicka W, Wujtewicz MA, Kawecka A, Lasek J, Wujtewicz M. Influence of spinal anesthesia on corrected QT interval. Reg Anesth Pain Med. 2005;30:548–552. doi: 10.1016/j.rapm.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Veering BT, Cousins MJ. Cardiovascular and pulmonary effects of epidural anaesthesia. Anaesth Intensive Care. 2000;28:620–635. doi: 10.1177/0310057X0002800603. [DOI] [PubMed] [Google Scholar]
- 25.Simon L, Provenchére S, de Saint Blanquat L, Boulay G, Hamza J. Dose of prophylactic intravenous ephedrine during spinal anesthesia for cesarean section. J Clin Anesth. 2001;13:366–369. doi: 10.1016/s0952-8180(01)00284-7. [DOI] [PubMed] [Google Scholar]
- 26.Wright RG, Shnider SM, Levinson G, Rolbin SH, Parer JT. The effect of maternal administration of ephedrine on fetal heart rate and variability. Obstet Gynecol. 1981;57:734–738. [PubMed] [Google Scholar]
- 27.Shearer VE, Ramin SM, Wallace DH, Dax JS, Gilstrap LC., 3rd Fetal effects of prophylactic ephedrine and maternal hypotension during regional anesthesia for cesarean section. J Matern Fetal Med. 1996;5:79–84. doi: 10.1002/(SICI)1520-6661(199603/04)5:2<79::AID-MFM6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 28.Eisler G, Hjertberg R, Lagercrantz H. Randomised controlled trial of effect of terbutaline before elective caesarean section on postnatal respiration and glucose homeostasis. Arch Dis Child Fetal Neonatal Ed. 1999;80:F88–F92. doi: 10.1136/fn.80.2.f88. [DOI] [PMC free article] [PubMed] [Google Scholar]