Abstract
As major constituents of the mammalian lens, β-crystallins associate into dimers, tetramers, and higher-order complexes in order to maintain lens transparency and refractivity. A previous study has shown that dimerization of βB2- and βA3-crystallins is energetically highly favored and entropically driven. While heterodimers further associate into higher order complexes in vivo, a significant level of reversibly associated tetrameric crystallin has not been previously observed in vitro. In order to enhance our understanding of the interactions between β-crystallins, this study characterizes the association of βB1-crystallin, a major component of large β-crystallin complexes (β-high) with itself and with βA3-crystallin. Mouse βB1- and human βA3-crystallins were expressed in E. coli and purified chromatographically. Their association was then characterized using size-exclusion chromatography, native gel electrophoresis, isoelectric focusing, and analytical sedimentation equilibrium centrifugation. When present alone, each β-crystallin associates into homodimers but no tetramer formation is seen. Upon mixing, heterocomplex formation between βB1- and βA3-crystallins is observed using size-exclusion chromatography, native gel electrophoresis, isoelectric focusing, and sedimentation equilibrium. In contrast to results previously obtained upon mixing βB2- and βA3-crystallins, mixed βB1- and βA3-crystallins show a dimer-tetramer equilibrium with a Kd of 1.1 μM, indicating that these two β-crystallins associate predominantly into heterotetramers in vitro. Thus, while each purified β-crystallin associates only into homodimers and mixed βB2- and βA3-crystallins form a mixture of homo- and heterodimers, mixed βB1- and βA3-crystallins associate predominantly into heterotetramers in equilibrium with heterodimers. These findings suggest a unique role for βB1-crystallin in promoting higher-order crystallin association in the lens.
Crystallins, which exist at extremely high concentrations in the eye lens, are structural proteins critical for transmitting and focusing light. The human lens contains α-, β-, and γ-crystallins, of which β-crystallins, taken as a whole, represent the greatest part. Seven different subtypes of β-crystallins have been identified in the human lens, four of which are relatively acidic (βA1, βA2, βA3, and βA4), and three of which are relatively basic (βB1, βB2, and βB3). β- and γ-crystallins, which have highly similar core sequences, form the βγ-crystallin superfamily. Structurally, both β- and γ-crystallins contain two homologous domains connected by a short connecting sequence. Each domain contains two Greek-key motifs, and each motif comprises four antiparallel β-sheets (1). β-Crystallins contain N-terminal extensions ranging in length from 12 to 57 amino acids. In addition, the basic β-crystallins contain C-terminal extensions ranging from 11 to 16 amino acids. β-Crystallins are highly conserved across species with βA3-crystallin being over 95% identical between mouse and human. In contrast to β-crystallins, β-crystallins have either no terminal extensions or short extensions containing only a few amino acids (2).
β-Crystallins are known to associate into dimers, tetramers, and higher-order complexes in vivo. Size-exclusion chromatography of bovine or human lens extracts shows three size classes of β-crystallin complexes: βH (160-200 kDa, primarily octamers), βL1 (70-100 kDa, primarily tetramers), and βL2 (46-50 kDa, primarily dimers) (3;4). The distribution of β-crystallins among different size classes is dependent on protein concentration, pH, and ionic strength (5;6). Despite the sequence homology between β- and γ-crystallins, γ-crystallins are observed in the lens strictly as monomers, which have prompted investigations into the role of terminal extensions in crystallin association. Previous studies have shown that although the terminal arms of β-crystallins are not required for association, the N-terminal extension of βA3-crystallin assists in self-association into homodimers, while the N-terminal extension of βB2-crystallin actually decreases association into homodimers (7;8). Several studies have also demonstrated a correlation between the length of the N-terminal extension of βB1-crystallin in lens extracts and the size class in which it migrates on size exclusion chromatography (9-11). Bateman et al. have previously shown that a truncated form of βB1- and βA3-crystallin migrate at a size of approximately 100 kDa on gel permeation chromatography, and have a similar size as estimated by laser light scattering.(12)
While β-crystallins are known to form oligomers in vivo, a significant level of tetramers or higher-order complexes generally has not been observed in previous in vitro studies. This can be explained in part by the lower protein concentration being examined in vitro, compared with the extremely high concentration in the eye lens. It has previously been demonstrated that both mouse βB2-crystallin and human βA3-crystallin form reversible homodimers when present alone, and that these readily form heterodimers upon mixing and incubation at room temperature in the absence of denaturants (13). However, no heterotetramers or higher-order complexes were observed between these two crystallins. The current study employs a similar strategy to study the association properties of another pair of β-crystallins—mouse βB1- and human βA3-crystallins. When present alone, each protein associates into homodimers but neither forms homotetramers. Upon mixing in the absence of denaturants, heterocomplex formation between these two β-crystallin subtypes was observed by size-exclusion chromatography, native gel electrophoresis, isoelectric focusing, and sedimentation equilibrium. In contrast to the previous results obtained with βB2- and βA3-crystallins, both size-exclusion chromatography and sedimentation equilibrium of the mixture of βB1- and βA3-crystallins show a dimer-tetramer equilibrium, with a Kd of 1.1 μM calculated from the latter. These results show that mixed βB1- and βA3-crystallins associate predominantly into heterotetramers in vitro.
EXPERIMENTAL PROCEDURES
Expression and purification of βB1-crystallin
Mouse cDNA encoding βB1-crystallin (consistent with NCBI sequence NM_023695) was cloned into a pET-20b(+) vector (Novagen) utilizing the ATG codon of its Nde1 site (CATATG) as a start codon (generous gift of Dr. H. Mchaourab). The recombinant pET/βB1 plasmid was then used to transform competent BL21(DE3)pLysS cells according to the manufacturer’s protocol (Invitrogen). Bacterial cultures were grown to an OD600 of 0.5-0.6, and induced for 2 hours with 1 mM IPTG. The harvested cell pellet was resuspended in Buffer A (50 mM Tris-HCl, 1 mM EDTA, 0.15 M NaCl, 1 mM DTT, 50 μM TCEP, at pH 7.5) with added Complete Protease Inhibitors cocktail (Roche). Lysis was performed by sonication. The presence of βB1-crystallin was confirmed by SDS-PAGE. The lysate was centrifuged and the supernatant was dialyzed overnight against 2 liters of Buffer B (50 mM sodium phosphate, 1 mM EDTA, 1 mM DTT, and 50 μM TCEP, at pH 6.8). The dialysate was then loaded on a HiTrap SP FF cation exchange chromatography column (GE Healthcare), and eluted with a gradient of 0 to 1 M NaCl in Buffer B. Fractions containing B1-crystallinB1-crystallin were pooled, concentrated, and chromatographed on a Superdex 75 HR16/60 size-exclusion chromatography column with Buffer A. The Superdex 75 column was precalibrated with the following low-molecular-weight standards: bovine serum albumin, ovalbumin, chymotrypsinogen, ribonuclease A, and acetone (Amersham Biosciences; Sigma-Aldrich). Fractions containing βB1-crystallin were pooled with a final purity of >95% as assessed by SDS-PAGE.
Expression and purification of βA3-crystallin
Human cDNA encoding βA3-crystallin (consistent with NCBI sequence NM_005208) was cloned into pET-20b(+) utilizing the ATG codon of its Nde1 restriction site as start codon. Recombinant pET/βA3 plasmid was then used to transform competent BL21(DE3) cells according to manufacturer’s protocol (Invitrogen). Bacterial cultures were grown to an OD600 of 0.5-0.6, and induced for 2 hours with 0.5 mM IPTG. The harvested cell pellet was resuspended in Buffer A. Lysis was performed by sonication. The presence of βA3-crystallin was confirmed by SDS-PAGE and Western blots using antibodies that target the first Greek-key motif of βA3-crystallin (a.a. 37-68: DQENFQGKRMEFTSSCPNVSERNFDNVRSLKV) (6). The lysate was centrifuged and the supernatant was dialyzed overnight against 2 L of Buffer C (50 mM Tris-HCl, 1 mM EDTA, 1 mM DTT, and 50 μM TCEP, at pH 8.1). The dialysate was then loaded on a HiTrap DEAE FF anion exchange chromatography column (GE Healthcare), and eluted with a gradient of 0 to 1 M NaCl in Buffer C. Fractions containing βA3-crystallin were pooled, concentrated, and eluted on a Superdex 75 HR16/60 size-exclusion chromatography column with Buffer A. Fractions containing βA3-crystallin were pooled and showed a final purity of >95% as assessed by SDS-PAGE.
Association of βB1- and βA3-crystallins
Purified βB1- and βA3-crystallins in Buffer A were adjusted to three sets of concentrations according to Table 1 (approximately 0.5, 1, and 2 mg/mL each), to give a 1:1 molar ratio when present in equal volumes. Concentrations were estimated from A280 readings on a spectrophotometer. At each concentration, equal volumes of βB1- and βA3-crystallins were mixed and incubated at room temperature in Buffer C. Aliquots of 300 μL and 30 μL were taken at some or all of the following time points: 0, 0.5, 1, 2, 4, 6, 12, and 24 hours after mixing, and immediately frozen in ethanol-dry ice bath and stored at -80°C until ready for analyses.
Table 1.
Starting concentrations of βB1- and βA3-crystallins for association experiments.
| Starting concentration of each protein in mg/mL (μmol/L) |
A280 of βB1- crystallin, o.u.* |
A280 of βA3- crystallin, o.u. |
|---|---|---|
| 0.5 (18) | 0.54 | 0.48 |
| 1 (36) | 1.14 | 1.04 |
| 2 (72) | 2.09 | 1.85 |
o.u. = Arbitary optical units
Chromatography and Electrophoresis
Each 300 μL aliquot of the mixture of βB1- and βA3-crystallins was subsequently thawed and loaded on a Superdex 75 HR10/30 size-exclusion chromatography column (analytical grade) precalibrated with the same low-molecular-weight standards as mentioned before. Each sample was eluted with one bed volume (25 mL) of Buffer A and the A280 was monitored throughout the run. For SDS-PAGE analysis, selected fractions were electrophoresed on ReadyGels (Tris-HCl, 4-15% gradient; BioRad) in Tris/glycine/SDS buffer at 200 V for 30 minutes, using a Benchmark Protein Ladder (Invitrogen) as molecular weight markers. For native gel electrophoresis, the 30 μL aliquots were electrophoresed on ReadyGels in the same manner but in the absence of SDS.
Analytical Ultracentrifugation
Prior to centrifugation, purified proteins were partially unfolded by adding 10 mM DTT and 1 M urea to the samples, incubated at room temperature for 30 minutes, and subsequently refolded by dialysis against 2 L of Buffer A without DTT at 4°C for 24 hours. Only samples showing a single peak on size-exclusion chromatography and >95% purity on SDS-PAGE were used for analytical ultracentrifugation. Sample concentration was adjusted to 0.6 mg/mL. Centrifugation was done in a Beckman Optima XL-I analytical centrifuge. Absorption optics, an An-60 Ti rotor, and standard double-sector centerpiece cells were used. All analyses were performed using duplicate protein samples. Data were collected after 16 hours of centrifugation at 16,500 rpm at 20°C. The baselines were established by overspeeding at 45,000 rpm for another 4 hours. Equilibria profiles were analyzed by standard Optima XL-I Origin-based data analysis software. Solvent density was estimated as previously described (14).
Dissociation constants were determined by fitting either a monomer-dimer equilibrium curve (for βB1-crystallin) or a dimer-tetramer equilibrium curve (for βB1/βA3-heterocomplex) to the concentration gradient profile established at each temperature.
Isoelectric focusing
Samples taken at various time points after mixing of βB1- and βA3-crystallins were electrofocused on nondenaturing PhastGels IEF 3-9 (Pharmacia). One μL of sample was applied in each lane. PhastSystem (Pharmacia) was set up to run the following protocol: (1) Prefocusing at 2000 V, 2.5 mA, 3.5 W, 15°C for 75 V·h; (2) Sample application at 200 V, 2.5 mA, 3.5 W, 15°C for 15 V·h; (3) Focusing at 2000 V, 2.5 mA, 3.5 W, 15°C for 410 V·h. Broad-range pI 3-10 markers (Pharmacia) were applied as standards. After isoelectric focusing, gels were fixed with 20% trichloroacetic acid, stained with 0.02% PhastGel Blue R solution (coomassie) in 30% methanol and 10% acetic acid with 0.1% (w/v) CuSO4, and finally destained with 30% methanol and 10% acetic acid.
RESULTS
Expression of mouse βB1-crystallin
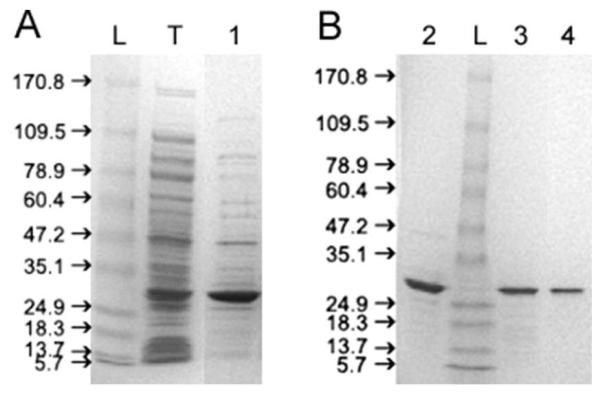
Mouse βB1-crystallin is expressed as soluble protein with high yield in E. coli. Figure 1 shows the SDS-PAGE of the sample following purification by ion-exchange (A) and size-exclusion chromatography. A discrete band at 28 kDa is seen with a final purity of >95% (Figure 1B). This band reacts strongly with anti-βH-crystallin antibodies on Western blot (data not shown). At 1 mg/mL, βB1-crystallin elutes on Superdex 75 column as a single peak with an apparent molecular weight of 37 kDa, intermediate between the predicted masses of monomeric (28 kDa) and dimeric (56 kDa) βB1-crystallin (Figure 2A). After incubation at room temperature for 24 hours, βB1-crystallin elutes again as a single peak, but at a slightly larger apparent size of 37.8 kDa (data not shown). This difference is small and of uncertain significance. The peaks are somewhat broad and slightly skewed, as is expected for a protein in rapid monomer-dimer equilibrium (7). Mass spectrometry shows the molecular mass to be 27,871.16 Da, consistent with the expected monomeric mass of 27,870 Da. The purified proteins were intact as assessed by SDS PAGE and no contaminating peptides were detected on mass spectrometry.
Figure 1.
SDS-PAGE showing stepwise purification of mouse βB1-crystallin: L- protein ladder; (A) T- total lysate of bacteria expressing βB1-crystallin; 1- sample after cation exchange; (B) 2- sample after size-exclusion chromatography; 3- sample after partial unfolding by urea and subsequent refolding by dialysis; 4- sample after second size-exclusion chromatography. A discrete band at 28 kDa is seen with a final purity of >95% (Figure 1B, lane 4).
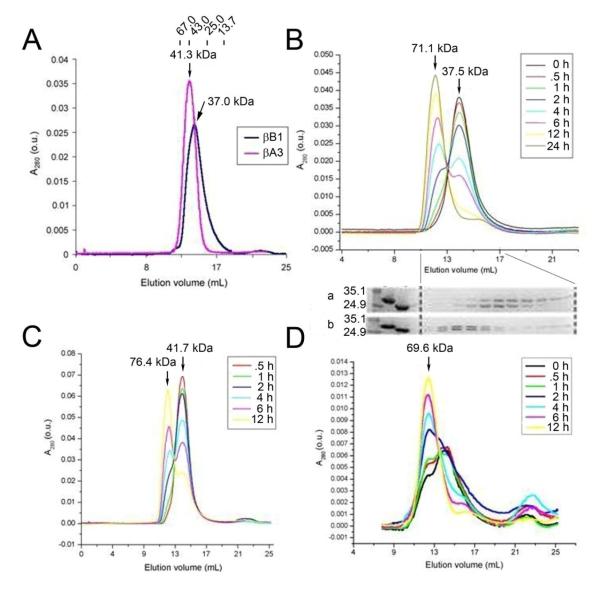
Figure 2.
Size-exclusion chromatography of mouse βB1-crystallin and human βA3-crystallins. Panel A. Size-exclusion chromatography of mouse βB1-crystallin and human βA3-crystallin, each at 1 mg/mL. The elution points of molecular weight standards (albumin 67.0 kDa, ovalbumin 43.0 kDa, chymotrypsinogen 25.0 kDa, and ribonuclease A 13.7 kDa) are shown at the top for reference. Molecular weights of βB1- and βA3-crystallins were calculated to be 37.0 kDa and 41.3 kDa, respectively. Panel B. Size-exclusion chromatography of βB1- and βA3-crystallins mixed at 1 mg/mL. Protein concentration was monitored by absorbance at 280 nm. Chromatograms obtained for each time point after initial mixing of the two crystallins are overlaid to show the trend. Molecular weights of the two peaks at different time points are calculated from the standard curve of the sizing column and averaged to be 71.1 kDa and 37.5 kDa, respectively. A stepwise increase in the higher-molecular-weight species (71.1 kDa) and a stepwise decrease in the lower-molecular-weight species (37.5 kDa) were observed. SDS-PAGE of the fractions from chromatography runs (a) 0 hr and (b) 24 hr are aligned on the bottom to show contents of the peaks. The first lane refers to protein markers (35.1 and 24.9 kDa). The second and third lanes refer to βB1- and βA3-crystallin standards, respectively. Panels C and D. Size-exclusion chromatography of βB1- and βA3-crystallins mixed at (C) 2 mg/mL and (D) 0.5 mg/mL. Averaged molecular weight of each peak is indicated, except for the lower-molecular-weight species in (D), which is indistinct. The small peak observed at the end of each run corresponds to a molecular mass that is lower than the column volume, and is therefore not analyzed.
Expression of human βA3-crystallin
Human βA3-crystallin was expressed as soluble protein with high yield in E. coli as previously described (7). After purification by ion-exchange and size-exclusion chromatography as previously described (15), SDS-PAGE shows a discrete band at 25 kDa with a final purity of >95%. This band reacts strongly with anti-βA3 antibodies on Western blots (data not shown). At 1 mg/mL, βA3-crystallin elutes on Superdex 75 column as a single peak with an apparent molecular weight of 41.3 kDa (Figure 2A). This is intermediate between the predicted masses of monomeric (25 kDa) and dimeric (50 kDa) βA3-crystallin. After incubation at room temperature for 24 hours, βA3-crystallin elutes as a single peak and shows minimal if any increase in its apparent molecular size (data not shown). The purified proteins were intact as assessed by SDS PAGE and no contaminating peptides were detected on mass spectrometry.
Size-exclusion chromatography of mixed βB1- and βA3-crystallins
When βB1- and βA3-crystallins are mixed at 36 μM (equivalent to approximately 1 mg/mL) each, allowed to stand at room temperature for varying periods of time, and chromatographed on a Superdex 75 column, one or two peaks are observed depending on the incubation time. These two distinct peaks have averaged apparent molecular masses of 71.1 kDa and 37.5 kDa, respectively (Figure 2B). At longer incubation times there is a clear trend towards an increasing amount of the higher-molecular-weight species (71.1 kDa), coupled with a decreasing amount of the lower-molecular-weight species (37.5 kDa) with an initial half life of about 5 hours. SDS-PAGE shows that at the beginning of the incubation (0 hour), the lower-molecular-weight peak comprises βB1- and βA3-crystallins that are asymmetrically distributed with βA3-crystallin appearing slightly before βB1-crystallin (Figure 2B-gel a). At the end of the incubation (24 hours), the higher-molecular-weight peak comprises samples with a 1:1 ratio of the two crystallins in the high molecular weight peak and some excess βB1-crystallin in smaller molecular weight fractions (Figure 2B-gel b, confirmed by scanning of the electrophoresed bands, data not shown).
When the initial concentration is doubled to 72 μM (equivalent to approximately 2 mg/mL), a similar trend is observed, with both species showing slightly higher molecular masses (76.4 and 41.7 kDa, respectively) (Figure 2C). A similar trend is also observed when the initial concentration is lowered to 18 μM (equivalent to approximately 0.5 mg/mL), but the peaks becomes less distinct as the levels approach the detection limit of the monitor (Figure 2D). A peak that corresponds to the higher-molecular-weight species has an averaged molecular weight of 69.6 kDa, which is slightly lower than that observed at higher concentrations, suggesting a rapid dimer-tetramer equilibrium for βB1-crystallin similar to that of βA3-crystallin monomers and dimers described in (7).
Native gel electrophoresis of mixed βB1- and βA3-crystallins
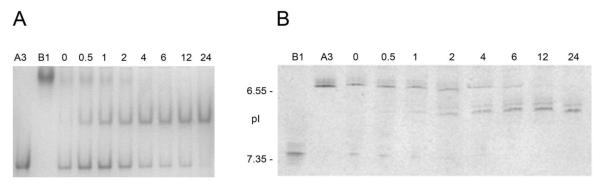
Figure 3A shows native gel electrophoresis of βB1- and βA3-crystallin samples mixed at 1 mg/mL. The migration of βB1-crystallin is markedly retarded, with most of the protein retained at the loading position of the gels. Conversely, βA3-crystallin migrates well into the gels. Both concentrations display qualitatively similar patterns. No interaction between βB1- andβA3-crystallins is observed at time 0, followed by increasing formation of the intermediate species, and decreasing amounts of the starting proteins at successive time points. The most prominent intermediate band is located closer to the βB1-crystallin position than to the βA3-crystallin position (indicated by black arrow).
Figure 3.
Native gel electrophoresis and isoelectric focusing of βB1- and βA3-crystallins. Panel A. Native gel electrophoresis of βB1- and βA3-crystallins mixed at 1 mg/mL each. The black arrow shows the major association product increasing towards end of the incubation. B1:βB1-crystallin only; A3: βA3-crystallin only; 0-24: aliquots taken at corresponding time points (in hours) after initial mixing of the two crystallins. Panel B. Isoelectric focusing of mixture of βB1- and βA3-crystallins at 1 mg/mL. Location of two pI markers, human carbonic anhydrase B (pI 6.55) and horse myoglobin-basic band (pI 7.35), are indicated on the left. B1: βB1-crystallin; A3: βA3-crystallin; 0-24: aliquots taken at corresponding time points (in hours) after mixing.
Isoelectric focusing of mixed βB1- and βA3 crystallins
Figure 3B shows that βB1-crystallin electrofocuses at an isoelectric point slightly lower than 7.35, consistent with its predicted pI of 7.28. βA3-Crystallin electrofocuses slightly lower than pI 6.55, also consistent with its predicted pI of 6.20. Isoelectric focusing immediately after mixing essentially produces an overlay of the two patterns observed with the individual proteins. At increasing incubation times, less protein is observed at the pI’s of the individual crystallins, while more protein is found at an intermediate pI, with two additional lighter and slightly more acidic bands also appearing as the incubation progresses. Formation of the intermediate species becomes noticeable by 0.5 hour after mixing, and appears to be approaching equilibrium at 12 hours, at which point most protein is present as the intermediate species.
Sedimentation equilibrium of βB1-crystallin
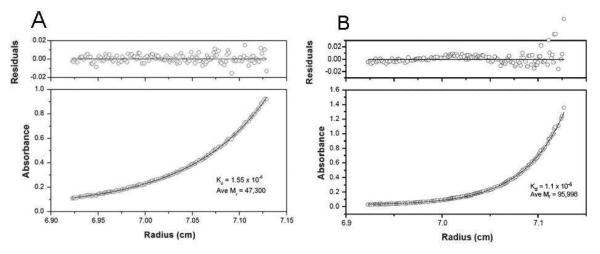
Sedimentation equilibrium analysis of purified mouse βB1-crystallin shows that the experimental values fit closely with the predicted monomer-dimer model at 20°C (Figure 4A). Assuming a monomer-dimer equilibrium, the dissociation constant (Kd) is 1.55 ± 0.20 μM and weight-average molecular weight (Mr) is determined to be 47,295 ± 276 Da. Comparison of the average Kd and Mr of mouse βB1-, βA3-, and βB2-crystallins is shown in Table 2.
Figure 4.
Sedimentation equilibrium of purified mouse βB1-crystallin and of the mouse βB1- and human βA3-crystallin complex are shown. Panel A. Sedimentation equilibrium of purified mouse βB1-crystallin at 0.6 mg/mL and 20°C. The absorbance (280 nm) gradient in the ultracentrifuge cell after attaining sedimentation equilibrium is shown in the bottom panel. The solid line indicates the predicted monomer-dimer association model, and the open circles represent the experiment values. The top panel shows the difference between the predicted and the experimental values as a function of radial position (residuals). Panel B. Sedimentation equilibrium of the mouse βB1- and human βA3-crystallin complex at 0.6 mg/mL at 20°C. The solid lines indicate the predicted heterodimer-heterotetramer association model, and the open circles represent the experiment values.
Table 2.
Comparison of homodimer dissociation constants (Kd) and average molecular weight (Mr) of mouse βB1-, βA3-, and βB2-crystallins determined by sedimentation equilibrium at 20°C.
| Protein | Kd,μM | Average Mr, kDa | Predicted Mr of dimer, kDa |
|---|---|---|---|
| βB1 | 1.55 | 47 | 56 |
| βA3* | 0.8 | 47 | 50 |
| βB2* | 5.0 | 39 | 46 |
Values previously determined (13).
Sedimentation equilibrium of βB1/βA3 heterotetramer
Figure 4B shows that at 20°C, βB1- and βA3-crystallins form heterocomplexes that fit well with the predicted dimer-heterotetramer equilibrium, with a Kd of 1.1 μM. The average molecular weight of the complex is determined to be 95,998 ± 714.9 Da at 20°C, which is close to the predicted mass of the heterotetramer containing two subunits of each crystallin (106.2 kDa).
DISCUSSION
Here we describe the association of mouse βB1- and human βA3-crystallins. When present alone, each protein associates into homodimers but neither forms homotetramers, similar to results obtained with βB2- and βA3-crystallins. However, in contrast to the previous results obtained from βB2- and βA3-crystallins, mixed βB1- and βA3-crystallins show a dimer-tetramer equilibrium, with a Kd of 1.1 μM. Thus mixed βB1- and βA3-crystallins associate predominantly into heterotetramers in vitro.
Mouse βB1-crystallin was faithfully expressed with high yield in BL21(DE3)pLysS, which is the same system used previously for expressing human βB1-crystallin by Lampi et al. (16). The identity of the expressed βB1-crystallin is supported by its correct molecular mass on SDS-PAGE and mass spectrometry. Human βA3-crystallin was previously expressed using the baculovirus system (6). This study utilized the bacterial system BL21(DE3), which is a more convenient and equally productive method for the expression of relatively stable proteins, such as human βA3-crystallin. The identity of the expressed βA3-crystallin is confirmed by its molecular mass on SDS-PAGE and its strong reactivity with antibodies that specifically target the first Greek-key motif of bovine βA3-crystallin (data not shown). While mouse and human βB1-crystallins are only 80.9% identical (90% similar), mouse and human βA3-crystallins share a 95.3% identity (100% similarity), suggesting that the use of human and mouse crystallins provides a reasonably accurate model of crystallin behavior, especially in the mouse lens. Also, while the conserved PAPA sequence is more evident in mouse βB1-crystallin, human βB1-crystallin is predicted to share similar structural characteristics in this region by both Chou-Fasman and Garnier-Robson algorithms (data not shown).
Size-exclusion chromatography of mouse βB1-crystallin at 1 mg/mL shows a somewhat broad and asymmetrical single peak with an apparent molecular weight that is intermediate between the monomeric and dimeric forms, consistent with a monomer-dimer equilibrium (17). An intermediate molecular weight is observed as monomers constantly associate and dissociate in a rapidly reversible manner as the protein is chromatographed (17). The homodimer formation observed in this study agrees with other reports of βB1-crystallin (16;18) and most other β-crystallins, including βA3- and B2-crystallins (6;13). However, while size exclusion chromatography in these studies has suggested that βB1-crystallin might behave as a dimer as concentrations increase above 10 mg/ml (18) or at 0.7 mg/ml (16), light scattering suggests molecular masses consistent with a dimer with a small population in aggregates of 264 kDa (16) or varying between 48 kDa and 125 kDa depending on the protein concentration (18). While the nature of the higher aggregates is unclear from the data presented, they do not appear to represent the reversible high affinity association described here. Rather, the data suggest either a stable high molecular weight complex co-existing with a lower dimeric form in some cases and a gradual smooth increase in molecular mass in others.
The stability of βB1-crystallin over time is supported by its molecular weight, which remains relatively constant after 24 hours of incubation at room temperature. Similar results were obtained for βA3-crystallin, which also displays persistent monomer-dimer equilibrium. Despite having a smaller predicted molecular size, βA3-crystallin shows a higher apparent molecular weight on size exclusion chromatography than βB1-crystallin. Delayed elution of βB1-crystallin from size exclusion chromatography has been reported previously(16;18) and has been attributed to interaction between βB1-crystallin and the column matrix, as is seen with amino-truncated βA3-crystallin,(8) . However, it is also consistent with the higher affinity for self-dimerization of βA3-crystallin relative to βB1-crystallin. The increase of the apparent molecular weight with increasing βB1-crystallin concentration on size exclusion chromatography suggests that the delayed elution of βB1-crystallin is due to reversible monomer-dimer equilibrium rather than interactions with the column or unusual effects of an unusual shape of the molecule, or perhaps both might contribute to some degree.
Sedimentation equilibrium of mouse βB1-crystallin at 0.6 mg/mL and 20°C again confirms a monomer-dimer equilibrium. In contradistinction to some previous reports,(18) no evidence for homotetramer formation was seen with analytical ultracentrifugation or size exclusion chromatography in these studies. The apparent average molecular weight estimated from sedimentation equilibrium at 20°C (47 kDa) is significantly higher than that estimated from size-exclusion chromatography (37 kDa). This discrepancy may be attributed to the fact that the association of crystallins is dependent on protein concentration, but also might reflect a tendency of βB1-crystallin to stick to the gel matrix in a size-exclusion column as discussed above. While the sedimentation study does not involve any elution buffer, size-exclusion chromatography subjects protein samples to dilution by the running buffer. As protein concentration drops, the monomer-dimer equilibrium is expected to shift towards monomer, thus resulting in a lower apparent molecular weight.
When mouse βB1-crystallin and human βA3-crystallin are incubated together at room temperature, heterocomplex formation is readily appreciable within 30 minutes by size-exclusion chromatography, native gel electrophoresis, and isoelectric focusing. In Figure 2, the higher-molecular-weight species that increases in amount with incubation time represents a dimer-tetramer equilibrium, as it corresponds to an apparent molecular weight that is intermediate between that of a dimer (either homodimer or heterodimer) and that of a tetramer. A number of observations suggest that this peak represents tetramer rather than trimer formation. As is seen with βA3- and βB1-crystallins in isolation, the apparent molecular weight increases towards the predicted tetramer size with increasing protein concentration.
SDS-PAGE of the eluted fractions shows that this peak contains equal amounts of βB1- and βA3-crystallins (Figure 2B). This 1:1 ratio suggests that each tetramer is composed on average of two subunits of βB1-crystallin and two subunits of βA3-crystallin. Conversely, the lower-molecular-weight species corresponds to a monomer-dimer equilibrium that decreases in amount over time. SDS-PAGE of this peak shows an asymmetric distribution of βB1- andβA3-crystallins, suggesting lack of interaction at the beginning of the incubation, and perhaps a tendency of βB1-crystallin to stick to the column matrix. In theory, three types of dimers can be present: βB1-homodimers (56 kDa), βA3-homodimers (50 kDa), and βB1/βA3-heterodimers (53 kDa). It is likely that the proximity in molecular masses of these three species has exceeded the resolution capacity of the Superdex 75 column, resulting in a broad single peak. While the exact mechanism of heterotetramer formation is unknown, it seems likely that it is via the association of two βB1/βA3-heterodimers rather than association of a βB1-homodimer with a βA3-homodimer. Ultimately, one would expect the mixture to reach a steady state of heterodimer-heterotetramer equilibrium. These data are in agreement with those of Bateman et al.(12). As expected, the final amount of heterotetramer formed is dependent on crystallin concentration. As protein concentration increases, the percentage of protein at the molecular masses representing both monomer-dimer and dimer-tetramer equilibria increase, and vice versa (Figures 2B-2D). This indicates that higher concentrations shift the equilibria towards association.
Native gel electrophoresis, which separates proteins in their native states, provides additional information on the shapes of the complexes. Of interest, the migration of βB1-crystallin by itself is markedly retarded with some protein not entering the gel (Figure 3A). One possible explanation is that the N-terminal extension of βB1-crystallin, being the longest among all β-crystallins, severely hinders the migration of monomeric/dimeric βB1-crystallin through the polyacrylamide gel matrix. The intermediates formed over time, representing heterocomplex formation with βA3-crystallin, migrate further down the gel compared to βB1-crystallin alone. This suggests the interaction between βB1- and βA3-crystallins gives rise to a conformation that is more compact and thus penetrates the matrix more easily. One possibility is that the N-terminal arm of βB1-crystallin may be involved in holding the heterocomplex in place and thus becomes less flexible in the tetramer (19), protruding less and allowing migration of the complex into the gel. Alternatively, the βB1-crystallin N-terminal arm might interact with the gel matrix less in heterotetramers, which would also allow the heterocomplex to migrate further than the βB1-crystallin dimer.
Heterocomplex formation is also confirmed by isoelectric focusing. As expected, the heterocomplex has a pI that is intermediate between that of βB1-crystallin and of βA3-crystallin (Figure 3B). While this technique does not distinguish between heterodimer and heterotetramer formation, the absence of homodimers or homotetramers, which would have identical isoelectric points, after longer incubation times suggests that most of the intermediate band is composed of heterotetramers. This can be seen in comparison to isoelectric focusing of mixed βB2- and βA3-crystallins, in which the heterodimer and two homodimers coexist in a 2-1-1 ratio (13). That is, at equilibrium there is twice as much heterodimer as there is of each homodimer, suggesting that none of the three possible dimers is energetically favored over the other.
Finally, analytical ultracentrifugation provides thermodynamic characterization of the heterocomplex equilibrium. The experimental data fit well with the expected gradient of the heterodimer-heterotetramer equilibrium, with a Kd of 1.1 μM. The observed molecular weight (96 kDa) is consistent with the predicted molecular weight of the heterotetramer consisting of two βB1- and two βA3-crystallin subunits (106.2 kDa), but significantly higher than the apparent molecular weight estimated from size-exclusion chromatography (69.6-76.4 kDa). Again, this may be due to the dilution effect during chromatography or adherence of the βB1-crystallin protein to the size exclusion chromatography gel matrix or both.
The formation of heterotetramers by mixed βB1- and βA3-crystallin contrasts with the lack of tetramers seen under similar conditions with mixed βB2- and βA3-crystallin (7;8). However, this is consistent with the high βB1-crystallin content observed in βH aggregates synthesized in vivo (5;20). βB2-crystallin, on the other hand, is present in all size-classes and is the primary constituent of βL2-crystallin (20), suggesting βB2-crystallin might have a low affinity for higher-order association with other β-crystallins. These observations might relate to the structural difference between βB2- and βB1-crystallins. The major factor that distinguishes βB1-crystallin from other β-crystallins, and especially basic β-crystallins, is its extremely long N-terminal extension containing 57 residues including a PAPA sequence (21). We hypothesize thatβB1-crystallin may promote higher-order complex formation with other β-crystallins in the lens through the action of its long N-terminal extension. While it cannot, by itself, account for the formation of βH-crystallin oligomers, this does agree with the presence of βB1-crystallin preferentially in the βH complexes and truncated βB1-crystallins in βL1 and βL2 complexes previously described (9).
It is important to realize that under physiologic conditions, where protein concentration exceeds 300 mg/mL, higher-order association between β-crystallins would be favored. Therefore, βB1-crystallin might have a crucial role in associating with other β-crystallins in higher order complexes. We have not been able to measure the dissociation constant of the putative βB1 βA3-crystallin heterodimer. However, if it has a similar dissociation constant to the βB1 homodimer the fraction of heterotetramer at 300 mg/ml would be greater than 99.99%, and this fraction would still approach 99.9% at 1 mg/ml. Thus, as has been previously suggested, the rapid interchange of crystallins might have more physiological implications than the existence of monomer or even dimer forms of the protein. This being said, the findings in this study have implications in cataractogenesis of the aging lens where N-terminal truncation of βB1-crystallin has been well documented (10;22). In this regard, the size distribution of β-crystallins is dependent on the age of the lens, which is itself correlated with crystallin modifications including truncation of the terminal arms (10;11). Finally, the lack of homotetramer formation in vitro indicates that higher-order association between different β-crystallin subtypes is favored over self-association. This supports a previous suggestion that acidic β-crystallins may preferentially associate with basic β-crystallins (23), although this may well result from heterotetramer/ heterodimer equilibrium with βB1- and βA3-crystallins as opposed to the heterodimer in which βB2- and βA3-crystallins associate into βB2 homodimers:βB2-βA3 heterodimers:βA3 homodimers with a roughly 1:2:1 ratio (13;15).
In summary, this study has demonstrated for the first time reversible spontaneous in vitro formation of heterotetramers by β-crystallins. When present alone under these conditions, βB1- and βA3-crystallin associate into homodimers but neither forms homotetramers. Upon mixing under physiological conditions, heterocomplex formation between these two β-crystallin subtypes was observed by size-exclusion chromatography, native gel electrophoresis, isoelectric focusing, and sedimentation equilibrium. In contrast to the previous results obtained from βB2- and βA3-crystallins, which did not show tetramer formation, analytical centrifugation shows a dimer-tetramer equilibrium with a Kd of 1.1 μM, suggesting that βB1- and βA3-crystallins associate predominantly into heterotetramers in vitro. Although we suspect that heterotetramers are formed preferentially through the interaction of two heterodimers (βB1/βA3 + βB1/βA3) these studies are unable to discriminate this mechanism from that involving the interaction between two homodimers (βB1/βB1 + βA3/βA3) or mixtures of both hetero- and homodimers. Future studies will address this question, as well as elucidating the molecular mechanisms of βB1-crystallin association into both dimers and heterotetramers.
References
- 1.Wistow G, Turnell B, Summers L, Slingsby C, Moss D, Miller L, Lindley P, Blundell T. X-ray analysis of the eye lens protein gamma-II crystallin at 1.9 A resolution. J. Mol. Biol. 1983;170:175–202. doi: 10.1016/s0022-2836(83)80232-0. [DOI] [PubMed] [Google Scholar]
- 2.Hejtmancik JF, Piatigorsky J. Lens Proteins and their Molecular Biology. In: Alpert DM, Jakobiec FA, Azar DT, Gragoudas ES, editors. Principles and Practice of Ophthalmology. W.B.Saunders Co.; Philadelphia: 2000. pp. 1409–1428. [Google Scholar]
- 3.Bindels JG, Koppers A, Hoenders HJ. Structural aspects of bovine beta-crystallins: physical characterization including dissociation-association behavior. Exp. Eye Res. 1981;33:333–343. doi: 10.1016/s0014-4835(81)80056-5. [DOI] [PubMed] [Google Scholar]
- 4.Zigler JS, Jr., Horwitz J, Kinoshita JH. Human beta-crystallin. I. Comparative studies on the beta 1, beta 2 and beta 3-crystallins. Exp. Eye Res. 1980;31:41–55. doi: 10.1016/0014-4835(80)90089-5. [DOI] [PubMed] [Google Scholar]
- 5.Siezen RJ, Anello RD, Thompson JD. Interactions of Lens Proteins. Concentration dependence of beta-crystallin aggregation. Exp. Eye Res. 1986;43:293–303. doi: 10.1016/s0014-4835(86)80067-7. [DOI] [PubMed] [Google Scholar]
- 6.Hope JN, Chen HC, Hejtmancik JF. BetaA3/A1-crystallin association: role of the amino terminal arm. Protein. Eng. 1994;7:445–451. doi: 10.1093/protein/7.3.445. [DOI] [PubMed] [Google Scholar]
- 7.Sergeev YV, Wingfield PT, Hejtmancik JF. Monomer-dimer equilibrium of normal and modified beta A3-crystallins: experimental determination and molecular modeling. Biochemistry. 2000;39:15799–15806. doi: 10.1021/bi001882h. [DOI] [PubMed] [Google Scholar]
- 8.Sergeev YV, Hejtmancik JF, Wingfield PT. Energetics of Domain-Domain Interactions and Entropy Driven Association of beta-Crystallins. Biochemistry. 2004;43:415–424. doi: 10.1021/bi034617f. [DOI] [PubMed] [Google Scholar]
- 9.Ajaz MS, Ma Z, Smith DL, Smith JB. Size of human lens beta-crystallin aggregates are distinguished by N-terminal truncation of betaB1. J. Biol. Chem. 1997;272:11250–11255. doi: 10.1074/jbc.272.17.11250. [DOI] [PubMed] [Google Scholar]
- 10.Lampi KJ, Ma Z, Hanson SR, Azuma M, Shih M, Shearer TR, Smith DL, Smith JB, David LL. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp. Eye Res. 1998;67:31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- 11.Ma Z, Hanson SR, Lampi KJ, David LL, Smith DL, Smith JB. Age-related changes in human lens crystallins identified by HPLC and mass spectrometry. Exp. Eye Res. 1998;67:21–30. doi: 10.1006/exer.1998.0482. [DOI] [PubMed] [Google Scholar]
- 12.Bateman OA, Sarra R, Van Genesen ST, Kappe G, Lubsen NH, Slingsby C. The stability of human acidic beta-crystallin oligomers and hetero-oligomers. Exp. Eye Res. 2003;77:409–422. doi: 10.1016/s0014-4835(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 13.Hejtmancik JF, Wingfield P, Chambers C, Russell P, Chen H-C, Sergeev YV, Hope JN. Association properties of beta-B2- and betaA3-crystallin: ability to form dimers. Protein. Eng. 1997;10:1347–1352. doi: 10.1093/protein/10.11.1347. [DOI] [PubMed] [Google Scholar]
- 14.Laue TM, Shah BD, Ridgeway TM, Pelletier SL. Computer-aided interpretation of analytical sedimentation data for proteins. In: Harding SE, Rowe AJ, Horton JC, editors. Analytical Ultracentrifugation in Biochemistry and Polymer Science. Royal Society for Chemistry; Cambridge, United Kingdom: 1992. pp. 90–125. [Google Scholar]
- 15.Hope JN, Chen H-C, Hejtmancik JF. Aggregation of betaA3-crystallin is independent of the specific sequence of the domain connecting peptide. J. Biol. Chem. 1994;269:21141–21145. [PubMed] [Google Scholar]
- 16.Lampi KJ, Oxford JT, Bachinger HP, Shearer TR, David LL, Kapfer DM. Deamidation of human beta B1 alters the elongated structure of the dimer. Exp. Eye Res. 2001;72:279–288. doi: 10.1006/exer.2000.0950. [DOI] [PubMed] [Google Scholar]
- 17.Winzor DJ, Scheraga HA. Studies of chemically reacting systems on sephadex: chromatographic demonstration of the Gilbert Theory. Biochemistry. 1963;2:1263–1267. doi: 10.1021/bi00906a016. [DOI] [PubMed] [Google Scholar]
- 18.Bateman OA, Lubsen NH, Slingsby C. Association behaviour of human betaB1-crystallin and its truncated forms. Exp. Eye Res. 2001;73:321–331. doi: 10.1006/exer.2001.1038. [DOI] [PubMed] [Google Scholar]
- 19.Lapatto R, Nalini V, Bax B, Driessen H, Lindley PF, Blundell TL, Slingsby C. High resolution structure of an oligomeric eye lens beta-crystallin: loops, arches, linkers and interfaces in betaB2 dimer compared to a monomeric gamma-crystallin. J. Mol. Biol. 1991;222:1067–1083. doi: 10.1016/0022-2836(91)90594-v. [DOI] [PubMed] [Google Scholar]
- 20.Slingsby C, Bateman OA. Rapid separation of bovine beta-crystallin subunits beta B1, beta B2, beta B3, beta A3 and beta A4. Exp. Eye Res. 1990;51:21–26. doi: 10.1016/0014-4835(90)90165-q. [DOI] [PubMed] [Google Scholar]
- 21.Hejtmancik JF, Thompson MA, Wistow G, Piatigorsky J. cDNA and deduced protein sequence for the beta B1-crystallin polypeptide of the chicken lens: Conservation of the PAPA sequence. J. Biol. Chem. 1986;261:982–987. [PubMed] [Google Scholar]
- 22.David LL, Lampi KJ, Lund AL, Smith JB. The sequence of human betaB1-crystallin cDNA allows mass spectrometric detection of betaB1 protein missing portions of its N-terminal extension. J. Biol. Chem. 1996;271:4273–4279. doi: 10.1074/jbc.271.8.4273. [DOI] [PubMed] [Google Scholar]
- 23.Slingsby C, Bateman OA. Qarternary interactions in eye lens beta-crystallins: basic and acidic subunits of beta-crystallins favor heterologous association. Biochemistry. 1990;29:6592–6599. doi: 10.1021/bi00480a007. [DOI] [PubMed] [Google Scholar]