Abstract
Background
Premenstrual dysphoric disorder (PMDD) was included as a provisional diagnostic category in the appendices of Diagnostic and Statistical Manual of Mental Disorders (DSM)-III-R (then called late luteal phase dysphoric disorder) and remained as an appendix in DSM-IV. Our study aimed to determine the prevalence of PMDD using all four DSM-IV research diagnostic criteria in a representative sample of women of reproductive age in the United States.
Method
Data were collected in the homes of women between the ages of 13 and 55 years in two urban and two rural sites using a random sampling procedure developed by the National Opinion Research Center. Women completed daily symptom questionnaires and provided urine specimens each day for two consecutive ovulatory menstrual cycles (ovulation was estimated for women taking oral contraceptives) and were screened for psychiatric disorders by trained interviewers. Symptoms were counted toward a diagnosis of PMDD if they worsened significantly during the late luteal week during two consecutive ovulatory menstrual cycles, occurred on days in which women reported marked interference with functioning, and were not due to another mental disorder.
Results
In the final analysis, 1246 women who had had at least one menstrual cycle and were neither naturally nor surgically menopausal nor pregnant were selected. Of the women in the study, 1.3% met criteria for the diagnosis as defined in DSM-IV.
Conclusions
The prevalence of PMDD is considerably lower than DSM-IV estimates and all but one of the estimates obtained from previous studies when all DSM-IV diagnostic criteria are considered. We suggest a new process for diagnosing PMDD based on our findings.
Keywords: Premenstrual dysphoric disorder, prevalence
Introduction
The idea has existed since the late 1970s that there is a subtype of premenstrual syndrome (PMS) that is primarily distinguished by severe debilitating mood disturbance. The condition, first called late luteal phase dysphoric disorder, was included as a provisional diagnostic category in the appendices of Diagnostic and Statistical Manual of Mental Disorders (DSM)-III-R (APA, 1987). It remained as an appendix in DSM-IV, after being renamed premenstrual dysphoric disorder (PMDD) (APA, 1997).
PMDD is defined by a set of four research criteria, all of which must be met to confirm the diagnosis (APA, 1997). Eleven possible symptoms are listed for the disorder, representing physical changes, changes in sleep, appetite, energy level, and interest in usual activities, difficulty in concentrating, anger/irritability, affective lability, sense of being overwhelmed, depressed mood, and anxiety, The first criterion is that at least five of the 11 possible symptoms, one of which is affective, must be present for most of last week of the luteal phase and be absent in the week post-menses. The second criterion is that PMDD must interfere markedly with school, work, or interpersonal relationships and the third that its symptoms cannot represent an exacerbation of another psychiatric disorder. The fourth criterion requires that the first three criteria must be confirmed by prospective daily ratings of symptoms for two consecutive menstrual cycles.
Although a few prior studies have attempted to determine the prevalence of PMDD, they either failed to take into account all four research criteria that define the disorder, or derived potentially biased prevalence estimates, because they did not use probability sampling. Samples from restricted age ranges or those made up exclusively of women seeking treatment for premenstrual symptoms, for example, likely would yield prevalence estimates that fail to represent the full range of women of reproductive age.
Rivera-Tovar & Frank (1990) reported a 30% increase in prospectively rated symptoms between post-menstrual and late luteal weeks of the cycle in 4.6% of 217 female university college students, but did not assess other psychiatric disorders. Hurt et al. (1992) followed the symptoms of 670 women seeking treatment for premenstrual complaints and found 14–45% to meet criteria for the disorder, depending on the method of assessing post-menstrual to premenstrual symptom change used. Cohen et al. (2002) prospectively measured the premenstrual symptoms of 513 women aged 36–44 years for one menstrual cycle for whom data on current psychiatric morbidity were available. The diagnosis was confirmed in 6.4% of women.
In Germany, Wittchen et al. (2002) found that 5.3% of a group of 1251 young women (aged 14–24 years) met criteria for PMDD. Instead of participants rating symptoms daily, however, they reported symptoms experienced over the previous 12 months to trained interviewers. Participants did undergo psychiatric diagnostic testing. Using a random sample of 83 women drawn from the National Registry of Iceland, Sveindottir & Backstrom (2000) found 2–6% to meet criteria for PMDD. Women completed daily symptom diaries for at least one menstrual cycle, but did not undergo psychiatric diagnostic testing. Banerjee et al. (2000) followed the symptoms of 62 non-treatment-seeking women in India for two menstrual cycles and found 6.4% to meet the diagnosis of PMDD, yet no psychiatric testing was done. In Japan, Takeda et al. (2006) found 1.2% of 1152 women aged 20–49 years recruited from a cancer-screening clinic to meet the diagnosis of PMDD. Women completed daily symptoms and were asked whether symptoms interfered with functioning. The authors do not report for how long women were followed, however, and no psychiatric testing was done.
In a pilot to the present study to test methods to operationalize all four DSM-IV criteria for PMDD in a sample of women aged 13–55 years checking into a variety of out-patient clinics, Gehlert & Hartlage (1997) followed the symptoms of 99 women for two consecutive ovulatory menstrual cycles. Women rated their symptoms each day as well as to what extent how they felt that symptoms interfered with functioning at home, school, or work. Past and present psychiatric disorders were measured and phase of cycle was confirmed by researchers analyzing urine samples for luteinizing hormone (LH) using ovulation predictor kits. Depending upon the method of determining post-menstrual to premenstrual change, 1.0–7.1% of women met diagnostic criteria for PMDD.
Although the study by Gehlert & Hartlage (1997) provided a preliminary design for addressing all four DSM-IV criteria for PMDD, its sample was small and made up of volunteers seeking treatment for medical problems. The present study followed a randomly selected sample of 1246 rural and urban women in their homes, using the methods successfully employed by Gehlert & Hartlage (1997) to determine the prevalence of PMDD.
Method
Participants
Participants were women randomly selected from two rural and two urban sites. Participants were selected as follows.
The 12 800 housing units deemed necessary to provide usable data from 2600 women were randomly selected from census data by the National Opinion Research Center (NORC) at the University of Chicago in four geographic areas: Chicago and DeKalb County in Illinois and St Louis and Franklin County in Missouri. The four areas were selected in part for feasibility reasons because the primary author was located in Chicago and has extensive professional ties in the Missouri sites. In addition, the two rural sites are equidistant from the urban sites.
Letters explaining the study and soliciting participation were mailed to each housing unit selected. Women were told that the study's purpose was to understand changes in women's health and wellness through time.
-
Trained field interviewers visited each housing unit to screen for eligible women after letters were sent [i.e. women aged 13–55 years who had had at least one menstrual cycle and were neither naturally (i.e. no menstrual period for 1 year) nor surgically menopausal nor pregnant] and, if present, enroll one selected by a formula into the study. An attempt was made to enroll equal numbers of women from the four sites, two of which are considered rural and two urban. Based on data from the 2000 US census, both Chicago, Illinois and St Louis, Missouri fit the Census Bureau's definition of an urbanized area (US Census Bureau, 1995). Both DeKalb County, Illinois and Franklin County, Missouri, can be considered rural, because none of their population live within urbanized areas (US Census Bureau, 1995).
Women enrolled in the study represented residents of the four geographic areas on key demographic variables (see Table 1). In addition, participants from the four geographic areas represented 2000 Decennial Census data (US Census Bureau, 2002) on a range of demographic variables, such as race, socio-economic status, and age. For example, the racial breakdown of the Franklin County sample (97.2% White, 1.2% Latino, 0.6% Black, 0.6% native American, and 0.3% Asian) closely mirrors that of the county as a whole (97.0% White, 0.7% Latino, 0.9% Black, 0.2% native American, 0.3% Asian, and 0.8% some other race) and the breakdown for the Chicago site (36% Black, 40.8% White, 20.4% Latino, 2.0% Asian, 1.0% native American, and 1.0% some other race) mirrors that of Chicago as a whole (36.4% Black, 31.3% White, 26.0% Latino, 4.3% Asian, 0.1% native American, and 1.8% some other race) (US Census Bureau, 2002).
Table 1. Sample characteristics.
| St Louis, MO (n=208) |
Franklin County, MO (n=324) |
Chicago, IL (n=250) |
DeKalb County, IL (n=464) |
Total unweighted (n=1246) |
Total weighted (n=1246) |
|
|---|---|---|---|---|---|---|
| Race, no. (%)a | ||||||
| Black | 66 (31.9) | 2 (0.6) | 90 (36.0) | 12 (2.6) | 170 (13.7) | 429 (34.5) |
| White | 132 (63.8) | 315 (97.2) | 102 (40.8) | 428 (92.6) | 977 (78.6) | 519 (41.7) |
| Hispanic | 5 (2.4) | 4 (1.2) | 51 (20.4) | 14 (3.0) | 74 (6.0) | 264 (21.2) |
| Native American | 1 (0.5) | 2 (0.6) | 1 (0.4) | 3 (0.6) | 7 (0.6) | 4 (0.3) |
| Asian/Pacific | 3 (1.4) | 1 (0.3) | 5 (2.0) | 4 (0.9) | 13 (1.0) | 26 (2.1) |
| Other | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.2) | 2 (0.2) | 3 (0.2) |
| Marital status, no. (%) | ||||||
| Married | 86 (42.6) | 222 (70.0) | 115 (47.1) | 296 (66.8) | 719 (59.6) | 516 (42.3) |
| Widowed/divorced/separated | 24 (11.9) | 32 (10.1) | 23 (9.4) | 38 (8.6) | 117 (9.7) | 132 (10.8) |
| Single | 64 (31.7) | 51 (16.1) | 82 (33.6) | 88 (19.9) | 285 (23.6) | 475 (38.9) |
| Living with someone | 28 (13.9) | 12 (3.8) | 24 (9.8) | 21 (4.7) | 85 (7.0) | 98 (8.0) |
| Age | ||||||
| Mean (S.D.), years | 33.40 (9.04) | 33.51 (9.03) | 33.51 (8.90) | 34.26 (8.90) | 33.77 (8.96) | 32.77 (10.39) |
| Annual family income, no. (%)a | ||||||
| $19999 or lower | 43 (22.3) | 29 (10.1) | 65 (28.8) | 58 (13.7) | 195 (17.3) | 345 (30.6) |
| $20000–59999 | 109 (56.5) | 187 (65.4) | 110 (48.7) | 226 (53.6) | 632 (56.1) | 561 (49.8) |
| $60000 or higher | 41 (21.2) | 70 (24.5) | 51 (22.6) | 138 (32.7) | 300 (26.6) | 220 (19.5) |
| Employment status, no. (%)a | ||||||
| Student | 24 (12.1) | 39 (12.8) | 32 (13.2) | 53 (12.3) | 148 (12.6) | 228 (18.8) |
| Unemployed | 8 (4.0) | 11 (3.6) | 9 (3.7) | 8 (1.9) | 36 (3.1) | 54 (4.4) |
| Employed | 150 (75.4) | 196 (64.3) | 156 (64.2) | 324 (75.2) | 826 (70.1) | 737 (60.8) |
| Homemaker | 17 (8.5) | 59 (19.3) | 46 (18.9) | 46 (10.7) | 168 (14.3) | 194 (16.0) |
| Education, no. (%)a | ||||||
| Less than high school | 28 (13.8) | 47 (14.9) | 49 (20.1) | 38 (8.6) | 162 (13.4) | 303 (24.8) |
| High school graduates | 26 (12.8) | 81 (25.6) | 39 (16.0) | 66 (14.9) | 212 (17.6) | 183 (15.0) |
| Some college | 65 (32.0) | 116 (36.7) | 68 (27.9) | 182 (41.1) | 431 (35.7) | 349 (28.6) |
| College graduate or more | 84 (41.4) | 72 (22.8) | 88 (36.1) | 157 (35.4) | 401 (33.3) | 386 (31.6) |
| Oral contraceptive use, no. (%)a | ||||||
| Non-user | 154 (74.4) | 244 (75.3) | 185 (74.3) | 322 (69.4) | 905 (72.7) | 951 (76.4) |
| User | 53 (25.6) | 80 (24.7) | 64 (25.7) | 142 (30.6) | 339 (27.3) | 293 (23.6) |
S.D., Standard deviation.
The sum does not add to the total (1246) because of missing responses.
Measures
Participants completed a daily checklist derived from the 11 symptoms for PMDD listed in DSM-IV. Compound symptoms (e.g. ‘hypersomnia or insomnia’) were separated into sub-symptoms, yielding 24 sub-symptoms or items. Positive items (e.g. ‘felt energetic’) were added to discourage response set (Sudman & Bradburn, 1982), resulting in a total of 33 items in the checklist. Sub-symptoms were rated on a six-point rating scale (from 0=‘I did not experience the symptom or emotion at all’ to 6=‘I experienced the symptom or emotion very severely’).
The checklist also asked women if the symptoms experienced on that day interfered with (1) functioning at home, work, or school, (2) social activities or (3) relationships with co-workers or family. Interference was explored separately for the three domains and for ability to interact with others and to get things done, yielding six separate questions. Specifically, participants were asked the extent to which they thought that the symptoms and emotions listed interfered with: (1) relationships with people at home, relationships with people at work, and relationships with people at school and (2) ability to get things done at home, work, and school. All were rated on a six-point scale (from 1=‘not at all’ to 6=‘extremely’).
Day of menses onset and LH surge data were used to define phase of cycle. Women provided daily urine samples, which were analyzed to provide an objective marker of phase of cycle in women not taking oral contraceptives. The first day during a cycle in which a urine enzyme immunoassay for LH [Ovukit; Quidel, San Diego, CA, USA; LH detection threshold 35–40 mIU/ml (milli International Unit per milliliter)] yielded a positive result was considered to be the onset of the preovulatory LH surge. For women taking oral contraceptives, the premenstrual phase was defined by determining the week prior to the onset of menses.
Women underwent psychiatric testing for past and present Axis I disorders using instruments appropriate for their age. Women aged 13–17 years were given the Schedule for Affective Disorder and Schizophrenia for School-age Children – Epidemiologic version 5 (K-SADS-E; Chambers et al. 1985). Women between the ages of 18–55 years underwent testing using the Structured Clinical Interview for DSM-IV Axis I Disorders-Non-Patient Edition (SCID-I/NP; First et al. 1996). Axis II disorders were assessed using the International Personality Disorder Examination (IPDE; Loranger et al. 1991). Data on age, race/ethnicity, education, income, employment and marital status were also collected and women were also asked to recall whether or not they had experienced each of the symptoms of PMDD in the past year.
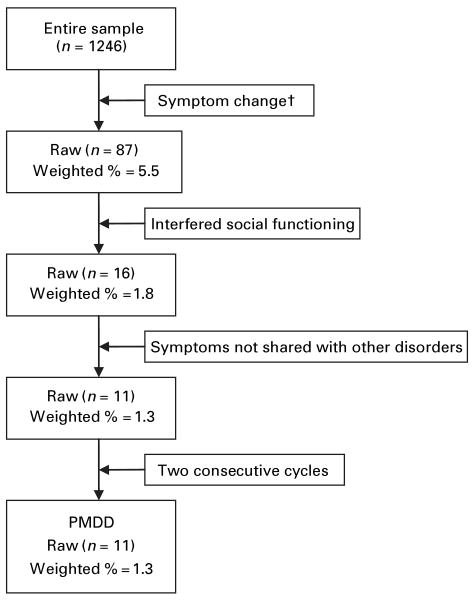
Fig. 1 demonstrates the number of women in the study sample who were excluded as each of the four criteria was added to the mix. Of the women in the weighted sample, 5.5% met the diagnosis based on mood change alone; 1.8% of the weighted sample met the diagnosis when interference with functioning was added. The prevalence rate dropped to 1.3% when PMDD symptoms which were also symptoms of another disorder were no longer considered. Including the fourth criterion of two consecutive ovulatory cycles produced no change in the rate of prevalence.
Fig. 1.
Diagram of premenstrual dysphoric disorder (PMDD) diagnostic procedure. † By using the effect size method.
Procedures
Women were enrolled on the first day of a menstrual cycle, based on information obtained during recruitment and orientation to the study. After being oriented to the study in their homes, participants completed daily questionnaires and provided daily urine samples. These were retrieved every 2 weeks by research assistants assigned to specific participants. Women were given contact information for the research assistants with whom they were working and encouraged to contact them with any questions or concerns about the study. Demographic data and items about previous experience with PMDD were collected in a face-to-face interview at the time of exit from the study by research assistants initially assigned to specific participants.
Psychiatric diagnostic testing was completed by a separate set of research assistants who received special training in administering the instruments. All were administered during what was determined to be the post-menstrual phase of the cycle. Research assistants who received extensive training conducted the assessments. Inter-rater reliability scores were high (e.g. κ=0.94, p=0.03 on the SCID-I/NP).
Analysis
Because we attempted to enroll equal numbers of women from four sites, relative weights inversely proportional to the sampling fraction were calculated by considering the segment selection probability and housing unit selection within each household as originally planned by NORC. Next, post-stratification adjustment was performed with the control totals of eligible women from the Census 2000 by site, age, and race/ethnicity, and final weights were normalized to 1246.
The determination of which women met the diagnosis of PMDD was made using a stepwise process. Daily rating data from seven post-menses follicular days were compared with data obtained during the late luteal phase (here defined as the week before the onset of menses). Symptom severity was evaluated by two methods (Schnurr, 1988; Eckerd et al. 1989) used in previous studies (Hurt et al. 1992; Gehlert & Hartlage, 1997; Banerjee et al. 2000; Sveindottir & Backstrom, 2000; Cohen et al. 2002; Wittchen et al. 2002; Takeda et al. 2006), so that results could be compared with these studies. The first, the absolute severity method (Schnurr, 1988), requires a rating >1 for no more than 2 days of the post-menstrual phase and a rating >3 for at least one premenstrual-phase day. The effect size method (Eckerd et al. 1989) requires that a subject's post-menstrual-to-premenstrual change be greater than the standard deviation of symptom change across the two cycles.
The presence and severity of symptom change was determined using the most predictive sub-symptom item for each of 11 PMDD symptoms measured. The selection of which sub-symptom to use was based on previous work by the authors using exploratory principal components analysis with Varimax (orthogonal) rotation to determine how sub-symptoms loaded onto symptoms (Gehlert et al. 1999). Specifically, the sub-symptom item with highest factor loading in its respective symptom was used.
The second criterion, marked interference with functioning, was measured by measuring whether sub-symptoms that met the first criterion occurred on days in which participants reported marked impairment in functioning in (1) functioning at home, work, or school, (2) social activities or (3) relationships with co-workers or family. Only sub-symptoms that occurred on days in which functioning was impaired were counted toward the diagnosis of PMDD.
The third criterion for the diagnosis of PMDD is that the disturbance not be merely an exacerbation of another psychiatric condition. We did not count a symptom toward the diagnosis of PMDD if it was also a symptom of a current psychiatric disorder. This was determined using the results of diagnostic testing using the SCID-I/NP, IPDE and K-SADS-E.
Results of prospective daily symptom ratings and urine testing were used to meet the fourth criterion, that the first three criteria be confirmed by prospective daily ratings over two consecutive cycles. A diagnosis of PMDD was based on the number of symptoms that survived the four criteria, namely a woman was said to have PMDD for one of the two methods of measuring symptom severity if she had at least five of the 11 symptoms of the diagnosis, at least one of which was from among the first four symptoms.
In order to determine how many women would meet the diagnosis of PMDD if retrospective self-reports alone were used, symptoms reported as having been experienced in the past year were counted to determine if women had at least five of the 11 symptoms of the diagnosis, at least one of which was from the first four symptoms.
Results
Of the 12 800 addresses systematically selected by the NORC, 9867 were determined to be valid housing units, and the screener response rate among those units was 78.85%. Women meeting eligibility for the study were found in 2696 housing units, and 1784 agreed to participate in the study (66.17%). During the study, 378 participants dropped out (21.2%), 116 (6.5%) became ineligible (e.g. because of absence of menstruation, pregnancy, moving from the area), and 44 (2.5%) subjects' data were unusable due to significantly missing responses in daily ratings. As a result, 1246 cases were used in the final analysis, yielding a completion rate of 69.8%, which is comparable with the completion rates of other studies in which women completed daily symptom diaries (Takeda et al. 2006). We compared women who completed the study with those not, and found them to differ only on race. White women were more likely than non-white to complete the study (χ2=6.12, p=0.01).
Responses of the 1246 women were analyzed using the two methods of symptom change evaluation. When the absolute severity method was used, the weighted prevalence rate of the method is 1.0% (see Table 2), and the lower bound of 95% confidence interval (CI) around the prevalence estimate was 0.004 and the upper bound was 0.015, with a standard error of 0.003. When the effect size method was used, 11 women met the diagnostic criteria for PMDD, yielding a 1.3% weighted prevalence rate. The lower bound of 95% CI around the prevalence estimate was 0.001 and the upper bound was 0.025, with a standard error of 0.006. Two of the participants who met the diagnostic criteria with the absolute severity method also met them when the effect size method was used. Compared with the 1.3% prevalence rate using the four DSM-IV criteria for PMDD, 54% of women met the diagnosis of PMDD if retrospective reports of symptoms alone were used. The women who met DSM-IV research criteria for PMDD represent four races/ethnicities and a range of other demographic variables (see Table 2).
Table 2. Characteristics of subjects with PMDD.
| Site | Race | Age (yr) |
Marital status | Income ($, thousands) |
Employment | Oral contraceptive | Method of analysis | Retrospective diagnosis |
|---|---|---|---|---|---|---|---|---|
| DeKalb County, IL | White | 29 | Married | 30–40 | Employed | No | Absolute severity | Yes |
| St Louis, MO | White | 30 | Married | 50–60 | Employed | Yes | Effect size | Yes |
| Chicago, IL | Hispanic | 30 | Married | 50–60 | Employed | No | Effect size | Yes |
| Franklin County, MO | White | 33 | Married | 40–50 | Employed | No | Effect size | No |
| DeKalb County, IL | White | 34 | Married | 50–60 | Homemaker | No | Effect size | Yes |
| Chicago, IL | Hispanic | 34 | Married | >60 | Student | No | Effect size, absolute severity | Yes |
| Chicago, IL | Black | 39 | Married | 30–40 | Employed | No | Effect size | Yes |
| Chicago, IL | Black | 39 | Single | >60 | Employed | Yes | Effect size | No |
| Franklin County, MO | White | 40 | Married | 30–40 | Homemaker | No | Absolute severity | Yes |
| DeKalb County, IL | Native American | 42 | Married | >60 | Employed | No | Effect size | Yes |
| Franklin County, MO | White | 45 | Married | 50–60 | Employed | No | Absolute severity | Yes |
| St Louis, MO | White | 46 | Divorced | 10–20 | Employed | No | Effect size | Yes |
| Chicago, IL | Black | 47 | Married | 40–50 | Employed | No | Effect size, absolute severity | Yes |
| St Louis, MO | White | 47 | Married | 40–50 | Employed | No | Effect size | Yes |
PMDD, Premenstrual dysphoric disorder.
PMDD was originally constructed to occur over the natural physiological menstrual cycle. We included women taking oral contraceptives in our sample, however, because women taking oral contraceptives met diagnostic criteria for PMDD in our pilot study (Gehlert & Hartlage, 1997). In the present study, we calculated prevalence rates separately for women who were and were not taking oral contraceptives. Among the 951 women who were not taking oral contraceptives, 14 (1.5%) met the diagnosis, compared with 0.8% for the 293 women taking oral contraceptives. Therefore, the prevalence rate of 1.3% of women who met the diagnosis in the sample as a whole did not differ significantly from the prevalence rate of 1.5% for women not taking oral contraceptives.
Discussion and conclusions
When all criteria outlined in DSM-IV are considered in a sample that is representative of rural and urban women in Missouri and Illinois, the prevalence of PMDD is considerably lower than DSM-IV estimates (3–5%) and all but one of the estimates obtained from previous studies that failed to take into account all of the four diagnostic criteria outlined for the proposed disorder in DSM-IV in representative samples of women (from 1.2% to 45.0%). In general, studies that used more representative samples and operationalized more of the four DSM-IV criteria yielded lower prevalence rates than studies that attended to fewer or no criteria or used small or convenience samples.
Because so few women in the sample met the DSM-IV diagnosis for PMDD, attempts to describe the group of women with PMDD in detail would be ill advised. Although one cannot say who is included in the group, it is safe to say that no demographic group seems to be excluded from the diagnosis. Women from four racial groups and a range of social backgrounds met the diagnosis of PMDD, as did women who did and did not take oral contraceptives (see Table 2).
That the prevalence obtained through prospective recording of symptom severity using more rigorous criteria is much lower than the rate (54%) obtained by asking study participants to recall whether they experienced symptoms in the past year raises very practical questions about diagnosing PMDD in clinical settings. Although retrospective approaches are seductive in their efficiency, they almost certainly result in over-diagnosis of the disorder. In addition to psychological and social challenges conferred by having a psychiatric diagnosis (Gray, 2002), over-diagnosis of PMDD may lead to unnecessary use of medications.
Some researchers have suggested ways in which the current diagnostic criteria for PMDD might be relaxed. Halbreich et al. (2003), for example, have argued that symptom counts are arbitrary under the current diagnostic criteria, and suggest that the prevalence of clinically relevant PMDD is probably higher than DSM-IV estimates. Although our data do not suggest that this is the case, a clear and consistent diagnostic protocol is needed that balances rigor with feasibility. Our data do suggest that PMDD exists, albeit in low numbers. If PMDD remains in diagnostic limbo, the few women who have the disorder will be disserved (e.g. not receive or be reimbursed for treatment or be unable to legitimately take time from work when they are markedly impaired by symptoms). Yet if the diagnosis is made without due care, the disservice might come from women being diagnosed with a disease in error.
In our study, the prevalence rate of PMDD was 5.5% when symptom change alone was considered. It dropped to 1.8% when interference was considered simultaneously. The rate dropped slightly, to 1.3%, when symptoms shared with other disorders were excluded from consideration and did not change when the fourth criterion (i.e. must occur two consecutive ovulatory cycles) was added. We thus suggest a compromise for clinical diagnosis of PMDD, in which women complete a daily diary of symptoms and interference with functioning between visits with their providers and psychiatric diagnostic testing is done on those women whose prospective symptom profiles suggests that they have PMDD. This approach is feasible, yet is based on empirical evidence about which DSM criteria are likely to contribute to a diagnosis of the condition.
Acknowledgments
The authors wish to acknowledge the support of the National Institutes of Mental Health through grant number R101MH55226 to S.G. and grant number R10MH55221 to S.A.H.
Footnotes
Declaration of Interest: None.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. 3rd. American Psychiatric Association; Washington, DC: 1987. revised. [Google Scholar]
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington, DC: 1997. revised. [Google Scholar]
- Banerjee N, Roy KK, Takkar D. Premenstrual dysphoric disorder – a study from India. International Journal of Fertility and Women's Medicine. 2000;45:342–344. [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semistructured interview. Test–retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Archives of General Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Otto MW, Sweeney BH, Liberman RF, Harlow BL. Prevalence and predictors of premenstrual dysphoric disorder (PMDD) in older premenopausal women. The Harvard Study of Moods and Cycles. Journal of Affective Disorders. 2002;70:125–132. doi: 10.1016/s0165-0327(01)00458-x. [DOI] [PubMed] [Google Scholar]
- Eckerd M, Hurt S, Severino S. Late luteal phase dysphoric disorder: relationship to personality disorders. Journal of Personality Disorders. 1989;4:338–344. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis 1 Disorders – Non-Patient Edition (SCID I/NP) New York State Psychiatric Institute, Biometrics Research; New York, NY: 1996. version 2.0. [Google Scholar]
- Gehlert S, Chang CH, Hartlage S. Symptom patterns of premenstrual dysphoric disorder as defined in the Diagnostic and Statistical Manual of Mental Disorders – IV. Journal of Women's Health. 1999;8:75–85. doi: 10.1089/jwh.1999.8.75. [DOI] [PubMed] [Google Scholar]
- Gehlert S, Hartlage S. A design for studying the DSM-IV research criteria of premenstrual dysphoric disorder. Journal of Psychosomatic Obstetrics and Gynaecology. 1997;18:36–44. doi: 10.3109/01674829709085567. [DOI] [PubMed] [Google Scholar]
- Gray AJ. Stigma in psychiatry. Journal of the Royal Society of Medicine. 2002;95:72–76. doi: 10.1258/jrsm.95.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28(Suppl 3):1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Hurt SW, Schnurr PP, Severino SK, Freeman EW, Gise LH, Rivera-Tovar A, Steege JF. Late luteal phase dysphoric disorder in 670 women evaluated for premenstrual complaints. American Journal of Psychiatry. 1992;149:525–530. doi: 10.1176/ajp.149.4.525. [DOI] [PubMed] [Google Scholar]
- Loranger A, Hirschfeld R, Sartorius N, Regier D. The WHO/ADAMHA international pilot study of personality disorders: background and purpose. Journal of Personality Disorders. 1991;5:296–306. [Google Scholar]
- Rivera-Tovar AD, Frank E. Late luteal phase dysphoric disorder in young women. American Journal of Psychiatry. 1990;147:1634–1636. doi: 10.1176/ajp.147.12.1634. [DOI] [PubMed] [Google Scholar]
- Schnurr PP. Some correlates of prospectively defined premenstrual syndrome. American Journal of Psychiatry. 1988;145:491–494. doi: 10.1176/ajp.145.4.491. [DOI] [PubMed] [Google Scholar]
- Sudman S, Bradburn NM. Asking Questions: A Practical Guide to Questionnaire Design. 1st. Jossey-Bass; San Francisco: 1982. [Google Scholar]
- Sveindottir H, Backstrom T. Prevalence of menstrual cycle symptom cyclicity and premenstrual dysphoric disorder in a random sample of women using and not using oral contraceptives. Acta Obstetricia et Gynecologica Scandinavica. 2000;79:405–413. doi: 10.1080/j.1600-0412.2000.079005405.x. [DOI] [PubMed] [Google Scholar]
- Takeda T, Tasaka K, Sakata M, Murata Y. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in Japanese women. Archive of Women's Mental Health. 2006;9:209–212. doi: 10.1007/s00737-006-0137-9. [DOI] [PubMed] [Google Scholar]
- US Census Bureau. Urban and rural definitions. [21 May 2007];1995 http://www.census.gov/population/censusdata/urdef.txt.
- US Census Bureau. Profiles of general demographic characteristics. [21 May 2007];2002 http://factfinder.census.gov/home/saff/main.html.
- Wittchen HU, Becker E, Lieb R, Krause P. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychological Medicine. 2002;32:119–132. doi: 10.1017/s0033291701004925. [DOI] [PubMed] [Google Scholar]