Summary
Prior case-control studies reported that levels of the soluble form of the endothelial protein C receptor (sEPCR) were strongly controlled by the PROCR 6963A/G polymorphism and higher levels were a risk factor for venous thromboembolism (VTE). We sought to prospectively examine the association of sEPCR and the 6963A/G polymorphism with the incidence of VTE. The Longitudinal Investigation of Thromboembolism Etiology (LITE) pooled data from the Cardiovascular Health Study (CHS) and the Atherosclerosis Risk in Communities (ARIC) Study on men and women aged ≥45 years. A nested case-control study of 458 incident VTE and 1,038 controls was performed. sEPCR levels were distributed trimodally according to 6963A/G polymorphism. Adjusting for age, sex and race, there was no overall association between sEPCR level and VTE: odds ratio (OR) [95% confidence interval] for highest vs lowest quartile=1.17[0.86–1.59]. However, higher sEPCR was associated with VTE in non-whites (OR=1.84[1.05–3.22]) and women (OR=1.51[1.01–2.26]). The 6963A/G polymorphism was not associated with VTE risk (OR=0.93[0.70–1.25]). In conclusion, sEPCR levels and the PROCR 6963A/G polymorphism were not associated overall with increased risk of VTE.
Keywords: epidemiology, follow-up study, risk factors, thrombophilia, venous thrombosis
Introduction
The protein C system provides an anticoagulant function by inactivating activated factors V and VIII (Va and VIIIa, respectively) to reduce thrombin formation (Dählback and Villoutreix 2005). Protein C is activated by the thrombin-thrombomodulin complex, and that activity is further enhanced by binding protein C to the endothelial protein C receptor (EPCR), which exists primarily on endothelial cells of large blood vessels. EPCR also circulates as a soluble form (sEPCR) with similar affinity to protein C and activated protein C, and this molecule inhibits activated protein C (Van de Wouwer, et al 2004). Some recent studies have shown that EPCR and sEPCR bind to activated factor VII and reduce its activity (Ghosh, et al 2007, López-Sagaseta, et al 2007). sEPCR, as well as the protein C system, is also considered to play an important role in the inflammatory process, and thereby may impact thrombotic diseases (Esmon 2006). Therefore, a higher level of sEPCR has been hypothesized to increase the risk of thrombosis.
A few prior case-control studies reported that sEPCR was distributed trimodally, and levels of sEPCR were strongly controlled by the A3 haplotype of the PROCR (EPCR) gene (Medina, et al 2004, Saposnik, et al 2004, Uitte de Willige, et al 2004). Saposnik et al (2004) reported the A3 haplotype of PROCR was associated with increased risk of venous thromboembolism (VTE), presumably due to decreased efficiency of the protein C system in A3 carriers. Another study reported that higher sEPCR concentration, but not the A3 haplotype, was a risk factor for VTE (Uitte de Willige, et al 2004). A 6936A/G or 4600A/G polymorphism seems to explain the A3 (H3) haplotype among whites (Medina, et al 2004, Saposnik, et al 2004, Uitte de Willige, et al 2004).
Although these traditional case-control studies were well-designed, to confirm their results in a prospective study is of interest because the association may differ by use of pre-VTE or post-VTE values of sEPCR. It is also of interest whether previous findings, shown in mainly white populations, can be replicated in non-white populations. We therefore conducted a nested case-control study to examine the association of sEPCR and the 6963A/G polymorphism of PROCR with incident VTE in a large biracial cohort study – the Longitudinal Investigation of Thromboembolism Etiology (LITE). Our hypothesis was that higher sEPCR and the 6963A/G polymorphism of PROCR would be risk factors for incident VTE.
Materials and methods
Study population
The LITE Study includes a nested case-control study of incident VTE in 2 pooled,community-based cohorts: the Atherosclerosis Risk in Communities (ARIC) Study (The ARIC Investigators 1989) and the Cardiovascular Health Study (CHS) (Fried, et al 1991). Details of LITE have been described elsewhere (Cushman, et al 2004). In brief, 15,792 men and women (11,478 whites and 4,314 non-whites) aged 45 to 64 years enrolled in the ARIC Study in 1987–1989 and 5,201 men and women (4,925 whites and 276 non-whites) aged ≥65 years enrolled in the CHS in 1989–1990 underwent assessments of cardiovascular risk factors. An additional 687 African Americans were recruited to CHS using similar methods in 1992–1993. Blood was drawn from fasting participants in the morning at baseline. Samples were promptly centrifuged for 3,000g-min, and stored in −70°C freezers. Baseline cardiovascular risk factors and additional hemostatic factors included in this paper were measured comparably in ARIC and CHS, as described elsewhere (Cushman, et al 1995, Folsom, et al 2002a, b, Folsom, et al 1997, Ohira, et al 2007, Tsai, et al 2002a, Tsai, et al 2002b). Informed consent was obtained, as approved by the Institutional Review Committees at each study centre.
Nested case-control design
A nested case-control design was employed to study associations between VTE incidence and parameters measured in stored blood specimens. Potential cases of VTE were identified from baseline through December 31, 2002, after a median follow up of 12.7 years in ARIC and 9.8 years in CHS. The ARIC cohort was followed by clinic visits every 3 years up to 1998, annual telephone calls, and surveillance of community hospitals. In CHS, follow-up involved alternating annual clinic visits and telephone calls every 6 months up to 1999. After clinic visits ended, telephone calls continued to identify all hospitalizations. Hospital records were obtained and VTE events validated by two physicians, as previously reported (Cushman, et al 2004). A diagnosis of DVT or pulmonary embolism (PE) required positive imaging tests. Cases were classified as incident (no self-reported VTE history before baseline) or recurrent (self-reported VTE history before baseline), and idiopathic (no obvious cause) or secondary (associated with cancer, major trauma, surgery, marked immobility, etc.).
Controls were selected at random using incidence-density sampling from the ARIC and CHS cohorts. Two controls per case were selected, frequency-matched to the cases by age (5-year groupings), gender, race (white, non-white), follow-up time (case's event date within 2 years of control's assigned date), and study (ARIC, CHS). Selection yielded 676 controls for the 338 cases in ARIC and 421 for the 210 cases in CHS.
Measurements
After selection of cases and controls, stored DNA and baseline plasma were retrieved from −70°C storage freezers. Baseline samples were preferred and used in the majority of instances. Rarely, if baseline plasma samples were limited, previously thawed, or exhausted for a participant, a sample was retrieved from the plasma repository for the next visit (approximately 3 years after baseline); if neither sample was available, it was considered missing.
The plasma sEPCR levels were measured by an in-house enzyme-linked immunosorbent assay using antibodies kindly provided by Dr Charles Esmon, Oklahoma Medical Research Foundation. Expected values for EPCR in normal, healthy individuals were 65.3 – 197.3 ng/ml. Analytical variance was 3.1%, while the within-person variance was 7.5% and the between-person variance was 44.5%. The intra-assay CV ranged from 3.3 to 6.4%.
The A/G polymorphism at position 6936 in the PROCR gene was genotyped (Saposnik, et al 2004). ABO genotypes were ascertained through genotyping of 4 single nucleotide polymorphisms (G261del, A297G, G703A and C526G) of the ABO gene (Ohira, et al 2007). Presence or absence of F5 (Factor V Leiden; A1691G, R506Q) (de Ronde and Bertina 1994) and the F2 (prothrombin) G20210A polymorphism (Poort, et al 1996) were also assessed. Factor VIII coagulant activity (FVIII:C) was measured as previously reported (Cushman, et al 1995, Folsom, et al 1997). Plasma protein C level was only available in the ARIC sample and was measured as reported (Folsom, et al 2002c). Body mass index (BMI) was calculated as weight (kg)/height (m)2. Diabetes mellitus was defined at baseline as a fasting glucose of ≥7 mmol/l, a history of physician-diagnosed diabetes, or use of diabetes medication.
Statistical analysis
In total, 548 VTE cases and 1,097 controls were selected. We excluded subjects who were taking warfarin at baseline (n=20). We further excluded those who were missing sEPCR (n=129), or those without consent to use DNA (n=48) or who were missing PROCR genotypes (n=65). Thus, we included 458 cases and 1,038 controls for sEPCR analyses, and 496 cases and 1,016 controls for genotype analyses.
Quartiles of sEPCR were based on the distribution in the combined ARIC and CHS control groups. Differences among the quartiles of sEPCR or the categories of 6963A/G polymorphism in age-, sex-, and race-adjusted mean values or prevalences of potential confounding factors at baseline were calculated among controls using analyses of covariance. Odds ratios (OR) and 95% confidence intervals (CI) of VTE in relation to quartiles of sEPCR or to 6963A/G genotype were calculated using logistic regression models. Adjustment was made primarily for age (at baseline, continuous), sex and race (white or non white). Further adjustment for potential risk factors, i.e. BMI (continuous), diabetes status (yes, no), and ABO blood type (O or non-O) was made for total VTE, as these were reported to be associated with VTE in this LITE sample (Ohira, et al 2007, Tsai,, et al 2002a). As a supplemental analysis, for ARIC only, protein C level (dichotomous values with cut-off point of 2.3 mg/L, the 5th percentile for whole ARIC sample (Folsom, et al 2002c) was added to the age, sex and race-adjusted model. Unconditional logistic regression analyses for subgroups of cases (idiopathic versus secondary VTE) were performed using all controls as the comparison group. Interactions were examined for VTE by cross-product terms in the logistic regression models. All probability values for statistical tests were two-tailed and values of p<0.05 were regarded as statistically significant.
Results
In ARIC, 272 individuals with VTE were identified for sEPCR analyses, 248 incident and 24 recurrent: 118 idiopathic and 154 secondary. Among 186 individuals with VTE events in CHS, 173 were incident and 13 recurrent, 76 were idiopathic and 110 secondary. Of the 458 events, 312 had deep venous thrombosis (DVT) only and 146 had a PE with or without DVT.
Age was correlated positively with sEPCR levels (Table 1). Whites and men had higher levels of sEPCR. Prevalence of non-O blood type was associated slightly with higher sEPCR levels. Sixty-six percent of persons in the highest quartile of sEPCR had the GG genotype at position 6963, whereas only 1% or less of persons in the other sEPCR quartiles had that genotype. BMI, prevalence of diabetes, FVIII:C, F5 and F2 genotype were unrelated to sEPCR quartiles. These parameters were not associated with the 6963A/G polymorphism, except for blood type (51% of AA carriers had non-O blood type versus 59% of AG/GG carriers). Protein C level in ARIC was positively associated with sEPCR quartiles, and also with the 6963A/G polymorphism (mean protein C =3.1 mg/L in AA vs 3.4 mg/L in AG/GG carriers, p<0.001).
Table 1.
Age-, race-, and sex- adjusted baseline characteristics of controls according to quartiles of soluble endothelial protein C receptor (EPCR) levels: LITE.
| Quartiles of Soluble Endothelial Protein C Receptor (ng/ml)* |
|||||
|---|---|---|---|---|---|
| 65.4–121.7 | 121.9–139.8 | 140.1–169.5 | 170.4–1381 | p for difference overall | |
| No. of controls† | 259 | 259 | 260 | 260 | |
| Age (years)† | 59.7 | 61.8 | 64.3 | 63.5 | <0.001 |
| CHS (%)† | 24 | 35 | 46 | 43 | <0.001 |
| White (%)† | 62 | 79 | 75 | 77 | <0.001 |
| Men (%)† | 33 | 47 | 46 | 48 | <0.001 |
| Body mass index | 27.2 | 27.6 | 27.7 | 27.4 | 0.71 |
| Diabetes (%) | 11 | 8 | 13 | 14 | 0.15 |
| Blood type non-O, % | 46 | 55 | 50 | 59 | 0.02 |
| Factor VIII (%) | 124 | 126 | 131 | 131 | 0.16 |
| Protein C (mg/L)‡ | 3.1 | 3.0 | 3.2 | 3.4 | <0.001 |
| F5 AA/AG genotype (%) | 3 | 4 | 3 | 2 | 0.76 |
| F2 AA/AG genotype (%) | 3 | 2 | 2 | 1 | 0.31 |
| PROCR AG/GG genotype (%) | 0 | 1 | 1 | 66 | <0.001 |
Quartiles based on the distribution in combined Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health Study (CHS) controls.
Unadjusted
Protein C was available only for the ARIC subsample (n=642 for ARIC controls).
Table 2 presents unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) of VTE according to quartiles of sEPCR. Overall, no association was observed between sEPCR and VTE. However, the OR (95%CI) for the highest quartile of sEPCR versus the lowest quartile was 1.84 (1.05–3.22) for non-whites, but 0.96 (0.66–1.39) for whites (p for race interaction =0.07) and was 1.51 (1.01–2.26) for women but 0.81 (0.50–1.31) for men (p for sex interaction =0.08). The ARIC sample showed a more prominent association compared with the CHS sample (p for interaction =0.05), due possibly to higher proportion of younger persons (baseline ages 45–64 years in ARIC vs 65–94 years in CHS) and non-whites (31% in ARIC vs 21% in CHS). No associations were observed for any subtype of VTE (ie. idiopathic or secondary). All these associations were unchanged or only slightly attenuated after adjustment for BMI, diabetes, and ABO blood type (data not shown). For ARIC, further adjustment for protein C levels did not alter the association shown in Table 2 (OR for highest vs lowest quartile =1.37[0.93–2.02], not shown in tables). As a previous study had suggested that low sEPCR levels reduced VTE risk (Uitte de Willige, et al 2004), we examined VTE risk for the lowest 10 percentile of sEPCR (<107.4 ng/ml) compared with higher levels, and no association was observed (age, sex and race-adjusted OR=0.85 [0.57–1.26] compared with sEPCR≥107.4 ng/ml, data not shown).
Table 2.
Odds ratios (OR) and 95% confidence intervals (CI) of venous thromboembolism (VTE) according to quartiles of soluble endothelial protein C receptor levels: LITE 1987–2002.
| Soluble Endothelial Protein C Receptor (ng/ml)* | ||||
|---|---|---|---|---|
| 65.4–121.7 | 121.9–139.8 | 140.1–169.5 | 170.4–1381 | |
| Total (case n/control n) | 112/259 | 118/259 | 95/260 | 133/260 |
| Unadjusted OR (95% CI) | 1.0 | 1.05 (0.77–1.44) | 0.85 (0.61–1.17) | 1.18 (0.87–1.60) |
| Age-, race-, and sex-adjusted OR (95% CI) | 1.0 | 1.05 (0.77–1.44) | 0.83 (0.60–1.15) | 1.17 (0.86–1.59) |
| Multivariable adjusted OR† (95% CI) | 1.0 | 1.06 (0.75–1.48) | 0.88 (0.62–1.25) | 1.07 (0.77–1.49) |
| ARIC (case n/control n) | 77/196 | 65/168 | 51/140 | 79/148 |
| Age-, race-, and sex-adjusted OR (95% CI) | 1.0 | 0.99 (0.66–1.46) | 0.93 (0.61–1.41) | 1.36 (0.93–1.99) |
| CHS (case n/control n) | 35/63 | 53/91 | 44/120 | 54/112 |
| Age-, race-, and sex-adjusted OR (95% CI) | 1.0 | 1.07 (0.62–1.83) | 0.68 (0.39–1.17) | 0.89 (0.52–1.51) |
| Non-white (case n/control n) | 35/99 | 26/55 | 26/65 | 39/59 |
| Age-, race-, and sex-adjusted OR (95% CI) | 1.0 | 1.31 (0.71–2.41) | 1.10 (0.61–2.01) | 1.84 (1.05–3.22) |
| White (case n/control n) | 77/160 | 92/204 | 69/195 | 94/201 |
| Age-, race-, and sex-adjusted OR (95% CI) | 1.0 | 0.93 (0.64–1.34) | 0.72 (0.49–1.06) | 0.96 (0.66–1.39) |
| Men (case n/control n) | 46/85 | 58/123 | 42/120 | 55/125 |
| Age-, race-, and sex-adjusted OR (95% CI) | 1.0 | 0.88 (0.55–1.43) | 0.64 (0.39–1.07) | 0.81 (0.50–1.31) |
| Women (case n/control n) | 66/174 | 60/136 | 53/140 | 78/135 |
| Age-, race-, and sex-adjusted OR (95% CI) | 1.0 | 1.16 (0.76–1.76) | 0.99 (0.64–1.52) | 1.51 (1.01–2.26) |
| Idiopathic VTE (case n/control n) | 50/259 | 53/259 | 38/260 | 53/260 |
| Age-, race-, and sex-adjusted OR (95% CI) | 1.0 | 1.08 (0.70–1.66) | 0.75 (0.47–1.20) | 1.07 (0.69–1.64) |
| Secondary VTE (case n/control n) | 62/259 | 65/259 | 57/260 | 80/260 |
| Age-, race-, and sex-adjusted OR (95% CI) | 1.0 | 1.03 (0.70–1.53) | 0.89 (0.59–1.34) | 1.26 (0.86–1.83) |
Quartiles based on the distribution in combined Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health Study (CHS) controls.
Adjusted for age, race, sex, body mass index, diabetes and ABO blood type.
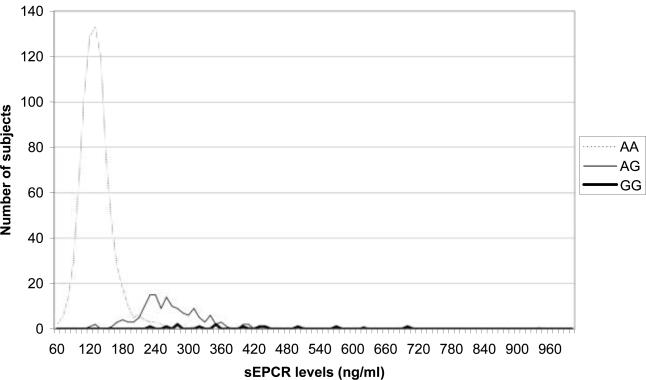
The genotype frequencies of the PROCR 6963A/G polymorphism were 86% for AA, 12% for AG and 2% for GG in non-white controls; and 82%, 17%, and 1% for white controls. The distribution of sEPCR was strongly associated with 6963A/G polymorphism (Figure 1). Median values of sEPCR were 134 ng/ml for AA genotype and 265 ng/ml for AG/GG genotype. However, the 6963A/G polymorphism was not associated with VTE (Table 3). There also was no association of the 6963A/G polymorphism with VTE upon stratification for each variable in Table 2. Inclusion of protein C level in ARIC had no impact on the PROCR-VTE association (not shown).
Distribution of sEPCR levels in control subjects according to the 6963A/G genotype (n=972)
Table 3.
Adjusted odds ratios (OR) and 95% confidence intervals (CI) of venous thromboembolism (VTE) according to PROCR 6963A/G genotype : LITE 1987–2002.
| 6963A/G genotype |
|||
|---|---|---|---|
| AA | AG | GG | |
| Number of controls | 844 | 158 | 14 |
| Number of cases | 417 | 72 | 7 |
| Age-, race-, and sex-adjusted OR | 1.0 | 0.93 (0.70–1.25) | |
| Multivariable adjusted OR* | 1.0 | 0.86 (0.63–1.16) | |
Adjusted for age, race, sex, diabetes, body mass index, and ABO blood type.
Discussion
Contrary to our hypothesis, there was no overall association between sEPCR and VTE risk in the LITE sample. The 6963A/G polymorphism of PROCR was associated with sEPCR levels, but the polymorphism did not affect VTE risk.
To our knowledge, this is the first prospective study investigating the association of sEPCR with VTE. The major advantage of a prospective nested case-control study is that the blood was sampled before VTE occurrence, in contrast with a traditional case-control study. Although we included a few subjects with a history of VTE in the analysis, exclusion of these subjects did not alter the null result (not shown). Therefore, the possibility of VTE affecting sEPCR should have been avoided. On the other hand, blood was collected years before many of the VTE events occurred and may not reflect levels just before VTE. Yet, as shown here, sEPCR level is largely controlled by the 6963A/G polymorphism which was not associated with VTE risk.
There has been one case-control study from the Netherlands, the Leiden Thrombophilia Study, which examined the relationship between sEPCR and VTE (Uitte de Willige, et al 2004). The authors reported a 1.9-fold higher OR for the highest quartile of sEPCR (≥137 ng/ml) compared with the lowest quartile (<81 ng/ml), and the sEPCR haplotype was not associated with VTE risk. In that study there was no dose-response relationship, suggesting that only the lowest sEPCR quartile had reduced VTE risk (OR=0.6 [0.4–0.8] compared with other quartiles pooled). When VTE risk was examined for the lowest 10th percentile of sEPCR (<107.4 ng/ml), we still observed no association (OR=0.85 [0.57–1.26] compared with sEPCR ≥107.4 ng/ml), even though LITE had a similar sample size to the Leiden study. Taking our results and the positive association for sEPCR in the Leiden study (where sEPCR was measured after incident VTE), together with the null results in both studies for the genotype that was highly correlated with sEPCR levels, it is suggested that sEPCR levels might reflect damage to venous endothelium at or after a VTE event. This might explain why sEPCR was not associated with VTE overall in the present study.
sEPCR levels were generally higher in the present study than in prior studies. Specifically, the median value of sEPCR in the ARIC controls was 135 ng/ml, which was higher than in the Leiden controls (median =101 ng/ml, mean age = 45 years), although the genotype distribution was similar in controls in the two studies (21% for AG and 2% for GG genotype in Leiden (Uitte de Willige, et al 2004) versus 16% and 1%, respectively, in LITE). The Leiden study also reported that the median value of sEPCR was higher among healthy controls aged ≥ 45 years than those <45 years (104 vs 96 ng/ml, p=0.04). The distribution of sEPCR in the study reported by Saposnik et al (2004), which also involved younger subjects (aged 18–35 years), was also lower than in the present study. This difference in sEPCR level between the present and previous studies is most likely due to assay calibration differences and possibly the older age of LITE participants. We did not replicate an association between the 6963A/G polymorphism, which has been shown to represent the A3 haplotype among whites, and risk of VTE. A prior case-control study (Saposnik et al 2004) found a 1.8-fold greater risk of VTE with one or two copies of the A3 haplotype, whereas two other case-control studies found no association between A3 haplotype and VTE (Medina, et al 2004, Uitte de Willige, et al 2004). Taken together with the weak association between sEPCR and VTE, we conclude that the 6963A/G polymorphism is associated with plasma sEPCR levels, but not with VTE either via, or independently of, sEPCR levels.
One strength of the present study is the biracial cohorts. Prior studies involved mostly white populations and no prior studies evaluated the association of sEPCR or A3 haplotype with VTE among non-whites specifically. Another strength is that we had information on sEPCR genotype, intermediate phenotype, and clinical phenotype (VTE). Study limitations are as follows. First, the LITE study included only clinically ascertained hospitalized VTE, and some outpatient VTE cases may have been missed. However, this may improve the specificity of our VTE classification, and outpatient treatment was not common during the case ascertainment period of LITE, so this may not significantly alter the findings. Second, although a previous study of mostly white subjects showed that the 6963A/G polymorphism reflected the A3 haplotype (Saposnik, et al 2004), we do not have information whether this polymorphism reflects the A3 haplotype in non-white subjects. Third, protein C levels were available only in ARIC, which were correlated with sEPCR levels, and ARIC previously reported that very low levels of protein C were associated with higher risk of VTE (Folsom, et al 2002c). However, adjustment for protein C levels did not alter the associations for the ARIC sample. Lastly, the number of cases for stratified analyses was relatively small. Our findings for a sEPCR-VTE association among younger persons, non-whites and women were not definitive, since the interaction terms were not significant. In addition, it is possible that we failed to detect a weak association of the 6963A/G polymorphism on VTE, since the prevalence of GG homozygotes was quite rare. A larger study involving younger persons, women and non-whites is needed to confirm these findings.
In conclusion, we confirmed that the PROCR 6963A/G polymorphism is strongly associated with sEPCR levels. However, we observed no association of 6963A/G polymorphism or greater sEPCR level with increased risk of VTE overall.
Acknowledgments
The authors thank the staff and participants of ARIC and CHS projects for their important contributions over many years. We acknowledge the contribution of reagents from Dr Charles Esmon, Oklahoma Medical Research Foundation. This study was funded by National Heart, Lung, and Blood Institute grant R01 HL59367 (LITE), contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022 (ARIC), and contracts N01-HC-85079 to N01-HC-85086 (CHS). Kazumasa Yamagishi was supported by Kanae Foundation for the Promotion of Medical Science, Tokyo, Japan, and Mary Cushman by the Leducq Foundation, Paris, France.
References
- Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clinical Chemistry. 1995;41:264–270. [PubMed] [Google Scholar]
- Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. American Journal of Medicine. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Dählback B, Villoutreix BO. The anticoagulant protein C pathway. FEBS Letters. 2005;579:3310–3316. doi: 10.1016/j.febslet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- de Ronde H, Bertina RM. Laboratory diagnosis of APC-resistance: a critical evaluation of the test and the development of diagnostic criteria. Thrombosis and Haemostasis. 1994;72:880–886. [PubMed] [Google Scholar]
- Esmon CT. The endothelial protein C receptor. Current Opinion in Hematology. 2006;13:382–385. doi: 10.1097/01.moh.0000239712.93662.35. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Wu KK, Rosamond WD, Sharrett AR, Chambless LE. Prospective study of hemostatic factors and incidence of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 1997;96:1102–1108. doi: 10.1161/01.cir.96.4.1102. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Cushman M, Tsai MY, Aleksic N, Heckbert SR, Boland LL, Tsai AW, Yanez ND, Rosamond WD. A prospective study of venous thromboembolism in relation to factor V Leiden and related factors. Blood. 2002a;99:2720–2725. doi: 10.1182/blood.v99.8.2720. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Cushman M, Tsai MY, Heckbert SR, Aleksic N. Prospective study of the G20210A polymorphism in the prothrombin gene, plasma prothrombin concentration, and incidence of venous thromboembolism. American Journal of Hematology. 2002b;71:285–290. doi: 10.1002/ajh.10229. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Aleksic N, Wang L, Cushman M, Wu KK, White RH. Protein C, antithrombin, and venous thromboembolism incidence: a prospective population-based study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002c;22:1018–1022. doi: 10.1161/01.atv.0000017470.08363.ab. [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Annals of Epidemiology. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Pendurthi UR, Steinoe A, Esmon CT, Rao LV. Endothelial cell protein C receptor acts as a cellular receptor for factor VIIa on endothelium. Journal of Biological Chemisty. 2007;282:11849–11857. doi: 10.1074/jbc.M609283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Sagaseta J, Montes R, Puy C, Díez N, Fukudome K, Hermida J. Binding of factor VIIa to the endothelial cell protein C receptor reduces its coagulant activity. Journal of Thrombosis and Haemostasis. 2007;5:1817–1824. doi: 10.1111/j.1538-7836.2007.02648.x. [DOI] [PubMed] [Google Scholar]
- Medina P, Navarro S, Estelles A, Vaya A, Woodhams B, Mira Y, Villa P, Migaud-Fressart M, Ferrando F, Aznar J. Contribution of polymorphisms in the endothelial protein C receptor gene to soluble endothelial protein C receptor and circulating activated protein C levels, and thrombotic risk. Thrombosis and Haemostasis. 2004;91:905–911. doi: 10.1160/TH03-10-0657. [DOI] [PubMed] [Google Scholar]
- Ohira T, Cushman M, Tsai MY, Zhang Y, Heckbert SR, Zakai NA, Rosamond WD, Folsom AR. ABO blood group, other risk factors and incidence of venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) Journal of Thrombosis and Haemostasis. 2007;5:1455–1461. doi: 10.1111/j.1538-7836.2007.02579.x. [DOI] [PubMed] [Google Scholar]
- Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3'-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698–3703. [PubMed] [Google Scholar]
- Saposnik B, Reny JL, Gaussem P, Emmerich J, Aiach M, Gandrille S. A haplotype of the EPCR gene is associated with increased plasma levels of sEPCR and is a candidate risk factor for thrombosis. Blood. 2004;103:1311–1318. doi: 10.1182/blood-2003-07-2520. [DOI] [PubMed] [Google Scholar]
- The ARIC Investigators The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. American Journal of Epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Archives of Internal Medicine. 2002a;162:1182–1189. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Tracy RP, Aleksic N, Folsom AR. Coagulation factors, inflammation markers, and venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE) American Journal of Medicine. 2002b;113:636–642. doi: 10.1016/s0002-9343(02)01345-1. [DOI] [PubMed] [Google Scholar]
- Uitte de Willige S, Van Marion V, Rosendaal FR, Vos HL, de Visser MC, Bertina RM. Haplotypes of the EPCR gene, plasma sEPCR levels and the risk of deep venous thrombosis. Journal of Thrombosis and Haemostasis. 2004;2:1305–1310. doi: 10.1046/j.1538-7836.2004.00855.x. [DOI] [PubMed] [Google Scholar]
- Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: integrated to regulate coagulation and inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:1374–1383. doi: 10.1161/01.ATV.0000134298.25489.92. [DOI] [PubMed] [Google Scholar]