Abstract
Background
The high mechanical index (MI) impulses from a diagnostic ultrasound (DUS) transducer may be a method of recanalizing acutely thrombosed vessels if the impulses are applied only when microbubbles (MB) are channeling through the thrombus.
Methods and Results
In 45 pigs with acute left anterior descending thrombotic occlusions, a low MI pulse sequence scheme (CPS) was utilized to image the myocardium and guide the delivery of high MI (1.9 MI) impulses during infusion of either platelet-targeted intravenous MBs (PTMB), or non-targeted MBs (NTMB). A third group received no DUS and MB. All groups received half dose recombinant pro-urokinase, heparin and aspirin. CPS examined for replenishment of contrast within the central portion of the risk area (RA), and guided the application of high MI impulses. Angiographic recanalization rates, resolution of ST segment elevation on EKG, and wall thickening were analyzed. Pigs receiving PTMB had more rapid replenishment of the central portion of the risk area (80% versus 40% for NTMB; p=0.03) and higher epicardial recanalization rates (53% versus 7% for pro-urokinase alone; p=0.01). Replenishment of contrast within the RA (whether with PTMB or NTMB) was associated with both higher recanalization rates and even higher rates of ST segment resolution (82% versus 21% for pro-urokinase alone; p=0.006). ST segment resolution occurred in six pigs (40%) treated with MB who did not have epicardial recanalization, of which five had recovery of wall thickening.
Conclusions
Intravenous PTMB combined with brief high MI DUS impulses guided by CPS improves both epicardial recanalization rates and microvascular recovery.
Keywords: Coronary disease, Ultrasonics, Thrombolysis, Microbubbles
Introduction
Coronary thrombosis on a ruptured coronary plaque is the main pathophysiologic event that leads to acute coronary syndromes (1–3). Current recanalization therapies in these disease states include pharmacological thrombolysis and percutaneous coronary intervention (PCI), both of which have improved the prognosis of patients with STEMI (4–6). Each of these therapeutic interventions, however, has significant limitations. The time required to open a coronary vessel successfully with PCI is, even at the most experienced centers, 90 minutes after presentation to the Emergency Department (7), during which extensive myocardial necrosis may have already occurred. Reperfusion using thrombolytics is most effective if given within the first hour after the onset of symptoms in STEMI, but effective epicardial recanalization is achieved in less than 60% of patients (8). Furthermore, the doses of thrombolytics utilized in clinical trials have increased the risk for intracerebral hemorrhage, even if patients with previous stroke are excluded (9, 10). Finally, neither percutaneous coronary interventions or thrombolytic agents have reduced the risk for microvascular no reflow (11, 12), a phenomenon in which there is a persistent perfusion abnormality within the risk area (RA) even after epicardial recanalization. This phenomenon correlates with lack of ST segment resolution on the 12 lead EKG, and is associated with post-infarction complications (13, 14).
Therapeutic ultrasound and intravenous microbubbles have been utilized to recanalize acute intravascular thrombi in peripheral vessels (15–23). Recently, we have demonstrated that a diagnostic ultrasound transducer has the capability to recanalize deeply located intravascular thrombi, if the high mechanical index (MI) impulses from this transducer are applied only when microbubbles are visualized channeling through the thrombus, using a low MI microbubble sensitive pulse sequence scheme as a guide (24). The ability of these guided high MI impulses to recanalize acutely thrombosed coronary arteries may be impaired by the paucity of intravenous microbubbles reaching an occluded coronary vessel, and the inability to directly visualize the small thrombosed coronary lumen. To overcome this limitation, we hypothesized that glycoprotein 2b/3a targeted microbubbles (PTMB) could be utilized to increase the avidity of microbubbles to thrombus in both the coronary artery and downstream microvasculature within the risk area. These microbubbles could then be visualized by examining for contrast within the central portion of the risk area, which would then guide the delivery of high MI impulses. The purpose of study was to determine whether a low MI microbubble sensitive imaging system could be utilized to guide high MI impulses from the same diagnostic transducer during a PTMB infusion, and improve both epicardial and microvascular flow in a pig model of acute coronary thrombosis.
Materials and Methods
Animal Preparation
There were nine pigs that died from refractory ventricular arrhythmias following left anterior descending thrombosis and thus were not randomized. Forty-five pigs completed the randomized study protocol. The mean weight of the 45 pigs was 36±4 kg. The study was compliant with the Guidelines of the Institutional Animal Care and Use Committee (IACUC) and the Standards in the Guide for the Care and Use of Laboratory Animals. The animals were preanesthetized with an intramuscular mixture of Telazol (4.4 mg/kg), Ketamine (2.2 mg/kg), and Xylazine (2.2 mg/kg). Atropine (0.05mg/kg) given IM was used to dry oral-tracheal secretions and prevent bradycardia during intubation or surgery. Following placement of a venous line in an ear vein (lateral or medial auricular vein), the animal was intubated and isoflurane anesthesia (induction at 4%, maintained at 1.0 to 1.8%) was administered. The oxygen mixture was kept at <24% (slightly greater than room air) during intravenous ultrasound contrast infusions. The animal was intubated and placed on a respirator at a volume of 10cc/kg of air and at a rate of 15 BPM.
Two femoral artery and two venous catheters were placed for hemodynamic monitoring and injections of microbubbles. The pig-tail catheter was advanced over a guide wire with fluoroscopic guidance in a retrograde fashion from the femoral artery into the left ventricle and left atrium. The placement was confirmed by the typical left atrial pressure waveform (a and v waves) on the pressure recordings. An 8 Fr guide catheter was introduced into the left main for digital angiograms and for balloon catheter insertion. Heart rate and oxygen saturation were also monitored throughout entire experiment. Low dose intravenous dobutamine (1–3 u/kg/min) was used to maintain systolic arterial pressure >90 mm Hg during the acute coronary thrombotic occlusions. Lidocaine boluses (40 mg IV followed by 20 mg IV boluses at periodic intervals) and a continuous lidocaine infusion (2–4 mg/min, IV) were used in all animals to control arrhythmias. Isolated ventricular ectopic beats were observed in all animals following thrombotic occlusion.
Ultrasound System
A Siemens Acuson Sequoia with 4V1c transducer (1.5 MHz, Siemens Ultrasound Solutions, California) was used for all studies. The MI was adjusted for two settings: the low MI (0.2 MI using Contrast Pulse Sequencing or CPS) for imaging of microbubbles within the RA of the myocardial microcirculation, and the high MI setting (1.9) for therapeutic applications over the RA and epicardial location of the LAD. These two MI settings were alternated using a foot pedal.
Microbubbles
Two formulations of lipid-encapsulated microbubbles with perfluorcarbon gas were provided by ImaRx Therapeutics, Inc. (Tucson, AZ): MRX-835, which are non-targeted microbubbles (NTMB) and a platelet glycoprotein 2b/3a targeted microbubble (PTMB) termed MRX-802. The targeting ligand was synthesized and purified using standard peptide chemistry techniques utilizing a Fmoc- strategy. Briefly, MRX-802 was formulated using phospatidylcholine, phosphatidic acid, phosphatidylethanolaminepolyethyleneglycol5000, and N,N'diaminobutyryl-a-amino, w-carboxy-polyethyleneglycol3400 (cyclo-CRGDWPC)-OH. The lipids and bioconjugate were dispersed in a mixture of propyleneglycol, glycerol, and phosphate buffered saline with intermittent heating to 50 degrees C. Both formulations (MRX 835 and 802) were then placed into 2 mL vials, sealed, and purged with perfluorobutane (Fluoromed, Round Rock, Texas). Samples were then activated by agitation on a modified dental amalgamator operating at approximately 4000 RPM. The mean diameter of the microbubbles was measured to be 1.0 ± 0.1 microns, with concentration of 1.5–3.0 × 1010/milliliter. The microbubble infusion was prepared by diluting 2 ml of the MRX-835 or MRX-802 in 80 ml of 0.9% saline, and infusing at a rate of 2.5 ml/min for 30 minutes.
Angiography
Angiography was carried out with a cardiovascular mobile digital C-arm system (GE Healthcare, Salt Lake City, UT) at 30, 60 and 90 minutes post treatment. If recanalization occurred, residual stenoses at the balloon injury site were quantified using a digital caliper, using a non-branching site proximal to the occlusion as the reference diameter. Angiographic recanalization was defined as evidence of contrast flow through the site of occlusion, and normal run-off of flow distal to the occlusion.
Electrocardiographic Measurements
Twelve-lead electrocardiograms were used to compute changes in ST-segment deviation before and after any treatment, using the PR segment as a reference point. The percent resolution of ST elevation with any randomized treatment was computed from the lead with the greatest degree of ST elevation immediately after angiographic occlusion occurred.
Measurements of wall thickening were obtained with the same diagnostic ultrasonic probe used for contrast imaging. A parasternal short-axis view of the left ventricle was obtained at the distal papillary muscle level using tissue harmonic imaging. Percent wall thickening was calculated as the difference in end-systolic wall thickness and end-diastolic wall thickness within the central portion of the RA divided by end-diastolic wall thickness. Improvement in wall thickening was defined as a >10% increase in this measurement following randomized treatment.
Protocol
A thrombotic occlusion in the left anterior descending artery (LAD) was created using a simulation of the Triad of Virchow as previously described (25). All occlusions had to persist for a total of 20 minutes before randomized treatments began. In nine animals, refractory ventricular tachycardia, unresponsive to lidocaine infusion or repeated DC cardioversions, occurred following left anterior descending thrombotic occlusion. These animals were therefore not part of the randomized treatment groups. At 20 minutes following initial angiographic occlusion, a baseline angiogram was obtained, followed by a 12 lead EKG and baseline assessment of wall thickening (WT) using a short axis view of the left ventricle obtained with the diagnostic transducer (Siemens Acuson; Mountain View, CA). At this point, all pigs received 650 milligrams of aspirin via a nasogastric tube, an intravenous bolus of heparin (80 mg/kg) and 50,000 U/kg of recombinant pro-urokinase (Abbott Laboratories, Abbott Park, IL). According to the package insert, this is one-half the recommended dose for systemic fibrinolysis. The pro-urokinase was given as an initial 25,000 Unit/kg bolus over one minute, followed by the remainder given as an infusion over twenty-nine minutes. The pigs were randomized to: (a) half dose recombinant pro-urokinase only; (b) half dose recombinant pro-urokinase with a continuous intravenous infusion (IV) of NTMB, or (c) half dose recombinant pro-urokinase with a continuous infusion of IV PTMB.
In pigs receiving microbubbles, CPS was utilized to visualize the left ventricular short axis until evidence of contrast within the central portion of the RA was observed. At this point, the MI was increased to 1.9 and applied at a 20 Hertz frame rate while scanning from apex to base in the short axis plane. Then the MI was switched back to <0.3 and replenishment imaging repeated. If no replenishment was observed anywhere within this central portion, high MI impulses were repeated within 40 seconds. This sequence of high and low MI imaging was repeated for thirty minutes.
At the 30, 60 and 90 minutes into treatment, a diagnostic angiogram and 12 lead EKG were repeated. At 60 minutes, WT measurements were repeated within the RA and compared to pre-treatment WT.
Grading of myocardial contrast replenishment within the RA following high MI impulses
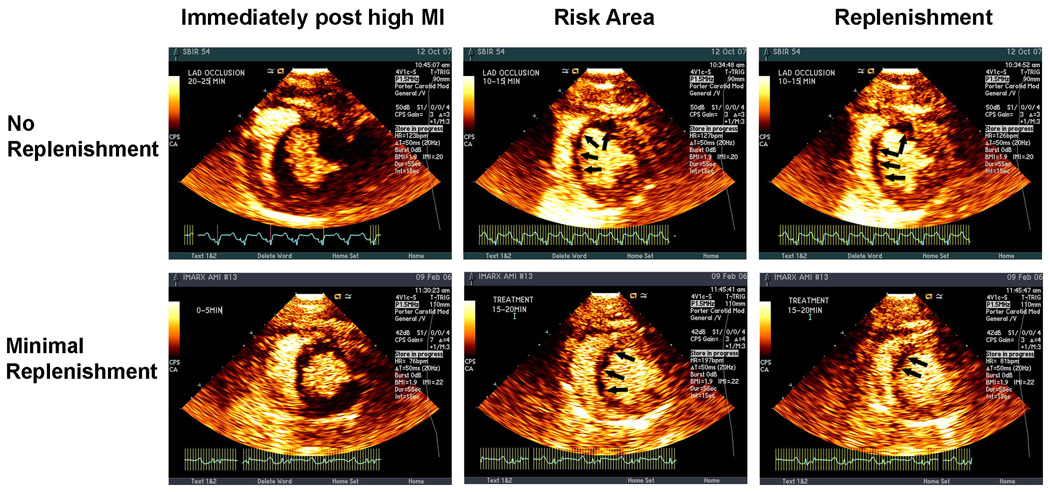
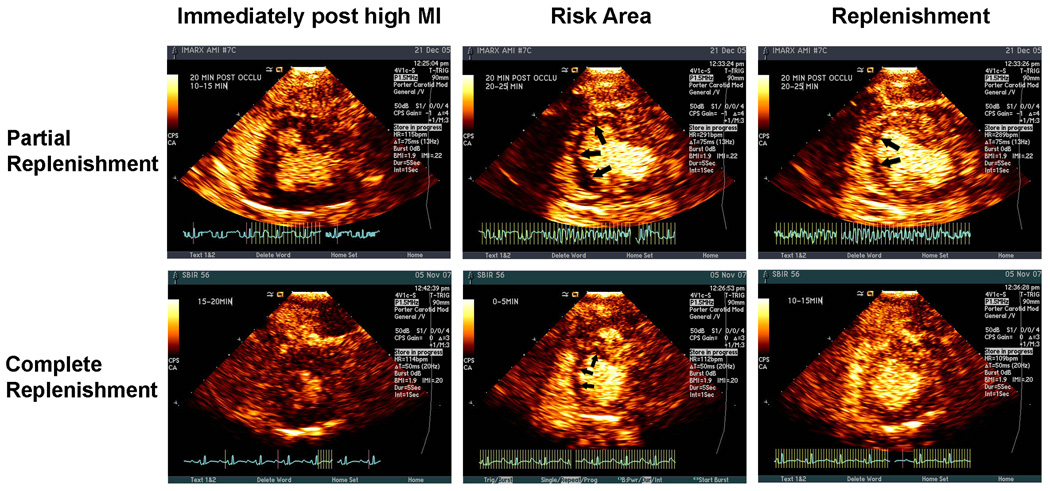
Similar to the methods described by Coggins et.al. (26), the size of the planimetered perfusion defect at the papillary muscle level within three seconds of the completed high MI impulse was defined as the RA. Subsequent to this, replenishment of the RA was noted in four different patterns (Figure 1 and Figure 2): a) none= no replenishment seen within either the central or lateral portions of the RA at 20–25 seconds following the high MI impulses (Figures 1); b) lateral replenishment only=replenishment of myocardial contrast only within the lateral portions of the RA (Figure 1); c) partial replenishment=replenishment of myocardial contrast within the sub-epicardial portion of the central RA as well as laterally (Figure 2); d) complete replenishment=complete transmural opacification of the RA following high MI impulses (Figure 2). The central portion of the RA was arbitrarily defined as that portion of the RA which did not enhance from in-growth along the lateral margins following high MI impulses. This central portion either filled along the epicardial rim only (Figure 2 top panels) or filled completely (Figure 2 bottom panels). Early central RA replenishment was defined as either partial or complete contrast appearance within the central portion of the RA by 15 minutes into treatment with microbubbles. These replenishment patterns were graded by an experienced reviewer blinded to the type of microbubbles used, angiographic results, and EKG or WT changes.
Figure 1.
The upper panel demonstrates an example of no replenishment within the lateral or central portions of the RA following a high MI impulse (left panel) at 10 minutes into treatment. The lower panel shows an example of replenishment only in the lateral portions of the RA following a high MI impulse at 15 minutes into treatment. Images were obtained after contrast plateau intensity had been reached (right panel).
Figure 2.
The upper panel shows an example of partial replenishment of the central portion of the RA at the plateau intensity following a high MI impulse (left panel). , again at 20 minutes into treatment The lower panel shows an example of complete replenishment of the RA (central and lateral portions) following the delivery of high MI impulses (left panel) at 10 minutes into treatment.
Statistical Analysis
All data are expressed as mean values ± SD or number and percentages. Chi-square tests were used to compare differences in recanalization rates, EKG resolution, and wall thickening improvement at 30, 60, and 90 minutes following documented occlusion. This test was also used when comparing partial or complete contrast replenishment within the RA at 5, 10–15, and 25–30 minutes into treatment to pigs that had only no or lateral RA replenishment at this point into treatment. One-way ANOVA was used for comparisons of quantitative WT measurements and EKG ST segment changes. The Bonferroni method was used to adjust p-values for multiple comparisons made at multiple time points during the experiment. A p-value <0.05 was considered statistically significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Hemodynamic Comparisons
Table 1 summarizes the hemodynamic, heart rate and oxygen saturation measurements in the three groups following coronary occlusion just prior to treatment, and at sixty minutes following initiation of treatment. There were no differences among the three groups in these parameters.
Table 1.
Hemodynamic Characteristics
| lytic therapy alone | Non-targeted MB | Targeted MB | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Heart rate (beat/minute) | 103±19 | 107±22 | 104±15 | 106±17 | 115±20 | 112±16 |
| Systolic arterial pressure (mmHg) |
97±13 | 94±18 | 92±16 | 87±11 | 108±11 | 104±7 |
| Diastolic arterial pressure (mmHg) |
61±13 | 58±16 | 59±10 | 54±9 | 66±9 | 66±8 |
| SpO2 (%) | 99±1 | 98±3 | 97±4 | 97±3 | 98±2 | 95±8 |
Angiographic Recanalization, ST Segment Resolution, and WT responses in the Three Different Treatment Groups
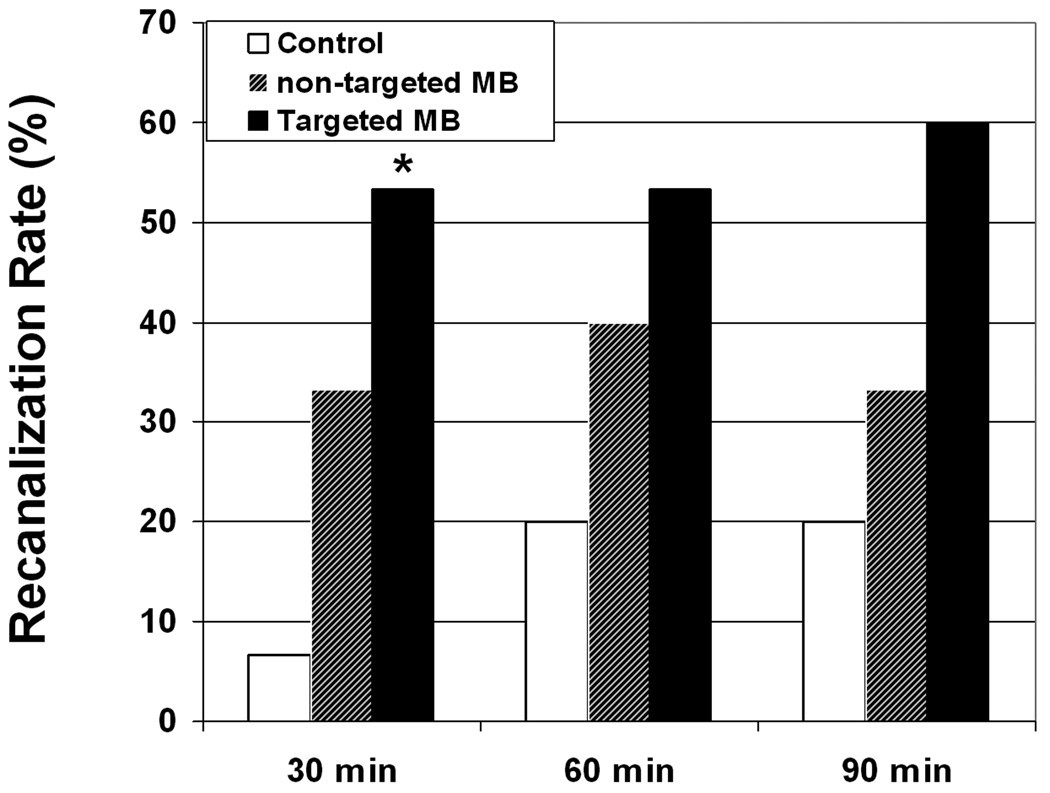
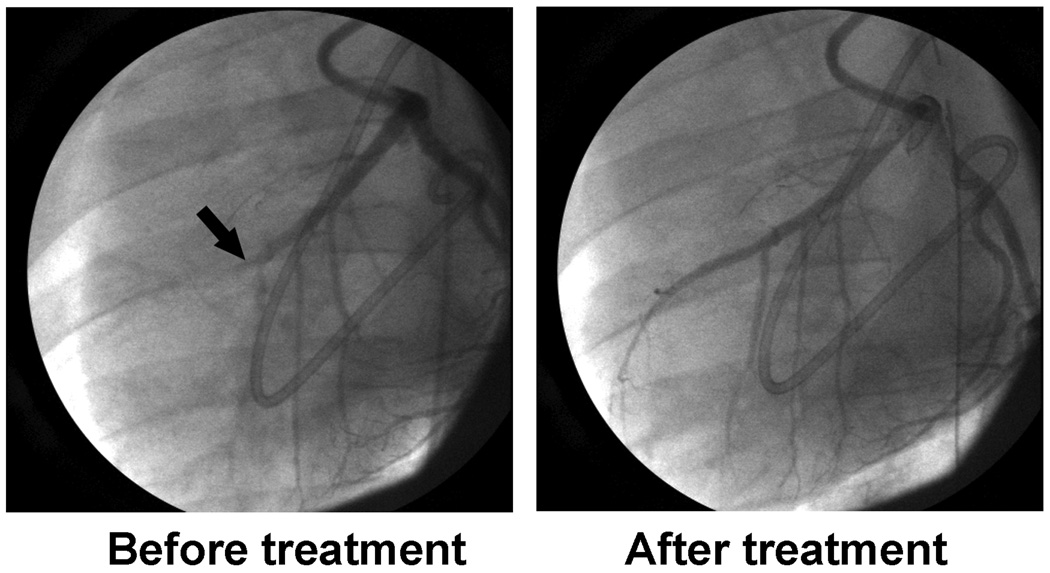
The pigs who had guided diagnostic ultrasound treatment regimen with PTMB had a 53% recanalization rate at 30 minutes (p=0.02 compared to half dose lytic therapy alone), and 53% and 60% recanalization rates at 60 and 90 minutes (Figure 3). In comparison, the pigs randomized to guided diagnostic ultrasound and NTMB with half dose lytics had recanalization rates of 33%, 40% and 33% at 30, 60 and 90 minutes after initiation of treatment. Recanalization rates for pro-urokinase alone were 7%, 20% and 20% at these same time periods. A residual 70% diameter or greater stenosis was present following reperfusion in four of the five (80%) recanalized vessels treated with NTMB, in six of the eight (75%) recanalized vessels treated with PTMB, and all three of the recanalized vessels of the pigs treated with half dose lytic therapy alone. Figure 4 is an example of a coronary angiogram of a recanalized left anterior descending artery after 30 minutes of treatment with IV PTMB.
Figure 3.
Pigs randomized to diagnostic ultrasound and targeted IV microbubbles (MB) had significantly higher recanalization rates than control (lytic therapy alone) at 30 minutes of treatment. Non-targeted IV microbubbles achieved a similar recanalization rate (50%) at 60 minutes.
*p=0.02 compared to control
Figure 4.
Angiograms of a left anterior descending artery before and after 30 minutes of intermittent high MI diagnostic ultrasound (guided by CPS) and intravenous platelet-targeted microbubbles.
WT within the central portion of the RA and maximal ST segment deviation were similar between groups prior to treatment (Table 2). Follow-up 12 lead EKG’s were not available in two pigs: one pig in the PTMB group, and one pig in the pro-urokinase only group who died before the 12 lead EKG could be obtained, leaving 14 for comparison in each of these groups. ST segment resolution occurred in nine of the 14 pigs (64%) treated with PTMB and nine of 15 treated with NTMB (60%), compared to 3 of 14 control pigs (21%). WT within the central RA increased in nine pigs treated with PTMB (60%), eight pigs (53%) in the NTMB group, compared to five pigs (36%) in the control group. Figure 5 demonstrates ST segment resolution observed in a pig randomized to diagnostic ultrasound and PTMB, even though no epicardial recanalization occurred in this particular pig.
Table 2.
Wall thickening before and following treatment
| Lytic therapy alone | DUS + Non- targeted MB+Lytic |
Targeted MB+DUS+ Lytic |
|
|---|---|---|---|
| Baseline | 3 ± 6 % | 3 ± 9 % | 6 ± 9 % |
| 60 minutes | 9 ± 8 % | 17 ± 12 %* | 17 ± 9 %† |
p=0.03 compared to lytic therapy alone
p=0.01 compared to lytic therapy alone.
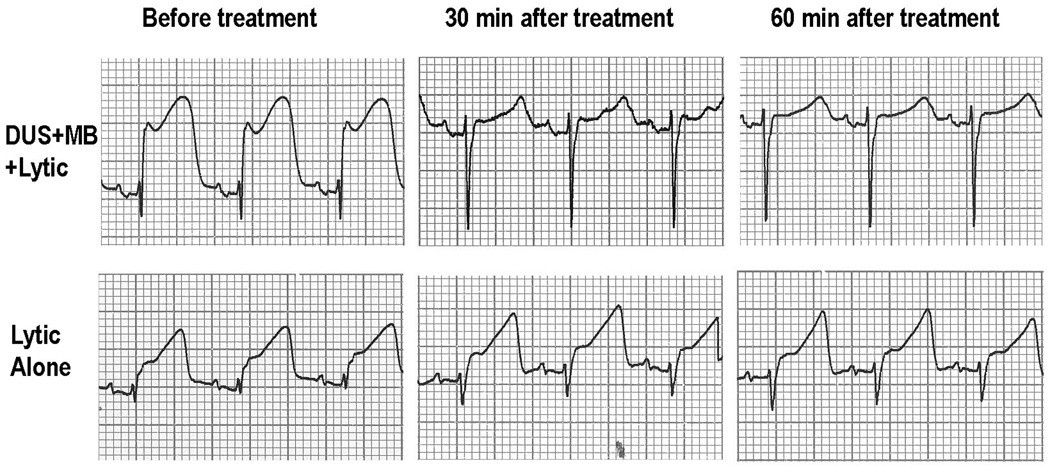
Figure 5.
Example of the improvement in the electrocardiogram observed in a pig after ultrasound and targeted microbubbles with lytic. Note that the ST-segment elevation observed at 0 mibutes almost totally resolves after 30 minutes of treatment with diagnostic ultrasound (DUS) and microbubbles (MB). In comparison, ST-segment elevation almost no changes after 30 minutes of lytic alone treatment.
Analysis of Myocardial Contrast Replenishment in the Pigs Randomized to Non-targeted versus Platelet-targeted Microbubbles
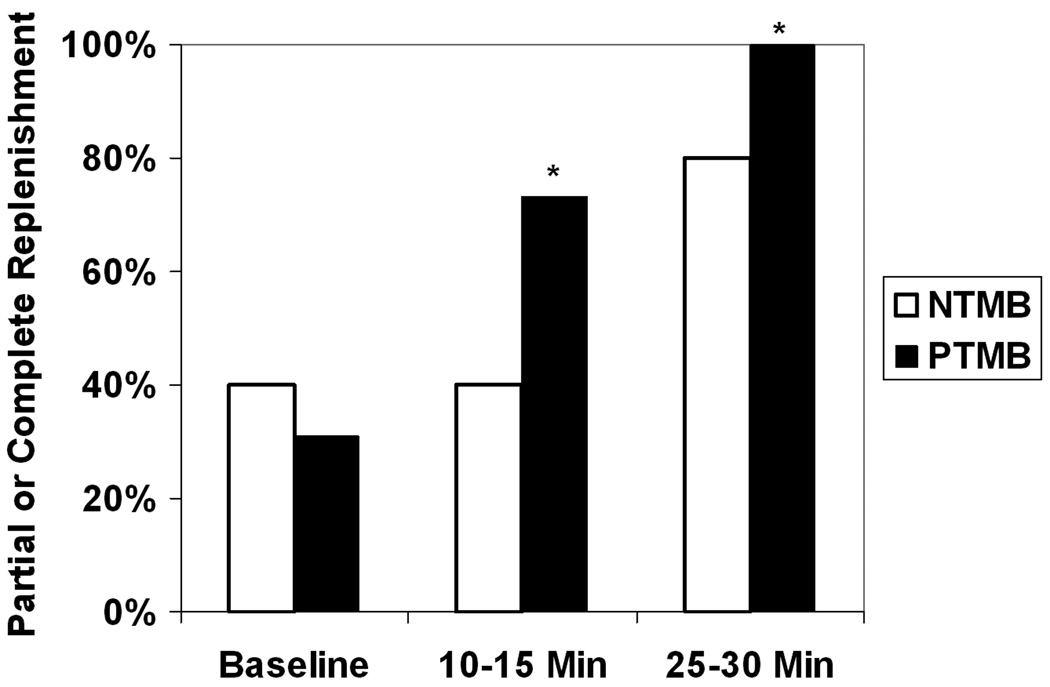
The baseline RA was 2.38±0.83 cm2 in the pigs treated with PTMB, versus 2.49±0.42 cm2 in the pigs treated with NTMB (p=0.64). The pigs who received PTMB exhibited more rapid replenishment of myocardial contrast within the central RA over the time course of treatment, when compared to pigs receiving NTMB (Figure 6). Although there were no differences in central RA replenishment at the initiation of treatment, partial or complete replenishment of myocardial contrast was observed in 12 of 15 pigs treated with PTMB at 10 minutes of treatment, versus six of 15 pigs receiving non-targeted microbubbles (p=0.04). At 30 minutes, all pigs receiving PTMB had partial or complete central RA replenishment (p=0.04 compared to NTMB).
Figure 6.
The percentage of pigs who exhibited partial or complete replenishment of myocardial contrast within the central portion of the risk area when randomized to receive guided diagnostic ultrasound and either non-targeted or platelet-targeted microbubbles following left anterior descending coronary thrombotic occlusion. *p=0.03
Regardless of whether the microbubbles were targeted or non-targeted, early central RA replenishment was associated with a higher epicardial recanalization rate and even higher rate of ST segment resolution. Pigs who had early RA replenishment had a 56 % epicardial recanalization rate at 30 minutes, compared to 25 % who did not have early central RA replenishment, and 7% for pro-urokinase alone (p=0.03). ST segment resolution at 60 minutes occurred in 14 pigs (82%) who had early central RA replenishment, compared to five of 12 pigs (42%) who did not, and three of 14 pigs (21%) receiving pro-urokinase alone (p=0.01). WT improved in 13 of the 18 pigs (72%) with early RA replenishment, compared to five of 12 (42%) who did not, and five of 14 pigs (37%) receiving pro-urokinase alone (p= 0.08).
In the 14 pigs treated with PTMB or NTMB that had epicardial recanalization at 60 minutes, 13 (93%) had ST segment resolution and WT recovery. However, in the 16 pigs treated with PTMB or NTMB that did not have epicardial recanalization by 60 minutes, ST segment resolution still occurred in six (38%). Five of these six pigs also had recovery of wall thickening.
Discussion
In this study, we demonstrated that IV PTMB combined with guided applications of high MI impulses from a diagnostic transthoracic ultrasound transducer significantly improved epicardial recanalization rates and microvascular recovery following acute coronary thrombotic occlusion. Furthermore, ST segment resolution and wall thickening improvement occurred even in the absence of epicardial recanalization, indicating that the beneficial effects of ultrasound at the capillary level may occur even in the absence of restored epicardial flow. This could have important implications for improving current pharmacologic and interventional treatment regimens in acute myocardial infarction.
Mechanism for Improved Early Recanalization
In vivo studies have shown in acute peripheral artery or dialysis graft thromboses that the addition of intravenous microbubbles increases the effectiveness of ultrasound in dissolving the thrombus (18, 23, 27, 28). Although the ultrasound we used for this study is routinely used for diagnostic imaging, the frequency and MI emitted from this transducer have been shown to induce cavitational activity in the presence of microbubbles (24, 29). Cavitation has generally been classified into two sub-types: stable and inertial, with the stable form being induced at a lower peak negative pressure (30). This stable form of cavitation induces microstreaming, and appears to be the main mechanism by which ultrasound potentiates thrombolysis in vitro (30, 31). Recent data has demonstrated that cavitation must be achieved for significant thrombolysis to occur in the presence of intravenously infused microbubbles (24).
Microvascular Recovery
In acute coronary syndromes, thrombi from within the epicardial vessel embolize downstream leading to microthombi that can reduce capillary perfusion even after epicardial recanalization (32, 33). ST segment resolution has served as an important marker of microvascular recovery in acute myocardial infarction (13, 34). In our study, ST segment resolution occurred in over 80% of the pigs exhibiting early central RA contrast replenishment, irrespective of whether treatment was with PTMB or NTMB, and even though epicardial recanalization was observed in 56% of these pigs. There are at least two potential mechanisms by which diagnostic ultrasound and microbubbles may improve microvascular flow in this setting. Low frequency ultrasound alone has been shown to improve tissue perfusion distal to occluded coronary arteries, a phenomenon which appeared to be nitric oxide-mediated (35). Alternatively, the guided high MI impulses and microbubbles may be dissolving the microthrombi within the capillaries. Re-opening capillaries within the RA would potentially reduce capillary resistance, which would then facilitate either anterograde coronary flow or collateral flow to these regions (36).
Clinical Implications
Diagnostic ultrasound-facilitated thrombolysis could impact current therapies in several ways. First, it could improve the success rate of existing treatment regimens used in acute coronary syndromes. Ultrasound and microbubbles could potentially be administered outside the hospital prior to reaching interventional facilities where definitive treatment occurs. Even if epicardial recanalization is not achieved with ultrasound and microbubbles, the improvements in microvascular flow may limit infarct size and relieve symptoms. This ultrasound treatment regimen could also be applied to non-ST segment elevation infarctions and acute coronary syndromes. In this setting, thrombus on a ruptured plaque is still the primary pathophysiologic event, and microbubbles have already been utilized to diagnose these entities in the emergency department (37). The addition of ultrasound and microbubbles may also permit lower doses of fibrinolytic agents to be administered while still achieving an equivalent pharmacologic effect. Furthermore, the enhanced thrombolytic effects would be targeted to just the region being insonified, which could reduce the risk of bleeding at remote locations. Thirdly, unlike other treatment modalities, this study indicates that ultrasound and microbubbles have the potential to improve microvascular flow, which must be achieved if there is to be recovery of regional function within the RA (12).
Limitations of the Study
Although we were able to successfully recanalize the coronary arteries, there was still a significant residual stenosis noted in the epicardial vessel of a majority of pigs that recanalized. Since no underlying atherosclerotic disease was present in this model, this stenosis was most likely residual thrombus. The ischemia-reperfusion process is associated with endothelial activation producing receptors which further propagate both thrombus formation and leukocyte adherence and activation (38). Additional anti-platelet and anti-inflammatory agents may need to be added to counter this activation and prevent re-occlusion.
The diagnostic transducer used in this study has an elevation plane of approximately five millimeters, and thus we manually moved the sector from the apical short axis plane to the base during the high MI impulses, in order to cover the entire coronary artery and RA affected. This two dimensional approach may result in certain segments of the RA not completely exposed to these impulses. A three-dimensional application of high MI impulses may improve the likelihood that the entire volume of the RA and upstream coronary artery are being insonified.
Since ultrasound and microbubbles were part of the randomized therapeutic regime, we did not use myocardial contrast echocardiography as an end point examining microvascular perfusion. Although this is a preferred method to assess microvascular recovery, ST segment resolution has correlated closely with myocardial contrast measurements of microvascular perfusion in acute myocardial infarction (34, 39), and thus served as an independent marker that could be compared between the three treatment groups.
Conclusions/Future Directions
We have shown that intravenous PTMB combined with a diagnostic transthoracic ultrasound transducer rapidly improve microvascular flow to the risk area, as well as improve epicardial recanalization rates. The improvement in microvascular flow was observed even when epicardial recanalization did not occur, and correlated with improvements in wall thickening within the risk area. Although ultrasonic methods targeting coronary artery thrombus will be challenging, methods to improve targeting to the microcirculation are easier to achieve with transthoracic ultrasound. For example, a three dimensional ultrasound volumetric probe may increase microvascular coverage when applying the high MI impulses, and improve visualization of the RA (or risk volume) when in the low MI imaging mode. Additional targeting ligands can be added to the microbubbles, such as targeting to both glycoprotein 2b/3a receptor inhibitor and fibrin . This may increase the number of microbubbles adherent to microthrombi, resulting in greater amounts of thrombus fragmentation with the high MI impulses, leading to a higher rate of microvascular recovery.
Even without these modifications, the ultrasound settings used in this study are already within Food and Drug Administration limits, and even non-targeted microbubbles were partially effective at improving microvascular recovery. Therefore, it is possible that this supplemental treatment regimen could be tested in a clinical scenario with non-targeted commercially available microbubbles, to determine whether the addition of guided diagnostic ultrasound and intravenous microbubbles to fibrinolytic therapy will result in improved regional function and better clinical outcomes when compared to treatment regimens focused on just recanalizing the epicardial vessel.
CLINICAL PERSPECTIVE
Coronary thrombosis on a ruptured coronary plaque is the main pathophysiologic event that leads to acute coronary syndromes (ACS). Although current pharmacological therapies and interventional techniques have improved the prognosis of patients with ACS, each of these therapeutic interventions has significant limitations. In the present study, we demonstrate that diagnostic ultrasound and intravenous microbubbles can improve both microcirculatory and epicardial recanalization rates in acute coronary thromboses. Following acute left anterior descending thrombotic occlusions, intravenous platelet targeted microbubbles combined with brief high MI diagnostic transthoracic ultrasound transducer guided by a low MI pulse sequence scheme improved microvascular flow to the risk area, and increased epicardial recanalization rates. The improvement in microvascular flow was observed even when epicardial recanalization did not occur, and correlated with improvements in wall thickening within the risk area. The addition of ultrasound and microbubbles may permit lower doses of fibrinolytic agents to be administered while still achieving an equivalent pharmacologic effect. Furthermore, the enhanced thrombolytic effects would be targeted to just the region being insonified, which would reduce the risk of bleeding at remote locations. Since the ultrasound pressures and frequencies used in this study are already within Food and Drug Administration limits, this supplemental treatment regimen could be tested in ST segment elevation myocardial infarction, to determine whether guided diagnostic ultrasound and intravenous microbubbles will result in improved regional function and better clinical outcomes when compared to treatment regimens focused on just recanalizing the epicardial vessel.
Acknowledgments
We thank Elizabeth Stolze and Gretchen Fry for their expert technical assistance. We also thank Lynette M. Smith, MS, for her statistical support in this manuscript.
Funding Sources
This work was supported by the National Institutes of Health SBIR grant (2R44HL071433-02).
Footnotes
Disclosures
1. Thomas R. Porter, MD
Grant Support: Bristol Myers Squibb Medical Imaging Siemens Medical Solutions
Consultant: ImaRx Therapeutics, Inc.
REFERENCES
- 1.Waxman S, Mittleman MA, Zarich SW, Fitzpatrick PJ, Lewis SM, Leeman DE, Shubrooks SJ, Jr, Snyder JT, Muller JE, Nesto RW. Angioscopic assessment of coronary lesions underlying thrombus. Am J Cardiol. 1997;79:1106–1109. doi: 10.1016/s0002-9149(97)00056-8. [DOI] [PubMed] [Google Scholar]
- 2.Ueda Y, Asakura M, Hirayama A, Komamura K, Hori M, Komada K. Intracoronary morphology of culprit lesions after reperfusion in acute myocardial infarction: serial angioscopic observations. J Am Coll Cardiol. 1996;27:606–610. doi: 10.1016/0735-1097(95)00534-x. [DOI] [PubMed] [Google Scholar]
- 3.Thieme T, Wernecke KD, Meyer R, Brandenstein E, Habedank D, Hinz A, Felix SB, Baumann G, Kleber FX. Angioscopic evaluation of atherosclerotic plaques: validation by histomorphologic analysis and association with stable and unstable coronary syndromes. J Am Coll Cardiol. 1996;28:1–6. doi: 10.1016/0735-1097(96)00108-8. [DOI] [PubMed] [Google Scholar]
- 4.AIMS Trial Study Group. Effect of intravenous APSAC on mortality after acute myocardial infarction: Preliminary report of a placebo-controlled clinical trialLancet. Lancet. 1988;1:545–549. [PubMed] [Google Scholar]
- 5.ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomized trial of intravenous streptokinanse, oral aspirin, bothof neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. Lancet. 1988;2:349–360. [PubMed] [Google Scholar]
- 6.Zijlstra F, de Boer MJ, Hoorntje JC, Reiffers S, Reiber JH, Suryapranata H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med. 1993;328:680–684. doi: 10.1056/NEJM199303113281002. [DOI] [PubMed] [Google Scholar]
- 7.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr, Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) Circulation. 2004;110:588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy JW, Ritchie JL, Davis KB, Fritz JK. Western Washington randomized trial of intracoronary streptokinase in acute myocardial infarction. N Engl J Med. 1983;309:1477–1482. doi: 10.1056/NEJM198312153092402. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg I, Matetzky S, Halkin A, Roth A, Di Segni E, Freimark D, Elian D, Agranat O, Har Zahav Y, Guetta V, Hod H. Primary angioplasty with routine stenting compared with thrombolytic therapy in elderly patients with acute myocardial infarction. Am Heart J. 2003;145:862–867. doi: 10.1016/S0002-8703(02)94709-5. [DOI] [PubMed] [Google Scholar]
- 10.Jong P, Cohen EA, Batchelor W, Lazzam C, Kreatsoulas C, Natarajan MK, Strauss BH. Bleeding risks with abciximab after full-dose thrombolysis in rescue or urgent angioplasty for acute myocardial infarction. Am Heart J. 2001;141:218–225. doi: 10.1067/mhj.2001.112239. [DOI] [PubMed] [Google Scholar]
- 11.Bolli R, Triana JF, Jeroudi MO. Prolonged impairment of coronary vasodilation after reversible ischemia. Evidence for microvascular "stunning". Circ Res. 1990;67:332–343. doi: 10.1161/01.res.67.2.332. [DOI] [PubMed] [Google Scholar]
- 12.Ito H, Maruyama A, Iwakura K, Takiuchi S, Masuyama T, Hori M, Higashino Y, Fujii K, Minamino T. Clinical implications of the 'no reflow' phenomenon. A predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93:223–228. doi: 10.1161/01.cir.93.2.223. [DOI] [PubMed] [Google Scholar]
- 13.de Lemos JA, Braunwald E. ST segment resolution as a tool for assessing the efficacy of reperfusion therapy. J Am Coll Cardiol. 2001;38:1283–1294. doi: 10.1016/s0735-1097(01)01550-9. [DOI] [PubMed] [Google Scholar]
- 14.Novelty R, Beek AM, Hirsch A, Stoel MG, Hofman MB, Umans VA, Algra PR, Twisk JW, van Possum AC. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol. 2008;52:181–189. doi: 10.1016/j.jacc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Tachibana K, Tachibana S. Albumin Microbubble Echo-contrast Material as an Enhancer for Ultrasound Accelerated Thrombolysis. Circulation. 1995;92:1148–1150. doi: 10.1161/01.cir.92.5.1148. [DOI] [PubMed] [Google Scholar]
- 16.Porter TR, Leveen RF, Fox R, Kricsfeld A, Xie F. Thrombolytic enhancement with perfluorocoarbon-exposed sonicated dextrose albumin microbubbles. Am. Heart J. 1996;132:964–968. doi: 10.1016/s0002-8703(96)90006-x. [DOI] [PubMed] [Google Scholar]
- 17.Dhond MR, Nguyen TT, Dolan C, Pulido G, Bommer WJ. Ultrasound-enhanced thrombolysis at 20 KHz with air-filled and perfluorocarbon-filled contrast biospheres. J. Am. Soc. Echocardiogr. 2000;13:1025–1029. doi: 10.1067/mje.2000.107006. [DOI] [PubMed] [Google Scholar]
- 18.Xie F, Tsutsui JM, Lof J, Unger EC, Johanning J, Culp WC, Matsunaga T, Porter TR. Effectiveness of Lipid Microbubbles and Ultrasound in Declotting Thrombosis. Ultrasound Med Biol. 2005;31:979–985. doi: 10.1016/j.ultrasmedbio.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Alvarez-Sabín J. Microbubble administration accelerates clot lysis during continuous 2-MHz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–429. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 20.Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD. Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. Thromb Res. 2007;119:777–784. doi: 10.1016/j.thromres.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birnbaum Y, Luo H, Nagai T, Fishbein MC, Peterson TM, Li S, Kricsfeld D, Porter TR, Siegel RJ. Noninvasive in vivo clot dissolution without a thrombolytic drug: recanalization of thrombosed iliofemoral arteries by transcutaneous ultrasound combined with intravenous infusion of microbubbles. Circulation. 1998;97:130–134. doi: 10.1161/01.cir.97.2.130. [DOI] [PubMed] [Google Scholar]
- 22.Nishioka T, Luo H, Fishbein MC, Cercek B, Forrester JS, Kim CJ, Berglund H, Siegel RJ. Dissolution of thrombotic arterial occlusion by high intensity, low frequency ultrasound and dodecafluoropentane emulsion: an in vitro and in vivo study. J Am Coll Cardiol. 1997;30:561–568. doi: 10.1016/s0735-1097(97)00182-4. [DOI] [PubMed] [Google Scholar]
- 23.Tsutsui JM, Xie F, Johanning J, Lof J, Cory B, Thomas L, Matsunaga T, Unger E, Porter TR. Treatment of Deeply Located Acute Intravascular Thrombi with Low Frequency Guided Ultrasound and Intravenous Microbubbles. J Ultrasound Med. 2006;25:1161–1168. doi: 10.7863/jum.2006.25.9.1161. [DOI] [PubMed] [Google Scholar]
- 24.Xie F, Everbach C, Matsunaga T, Lof J, He A, Bennett RM, Porter TR. Detection of Intravascular Cavitational Activity during Treatment of Deep Vessel Thromboses with Diagnostic Ultrasound and Intravenous Microbubbles. Circulation. Suppl. 2007;116:II–646. [Google Scholar]
- 25.Porter TR, Kricsfeld D, Lof J, Everbach EC, Xie F. Effectiveness of transcranial and transthoracic ultrasound and microbubbles in dissolving intravascular thrombi. J Ultrasound Med. 2001;20:1313–1325. doi: 10.7863/jum.2001.20.12.1313. [DOI] [PubMed] [Google Scholar]
- 26.Coggins MP, Sklenar J, Le DE, Wei K, Lindner JR, Kaul S. Noninvasive prediction of ultimate infarct size at the time of acute coronary occlusion based on the extent and magnitude of collateral-derived myocardial blood flow. Circulation. 2001;104:2471–2477. doi: 10.1161/hc4501.098954. [DOI] [PubMed] [Google Scholar]
- 27.Culp WC, Porter TR, Xie F, Goertzen TC, McCowan TC, Vonk BN, Baxter BT. Microbubble potentiated ultrasound as a method of declotting thrombosed dialysis grafts: Experimental study in dogs. Cardiovasc Intervent Radiol. 2001;24:407–412. doi: 10.1007/s00270-001-0052-4. [DOI] [PubMed] [Google Scholar]
- 28.Culp WC, Porter TR, McCowan TC, Roberson PK, James CA, Matchett WJ, Moursi M. Microubble-augmentd ultrasound declotting of thrombosed arteriovenous dialysis grafts in dogs. J Vasc Interv Radiol. 2003;14:343–347. doi: 10.1097/01.rvi.0000058409.01661.b4. [DOI] [PubMed] [Google Scholar]
- 29.Porter TR, Everbach C, Kricsfeld D, Xie F. Myocardial cavitational activity during continuous infusion and bolus intravenous injections of perfluorocarbon-containing microbubbles. J Am Soc Echocardiogr. 2001;14:618–625. doi: 10.1067/mje.2001.112750. [DOI] [PubMed] [Google Scholar]
- 30.Miller DL. Particle gathering and microstreaming near ultrasonically activated gas-filled micropores. J Acoust Soc Am. 1988;84:1378–1387. doi: 10.1121/1.396636. [DOI] [PubMed] [Google Scholar]
- 31.Datta S, Coussios C-C, McAdory LE, Tan J, Porter T, De Courten-Myers G, Holland C. Correlation of cavitation with ultrasound enhancement of thrombolysis. Ultrasound Med Biol. 2006;32:1257–1267. doi: 10.1016/j.ultrasmedbio.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakuma T, Leong-Poi H, Fisher NG, Goodman NC, Kaul S. Further insights into the no-reflow phenomenon after primary angioplasty in acute myocardial infarction: the role of microthromboemboli. J Am Soc Echocardiogr. 2003;16:15–21. doi: 10.1067/mje.2003.44. [DOI] [PubMed] [Google Scholar]
- 33.Thillmanns H, Leinberger H, Neumann FJ, Steinhausen M, Parekh N, Zimmerman R, Dussel R, Kuebler W. Myocrdial microcirculation in the beating heart – In vivo microscopic studies. In: Spaan JAE, Bruscheke AVG, Gittenberger-de Groot AC, editors. Coronary Circulation. The Netherlands: Martin Nijhoff Publishers, Dordrecht; 1987. pp. 88–94. [Google Scholar]
- 34.Feldman LJ, Coste P, Furber A, Dupouy P, Slama MS, Monassier JP, Tron C, Lafont A, Faraggi M, Le Guludec D, Dubois-Randé JL, Steg PG. FRench Optimal STenting-2 Invest. Incomplete resolution of ST-segment elevation is a marker of transient microcirculatory dysfunction after stenting for acute myocardial infarction. Circulation. 2003;107:2684–2689. doi: 10.1161/01.CIR.0000070423.91346.45. [DOI] [PubMed] [Google Scholar]
- 35.Siegel RJ, Suchkova VN, Miyamoto T, Luo H, Baggs RB, Neuman Y, Horzewski M, Suorsa V, Kobal S, Thompson T, Echt D, Francis CW. Ultrasound energy improves myocardial perfusion in the presence of coronary occlusion. J Am Coll Cardiol. 2004;44:1454–1458. doi: 10.1016/j.jacc.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 36.Lee CW, Park SW, Cho GY, Hong MK, Kim JJ, Kang DH, Song JK, Lee HJ, Park SJ. Pressure-derived fractional collateral flow: a primary determinant of left ventricular recovery after reperfused acute myocardial infarction. J Am Coll Cardiol. 2000;15:949–955. doi: 10.1016/s0735-1097(99)00649-x. [DOI] [PubMed] [Google Scholar]
- 37.Leng Tong K, Kaul S, Wang X, Rinkevich D, Kalvaitis S, Belcik T, Lepper W, Foster WA, Wei K. Myocardial contrast echocardiography versus thrombolysis in myocardial infarction score in patients presenting to the emergency department with chest pain and a non-diagnostic electrocardiogram. J Am Coll Cardiol. 2005;46:920–927. doi: 10.1016/j.jacc.2005.03.076. [DOI] [PubMed] [Google Scholar]
- 38.Gawaz M, Neumann FJ, Dickfeld T, Reininger A, Adelsberger H, Gebhardt A, Schömig A. Vitronectin receptor (alpha(v)beta3) mediates platelet adhesion to the luminal aspect of endothelial cells: implications for reperfusion in acute myocardial infarction. Circulation. 1997;96:1809–1818. doi: 10.1161/01.cir.96.6.1809. [DOI] [PubMed] [Google Scholar]
- 39.Santoro GM, Valenti R, Buonamici P, Bolognese L, Cerisano G, Moschi G, Trapani M, Antoniucci D, Fazzini PF. Relation between ST-segment changes and myocardial perfusion evaluated by myocardial contrast echocardiography in patients with acute myocardial infarction treated with direct angioplasty. Am J Cardiol. 1998;82:932–937. doi: 10.1016/s0002-9149(98)00508-6. [DOI] [PubMed] [Google Scholar]