Abstract
Reduced glucose metabolism and astrocyte activation in selective areas of the brain are pathological features of Alzheimer’s disease (AD). The underlying mechanisms of low energy metabolism and a molecular basis for preventing astrocyte activation are not, however, known. Here we show that amyloid beta peptide (Aβ)-dependent astrocyte activation leads to a long-term decrease in hypoxia-inducible factor (HIF)-1α expression and a reduction in the rate of glycolysis. Glial activation and the glycolytic changes are reversed by the maintenance of HIF-1α levels with conditions that prevent the proteolysis of HIF-1α. Aβ increases the long-term production of reactive oxygen species (ROS) through the activation of nicotinamide adenine dinucleotide phosphate oxidase and reduces the amount of HIF-1α via the activation of the proteasome. ROS are not required for glial activation, but are required for the reduction in glycolysis. These data suggest a significant role for HIF-1α-mediated transcription in maintaining the metabolic integrity of the AD brain and identify the probable cause of the observed lower energy metabolism in afflicted areas. They may also explain the therapeutic success of metal chelators in animal models of AD.
Keywords: Alzheimer’s disease, amyloid beta peptide, astrocytes, glycolysis, hypoxia-inducible factor, rodents
Introduction
Astrocytes constitute the majority of the cells within the brain and play a fundamental role in its energy metabolism. Most agree that the amyloid beta peptide (Aβ) is involved in the pathogenesis of Alzheimer’s disease (AD). While the effect of Aβ on nerve cells has been extensively studied, less is known about its interaction with astrocytic glial cells. The effects of Aβ on astrocytes are likely to be of importance as there is a cytotoxic response in AD brain that may be mediated by toxins produced by activated astrocytes. Astrocytes are activated in AD, and Aβ can directly activate cultured astrocytes (Hu et al., 1998; Prat et al., 2000). In addition to astrocyte activation, there is a decrease in energy metabolism in AD brain relative to agematched controls (de Leon et al., 2007). Because astrocytes are major players in brain energy metabolism (for reviews, see Lian & Stringer, 2004; Schubert, 2005), it is important to examine the Aβ–astrocyte interaction in more detail with respect to both glial activation and energy metabolism.
We have previously shown that Aβ has a profound effect on nerve cell energy metabolism (Soucek et al., 2003). CNS cell lines and cortical cultures that are resistant to Aβ toxicity have an enhanced flux of glucose through both the glycolytic pathway and the hexose monophosphate shunt (HMS). The result is higher levels of reducing equivalents in the form of reduced nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is the electron source for the majority of the cell’s antioxidant enzymes and associated pathways. Glucose metabolism is in part regulated by the transcription factor hypoxia-inducible factor 1 (HIF-1; Semenza, 1999), and we showed that the Aβ-induced changes in nerve glucose metabolism are caused by the activation of HIF-1 (Soucek et al., 2003).
HIF-1 is a heterodimeric transcription factor comprised of two subunits, HIF-1α and HIF-1β (Wang et al., 1995). Under normoxic conditions, HIF-1α DNA binding and activation of gene transcription does not occur because of the proteolytic degradation of HIF-1α. Because iron is a co-factor required for HIF-1α breakdown, iron chelators mimic hypoxia and induce HIF-1α. HIF-1α mediates the adaptation of cells to hypoxia and hypoglycemia by upregulating the expression of genes involved in glucose transport and glycolysis (Semenza, 1999). Enhanced HIF-1 activity was initially implicated in neuroprotection against Aβ toxicity by the observations that iron chelators and heat shock both induce HIF-1 and protect cells from Aβ (Behl & Schubert, 1993; Schubert & Chevion, 1995). It was also shown that the metal chelator clioquinol reverses some aspects of AD-like pathology in a mouse model for AD (Cherny et al., 2001).
To determine if HIF-1α regulates Aβ-induced changes in astrocyte metabolism and activation, HIF-1α expression and energy metabolism were monitored. It is shown that unlike the response of cortical neurons to Aβ, the long-term exposure to Aβ decreases the rate of glycolysis. Aβ-induced activation occurs independently of HIF-1α, but sustained levels of HIF-1α prevent glial activation.
Materials and methods
Cell culture
Mouse and rat astrocytes were prepared from the cortex as described elsewhere (Parpura-Gill et al., 1997; Liu & Piasecki, 2001). Approximately 50 pregnant Sprague–Dawley rats (Jackson Laboratory, Bar Harbor, Maine, USA) and three C57 Black / 6 mice (University of California, San Diego, USA) were used. Animals were anesthetized with 1% isofluorene via inhalation before the removal of the embryos. Cells were initially plated at 1 × 107 cells per 100-mm tissue culture dish in Alpha medium (Gibco, Grand Island, NY, USA) plus 10% fetal calf serum. After reaching confluence they were dissociated and replated at 5 × 104 cells per 35-mm tissue culture dish in the same medium. Two weeks later the confluent dishes were used for the experiments. The cultures contained no nerve or microglia at this point as determined by immunofluorescence staining with anti-microtubule-associated protein (MAP)2 (2a + 2B; Sigma, Saint Louis, MO, USA; #M1406) and Iba-1 (Wako, Richmond, VA, USA; #019-19741), respectively. The culture medium was switched to N2 supplement (Gibco) 24 h before the experiments were initiated.
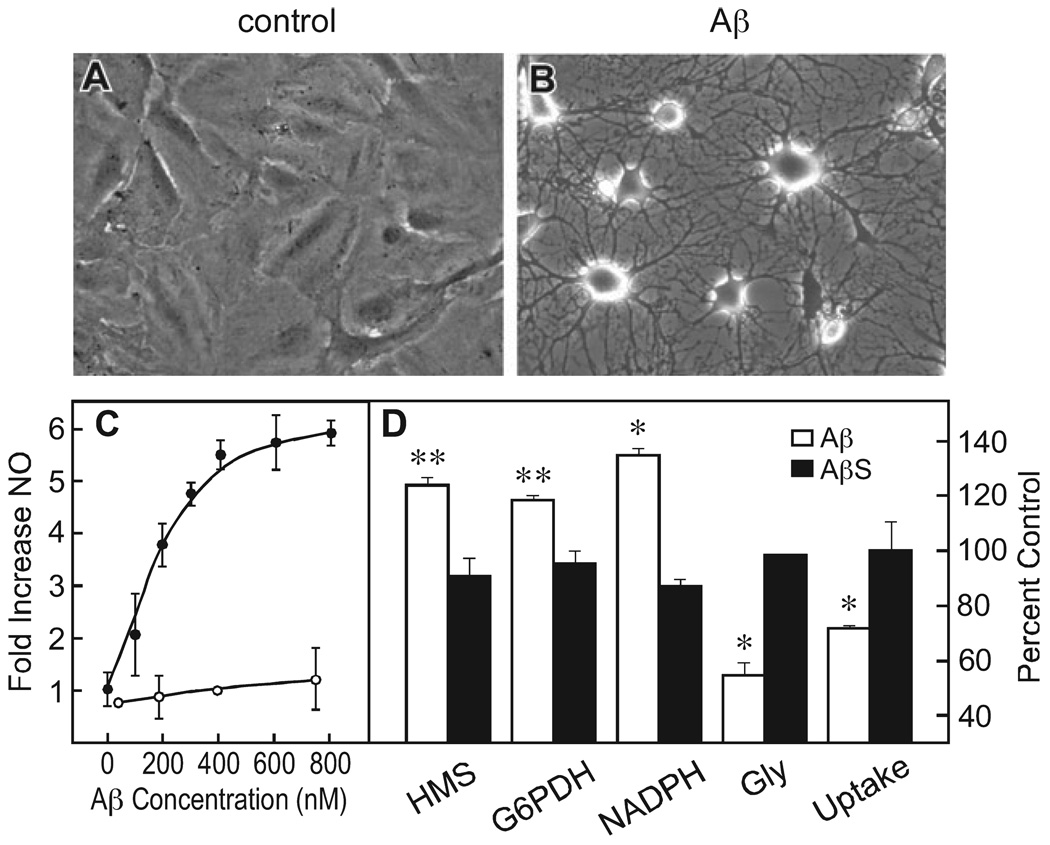
To assay glial activation based upon morphology, cultures were examined with high-contrast phase optics where the nuclei of individual cells are visible. In confluent cultures the cells are extremely flattened and the cytoplasm appears clear (Fig. 1A). Cells were scored as activated when there is an apparent contraction of the cytoplasm into phase dense ‘arms’ radiating from the area around the nucleus (Fig. 1B; Steinbach & Schubert, 1975). Activation was quantified by counting the number of activated cells per total number of cells in 10 randomly selected fields of 20–30 cells each. The data are presented as the mean plus or minus the standard error of the mean between the 10 fields examined.
FIG.1.
Amyloid beta peptide (Aβ)1–42 activates astrocytes and alters glucose metabolism. (A and B) Astrocytes were exposed to solvent alone (A), or to 1 µm Aβ1–42 (B) for 24 h and the morphological changes monitored by phase contrast microscopy. (C) Concentration-dependence of Aβ1–42-induced NO accumulation. Cells were exposed to increasing concentrations of Aβ1–42 or scrambled Aβ (AβS), and the accumulation of NO determined at 8 h relative to untreated controls.●–●, Aβ1–42; ○-○, AβS. (D) Aβ alters glucose metabolism. Glial cells were exposed to 1 µm Aβ1–42 or AβS for 24 h and the indicated activities monitored. In each experiment the values are shown as increases or decreases relative to controls (vehicle alone) as the mean plus or minus the standard error of the mean of three independent experiments. The following are the values for the control cells in each: deoxyglucose uptake 7.1 ± 0.5 nmols/min/ 106 cells; glycolysis 5.2 ± 0.2 nmols / min / 106 cells; hexose monophosphate shunt (HMS), 1.4 ± 0.1 nmols/min/106 cells; nicotinamide adenine dinucleotide phosphate (NADPH), 280 ± 20 pmols/mg protein; glucose-6-phosphate dehydrogenase (G6PDH), 124 ± 0.5 nmols/min/mg protein. *P < 0.0003; **P < 0.0001 by two-tailed t-test vs. vehicle (n = 3).
To determine the HIF-1α requirement for glial activation, C57 Black/6 mice harboring two alleles of exon 2 of HIF-1α flanked by a loxP site (HIF-1α f+/f+ or ‘floxed’) were crossed with GFAPCre mice, and 2-day-old offspring were used for cell isolation (Ryan et al., 2000; Bajenaru et al., 2002). Astrocyte cultures were prepared from the six embryos of one mouse as described above and screened by polymerase chain reaction for the HIF-1α null and wild-type genotypes as described in the original texts. Two of the six pups had HIF-1α deleted. Aβ1–42 (American Peptide) was dissolved in water at 1 mm and allowed to aggregate at 4°C for 24 h before use. Scrambled Aβ1–42 (AβS) has the same amino acid composition as Aβ1–42 but the sequence is random. In our hands the reverse Aβ1–42 sequence, which is frequently used as a control, can give false positive results because it has similar amphipathic properties to Aβ1–42 (Schubert et al., 1995). All animal studies have been approved by the Salk Institute’s Institutional Review Board.
Enzyme and glucose uptake assays
Two assays were used to assay glucose metabolism through the glycolytic pathways. The first was the formation of 3H2O from [5-3H]glucose (Ashcroft et al., 1972). The second assay involved the production of 14CO2 from d-[1-14C]glucose and d-[6-14C]glucose (NEN, Boston, MA, USA). They were used to measure net glucose utilization via the HMS and the citric acid cycle pathways, respectively, according to published procedures (Hyslop et al., 1988). Lactate production was not used because it does not directly reflect the distribution of glucose carbon through the HMS and citric acid cycles. To examine glucose uptake, cells were washed twice with glucose-free Dulbecco’s modified Eagle’s medium and incubated in this medium for 1 h at 37°C. [U-14C]Deoxyglucose was then added at 0.5 µCi/mL to a final concentration of 1.6 mm and uptake monitored over 20 min, during which time the uptake was linear. Uptake was stopped by washing the cells three times with ice-cold phosphate-buffered saline and dissolving the cells in 0.2 N NaOH for isotope counting. Uptake was completely inhibited by 10 mm phloretin, establishing the specificity of uptake through a glucose transporter. All enzymes were assayed by standard optical methods (Worthington, 1947). Cell cultures were washed two times with phosphate-buffered saline and lysed in the assay buffer by sonication. In all cases, care was taken to ensure that the reactions were linear and substrate dependent. NADPH was measured as described (Zhang et al., 2000).
Electrophoretic mobility shift assays (EMSAs) and Western blotting. Nuclear extracts were prepared and used for an EMSA specific for HIF-1. A 32P-labeled 24-bp oligonucleotide (5′-GCCCTACGTGCTGCCTC GCATGGC-3′) from the mouse EPO 3′ enhancer was used as a probe (Maxwell et al., 1999). The identity of the retarded bands was confirmed by adding antibodies specific for HIF-1α to the incubation mixture (Santa Cruz Biotechnology, Santa Cruz, CA, USA; #8089). A super shift of the retarded bands was observed (data not shown). In addition, cell extracts were incubated with a 100-fold excess of unlabeled wild type oligo or with an oligo mutated in the HRE region; the wild-type oligo eliminated the retarded band while the mutated oligo did not (data not shown). Western blotting for HIF-1α was done as described by Soucek et al., (2003), using rabbit HIF-1α antibody (Novus, Littleton, CO, USA; #19382). The antibody against the transferrin receptor (TR) is rat monoclonal R17-217 (Lesley & Schulte, 1984), a gift from Roberta Schulte, The Salk Institute.
Proteasome activity
The two main 26S proteasome proteolytic activities, trypsin-like and chymotrypsin-like activities were measured. Samples containing 20 µg of protein were incubated between 1 and 30 min at 37°C in 100 µL of (in mm): HEPES-HCl, 20 (pH 7.5); NaCl, 10; MgCl2, 1.5; EDTA, 1; sucrose, 250; dithiothreitol, 4; ATP, 2 containing 100 µm of substrate (Z-Ala-Arg-Arg-7-amino-4-methyl coumarin or Suc-Leu-Leu-Val-Tyr-7-amino-4-methyl coumarin). The reaction was monitored at 1-min intervals in a spectrofluorometer plate reader at 370 nm excitation and 430 nm emission wavelengths, and the rate constants calculated on the basis of total cellular protein. The data are presented as percent control rates.
Reactive oxygen species (ROS) and nitric oxide (NO) assays
ROS measurements were performed as described by Tan et al. (1998) by flow cytometry. ROS production was detected using 2′,7′-dichlorodihydrofluorescein diacetate (DCF). Briefly, DCF was added, and cells were incubated for 5 min. Cells were collected and washed once in HEPES buffer supplemented with 2% dialysed fetal bovine serum. Washed cells were resuspended in HEPES buffer and kept on ice until flow cytometric analysis. DCF data were collected with 475-nm excitation and 525-nm emission wavelengths and plotted using the data analysis program CELLQuest (Becton Dickinson, Mountain View, CA, USA). Data were analysed from 10 000 live cells as determined by the lack of propidium iodide fluorescence. NO was assayed by the measurement of nitrite (a stable oxidation product of NO) in the culture medium, the procedure of Hu et al. (1996). Briefly, nitrite levels were determined by mixing 100-µL aliquots of conditioned medium with 50 µL of 1% sulfanilamide in water plus 50 µL of 0.1% N-1-naphthylethylenediamine dihydrochloride in 5% phosphoric acid, and incubating for 10 min at room temperature. The absorbance at 540 nm was then measured by using a Molecular Probes plate reader.
Statistics
The data were analysed using GraphPad Instat (GraphPad Software, San Diego, CA, USA). For Western blots, data were normalized to actin and we used one-way ANOVA for experiments accessing dose-and time-dependent effects. For enzyme and viability assays data are normalized to vehicle-treated cultures analysed by one-way ANOVA. Tukey post hoc tests were used for multiple comparisons. In some cases unpaired two-tailed t-tests were used to assess differences between means. Null hypotheses were rejected at the 0.05 level.
Results
Aβ activates astrocytes and inhibits glycolysis
Because there is increased Aβ accumulation, altered glucose metabolism and astrocyte activation in the brains of patients with AD, we asked if there is a functional relationship between these three phenomena. Initially, the effect of Aβ1–42 on the activation of cultured astrocytes was assayed by morphological criteria and the release of NO. As previously reported (Hu et al., 1998; Akama & Van Eldik, 2000; Liu & Piasecki, 2001; Avasolla et al., 2004), 1 µm Aβ1–42 increases both morphological and biochemical astrocyte activation as defined by the activation of inducible NO synthetase (iNOS) and the release of NO. In their non-activated state cultured astrocytes are extremely flattened and relatively phase transparent (Fig. 1A). Upon activation they acquire long phase-dense processes via the shrinkage of the cytoplasm around extended elements of the cytoskeleton (Fig. 1B; Steinbach & Schubert, 1975). Aβ1–42 -induced astrocyte activation defined by NO release is concentration dependent, with an EC50 of 200–300 nmol (Fig. 1C). The scrambled form of Aβ1–42 (AβS), which has the same amino acid composition but a random sequence, is inactive.
We have previously shown that Aβ1–42 increases the flux of glucose through the glycolytic pathway in cortical neurons as well as the activity of the HMS (Soucek et al., 2003). The HMS is the cell’s major source of NADPH, a molecule that serves as a cofactor for many antioxidant enzymes and for maintaining high levels of glutathione, the cell’s major antioxidant. Because astrocytes are the most abundant cell type in the brain and therefore may share any toxic insults from Aβ with nerve cells, we asked if Aβ alters glial glycolysis. Several assays were used to distinguish differences in the flux of glucose through the HMS and the standard glycolytic pathway. Perhaps the best measures 14CO2 production from 14C-1-glucose and 14C-6-glucose. 14C-1-Glucose releases 14CO2 both from the citric acid cycle and via the shunt by the oxidative decarboxylation of 6-phosphogluconate to form ribose-5-phosphate. 14C-6-Phosphate releases 14CO2 from the citric acid cycle only. HMS activity is determined by the subtraction of 14CO2 derived from 14C-6-glucose from 14CO2 derived from 14C-1-glucose. Figure 1D shows that following 24 h exposure, Aβ increases the amount of glucose fluxing through the HMS by about 20% (t6 = 20, P = 0.0003). The increase in HMS activity was confirmed by measuring the activity of the rate-limiting enzymatic step in the HMS, glucose-6-phosphate dehydrogenase (G6PDH) and the levels of a product of the shunt, NADPH. Both of these parameters were increased after a 24 h exposure of astrocytes to Aβ, but not by the scrambled peptide (AβS; t6 = 7.4, P < 0.0003; t3 = 11.2, P < 0.0001, respectively).
In contrast to the enhancement of the HMS in cells exposed to Aβ relative to controls, the rate of glucose flux through the glycolytic pathway was significantly decreased by Aβ after 24 h (t6 = 14.1, P < 0.0001). Glycolysis was measured by two methods, the difference in the conversion of 14C-1 and 14C-6 glucose to 14CO2 as described above (Fig. 1D, Gly), and the conversion of 5-3H-glucose to 3H2O (not shown). By both criteria, there is about a 50% decrease in overall glucose flow through the glycolytic pathway, excluding the shunt. There was also about a 30% decrease in the uptake of 14C-Udeoxyglucose (Fig. 1D, uptake; t6 = 10.3, P < 0.0001). Together these data show that a 24 h exposure of rat astrocytes to Aβ1–42 decreases the rate of glycolysis, but enhances the flow of glucose through the HMS providing additional reducing equivalents in the form of NADPH. This is in contrast to primary cultures of cortical neurons where Aβ1–42 enhances the flux of glucose through both the glycolytic and HMS pathways twofold (Soucek et al., 2003).
Aβ modulates HIF-1α expression
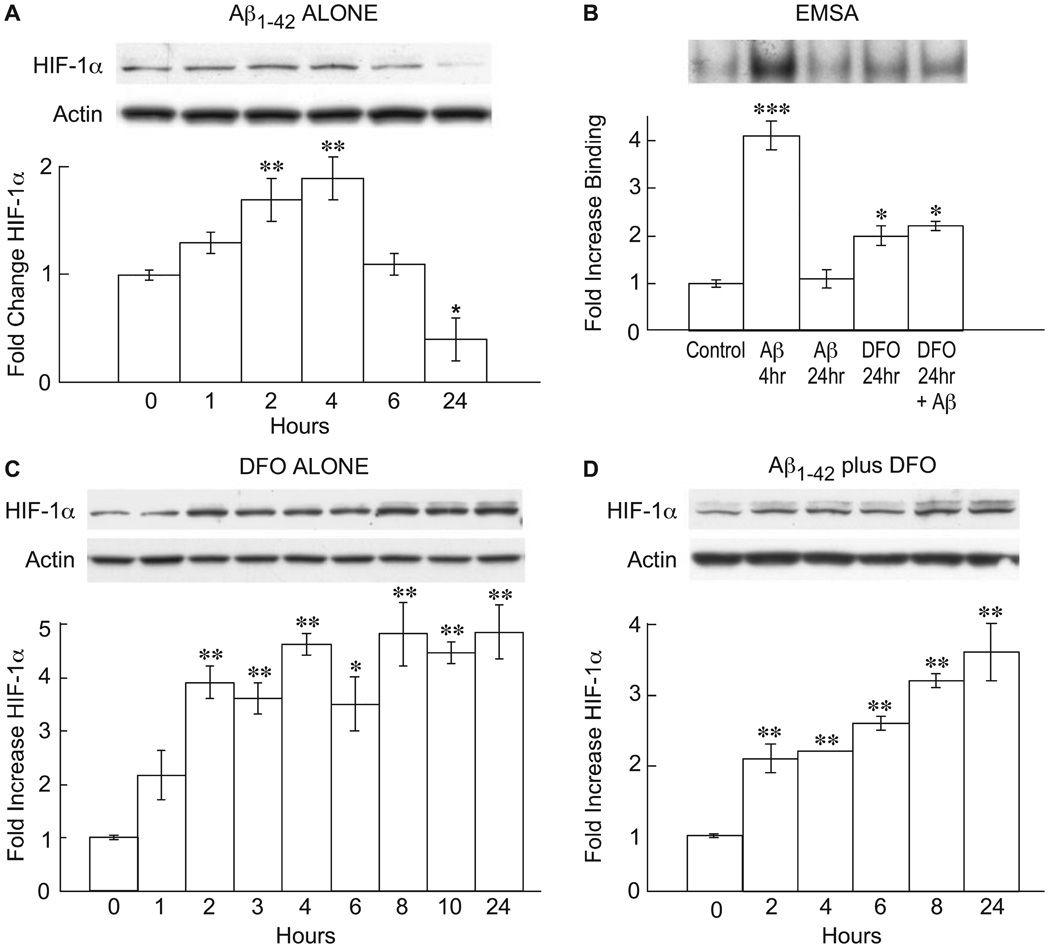
In nerve cells the increase in glycolysis caused by Aβ is mediated by the activation of the transcription factor HIF-1 (Soucek et al., 2003). To determine if astrocytes are also responsive to Aβ in this manner, cells were exposed to Aβ1–42 and the activation of HIF-1α monitored by Western blotting and EMSAs. Figure 2A shows a time course of HIF-1α stabilization as measured by Western blotting following Aβ exposure. There is a significant increase by 2 h followed by a return to control levels by 6 h, and an overall decrease of nearly 50% relative to control at 24 h (F4,11 = 15, P < 0.0001). Figure 2B shows an EMSA assay after 4 h of Aβ exposure demonstrating an increase in HIF-1α DNA-binding activity relative to vehicle alone, followed by a decline to baseline levels after 24 h (F3,8 = 17, P < 0.0001). Therefore, Aβ1–42 has the ability to activate astrocytes, alter glucose metabolism and cause a transient increase in the activity of the transcription factor HIF-1α followed by a large decline. What are the mechanistic relationships between these three activities?
FIG.2.
Amyloid beta peptide (Aβ) and desferoxamine (DFO) induce hypoxia-inducible factor (HIF)-1α. Rat astrocytes were exposed to Aβ, DFO or both, and HIF- 1α protein or electrophoretic mobility shift assay (EMSA) activity followed as a function of time. The fold-change is relative to control. Exactly 20 µg protein per lane was loaded. For Western blots the data are normalized to actin. (A) 1 µm Aβ1–42, anti-HIF-1α; (B) 1 µm Aβ and/or 50 µm DFO, EMSA assay; (C) 50 µm DMSO, anti-HIF-1α; (D) 1 µm Aβ plus 50 µm DFO, anti-HIF-1α. (A) *P < 0.01 by one-way ANOVA (n = 3); **P < 0.05 by one-way ANOVA vs. control (n = 3). (B) ***P < 0.001; *P < 0.01 by ANOVA (n = 3). (C) *P < 0.01; **P < 0.001 by ANOVA (n = 3). (D) **P < 0.001 by ANOVA (n = 3; Tukey’s post hoc test).
Because the enzyme that causes the breakdown of HIF-1α is iron dependent, iron chelators increase the stability of HIF-1α and enhance gene transcription. To determine if the activation of astrocytes by Aβ is mediated via the enhanced activity of HIF-1α, it was asked if iron chelators such as desferoxamine (DFO), which induce HIF-1α, mimic the effect of Aβ; cells that lack HIF-1α should not be activated. Figure 2C shows that DFO increases the stability of HIF-1α as assayed by Western blotting, with a maximum induction of about 4.5-fold at 8 h (F7,16 = 40, P < 0.001). However, unlike Aβ, DFO is able to maintain elevated HIF-1α protein levels for 24 h (Fig. 2C) and, as long as the cultures are viable, about 3 weeks (data not shown).
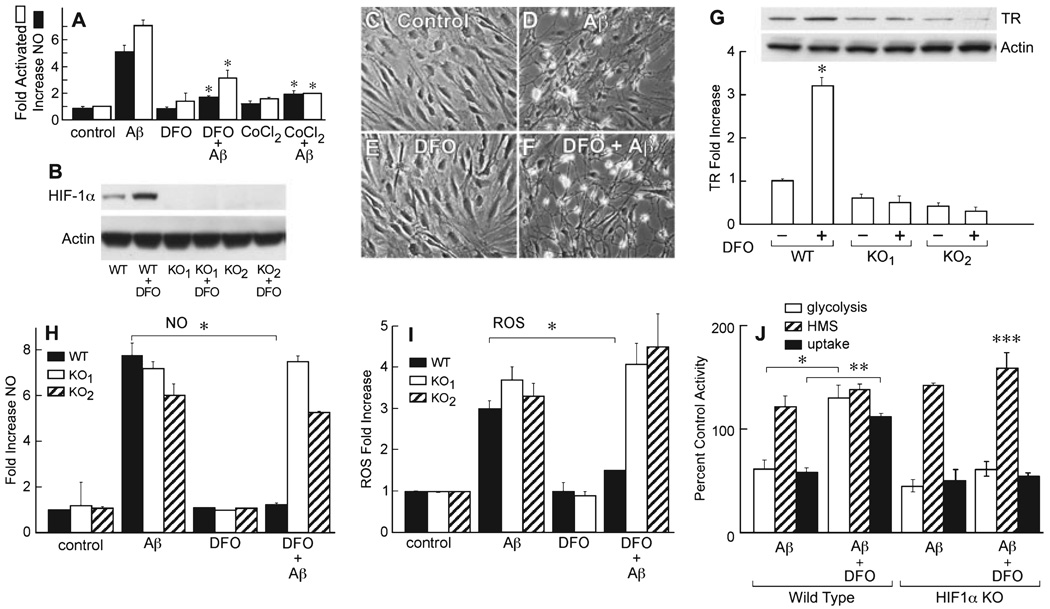
The HIF-1α-mediated response to low concentrations of Aβ in neurons is neuroprotective for it increases cellular-reducing equivalents by activating HMS, thereby protecting cells from higher toxic concentrations of Aβ (Soucek et al., 2003). Aβ also enhances the shunt in astrocytes (Fig. 1D), but activates astrocytes to produce potentially neurotoxic compounds such as NO. However, because the kinetic analysis of HIF-1α induction shows a transient induction with 1 µL Aβ1–42, but a robust sustained response with DFO (Fig. 2A–C), it is possible that the transient, Aβ-induced HIF-1α increase is a failed protective response, which is then overridden by other mechanisms associated with glial activation. To test this possibility, it was asked if two inducers of HIF-1α, DFO and CoCl2, maintain HIF-1α levels and block activation. Figure 2D shows that DFO maintains high levels of HIF-1α protein expression in the presence of Aβ1–42 (F5,12 = 45.9, P < 0.001), and Fig. 3A shows that DFO prevents the morphological and biochemical activation of rat astrocytes. CoCl2, that functions by a mechanism distinct from that of DFO (Yuan et al., 2003), also inhibits activation (Fig. 3A; F2,9 = 662, P < 0.01 for activation and F2,9 = 294, P < 0.01 for NO).
FIG.3.
The iron chelator desferoxamine (DFO) prevents amyloid beta peptide (Aβ)-induced activation in wild-type astrocytes, but does not prevent glial activation in hypoxia-inducible factor (HIF)-1α-deficient astrocytes. (A) Astrocyte activation is inhibited by DFO and CoCl2. Nitric oxide (NO) release (black bars) and morphological activation (white bars) were monitored at 8 h post-Aβ exposure, as described in Materials and methods. *Significantly different from Aβ alone for each condition (P < 0.001, n = 8), one-way ANOVA, Tukey’s post hoc test. (B–J) HIF-1α gene knockout glial cells can be activated by Aβ, but are not inhibited by DFO. Western blot of HIF-1α from control and HIF-1α-deleted cultures after 8 h. WT, wild-type cells control. KO1 and KO2, cells from two HIF-1α-deleted animals plus or minus 50 µm DFO. (C) Control, and (D) HIF-1α null cells exposed to 1 µm Aβ1–42 for 8 h. (E) HIF-1α null cells exposed to 50 µm DFO for 8 h alone. (F) HIF-1α null cells exposed to DFO and Aβ for 8 h. Wild-type cells from the same litter were activated by Aβ1–42, and activation was blocked by DFO (data not shown). The phase bright areas in the activated cells (D and F) are contracted cell bodies. (G) Increase in transferrin receptor (TR) amount at 8 h following exposure to 50 µm DFO in wild-type (WT) and cells from the two HIF-1α knockout mice (KO1 and KO2), *P < 0.0001, two-tailed P-value, unpaired t-test (n = 3). (H) NO production in wild-type (black) and KO1 (white) and KO2 (stripes) astrocytes 8 h post-Aβ exposure. Aβ1–42 and DFO concentrations were 1 µm and 50 µm, respectively. *P < 0.0002, two-tailed P-value, unpaired t-test (n = 3). (I) Reactive oxygen species (ROS) production in wild-type (black bars) and HIF-1α KO1 (white bars) and KO2 (stripes) mouse astrocytes determined after 8 h exposure to 50 µm DFO and/or 1 µm Aβ1–42. *P < 0.0016, two-tailed P-value. (J) A comparison of energy metabolism in wild-type and HIF-1α KO astrocytes 24 h after exposure to 50 µm DFO and/or 1 µm Aβ1–42. Glycolysis (white), uptake (black), hexose monophosphate shunt (HMS; hatched). *P < 0.001 for glycolysis; **P < 0.001 for uptake; ***P < 0.0001 for HMS (n = 4) independent cultures from the same animal, vs. control without Aβ, two-tailed t-test.
Astrocytes lacking HIF-1α are not rescued from activation by DFO
To determine if HIF-1α induction by Aβ is a failed protective response in astrocytes and not a necessary step in glial activation, it was asked if astrocytes derived from mice lacking the HIF-1α gene are activated by Aβ but not rescued by DFO. The Cre-LOX system was used to delete HIF-1α from astrocytes derived from embryos of a cross between mice with two alleles of HIF-1α flanked by loxP sites with GFAPCre transgenic mice (Ryan et al., 2000; Bajenaru et al., 2002). Of the six embryos assayed, two had the restriction fragment indicative of the HIF-1α knockout. Two additional criteria show that these cells contain no HIF-1α. Figure 3B shows by Western blotting that while HIF-1α is induced in wild-type cells by DFO, HIF-1α protein is not expressed in the HIF-1α cultures from the HIF-1α knockout mice, even in the presence of DFO. The TR is an HIF-1-responsive gene (Bianchi et al., 1999). Figure 3G shows that DFO does not induce an elevated expression of the TR in cultures in which HIF-1α has been deleted (t6 = 19.7, P < 0.0001). However, Aβ1–42 still activates cells morphologically in which HIF-1α has been deleted (Fig. 3C and D), and this effect is not inhibited by DFO (Fig. 3E and F). The inability of DFO to prevent astrocyte activation was also reflected in NO production (Fig. 3H; t6 = 26.7, P < 0.0002). DFO inhibits glial activation in wild type mouse astrocytes at 50 µm but not in the HIF-1α-null cells at 50, 100 or 200 µm (last two concentrations not shown).
To determine if HIF-1α indeed mediates the effect of DFO on the maintenance of glycolysis in the presence of Aβ, it was asked if glycolysis is responsive to DFO in the HIF-1α knockout cells. Figure 3J shows that while DFO rescues glycolysis (t6 = 15.7, P < 0.0001) and glucose uptake (t6 = 38, P < 0.001) in wild-type cells, it only maintains HMS activity in the HIF-1α knockout cells (t6 = 52.9, P < 0.0001). The probable reason for the maintenance of HMS activity is that ROS is still produced, inactivating glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and causing reverse flow of trioses through the shunt (Hyslop et al., 1988).
These data show that HIF-1α expression is not necessary for astrocyte activation but is necessary for DFO protection from activation, and importantly that DFO is not protective simply by its ability to limit ROS production or activity via the inhibition of Fenton chemistry as an iron chelator.
ROS induction is not sufficient for astrocyte activation
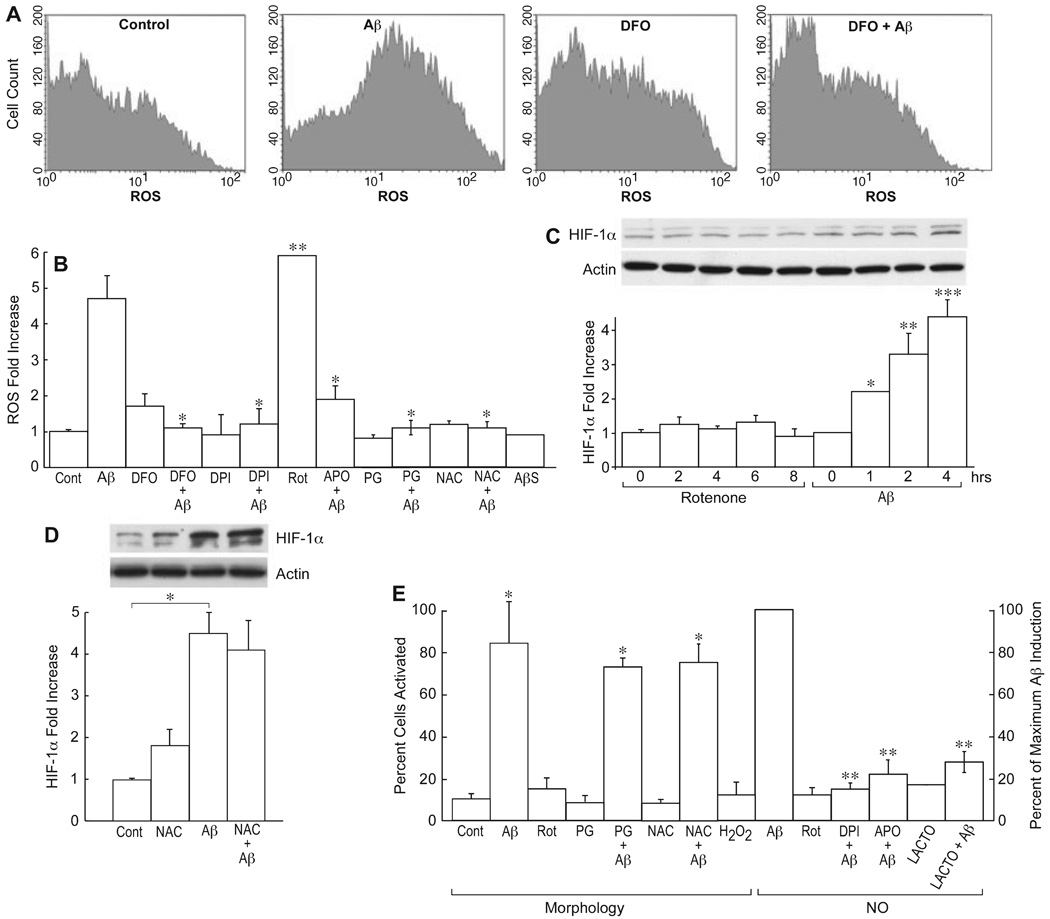
Because the exposure of neurons to Aβ increases production of ROS (Behl et al., 1994), a similar response may be elicited in astrocytes and be responsible for their activation. It has, in fact, been shown that Aβ activates NADPH oxidase in astrocytes (Abramov et al., 2004). To determine if ROS is produced from Aβ exposure and responsible for HIF-1α induction or activation, astrocytes were incubated with 1 µm Aβ1–42 for 8 h and ROS accumulation assayed by FACS using dichlorofluorescein. Figure 4A and B shows that Aβ1–42 but not scrambled Aβ1–42 increased ROS accumulation by 4.7-fold relative to control. In agreement with previous reports, this increase was blocked by the NADPH oxidase inhibitors diphenyliodonium (DPI) and apocynin (APO; Gao et al., 2003; Abramov et al., 2004) and the antioxidant propyl gallate (PG; F5,8 = 70.6, P < 0.001). In addition, a 30-min exposure of cells to DFO before the addition of Aβ resulted in the almost complete suppression of ROS accumulation caused by Aβ (Fig. 4A and B). To determine if increased ROS is sufficient to induce HIF-1α or activate glial cells, cells were exposed to the pro-oxidant rotenone (Rot), and glial activation and HIF-1α induction monitored. Figure 4C shows that the stimulation of intracellular ROS several-fold by Rot (Fig. 4B; t6 = 16.4, P < 0.0001) is not sufficient to cause HIF-1α induction (F3,15 = 42.2, P < 0.05 for Aβ alone). In addition, the antioxidants N-acetyl-l-cysteine (NAC), PG and vitamin E (not shown) are not able to block glial activation or HIF-1α activation by Aβ (t6 = 13.7; P < 0.001; Fig. 4D and E). Indeed, concentrations of vitamin E above 10 µm activated the glia, while H2O2 is ineffective. In HIF-1α knockout cells, the basal level of ROS is identical to wild-type cells, as is the Aβ-induced level (Fig. 3I). However, DFO does not block ROS production (t6 = 16.0, P < 0.0016) or glial activation (Fig. 3C–F, H and I). It can be concluded that Aβ stimulates both early HIF-1α activation and an increase in ROS production, but that ROS production per se is not responsible for HIF-1α induction or glial activation. If not ROS, what induces the HIF-1α protein?
FIG.4.
Reactive oxygen species (ROS)-dependent changes in the amyloid beta peptide (Aβ) response. (A) Glial cells were exposed to the indicated compounds for 8 h, and intracellular ROS determined by FACS using DCF. The single-cell fluorescence intensity is plotted vs. cell number (counts) for 10 000 cells. Aβ1–42, 1 µm, desferoxamine (DFO), 50 µm. (B) The ROS production shown in (A) presented as a histogram, plus ROS data under the same conditions and time for 1 µm rotenone (Rot), 1 µm diphenyliodonium (DPI), 100 µm propyl gallate (PG), 30 mm N-acetyl-l-cysteine (NAC) and 100 µm apocynin (APO), both individually and in combination with Aβ. *P < 0.001 one-way ANOVA vs. Aβ alone; **P < 0.0001, two-tailed P-value, unpaired t-test (n = 3) vs. vehicle alone. (C) Hypoxia-inducible factor (HIF)-1α protein was determined by Western blotting and quantified relative to actin as a function of time in the presence of 1 µm Rot or 1 µm Aβ1–42. *P < 0.05; **P < 0.01; ***P < 0.001 anova vs. 0 time (n = 4). (D) NAC does not inhibit HIF-1α expression. Cells were exposed to vehicle (cont), 30 mm NAC alone (NAC), 1 µm Aβ1–42 (Aβ) or both together (NAC + Aβ) for 4 h, and the HIF-1α amount determined by Western blotting. *P < 0.001, unpaired t-test (n = 4). (E) Glial activation was determined at 8 h by morphological criteria or by NO release. Rot, 1 µm, 100 µm H2O2, 100 µm PG, 30 mm NAC, 1 µm Aβ1–42, 1 µm DPI, 100 µm APO or 30 nm lactacystin (LACTO). *P < 0.01, significantly different from controls of vehicle alone in the morphology assay (n = 5) and **P < 0.001 from Aβ-induced NO release (n = 3) using ANOVA and Tukey’s post hoc test.
Aβ inhibits, then activates proteasome activity
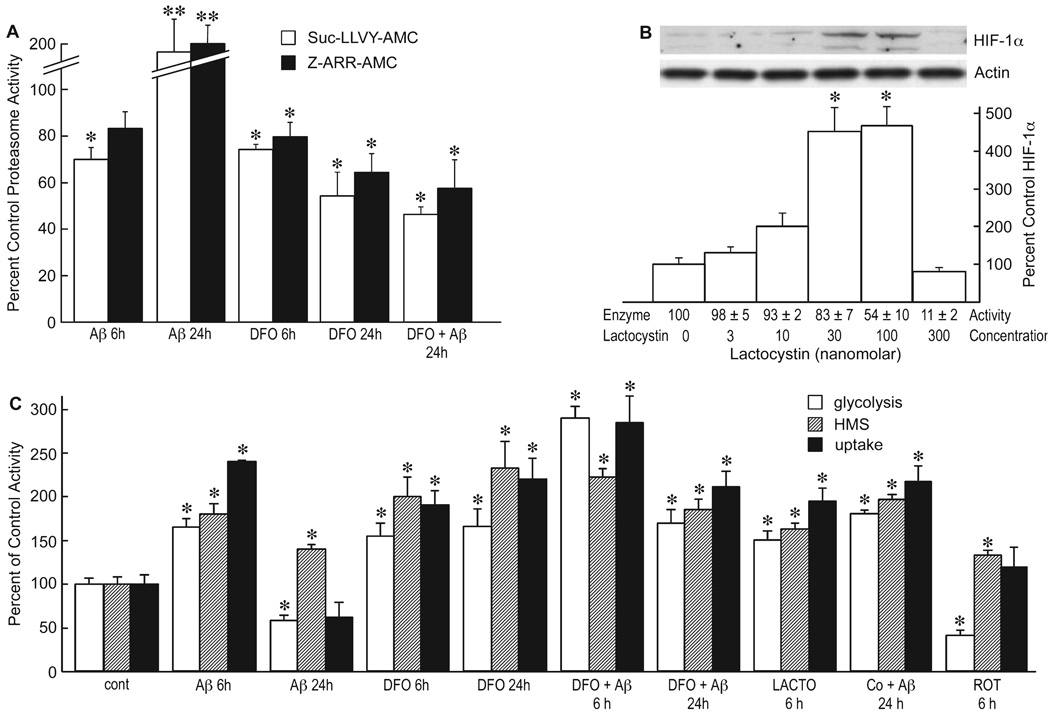
There are multiple pathways that lead to the induction of HIF-1α activity (Hewitson et al., 2007). With the apparent elimination of ROS as an inducer, an alternative is the inhibition of proteasome activity. The stability of HIF-1α is mediated by its breakdown by the proteasome, and inhibitors of proteasome activity should increase the stable expression of HIF-1α and therefore its activity, and the activation of proteasomes reduce HIF-1α. Because Aβ can inactivate proteasomes (Gregori et al., 1995; Lopez Salon et al., 2003), we asked if Aβ1–42 is able to alter proteasome activity in astrocytes. Figure 5A shows that the short-term treatment (6 h) of cells with Aβ1–42 inhibited both the chymotryptic (Suc-LLVY-AMC substrate) and tryptic (Z-ARR-AMC substrate) proteasome activities by between 20 and 30% (F7,16 = 16.9, P < 0.0001). DFO also inhibited these activities. In contrast to 6 h, a 24-h treatment with Aβ1–42 increased proteasome activity about twofold, while treatment with DFO and Aβ1–42 together reduced proteasome activity to levels slightly below those observed after 6 h. The data are consistent with those showing that Aβ1–42 causes a transient increase in HIF-1α expression during the first 6 h of exposure, followed by a net decrease at 24 h (Fig. 2A). The decrease in HIF-1α and the increase in proteasome activity after 24 h are both inhibited by DFO, and this inhibition is maintained as long as the cells are viable (data not shown).
FIG.5.
Amyloid beta peptide (Aβ) inhibits proteasome enzyme activity. (A) Glial cells were treated with 1 µm Aβ1–42 for 6 or 24 h in the presence or absence of 50 µm desferoxamine (DFO) and the proteasome activity of the cells assayed using Suc-LLVY-AMC (chymotrypsin substrate) or Z-ARR-AMC (trypsin substrate). The data are presented as the percent control (vehicle alone) activity *P < 0.05; **P < 0.001 ANOVA (n = 4). (B) Cells were exposed to increasing concentrations of lactacystin, and the expression of hypoxia-inducible factor (HIF)-1α was monitored by Western blotting and quantified as percent control. Proteasome activity (enzyme activity) was monitored using Z-ARR-AMC and the activity given at the bottom of the figure relative to untreated control *P < 0.001 vs. control, one-way ANOVA (n = 4). (C) Cells were treated with 1 µm Aβ1–42, 50 µm DFO, 30 nM lactacystin (LACTO), 100 µm cobalt, 100 µm PG or 1 µm rotenone (Rot) for the indicated times, and the rate of glycolysis (open bar), hexose monophosphate shunt (HMS) activity (hatched bar) or glucose uptake (solid bar) monitored. *P < 0.05 relative to control values at 6 or 24 h for each assay. One-way ANOVA (n = 3) and Tukey’s post hoc test.
To determine if proteasome inhibition is responsible for the transient increase in HIF-1α expression and glycolytic changes following Aβ exposure, it was asked if proteasome inhibitors increase HIF-1α expression and glycolysis, and inhibit short-term glial activation (they are toxic after 12–15 h). We initially observed that when cells were treated with 5 or 10 µm lactacystin, concentrations that are frequently used to inhibit proteasome activity (Kallio et al., 1999; Kaluz et al., 2006), there is a loss of HIF-1α expression. However, because Aβ1–42 inhibits proteasome activity only by 20–30%, we titrated lactacystin to lower concentrations, assaying both HIF-1α and proteasome activity at 4 h. Figure 5B shows HIF-1α expression and proteasome activity as a function of lactacystin concentration. Lactacystin inhibited proteasome activity and HIF-1α expression at concentrations above 300 nM, but induced HIF-1α expression at concentrations where there was a 20–50% inhibition of proteasome activity similar to that caused by Aβ1–42 (F2,6 = 126, P < 0.0001). These concentrations also inhibited the NO release indicative of activated cells, as did the NADPH oxidase inhibitors APO and DPI (F3,12 = 494, P < 0.0011; Fig. 4E). Is the Aβ1–42-induced, proteasome-dependent variation in HIF-1α expression also directly responsible for the observed changes in glucose metabolism?
To determine if the changes in HIF-1α expression correlate with changes in glucose metabolism, we used the three assays described in Fig. 1 that reflect different aspects of this activity. These assays include glucose uptake monitored by 14C-deoxyglucose, the HMS activity, and the overall glycolytic rate monitored by the release of CO2 from 14C-1 and 14C-6-glucose. Data were collected at 6 and 24 h in the presence of Aβ1–42 plus or minus DFO, and at 6 h with the proteasome inhibitor lactacystin or the pro-oxidant Rot. Figure 5C shows that all three indicators of glycolysis were upregulated at 6 h by conditions that increase HIF-1α expression, while there was a net decrease in glycolysis and glucose uptake at 24 h except in the presence of DFO (F9,20 = 234, P < 0.0001 for glycolysis; F9,20 = 100, P < 0.0001 for HMS; F9,20 = 248, P < 0.0001 for uptake). In agreement with the HIF-1α data, lactacystin at 30 nm also increased glycolysis while Rot inhibited glycolysis. As with DFO, cobalt also maintained glycolysis in the presence of Aβ after 24 h. It can be concluded that Aβ initially inhibits proteasome activity, allowing HIF-1α expression, followed by an activation of the proteasome and a reduction of HIF-1α expression at 24 h. DFO maintains both lower proteasome activity and HIF-1α expression in the presence of Aβ.
Discussion
The above data show that Aβ1–42 activates astrocytes and reduces the rate of glycolysis following long-term exposure, and that the pathological changes caused by Aβ are reversed by the stable induction of HIF-1α. In order to put these observations in context, we will briefly review what is known about glial activation and glial energy metabolism, followed by a discussion of how Aβ may mediate the various physiological responses of astrocytes. Finally, we will describe a mechanism for preventing the negative aspects of the Aβ-induced glial activation by the induction of HIF-1. A summary of the data is shown in Fig. 6.
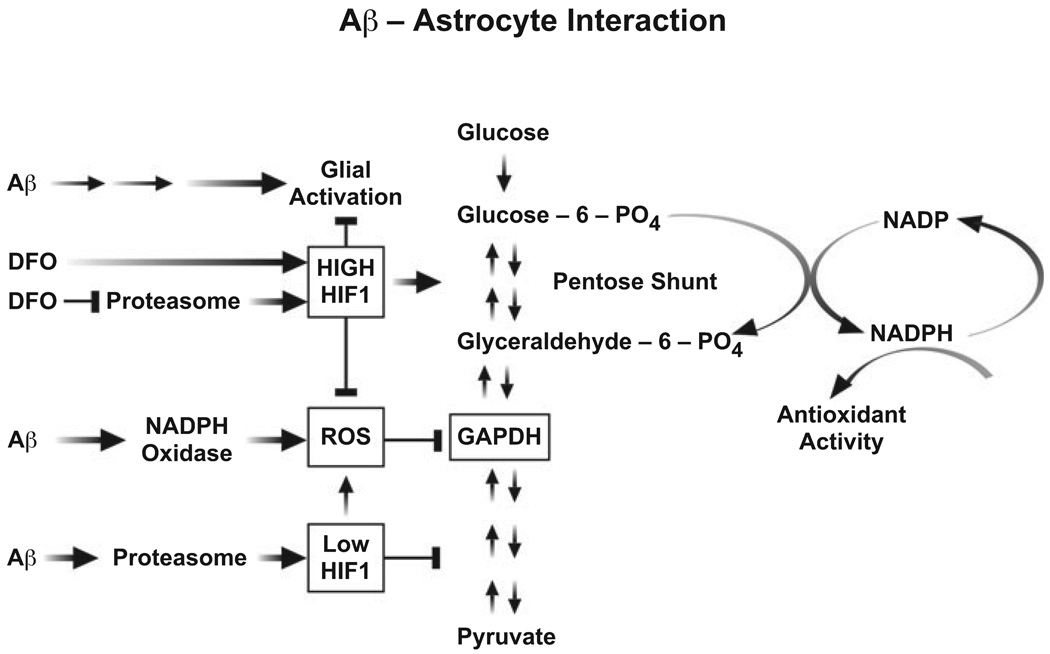
FIG.6.
Schematic of amyloid beta peptide (Aβ) interaction with astrocytes. Aβ activates astrocytes by an unknown mechanism, and at the same time causes nicotinamide adenine dinucleotide phosphate (NADPH) oxidase to produce reactive oxygen species (ROS). ROS inhibits glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity and glycolysis, causing the reverse flow of intermediates through the HMS, increasing the production of NADPH. Aβ also activates proteasome activity, lowering the amount of hypoxia-inducible factor (HIF)-1 after 24 h and decreasing glycolytic enzyme expression. In the presence of desferoxamine (DFO), HIF-1α is elevated by the ability of DFO to inhibit both pyrolyl hydroxylases and the proteasome, thus reducing glial activation and enhancing the pentose shunt, producing more NADPH to limit ROS accumulation. Large arrow activates; Bar inhibits.
Astrocytes play major roles in both normal and diseased brain (Dienel & Cruz, 2006; Wang et al., 2006). Under conditions of stress, trauma or disease, astrocytes can become activated, releasing a variety of toxic agents, including superoxide, NO and cytokines, and undergo changes in morphology to a more filamentous form (reactive gliosis; Wang et al., 2006). These responses are observed in AD brain (Maragakis & Rothstein, 2006) as well as in cultured astrocytes in the presence of Aβ (Gitter et al., 1995; Akama et al., 1998; Prat et al., 2000). In agreement with these data, Fig. 1 shows that Aβ1–42 induces both the morphological changes associated with glial activation and the release of NO. In addition, Aβ1–42 causes an increase in ROS accumulation (Fig. 4A and B). This pool of ROS is blocked by two inhibitors of NADPH oxidase, confirming published data that Aβ1–42 activates this enzyme in astrocytes (Abramov et al., 2004). Associated with glial activation and ROS production is a reduced rate of glycolysis and an elevated rate of glucose flux through the HMS (Fig. 1D). Conversely to astrocytes, in cortical neurons Aβ induces a transient increase in ROS and the sustained expression of HIF-1α, which in turn elevates both HMS and glycolytic activity. The exposure of astrocytes to Aβ leads to sustained ROS production, and an initial short-term increase in HIF-1α expression, followed by a decline after 24 h. What accounts for these differences in ROS and HIF-1α expression?
Although Aβ activates NADPH oxidase in both nerves and glia, it is likely that the production of ROS is greater in glia because of the ability of Aβ to sustain NADPH activation due to differences in membrane composition or signaling pathways. The transient increase in HIF-1α following Aβ is likely a protective response that fails in astrocytes and rapidly returns to baseline in the absence of much more potent HIF-1α inducers such as DFO or cobalt. In nerve cells HIF-1α levels and this protective response are maintained. There are multiple pathways that activate HIF-1α (Koh et al., 2008) and also a large number of signaling pathways that are altered by Aβ (Cappai & Barnham, 2008). This innate complexity makes the temporal interaction between the two pathways following Aβ exposure exceedingly difficult to define, but it is possible to rule out one potential mechanism of HIF-1α activation.
ROS per se is not the mechanism of transient HIF-1α induction in astrocytes because neither mitochondrial-derived ROS nor H2O2 increase HIF-1α, nor do antioxidants block HIF-1α induction (Fig. 4). Although antioxidants block ROS accumulations and inhibit HIF-1α activation in some experimental paradigms (Rayner et al., 2006), in other cell types HIF-1α activation occurs by a ROS-independent mechanism (Salnikow et al., 2000; Griguer et al., 2006). However, high ROS levels inhibit glycolysis, for Rot-induced mitochondrial-derived ROS are sufficient to inhibit glycolysis by 6 h (Fig. 5C), and Aβ-generated ROS inhibits glycolysis by 24 h (Fig 1D and Fig 5C). This inhibition is likely due to the inhibition of GAPDH, a glycolytic enzyme that is exceptionally sensitive to oxidative damage (Hyslop et al., 1988; Cumming & Schubert, 2005). In astrocytes, Aβ decreases GAPDH activity by 36 ± 7% (t6 = 7.2, P < 0.0005, n = 3) at 24 h. There is also a decrease in GAPDH activity in old AD transgenic mouse brains that have high concentrations of soluble Aβ (Soucek et al., 2003; Shalova et al., 2007), and an increase in HMS activity (Soucek et al., 2003). The long-term inhibition of glycolysis by the inhibition of GAPDH may also explain the sustained increase in HMS activity because of a backflow of upstream glycolytic intermediates through the HMS pathway (Hyslop et al., 1988).
There is, however, an apparent contradiction between the data in this manuscript demonstrating a net loss of glycolytic activity in astrocytes exposed to Aβ and our previous study showing that the amount and activity of some glycolytic enzymes, like GAPDH, in human AD brain are elevated relative to age-matched controls (Soucek et al., 2003). It would be expected that most GAPDH in AD brain is in astrocytes and, as argued here, that the reduction of astrocyte glycolysis caused by Aβ accounts for the reduced energy metabolism in AD brain as assayed by imaging techniques. There is at least one resolution to this conundrum. Because GAPDH is readily oxidized (Cumming & Schubert, 2005), perhaps it is extensively upregulated in AD brain as a compensatory mechanism, but other enzymes or substrates in the glycolytic pathway are rate-limiting in vivo, resulting in the observed decrease in glucose metabolism. AD develops over many years, and it is likely that both the composition and metabolism of the surviving cell population changes over time. The astrocyte data presented here predict that the changes in energy metabolism detected in mild cognitive impairment (MCI) patients may be primarily due to a reduction in astrocyte glycolysis, and as the disease progresses there may be additional contributing factors to the reduction in energy metabolism, such as nerve cell death and vascular damage (Perneczky et al., 2007). Our previous studies used only terminal AD brain, for MCI material is essentially impossible to obtain. Moreover, data from AD transgenic mice, which may represent an early stage of human AD, show that there is a reduction in GAPDH activity consistent with the astrocyte data presented here (Soucek et al., 2003; Shalova et al., 2007).
Astrocyte activation, defined in terms of the morphological change, and the synthesis and release of cytokines and NO, is an exceptionally complex process and poorly understood. It is mediated by a very large number of receptors and cell signaling pathways for various ligands, from viruses and lipids to growth factors (for reviews, see Raivich et al., 1999; Pekny & Nilsson, 2005). These include all forms of bioactive amyloid, including prion proteins (Liu & Piasecki, 2001). Changes in both ROS and calcium levels in response to Aβ are well documented in astrocytes (Abramov et al., 2004). Using cells in which the gene for HIF-1α is deleted, we show that HIF-1α expression is not necessary for glial activation, but it is necessary for the inhibition of astrocyte activation by the iron chelator and HIF-1α inducer DFO (Fig. 3). Cells lacking the HIF-1 gene are not rescued by DFO. While high intracellular ROS levels per se do not seem to be required for glial activation, it is possible that the combination of HIF-1 induction with low levels of ROS initiates one or more signaling pathways that block the morphological and gene expression patterns associated with astrocyte activation.
Aβ increases ROS in both nerves and glia, while HIF-1α is transiently induced followed by a permanent reduction of activity after 24 h Aβ exposure in astrocytes. In some cell types, ROS is thought to increase HIF-1 activity (Rayner et al., 2006; Block et al., 2007). However, our data show that ROS is an unlikely activator of Aβ-induced HIF-1α because other ROS-generating conditions do not induce HIF-1α, nor does the antioxidant NAC, which inhibits HIF-1α induction in some cells (Shatrov et al., 2003; Callapina et al., 2005), block it in astrocytes (Fig. 4). Instead, Aβ1–42 inhibits proteasome activity in astrocytes 6 h after exposure at a time when HIF-1α is activated, but enhances proteasome activity by 24 h (Fig. 5). These data are consistent with studies showing that Aβ can activate proteasomes after longer term exposure (Favit et al., 2000), but can also inhibit the proteasome at shorter times or different experimental conditions (Gregori et al., 1995; Chin et al., 2007). Concentrations of the proteasome inhibitor lactacystin that completely inhibit proteasome activity in astrocytes block HIF-1α activation in other cell types (Kallio et al., 1999; Kaluz et al., 2006). However, lactacystin is able to induce HIF-1α stabilization at concentrations that reproduce the limited proteasome inhibition seen with Aβ (Fig. 5B). Previous studies showing that proteasome inhibition decreases HIF-1α activity used 10–20-fold higher concentrations of lactacystin than were necessary to induce HIF-1α, suggesting that the drug may have different effects when used at high concentrations.
In summary, Fig. 6 outlines the probable events following the interaction of Aβ with astrocytes and the ability of inducers of HIF-1α to limit the process. Aβ activates NADPH-oxidase to produce superoxide. NADPH-oxidase can be activated by many amphiphilic peptides, and Aβ is a prototypical amphiphilic peptide (Schubert et al., 1995). ROS from NADPH oxidase then inhibits GAPDH and perhaps other glycolytic enzymes, leading to a reduction in glycolysis. Aβ also inhibits (2–6 h) then activates (24 h) proteasome activity, leading to an increase then a decrease in HIF-1α stability. How Aβ modulates proteasome activity is not known, but it is likely due to internalized Aβ that is either aggregated or broken down to more toxic fragments (Wyss-Coray et al., 2003). Because HIF-1 regulates glycolytic enzymes at the transcriptional level, the suppression of HIF-1 also additionally reduces glycolysis. Because astrocytes expend a great deal of energy in maintaining glutamate uptake systems and reduced glutathione levels (for review, see Schubert, 2005), the consequence of reduced amounts of ATP from glucose flux through respiration would be increased extracellular glutamate leading to glutamate toxicity and a more oxidative environment in the AD brain. However, in the presence of potent inducers of HIF-1α, such as the metal chelator DFO, HIF-1α activity is stabilized. DFO may function both by inhibiting pyrolyl-4-hydroxylases and by reducing proteasome activity (Fig. 5A). DFO is able to reduce ROS production and the inhibition of glycolysis by Aβ by enhancing the expression of genes encoding proteins in the glycolytic and HMS pathways and therefore providing a more reductive environment. This mechanism of cellular protection is consistent with the repeated observation that metal chelators prevent amyloid toxicity both in cultured cells (Schubert & Chevion, 1995) and in transgenic mouse models of AD (Cherny et al., 2001). It also explains the observation that energy metabolism is reduced in the areas of AD brain that are continually exposed to high levels of Aβ.
Acknowledgements
This work was supported by the Alzheimer’s Association, the Donald and Darleen Shiley Trust for Alzheimer’s Research, and the Bundy Foundation. The authors declare that they have no financial, personal or professional interests that could be construed to have influenced this paper.
Abbreviations
- AD
Alzheimer’s disease
- APO
apocynin
- Aβ
amyloid beta peptide
- AβS
scrambled Aβ
- DCF
2′,7′-dichlorodihydrofluorescein diacetate
- DFO
desferoxamine
- DPI
diphenyliodonium
- EMSA
electrophoretic mobility shift assay
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HIF
hypoxia-inducible factor
- HMS
hexose monophosphate shunt
- MCI
mild cognitive impairment
- NAC
N-acetyl-l-cysteine
- NADP(H)
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- PG
propyl gallate
- ROS
reactive oxygen species
- Rot
rotenone
- TR
transferrin receptor
References
- Abramov AY, Canevari L, Duchen MR. Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim. Biophys. Acta. 2004;1742:81–87. doi: 10.1016/j.bbamcr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J. Biol. Chem. 2000;275:7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- Akama KT, Albanese C, Pestell RG, Van Eldik LJ. Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc. Natl Acad. Sci. USA. 1998;95:5795–5800. doi: 10.1073/pnas.95.10.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft SJH, Weerasinghe LCC, Bassett JM, Randle PJ. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J. 1972;126:525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasolla K, Khan M, Singh AK, Singh I. Inflammatory mediator and beta-amyloid (25–35)-induced ceramide generation and iNOS expression are inhibited by vitamin E. Free Radic. Biol. Med. 2004;37:325–338. doi: 10.1016/j.freeradbiomed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Bajenaru ML, Zhu Y, Hedrick NM, Donahoe J, Parada LF, Gutmann DH. Astrocyte-specific inactivation of the neurofibromatosis 1 gene (NF1) is insufficient for astrocytoma formation. Mol. Cell. Biol. 2002;22:5100–5113. doi: 10.1128/MCB.22.14.5100-5113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behl C, Schubert D. Heat shock partially protects rat pheochromocytoma PC12 cells from amyloid beta peptide toxicity. Neurosci. Lett. 1993;154:1–4. doi: 10.1016/0304-3940(93)90156-f. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77:817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Tacchini L, Cairo G. HIF-1-mediated activation of transferrin receptor gene transcription by iron chelation. Nucleic Acids Res. 1999;27:4223–4227. doi: 10.1093/nar/27.21.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA, Yoneda T, Abboud HE. NAD(P)H oxidases regulate HIF-2alpha protein expression. J. Biol. Chem. 2007;282:8019–8026. doi: 10.1074/jbc.M611569200. [DOI] [PubMed] [Google Scholar]
- Callapina M, Zhou J, Schmid T, Kohl R, Brune B. NO restores HIF-1alpha hydroxylation during hypoxia: role of reactive oxygen species. Free Radic. Biol. Med. 2005;39:925–936. doi: 10.1016/j.freeradbiomed.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Cappai R, Barnham KJ. Delineating the mechanism of Alzheimer’s disease A beta peptide neurotoxicity. Neurochem. Res. 2008;33:526–532. doi: 10.1007/s11064-007-9469-8. [DOI] [PubMed] [Google Scholar]
- Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:641–642. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, Zhang XC, Gallo D, Cszimadia E, Bach FH, Lee PJ, Otterbein LE. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc. Natl Acad. Sci. USA. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming RC, Schubert D. Amyloid-beta induces disulfide bonding and aggregation of GAPDH in Alzheimer’s disease. FASEB J. 2005;19:2060–2062. doi: 10.1096/fj.05-4195fje. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Astrocyte activation in working brain: energy supplied by minor substrates. Neurochem. Int. 2006;48:586–595. doi: 10.1016/j.neuint.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Favit A, Grimaldi M, Alkon DL. Prevention of beta-amyloid neurotoxicity by blockade of the ubiquitin-proteasome proteolytic pathway. J. Neurochem. 2000;75:1258–1263. doi: 10.1046/j.1471-4159.2000.0751258.x. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. FASEB J. 2003;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Gitter BD, Cox LM, Rydel RE, May PC. Amyloid beta peptide potentiates cytokine secretion by interleukin-1 beta-activated human astrocytoma cells. Proc. Natl Acad. Sci. USA. 1995;92:10738–10741. doi: 10.1073/pnas.92.23.10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregori L, Fuchs C, Figueiredo-Pereira ME, Van Nostrand WE, Goldgaber D. Amyloid beta-protein inhibits ubiquitin-dependent protein degradation in vitro. J. Biol. Chem. 1995;270:19702–19708. doi: 10.1074/jbc.270.34.19702. [DOI] [PubMed] [Google Scholar]
- Griguer CE, Oliva CR, Kelley EE, Giles GI, Lancaster JR, Jr, Gillespie GY. Xanthine oxidase-dependent regulation of hypoxia-inducible factor in cancer cells. Cancer Res. 2006;66:2257–2263. doi: 10.1158/0008-5472.CAN-05-3364. [DOI] [PubMed] [Google Scholar]
- Hewitson KS, Lienard BM, McDonough MA, Clifton IJ, Butler D, Soares AS, Oldham NJ, McNeill LA, Schofield CJ. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J. Biol. Chem. 2007;282:3293–3301. doi: 10.1074/jbc.M608337200. [DOI] [PubMed] [Google Scholar]
- Hu J, Castets F, Guevara JL, Van Eldik LJ. S100 beta stimulates inducible nitric oxide synthase activity and mRNA levels in rat cortical astrocytes. J. Biol. Chem. 1996;271:2543–2547. doi: 10.1074/jbc.271.5.2543. [DOI] [PubMed] [Google Scholar]
- Hu J, Akama K, Krafft G, Chromy B, Eldik L. Amyloid-beta peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release. Brain Res. 1998;785:195–206. doi: 10.1016/s0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- Hyslop PA, Hinshaw DB, Halsey WA, Jr, Schraufstatter IU, Sauerhebet RD, Spragg RG, Jackson JH, Cochrane CG. Mechanisms of oxidant-mediated cell injury. J. Biol. Chem. 1988;263:1665–1675. [PubMed] [Google Scholar]
- Kallio PJ, Wilson WJ, O’Brien S, Makino Y, Poellinger L. Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin-proteasome pathway. J. Biol. Chem. 1999;274:6519–6525. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- Kaluz S, Kaluzova M, Stanbridge EJ. Proteasomal inhibition attenuates transcriptional activity of hypoxia-inducible factor 1 (HIF-1) via specific effect on the HIF-1alpha C-terminal activation domain. Mol. Cell. Biol. 2006;26:5895–5907. doi: 10.1128/MCB.00552-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MY, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem. Sci. 2008;33:526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- de Leon MJ, Mosconi L, Blennow K, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Tsui W, Saint Louis LA, Sobanska L, Brys M, Li Y, Rich K, Rinne J, Rusinek H. Imaging and CSF studies in the preclinical diagnosis of Alzheimer’s disease. Ann. NY Acad. Sci. 2007;1097:114–145. doi: 10.1196/annals.1379.012. [DOI] [PubMed] [Google Scholar]
- Lesley JF, Schulte RJ. Selection of cell lines resistant to anti-transferrin receptor antibody: evidence for a mutation in transferrin receptor. Mol. Cell. Biol. 1984;4:1675–1681. doi: 10.1128/mcb.4.9.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian XY, Stringer JL. Energy failure in astrocytes increases the vulnerability of neurons to spreading depression. Eur. J. Neurosci. 2004;19:2446–2454. doi: 10.1111/j.0953-816X.2004.03289.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Piasecki D. A cell-based method for the detection of nanomolar concentrations of bioactive amyloid. Anal. Biochem. 2001;289:130–136. doi: 10.1006/abio.2000.4928. [DOI] [PubMed] [Google Scholar]
- Lopez Salon M, Pasquini L, Besio Moreno M, Pasquini JM, Soto E. Relationship between beta-amyloid degradation and the 26S proteasome. Exp. Neurol. 2003;180:131–143. doi: 10.1016/s0014-4886(02)00060-2. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat. Clin. Pract. Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:203–204. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Parpura-Gill A, Beitz D, Uemura E. The inhibitory effects of beta-amyloid on glutamate and glucose uptakes by cultured astrocytes. Brain Res. 1997;754:65–71. doi: 10.1016/s0006-8993(97)00043-7. [DOI] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Perneczky R, Hartmann J, Grimmer T, Drzezga A, Kurz A. Cerebral metabolic correlates of the clinical dementia rating scale in mild cognitive impairment. J. Geriatr. Psychiatry Neurol. 2007;20:84–88. doi: 10.1177/0891988706297093. [DOI] [PubMed] [Google Scholar]
- Prat E, Baron P, Meda L, Scarpini E, Galimberti D, Ardolino G, Catania A, Scarlato G. The human astrocytoma cell line U373MG produces monocyte chemotactic protein (MCP)-1 upon stimulation with beta-amyloid protein. Neurosci. Lett. 2000;283:177–180. doi: 10.1016/s0304-3940(00)00966-6. [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res. Rev. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- Rayner BS, Duong TT, Myers SJ, Witting PK. Protective effect of a synthetic anti-oxidant on neuronal cell apoptosis resulting from experimental hypoxia re-oxygenation injury. J. Neurochem. 2006;97:211–221. doi: 10.1111/j.1471-4159.2006.03726.x. [DOI] [PubMed] [Google Scholar]
- Ryan HE, Poloni M, McNulty W, Elson D, Gassmann M, Arbeit JM, Johnson RS. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- Salnikow K, Su W, Blagosklonny MV, Costa M. Carcinogenic metals induce hypoxia-inducible factor-stimulated transcription by reactive oxygen species-independent mechanism. Cancer Res. 2000;60:3375–3378. [PubMed] [Google Scholar]
- Schubert D. Glucose metabolism and Alzheimer’s disease. Ageing Res. Rev. 2005;4:240–257. doi: 10.1016/j.arr.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Schubert D, Chevion M. The role of iron in beta amyloid toxicity. Biochem. Biophys. Res. Commun. 1995;216:702–707. doi: 10.1006/bbrc.1995.2678. [DOI] [PubMed] [Google Scholar]
- Schubert D, Behl C, Lesley R, Brack A, Dargusch R, Sagara Y, Kimura H. Amyloid peptides are toxic via a common oxidative mechanism. Proc. Natl Acad. Sci. USA. 1995;92:1989–1993. doi: 10.1073/pnas.92.6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Ann. Rev. Cell. Dev. Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- Shalova IN, Cechalova K, Rehakova Z, Dimitrova P, Ognibene E, Caprioli A, Schmalhausen EV, Muronetz VI, Saso L. Decrease of dehydrogenase activity of cerebral glyceraldehyde-3-phosphate dehydrogenase in different animal models of Alzheimer’s disease. Biochim. Biophys. Acta. 2007;1770:826–832. doi: 10.1016/j.bbagen.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Shatrov VA, Sumbayev VV, Zhou J, Brune B. Oxidized low-density lipoprotein (oxLDL) triggers hypoxia-inducible factor-1alpha (HIF-1alpha) accumulation via redox-dependent mechanisms. Blood. 2003;101:4847–4849. doi: 10.1182/blood-2002-09-2711. [DOI] [PubMed] [Google Scholar]
- Soucek T, Cumming R, Dargusch R, Maher P, Schubert D. The regulation of glucose metabolism by HIF-1 mediates a neuroprotective response to amyloid beta peptide. Neuron. 2003;39:43–56. doi: 10.1016/s0896-6273(03)00367-2. [DOI] [PubMed] [Google Scholar]
- Steinbach JH, Schubert D. Multiple modes of dibutyryl cyclic AMP-induced process formation by clonal nerve and glial cells. Exp. Cell Res. 1975;91:449–453. doi: 10.1016/0014-4827(75)90126-3. [DOI] [PubMed] [Google Scholar]
- Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J. Cell. Biol. 1998;141:1423–1432. doi: 10.1083/jcb.141.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl Acad. Sci. USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Wen LL, Huang YN, Chen YT, Ku MC. Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr. Pharm. Des. 2006;12:3521–3533. doi: 10.2174/138161206778343109. [DOI] [PubMed] [Google Scholar]
- Worthington C. Worthington Enzyme Manual. Frehold, NJ: Worthington Biochemical Corp.; 1947. [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat. Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Hilliard G, Ferguson T, Millhorn DE. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel-Lindau protein by direct binding to hypoxia-inducible factor-alpha. J. Biol. Chem. 2003;278:15911–15916. doi: 10.1074/jbc.M300463200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yu J, Stanton RC. A method for determination of pyridine nucleotides using a single extract. Anal. Biochem. 2000;285:163–167. doi: 10.1006/abio.2000.4701. [DOI] [PubMed] [Google Scholar]