Abstract
OBJECTIVE
Ischemia-reperfusion injury (IRI) continues to plague the field of lung transplantation resulting in suboptimal outcomes. In acute lung injury processes such as ventilator-induced injury, sepsis, or acute respiratory distress syndrome, extravascular fibrin has been shown to promote lung dysfunction and the acute inflammatory response. This study investigates the role of the fibrinolytic cascade in lung IRI and investigates the interplay between the fibrinolytic system and the inflammatory response.
METHODS
Mice lacking the plasminogen activator inhibitor-1 gene (PAI-1 knock out, PAI-1 KO; and thus increased lysis of endogenous fibrin) and wild-type mice underwent in-situ left lung ischemia and reperfusion. Fibrin content in the lung was evaluated by immunoblotting. Reperfusion injury was assessed by histology and physiologic parameters. Proinflammatory mediators were measured in bronchoalveolar lavage fluid and plasma using enzyme-linked immunosorbent assays.
RESULTS
Ischemia-reperfusion causes fibrin deposition in murine lungs. Less fibrin was seen in PAI-1 KO mice compared to wild-type mice subjected to the same ischemia-reperfusion conditions. By histologic criteria, more evidence of IRI was noted (thickening of the interstium, cellular infiltration in the alveoli) in the wild type than in PAI-1 KO mice. Physiologic parameters also revealed more IRI in the wild-type compared to PAI-1 KO mice. Cytokine and chemokines were elevated more in the wild-type group than the PAI-1 KO group.
CONCLUSIONS
Lung IRI triggers fibrin deposition in the murine lungs and fibrin creates a proinflammatory environment. Preventing fibrin deposition may reduce IRI and inflammation. This finding may lead to novel treatment strategies for ischemia-reperfusion.
Keywords: ischemia-reperfusion injury, lung transplantation, lung injury
INTRODUCTION
Lung transplantation currently is the preferred treatment option for a variety of end-stage pulmonary diseases. Remarkable progress has occurred through refinement in technique and improved understanding of transplant immunology and microbiology. Despite improvements in preservation, ischemia-reperfusion injury (IRI) continues to result in unpredictable sub-optimal outcomes in lung transplant recipients. The incidence of IRI ranges from 10-25% in most series (1, 2) and there is mounting evidence that IRI is associated with an increased risk of bronchiolitis obliterans development (3, 4).
IRI is a form of acute lung injury. It is well documented that increased coagulation and impaired fibrinolysis play an important role in the pathogenesis of the various forms of acute lung injury (5). Extravascular fibrin deposition has been shown to promote lung dysfunction, and furthermore intravascular thrombosis occurs in the acutely injured lung(6). Depression of fibrinolysis has been shown to increase detection of fibrin and these results mostly through amplification of plasminogen activator inhibitor-1 (PAI-1), which is a 52-kDa serine protease inhibitor that serves as the major plasma inhibitor of tissue-type plasminogen activator and urokinase-type plasminogen activator. Hypoxia has been shown also to increase PAI-1 and promote pulmonary vascular fibrin deposition(7). Figure 1 details the fibrinoltyic/PAI-1 pathway.
Figure 1. The fibrinolytic system.
This system is strictly regulated by plasminogen activators, plasminogen activator receptor, plasminogen activator inhibitors and α2-antiplasmin.
An important area of recent interest has been the interplay between coagulation and inflammation in acute lung injury and much has been written on this topic in acute lung injury from adult respiratory distress syndrome (ARDS), sepsis, and ventilator-induced lung injury (5). One concept that has emerged over the past decade is that coagulation and inflammation are linked and should not be considered different distinct pathways(5). Anticoagulants reduce inflammation and result in improved outcomes in animals models of sepsis and acute lung injury (5). Tissue fibrin is known to influence local alveolar inflammation in a variety of ways (6). Therefore, fibrinolysis may be useful for preventing inflammation during lung IRI.
This paper examines the role of changes in the fibrinolytic pathway in IRI by using PAI-1 KO mice in our mouse ischemia-reperfusion model. We try to investigate the association between fibrin deposition and the inflammatory response in our experimental model. We hypothesize that enhancing fibrin turnover will decrease inflammation and protect lung from in our IRI mouse model.
METHODS
Animal care
C57BL6 (Wild-Type; WT) and plasminogen-activator inhibitor 1 knock out (PAI-1 KO; also on a C57BL6 background) male mice (Jackson Laboratory, Bar Harbor, ME) (28-35 grams) were used for all studies and received humane care in accordance with “Principles of Laboratory Animal Care,” formulated by the National Society for Medical Research, and The Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Science and published by the National Institutes of Health. The study protocol was reviewed and approved by the Animal Care and Use Committee at the University of Virginia before experimentation.
Experimental Protocol of Ischemia-Reperfusion (IR)
Three experimental groups (wild type sham, wild-type IR, and PAI-1 KO IR) were compared using an in-vivo left hilar clamp model. Wild-type IR (WT- IR) and PAI-1 KO IR groups underwent 1-hour of left hilar clamping followed by two hours of reperfusion at room temperature. The wild type sham (WT-sham) group underwent chest opening and closure but no hilar clamping. A minimum of six mouse lungs were studied per group (6 mice with 1 lung each). In this model of IR lung injury, mice are anesthetized with inhalational isoflurane, intubated and connected to a volume-controlled ventilator (Harvard Apparatus Co, South Natick, MA) and ventilated with room air. Heparin (30U/kg) is given via right external jugular vein injection. The left hilum is approached via a left thoracotomy. The left 4th rib is cut with a cautery pen at about 2 mm lateral to the sternum. A 6-0 prolene suture is placed around the left hilum and both ends are threaded out through a piece of PE-10 tubing. The left hilum is occluded by pushing down the PE-tubing while a pulling tension on the suture. A small surgical clip is placed around the suture to keep the PE tubing in position. The left lung is rendered ischemic for one hour, reperfusion is then established by removing the PE tubing and the suture. The wound is suture-closed in layers. The mouse is allowed to awaken and recover in the cage. The WT-sham operated controls consist of the same peri-operative protocols as the mice (i.e., sedation, hydration, warming mattress) then performing a left thoracotomy with dissection of the hilum then closing the animals left thoracotomy site.
In Situ Buffer-Perfused Mouse Lung Model (to obtain physiologic parameters)
After two hours of reperfusion, one group of the mice in each set of experiments was re-anesthetized and pulmonary function evaluated by an in situ buffer-perfused mouse lung system. The isolated perfused mouse lung system (Hugo Sachs Elektronik, Germany) is utilized as previously described by our laboratory (8). An advantage of this system is that the lungs are not removed from the chest cavity, but instead the whole animal is placed in the chamber with an open chest. Briefly, the animal is anesthetized (pentobarbital sodium, 160 mg/kg, i.p.) and placed on the heated operating table, and a tracheotomy performed. The skin is incised in a median line from the abdomen to the neck. Positive-pressure ventilation is initiated before the thorax is opened, at 120 breaths/min, and ventilation pressure is controlled so that a tidal volume of approximately 15ml/kg is achieved with a positive end expiratory pressure of 2cm H2O. The abdominal wall is incised and the incision extended laterally on both sides to the lower extremities so that the vessels of the thigh become visible and accessible. The diaphragm is carefully removed and the thorax is opened by median sternotomy. The animals are exsanguinated by a cut through the renal vein 30 seconds after injection of 50 units of heparin. Krebs-Henseleit buffer that contains 2% albumin, 0.1% glucose and 0.3% HEPES is used for perfusing the lungs. The Krebs solution is prepared to mimic right ventricular (mixed venous blood) using a gas bubbling stone with titrated gases generating a pH of 7.40-7.45; pO2 60-70 mmHg; and a pCO2 50-60 mmHg. The buffered perfusate and isolated lung apparatus are maintained at 37°C. Using an operating microscope, the thymus is dissected cephalad exposing the great vessels. The pulmonary artery and left atrium are cannulated, and the perfusate flow is set to a final perfusion rate of 2 ml/min. The left ventricle is vented with a small incision at the apex of the heart. The mitral apparatus was carefully dilated and the left atrial cannula was passed through the mitral valve and into the left atrium. The placement of the pulmonary artery and left atrial cannulae is further confirmed by a pressure tracing generated by the Pulmodyn data acquisition system (Hugo Sachs Elekrtonik, Germany). Lungs are allowed to equilibrate for a 10 min stabilization period. The following parameters are measured and stored using the computer data acquisition software: 1) pulmonary compliance, 2) pulmonary artery pressure, and 3) airway resistance.
Western Blot
For these experiments, each mouse was injected with 20 units of heparin before being sacrificed. Immediately after harvest, the mouse’s left lung is placed in ice-cold homogenizing buffer (20mM Tris, 100 mM NaCl and 2.7 mg/ml heparin) and then homogenized by six, 10-second rounds of homogenization. Tissue extracts are incubated with 0. 32 unit of human plasmin (Sigma, St. Louis, MO) at 37°C for total 8 hours on a rotating shaker. After centrifugation, collected supernatants are transferred to a fresh tube and stored at -80°C freezer for analysis. The solubilized protein is then loaded onto a SDS-polyacrylamide gel for electrophoresis after the concentration of total protein was adjusted. Transferred membranes were blocked with 5 % nonfat skim milk for 2 hours, and incubated with murine monoclonal antibody against fibrin (American Diagnostic, Stamford, CT) for 16 hours at -4°C. After washing three times with Tris Buffered Saline (TBS) with 0.05% Tween 20, the membranes are incubated with peroxidase-conjugated secondary antibody for 2 hours. The membrane is washed three times with TBS and developed using Amersham ECL Western Blotting Detection Reagents.
Histology
Whole-lung tissue specimens were immediately fixed in 10% formalin. Following 24 hours they were embedded in paraffin and then cut and stained with hematoxylin & eosin (H&E) or stained by immunohistochemistry.
Immunohistochemical Staining
The experiments were performed as described previously (9, 10). A rat anti-mouse neutrophils antibody (AbD Serotec, Raleigh, NC) and a rat anti-mouse macrophage antibody (Mac-2, Accurate Chem, Westbury, NY) was used as a primary antibody. Alkaline phosphatase - conjugated anti-rat IgG (Sigma, St Louis MO) was used as secondary antibody. The signals were detected using Fast-Red (Sigma, St Louis MO). Purified normal rat IgG (eBioscience Inc, San Diego, CA) was used as negative controls. The sections were counterstained lightly with hematoxylin for viewing negatively stained cells. The number of neutrophils and macrophages per high power field were assessed via immunohistochemical staining of peripheral lung sections, 6 fields were counted per lung. Three individuals who count the positive stained cells were blinded to the condition being assessed. The average cell number was used for statistic analysis.
Plasma
The mice for blood withdrawing in this experiment were not used for obtaining physiologic parameters using the In Situ Buffer-Perfused Mouse Lung Model. After the animal is sacrificed, the blood is collected by left arterial puncture (approximately 0.80-1 ml per mouse) into tubes containing 50 ul of 169 mM EDTA. Plasma is collected by centrifugation (1500 × g, 5 min) and stored at -80°C for later cytokine analyses.
Bronchoalveolar lavage (BAL) fluid collection
BAL fluid was collected by washing with 2 separate aliquots of 1 ml of Ca2+ - and Mg2+ - free Hanks’ Balanced Salt Solution (HBSS, GibcoBRL, Grand Island, NY) through the trachea. The first wash was centrifuged and the BAL supernatant stored for cytokine and chemokine analysis. The second wash was centrifuged, and the cell pellet from the first wash was pooled with the cell pellet from the second.
Cytokine and Chemokine Analysis
Monocyte chemoattractant protein-1 (MCP-1), tumor necrosis factor- alpha (TNF-α), keratinocyte chemoattractant (KC or CXCL1), and macrophage inflammatory protein-2 (MIP-2) were chosen to be measured. All chemokine and cytokine measurement were performed simultaneously to reduce errors because of inter-assay variation. MCP-1, TNF-α and KC were measured by ELISA using matched antibody pairs (R&D System, Inc., Minneapolis, MN) as previously described(11). For lung homogenates, the left lungs were removed and immediately placed in 3.0 ml ice-cold homogenization buffer (1 tablet of protease inhibitor cocktail (Complete® Roche, Penzberg, Germany) with 0.05% triton X-100 (Sigma) in 50 ml PBS). Three, 10-second rounds of homogenization and sonication were followed by centrifugation (15,000 X for 15 min at 4°C). The supernatants are removed and stored at -80C for later cytokine/chemokine assay. Addtionally total RNA was extracted from the left lung by Trizol according to manufacturer protocols (Invitrogen). Real time PCR/RQ-PCR was carried out using TaqMan probe-based chemistry (Applied Biosystems) according to previous report (12). The primers and probe were designed against GenBank-published sequences in association with Primer Express (Applied Biosystems).
Data analysis
Statistical differences between results were determined using one-way ANOVA analysis of variance followed by Tukey’s Multiple Comparison Test for the comparison of more than two categorical variables or by T-test for two group comparison. Data were represented as the mean ± SE from six separate experiments. P value equal or less than 0.05 was considered to be significant.
RESULTS
We used mice null for PAI-1 (which would potentially decrease the net accrual of fibrin during ischemia-reperfusion) in a series of IR experiments and compared these to wild type IR mice. As can be seen in Figure 2, real-time PCR showed that PAI-1 mRNA was not present in the left lung of PAI-1 KO mice, while abundant PAI-1 mRNA in that of WT C57BL/6 mice (Figure 2A). Western analysis of lung tissue following IRI demonstrated fibrin deposition in the WT mice lungs and less deposition of fibrin in the PAI-1 KO mice (Figure 2B). These studies suggest deletion of PAI-1 enhances fibrinolysis under conditions of ischemia-reperfusion. As these immunoblots were performed on entire lung extractions, we can not determine from these experiments which compartment (intra-alveolar, intravascular, or extravascular) accounted for the decrease in fibrin deposition in PAI-1 KO mice. Additionally it could be a combination of compartments.
Figure 2. Expression of PAI-1 and fibrin in the WT and PAI-1 KO mice.
A). Expression of PAI-1 mRNA in WT and PAI-1 KO left lung as determined by Real-Time PCR. β-actin was used to normalize the PAI-1 mRNA expression. B) Western blot analysis of fibrin expression in the lung from WT-IR and PAI-1 KO IR mice. Fibrin concentration is substantially reduced in PAI-1 KO mice compared to WT.
Histology and immunohistochemistry
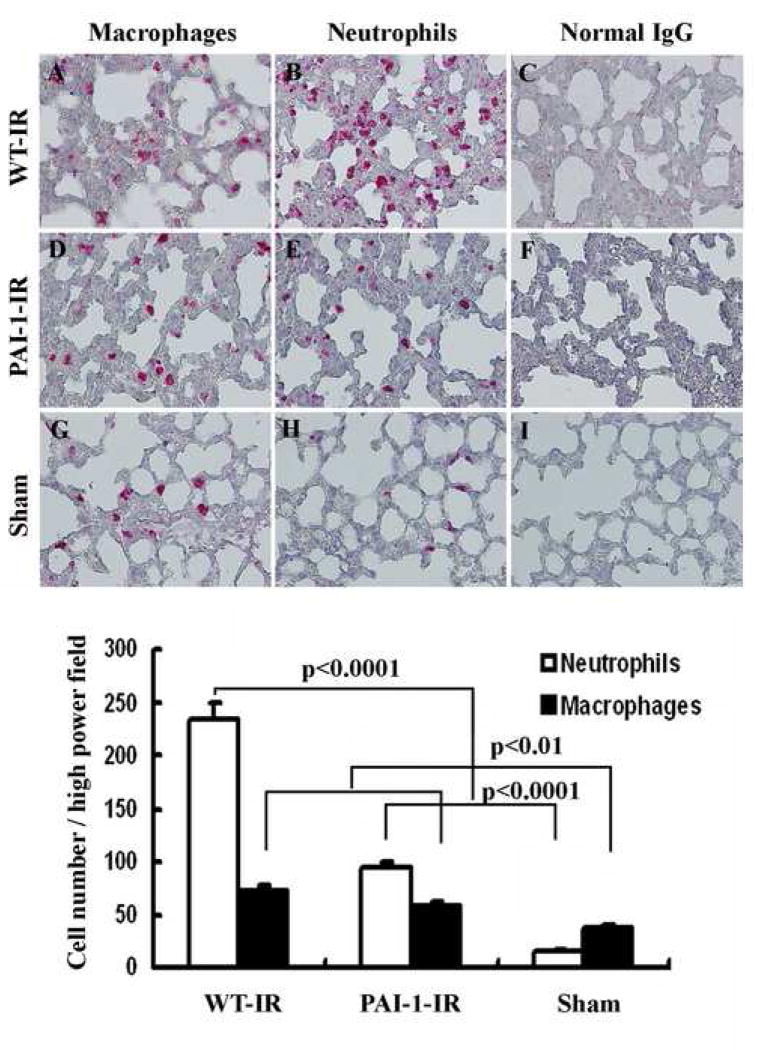
The histological samples of the left lung of WT- sham, WT-IR, and PAI-1 KO IR mice are shown in Figure 3. The interstium of the left lung in WT-IR and PAI-1 KO IR mice appeared thicker with more cellularity and edema than sham mice. The WT-IR mice had the most pronounced IRI. Immunohistochemical staining revealed that more neutrophil and macrophage infiltration was seen in the left lung of WT-IR compared with the PAI-1 KO IR and WT sham mice. These results indicate that plasminogen activator/PAI-1 system may play an important role in recruitment of inflammatory cells during lung transplantation.
Figure 3. Immunohistochemical staining of neutrophils and macrophages in mice lung.
Cells stained red indicate neutrophils or migratory macrophage infiltration. All sections were counterstained lightly with hematoxylin for viewing negatively stained cells. A, D, and G are stained with anti-Mac-2 antibody, B, E, and H are with anti-neutrophils antibody and C, F, and I are stained with normal rat IgG. All figures are 200 times magnifications. The bar graph shows the analysis of positive immunostaining of neutrophils and macrophages in the left lung of sham, WT-IR and PAI-1 KO IR. Data shown are the mean ± S.E. values for each group.
Physiologic data
As shown in Figure 4 there was a significant protection in pulmonary compliance, pulmonary artery pressures, and airway resistance in the PAI-1 KO IR mice compared to the WT-IR. The mean pulmonary compliance in the WT-sham group was 5.587 ± 0.547 μl/cmH2O which was not statistically different from the mean compliance in the PAI-1 KO IR group which was 4.292 ± 0.377 μl/cmH2O (p=0.08). These values were markedly better than the mean compliance, 2.552 ± 0.202 μl/cmH2O in the WT-IR mice lungs (p=0.003). The mean pulmonary artery pressures were markedly higher in the WT-IR mice, 12.53 cmH2O compared to both PAI-1 KO IR (9.23 cm H2O) and WT-sham (5.70 cm H2O) (p<0.0001). In terms of airway resistance the WT-IR was also significant worse, 2.240± 0.287 cm H2O/μl/sec compared to PAI-1 KO IR (1.240± 0.114 cm H2O/μl/sec) and Sham-IR (0.788± 0.038 cm H2O/μl/sec). Unlike pulmonary compliance were there was no significant difference between PAI-1KO IR and Sham IR, the protection compared to WT-IR was only partial with pulmonary artery pressure and airway resistance with PAI-1KO IR have higher pulmonary artery pressures and airway resistance compared to sham-IR (p<0.0001 and p=0.004, respectively). Taken together this data shows the ischemia reperfusion injury in PAI-1 KO IR mice was less when compared to WT-IR mice although there was still evidence of some injury. This data corroborates the histology and immunohistochemisty data.
Figure 4. Pulmonary compliance, pulmonary artery pressures, and airway resistance in the PAI-1 KO IR mice compared to the WT-IR.
These physiologic parameters show less IRI in the PAI-1 KO IR compared to WT-IR. A) Comparison of lung compliance in sham, PAI-1 KO IR and WT-IR mice. B) Comparison of pulmonary artery pressure in sham, PAI-1 KO IR and WT-IR mice. C) Comparison of airway resistance in sham, PAI-1 KO IR and WT-IR mice. Data shown are the mean ± S.E. values for each group. All p values are included in graph.
Chemokine/Cytokine Data
TNF-α in plasma samples was significantly lower in the PAI-1 KO IR (0.136 ± 0.007 ng/ml) compared to WT-IR (0.270±0.054 ng/ml); p =0.027. MCP-1 in plasma samples was also significantly lower in the PAI-1 KO IR (0.026± 0.0002 ng/ml) compared to WT-IR (0.039± 0.005 ng/ml); p = 0.017. MIP-2 trended lower in plasma samples in the PAI-1 KO IR mice compared to the WT-IR but this did not reach statistical significance (p=0.190), but in all the PAI-1 KO IR mice, MIP-2 levels were below detection limit of the ELISA. In the WT-IR, MIP-2 levels were detectable (0.604 ± 0.373 ng/ml) (see Figure 5A). KC levels were below the detection limits in the plasma samples in both groups. In BAL samples TNF-α KC, and MIP-2 were significantly lower in the PAI-1 KO IR (0.003± 0.0002 ng/ml, 0.007±0.016 ng/ml, and 0.029± 0.004ng/ml; respectively) compared to the WT-IR group (0.005±0.0006 ng/ml, 0.019± 0.002 ng/ml, and 0.078±0.0197 ng/ml; respectively) (p= 0.003, 0.0006, 0.027; respectively) (see Figure 5B). MCP-1 levels in the BAL were below detection limits in both groups. Taken together these data suggests that local abnormalities of fibrinolysis in ischemia-reperfusion affect the various inflammatory cytokines/chemokines locally, and these affects may also be reflected in the systemic circulation. We also performed ELISAs on whole lung homogenates as well as extracted total RNA from the left lung for real time PCR and there did not appear to be a significant different in any of the measured cytokines/chemokines (Figure 5C) which suggests the intra-alveolar inflammatory component may be most affected locally.
Figure 5. Proinflammatory mediators in plasma and BAL of ischemia/reperfusion injured mice.
A) ELISA analysis showing TNF-α and MCP-1 in the plasma of WT-IR and PAI-1 KO IR mice. B) ELISA analysis showing TNF-α, KC and MIP-2 in BAL of WT-IR and PAI-1 KO IR mice. C) Analysis showing mRNA expression of TNF-α, MCP-1, MIP-2 and KC in the left lung of WT and PAI-1 KO mice as determined by real time PCR. Data shown are the mean ± S.E. values for each group.
DISCUSSION
A substantial body of evidence supports the idea that coagulation, fibrinolysis, and extravascular fibrin deposition play a role in acute lung injury and subsequent repair (6). Deposition of alveolar fibrin is characteristic of a wide range of acute insults in lung models. In the acutely injured lung, intravascular thrombosis can also occur. Extravascular fibrin deposition promotes an acute inflammatory response, and lung dysfunction (6). Alveolar deposition is also characteristic of diffuse alveolar damage which occurs from various insults (6). Evidence supports transitional fibrin neomatrix involved in pathogenesis of acute inflammatory response in acute lung injury.
We have previously investigated the effects of hypoxia on the fibrinolytic system(7). Mice exposed to hypoxia were found to have an increase in plasma levels of PAI-1 and a decrease in tissue-type plasminogen activator and urokinase-type plasminogen activator. The colocalization studies with immunohistochemistry identified macrophages as the predominant source of increased PAI-1 within the hypoxic lung(7).
In our IRI model, we found decreased deposition of fibrin in the PAI-1 KO IR mice and a protective effect on injury in these mice. We do not know from these studies which compartment/s of the lung (intravascular, interstium or intra-alveolar) the decrease in fibrin is seen, but it does appear that with this decrease in fibrin there is a decrease in inflammation. We have previously reported on the interplay between the fibrinolytic system and inflammation in ischemia reperfusion injury. After ischemia reperfusion interleukin-10 null mice (interleukin-10 suppresses macrophage activation and down-regulates proinflammatory cytokine production) were found to have exaggerated pulmonary PAI-1 expression compared to mice with interleukin-10 (IL-10). These mice null for IL-10 showed poor postischemic function and survival compared to mice with IL-10(13). Furthermore in this study, recombinant IL-10 when given to mice null for IL-10 normalized the PAI/tissue-type plasminogen activator ratio, reduced pulmonary vascular fibrin deposition and rescued mice from lung injury.(13)
Farivar and colleagues(14) have also reported on the crosstalk between thrombosis and inflammation in a rat lung reperfusion injury model. In this paper they used a specific direct thrombin inhibitor, hirudin and showed it protected against IRI. They also suggested mechanistically that the protective effects of thrombin inhibition were a result of decreased inflammatory pathway activation. Thrombin exerts proinflammatory influences on neutrophils, endothelial cells, and macrophages and thus inhibition of thrombin with hirudin prevents this proinflammatory activation.
Our current findings are additive to our previous studies and those of Farivar and colleagues. The PAI-1 KO mice prevent fibrin accrual in the lung by increasing fibrinolysis. We saw less of an inflammatory response with less neutrophil infiltration, less cytokine and chemokine release, and improvement in physiologic parameters with the PAI-1 KO mice compared to controls.
The importance of PAI-1 in clinical lung transplantation is suggested by a study performed by Christie and colleagues(15). They found in plasma of human lung transplant recipients with severe ischemia reperfusion injury higher PAI-1 levels compared to patients without IRI. This increase in plasma PAI-1 levels has also been seen in ARDS patients compared to critically ill control patients (16).
The transitional fibrin that is found at the time acute lung injury has been linked to various pulmonary fibrotic conditions(6). Not only does tissue fibrin influence inflammation, it also affects tissue repair responses. The presence and severity of IRI is a major risk factor in the development of chronic allograft rejection(3, 4). Chronic allograft rejection in the lung is a fibro-obliterative process of the small airways. While this study does not investigate the role played by fibrin deposition in the subsequent development of chronic allograft lung rejection, it does suggest that one way IRI may increase the risk of development of chronic rejection is via alterations in the fibrinolytic system.
The current study focuses on impaired fibrinolysis. The protection seen with the PAI-1 KO mice was only partial but significant in our studies. We plan future studies to look at whether increased coagulation also contributes to IRI. In human lung transplant recipients that have severe IRI, evidence of enhanced coagulation is seen by decreased levels of protein C (15).
In summary, our study provides evidence to support the hypotheses that PAI-1 plays an important role during lung IRI injury via (1) decreasing fibrin deposition, (2) suppressing cytokine/chemokines involved in inflammation and chemotaxis, and (3) blocking inflammatory cells (such as neutrophil and macrophage) infiltration into the injured lung tissues. Our data indicate that the association between fibrinolysis and inflammation in our lung IRI model may provide potential novel therapeutic targets for lung transplantation. For example, treatment with angiotensin-converting enzyme (ACE) inhibitors quinapril (40mg) and ramipril (10 mg) have been reported to decrease plasma PAI-1 antigen and PAI-1 activity significantly, but without affecting the level and activity of tissue plasminogen activator in patients (17, 18). Therefore, ACE inhibitors could be administered to the donor to enhance fibrinolysis when heparin is given already as a routine prior to cold flush perfusion to prevent thrombosis. We will further investigate whether this early stage protection will attenuate bronchiolitis obliterans development during late stage of lung transplantation using our heterotopic tracheal transplantation model.
Footnotes
Presented in Part at the Twenty-eighth Annual Meeting and Scientific Sessions of the International Society for Heart and Lung Transplantation, Boston Massachusetts, April 9-12, 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meyers BF, de la Morena M, Sweet SC, Trulock EP, Guthrie TJ, Mendeloff EN, et al. Primary graft dysfunction and other selected complications of lung transplantation: A single-center experience of 983 patients. J Thorac Cardiovasc Surg. 2005;129(6):1421–1429. doi: 10.1016/j.jtcvs.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124(4):1232–1241. doi: 10.1378/chest.124.4.1232. see comment. [DOI] [PubMed] [Google Scholar]
- 3.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175(5):507–513. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 4.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73(4):1041–1047. doi: 10.1016/s0003-4975(01)03606-2. discussion 1047-1048. [DOI] [PubMed] [Google Scholar]
- 5.Ware LB, Camerer E, Welty-Wolf K, Schultz MJ, Matthay MA. Bench to bedside: targeting coagulation and fibrinolysis in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L307–311. doi: 10.1152/ajplung.00157.2006. [DOI] [PubMed] [Google Scholar]
- 6.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31(4 Suppl):S213–220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 7.Pinsky DJ, Liao H, Lawson CA, Yan SF, Chen J, Carmeliet P, et al. Coordinated induction of plasminogen activator inhibitor-1 (PAI-1) and inhibition of plasminogen activator gene expression by hypoxia promotes pulmonary vascular fibrin deposition. J Clin Invest. 1998;102(5):919–928. doi: 10.1172/JCI307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao M, Fernandez LG, Doctor A, Sharma AK, Zarbock A, Tribble CG, et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L1018–1026. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y-G, Xiao A-Z, Newcomer RG, Park HI, Kang T, Chung LWK, et al. Activation of pro-gelatinase B by endometase/matrilysin-2 promotes invasion of human prostate cancer cells. J Biol Chem. 2003;278(17):15056–15064. doi: 10.1074/jbc.M210975200. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y-G, Xiao A-Z, Park HI, Newcomer RG, Yan M, Man Y-G, et al. Endometase/matrilysin-2 in human breast ductal carcinoma in situ and its inhibition by tissue inhibitors of metalloproteinases-2 and -4: a putative role in the initiation of breast cancer invasion. Cancer Res. 2004;64(2):590–598. doi: 10.1158/0008-5472.can-03-1932. [DOI] [PubMed] [Google Scholar]
- 11.Nemzek JA, Siddiqui J, Remick DG. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Meth. 2001;255(12):149–157. doi: 10.1016/s0022-1759(01)00419-7. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Fattah R, Xiao A, Bomgardner D, Pease CS, Lopes MBS, Hussaini IM. Differential expression of HOX genes in neoplastic and non-neoplastic human astrocytes. J Pathol. 2006;209(1):15–24. doi: 10.1002/path.1939. [DOI] [PubMed] [Google Scholar]
- 13.Okada K, Fujita T, Minamoto K, Liao H, Naka Y, Pinsky DJ. Potentiation of endogenous fibrinolysis and rescue from lung ischemia/reperfusion injury in interleukin (IL)-10-reconstituted IL-10 null mice. J Biol Chem. 2000;275(28):21468–21476. doi: 10.1074/jbc.M002682200. [DOI] [PubMed] [Google Scholar]
- 14.Farivar A, Delgado M, McCourtie A, Barnes A, Verrier E, MS M. Crosstalk between thrombosis and inflammation in lung reperfusion injury. Ann Thorac Surg. 2006;81:1061–1067. doi: 10.1016/j.athoracsur.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 15.Christie JD, Robinson N, Ware LB, Plotnick M, De Andrade J, Lama V, et al. Association of Protein C and Type 1 Plasminogen Activator Inhibitor with Primary Graft Dysfunction. Am J Respir Crit Care Med. 2007;175(1):69–74. doi: 10.1164/rccm.200606-827OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moalli R, Doyle JM, Tahhan HR, Hasan FM, Braman SS, Saldeen T. Fibrinolysis in critically ill patients. Am Rev Respir Dis. 1989;140(2):287–293. doi: 10.1164/ajrccm/140.2.287. [DOI] [PubMed] [Google Scholar]
- 17.Brown NJ, Agirbasli M, Vaughan DE. Comparative Effect of Angiotensin-Converting Enzyme Inhibition and Angiotensin II Type 1 Receptor Antagonism on Plasma Fibrinolytic Balance in Humans. Hypertension. 1999;34(2):285–290. doi: 10.1161/01.hyp.34.2.285. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan DE, Rouleau JL, Ridker PM, Arnold JM, Menapace FJ, Pfeffer MA. Effects of ramipril on plasma fibrinolytic balance in patients with acute anterior myocardial infarction. HEART Study Investigators. Circulation. 1997 Jul 15;96(2):442–7. doi: 10.1161/01.cir.96.2.442. [DOI] [PubMed] [Google Scholar]