Abstract
BACKGROUND
Malignant brain tumors are among the most challenging to treat and at present there are no uniformly successful treatment strategies. Standard treatment regimens consist of maximal surgical resection followed by radiotherapy and chemotherapy. The limited survival advantage attributed to chemotherapy is partially due to low CNS penetration of antineoplastic agents across the blood-brain barrier (BBB).
OBJECTIVE
The objective of this paper is to review recent approaches to deliver anticancer drugs into primary brain tumors.
METHODS
Both preclinical and clinical strategies to circumvent the BBB are considered that includes chemical modification and colloidal carriers.
CONCLUSION
Analysis of the available data indicates that novel approaches may be useful for CNS delivery, yet an appreciation of pharmacokinetic issues, and improved knowledge of tumor biology will be needed to significantly impact drug delivery to the target site.
Keywords: blood brain barrier, drug delivery strategies, glioma, nanocarriers, targeted therapy
1. Introduction
Brain cancer treatment is still one of the biggest challenges in oncology. Brain tumors include a wide variety of neoplasms that can be primary or metastatic. The primary brain tumours are thought to be derived from glial cells or their progenitors and are generically classified as gliomas. The metastatic ones arise from systemic malignancies and then develop within the brain parenchyma [1,2]. Three major types of brain tumors are recognized by the World Health Organization, as a classification of gliomas: astrocytomas, oligodendrogliomas, and oligo-astrocytomas [3]. These tumors are further classified by subtypes (mainly for astrocytomas) and are graded from I to IV based on histology with grade IV being the most aggressive glioblastoma multiforme (GBM). Although histopathological analysis is still the standard way for the classification of gliomas, it is increasingly clear that different genetic subtypes exist, and that specific molecular changes are of prognostic significance. Molecular analysis has revealed that a gain of chromosome 7p and a loss of chromosome 10q are characteristic for GBM, whereas losses of 1p and 19q are most frequently detected in oligodendrogliomas [4].
Malignant astrocytomas constitute about 50% to 60% of primary brain tumors, with a peak incidence in the fifth or sixth decade of life that ranges from 5 to 8 per 100,000 inhabitants [5]. The incidence of brain tumors seems to be increasing, but it is unclear if this is due to environmental or genetic factors [6].
The standard treatment for brain tumors consists of maximal surgical resection, followed by radiotherapy and chemotherapy. However, despite continued research and new approaches, the prognosis for patients with malignant brain tumors is still extremely poor [7]. Thus, the median survival of patients with GBMs is only 20 weeks by surgical resection alone, 36 weeks by surgery and radiation, and inclusion of standard cytotoxic chemotherapy offers a minimal survival advantage, raising the median survival to 40-50 weeks [8].
In the last decades, despite advances in anticancer drug discovery and development, there has been little improvement on the prognosis of patients with brain cancer. Often, it has been found that promising agents for primary brain cancers in vitro have had little impact on disease in clinical trials [9]. These disappointing results can be at least in part explained by the inability to deliver therapeutic agents to the CNS across the blood-brain barrier (BBB) avoiding various resistance mechanisms and to reach the desired targets [10,11]. Moreover, it should be also taken into account that low-molecular weight chemotherapeutics do not achieve and maintain effective steady state concentrations within malignant glioma cells because of short blood half-lives [12].
Taking into account the high incidence and the unfavorable prognosis of brain tumors, a great deal of efforts have been made to identify the optimal agent(s) and valuable systems for the delivery of anticancer drugs to the CNS. It is now well established that a tumor must develop its own vascular network to grow and the neo-vasculature within tumors consists of vessels with increased permeability due to the presence of large endothelial cell gaps compared with normal vessels [13]. All of these features can be exploited for the development of BBB targeting anticancer drug delivery systems.
This paper deals with the various approaches which have been established for the treatment of primary CNS tumors. These tumors are characterized by a significant infiltrative capacity as their reappearance after resection usually occurs within 2 cm of the tumor margin. A number of review articles on this specific topic have been already published and summarize the progress made in this area [14-18]. This review primarily focuses on recent findings concerning the new strategies for delivering anticancer drugs to the CNS by chemical modification of drugs as well as by designing efficient targeted vectors (such as antibodies and protein carriers) or nanosystems (colloidal carriers) able to cross biological barriers as BBB in a controlled and non-invasive manner.
2. Standard chemotherapeutic treatment
2.1. Alkylating agents
For the treatment of primary brain tumors, many chemotherapeutic agents are in clinical use or trials [19]. Carmustine, lomustine and nimustine are the nitrosoureas which are frequently used in the treatment of malignant astrocytomas. They are alkylating agents and produce their cytotoxic effect by methylation of DNA mainly at the O6 position of guanine. The systemic toxicity of nitrosoureas consists of myelosupression, gastro-intestinal effects and nephrotoxicity. Due to a short blood half-life [12] as well as to a poor capability to cross the BBB, a limited distribution in the brain of nitrosoureas occurs and thus, a minimal benefit in terms of average survival was found for patients affected by brain tumors [20]. The efficacy of radiotherapy alone was compared with that of radiotherapy followed by procarbazine, lomustine and vincristine treatment. In each case, no significant difference in overall survival for patients with high grade astrocytomas was found [21].
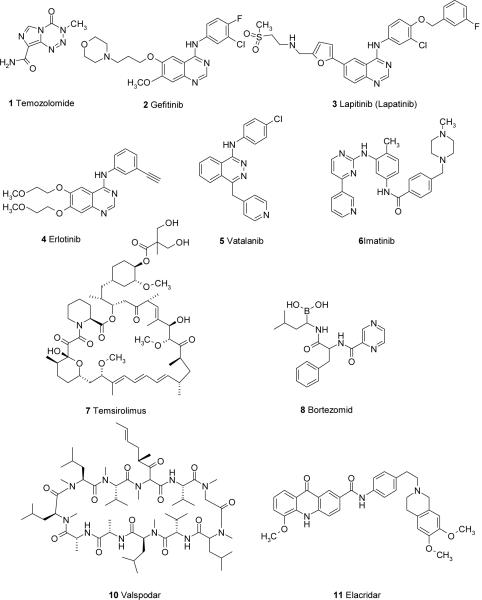
Temozolomide 1 (Figure 1) is one of the newest alkylating agents. It is characterized by high absolute oral bioavailability and good BBB penetration. However, temozolomide must be administered in high systemic doses to achieve therapeutic brain levels due to its short half-life of about 1.8 h in plasma [22]. Prolonged systemic administration is associated with side effects such as thrombocytopenia, nausea and vomiting. It has been approved by the FDA for the treatment of GBM and AAs showing a median survival time of 5.8 months [23]. Temozolomide is considered the current standard of care in the treatment of GBM. For the treatment of GBM, the protocol consists in a daily dose of 75 mg/m2 during the 6 weeks of radiation therapy, followed by the 5-day regimen over the following months [24]. When temozolomide was compared to the procarbazine in randomized studies, it resulted that survival rates were not statistically different between the groups treated with these alkylating agent, but there was a clear advantage in favor of temozolomide as for the progression-free survival (12.4 vs. 8.3 weeks) [25].
Figure 1.
To know the most effective regimen of combination chemotherapy in treating malignant gliomas, randomized phase III trials to compare the effectiveness of temozolomide alone to that of a combination of procarbazine, lomustine, and vincristine have also been carried out [26]. Besides the average survival time, it was also evaluated the quality of life that was maintained by the patient during the treatment and it seems that benefits are associated with the temozolomide treatment [27]. Based on these favorable characteristics of temozolomide, at present the combination of this agent with radiotherapy represents the standard treatment for newly diagnosed glioma patients improving the median survival time and quality of life [28].
Many other antineoplastic agents have been used for the treatment of CNS tumors including anthracyclines, platinum (II) complexes, paclitaxel, etoposide, irinotecan, topotecan and methothrexate. However, most of these chemotherapeutic agents do not penetrate into brain in appropriate amounts and high doses of drugs are required systemically for obtaining effective brain tumor concentrations.
2.2. Targeted Therapy
Currently, anticancer drugs tend to be cytotoxic compounds of limited specificity and their prolonged use results in lethal damage also for health cells. Therefore, the chemotherapeutic treatment of CNS tumors is associated with a severe systemic toxic side effects and thereby compromising the quality of patient life. Hence, the development of innovative anticancer drugs with reduced toxicity and targeted delivery methods for the treatment of malignant gliomas are required. Recently, an interesting approach that has undergone much investigation is the targeted therapy. The success of this strategy requires specifity or high selectivity of binding to tumor cells. This can occur exploiting over-expression of receptors by tumor cells or expression of receptors not found on normal brain tissues. Recent discoveries in molecular biology clarified that several pathways are involved in the oncogenic process in gliomas. These pathways include the involvement of the epidermal growth factor receptor (EGFR), the platelet-derived growth factor receptor (PDGFR), the vascular endothelial growth factor receptor (VEGFR) as well as the phosphoinositide 3' kinase (PI3K), the mammalian target of rapamycin (mTOR) and the Ras/Raf/MAPK proteins, among others. PI3K is frequently involved in oncogenesis of brain tumors and is upregolated from activity of growth factor receptor stimulation, including PDGFR, EGFR, VEGFR, fibroblast growth factor receptor, insulin growth factor-1 receptor and Ras. mTOR is involved in the control of cell homeostasis and growth through the regulation of p70s6k and 4E-BP1 proteins [16]. Therefore, novel targeted therapies are based on the use of inhibitors of these factors, ranging from small-molecules to even monoclonal antibodies. Clinical trials for the treatment of malignant gliomas with a single inhibitor or a combination of inhibitors as well as a combination of inhibitors with anticancer drugs have been carried out [9,26]. Among these new chemotherapeutic agents in targeted therapy it should be mentioned Gefitinib 2, Lapitinib 3, Erlotinib 4, Vatalanib 5, Imatinib 6, Temsirolimus 7, Bortezomid 8 and the recombinant humanized monoclonal antibody Bevacizumab 9 (Figure 1). Compounds 2-4 are EGFR inhibitors, compound 5 is a protein kinase C-beta and other angiogenesis pathway inhibitor, compound 6 is PDGR inhibitor, compound 7 is mTOR inhibitor, the monoclonal antibody 9 is a VEGFR inhibitor. Recent applications of the targeted chemotherapeutic strategies exploiting the mentioned targets are presented in Section 4.
3. Drug Transport at the Blood-Brain Barrier
3.1. Transport mechanisms at the Blood-Brain Barrier (BBB)
The physiological function of the BBB is to maintain brain homeostasis by selectively transporting nutrients and beneficial endogenous substances into the brain and excluding toxic metabolite or xenobiotics from the brain. The pivotal component of the BBB is a monolayer of brain capillary endothelial cells fused by tight junctions. Other components of the BBB including the astrocytic foot process, pericytes and perivascular macrophages within a basal lamina regulate and further strengthen the BBB [29,30]. In addition to tight junctions, the absence of fenestrations also contributes to the barrier property of brain endothelial cells. Furthermore, in contrast to vascular endothelial cells in other tissues, the low activity of pinocytosis and vesicular traffic further limits non-specific trans-endothelial transport with the exception of small lipid-soluble molecules [31].
Transport mechanisms at the BBB can be divided into two categories: passive diffusion and endogenous carrier-mediated transport. Passive diffusion is a process whereby drugs or endogenous substances travel across the BBB dependent upon along a concentration gradient from blood to brain, and the physicochemical properties of the drug. Qualitatively, drugs that passively diffuse through the BBB are generally lipophilic, often related to the octanol/water partition coefficient, and have a molecular weight of less than 400-500 Da. Numerous quantitative relationships have been cast to correlate BBB penetration to lipophilicity and molecular weight as well as other chemical structural features [32,33].
Endogenous transport systems at the BBB can be categorized into receptor-mediated endocytosis, carrier-mediated facilitated transport and/or ATP-dependent active transport. Receptor-mediated endocytosis is generally a three-step procedure that involves receptor mediated endocytosis at the luminal (blood) side followed by intracellular movement and exocytosis at the abluminal (brain) side of brain endothelial cell [34]. Several receptors are implicated in this process, including transferrin receptors, insulin receptors, lipoprotein-related protein 1 and lipoprotein-related protein 2 receptors as well as diphtheria toxin receptor[35]. Receptor-mediated endocytosis allows large molecules to be transported, which is potentially useful means to deliver anticancer biologics into the brain [36].
The carrier-mediated facilitated transport system refers to all the solute carrier (SLC) members that are composed of 43 families [37]. Each SLC member transports specific substrates including sugars, amino acids, oligopeptides, organic anions and organic cations. In so doing, SLC transporters play critical roles in various cellular physiological processes, such as importing or exporting nutrients, neurotransmitters and metabolites [38]. Among the SLC superfamily, organic cation transport (OCT) system (SLC21) and organic anion/cation transport system (SLC22) are of particular interests because of their roles in transporting anticancer drugs at the BBB. In contrast to ATP-binding cassette (ABC) transporter, organic anion and cation transporters exchange anions and cations following electrochemical gradients in an ATP-independent manner. Dependent upon the sub-cellular localization of these transporters (apical or basolateral) on the BBB, endogenous substrate or anticancer drugs can be transported into or pumped out of the brain [39].
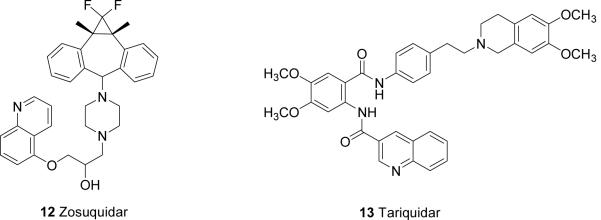
ATP-dependent active transport at the BBB is best characterized by P-Glycoprotein (P-gp/MDR1), breast cancer resistance protein (BCRP/ABCG2) and the multidrug resistance-associated protein (MRP) family. P-gp, a member of the ABC transporter superfamily, is a 170-kDa efflux pump expressed at the apical side of the BBB, which actively pumps variety of anticancer drugs including paclitaxel, topotecan and anthracyclines back into systemic circulation [40]. This active transport process is thought to be one of the underlying mechanisms of CNS anticancer drug resistance. Moreover, P-gp was found in resistant glioblastomas [41], suggesting a role of P-gp in limiting anticancer drug penetration into brain tumors in spite of the leaky nature of glioma vasculature. Indeed, data from our lab indicate that the disposition of anticancer drugs in brain tumor, where the integrity of the BBB is compromised, is affected by P-gp. Using a syngeneic intracerebral B16 brain tumor model, paclitaxel penetration in normal brain was significantly enhanced, and to a somewhat lesser extent in brain tumor in P-gp knockout mice in comparison to wild-type mice [42]. Preclinical and clinical studies have been done to explore the potential applications of P-gp inhibition to improve CNS penetration of anticancer drugs. However, disappointing results were obtained in clinical trials using first generation P-gp inhibitors due to toxicity issues [43]. Several novel P-gp inhibitors (e.g. valspodar 10, elacridar 11, zosuquidar 12, Figure 1) may improve the clinical outcome for this strategy [44]. BCRP is also expressed at the apical side of the BBB. Its substrates include cytotoxic compounds (mitoxantrone, topotecan, flavopiridol, methotrexate), sulfated conjugates of therapeutic drugs, and hormones (estrogen sulfate) [39]. In comparison to P-gp, the overlapping substrates profile and similar localization at the BBB suggest BCRP may limit BBB penetration of its substrates.
The MRP family is comprised of nine members (i.e. MRP1-9) that are efflux pumps and capable of transporting structurally diverse lipophilic anions [45]. It has been demonstrated that MRP1, MRP2, MRP4 and MRP5 are expressed at the apical side of the BBB, thus, these MRPs may be of particular interests with regard to their roles in chemoresistance at the BBB [46]. For instance, MRP1 is a glutathione and glucuronate conjugate pump which also confers resistance to anthracyclines, vinca alkaloids, epipodophyllotoxins, camptothecins and methotrexate [45]. In addition to prototypical MRP1 substrates such as E217βG, methotrexate and reduced folates, MRP4 and MRP5, responsible for transportting of cAMP and cGMP, are thought to be the resistance factors for nucleotide analogs drugs [45].
3.2. Alterations of the BBB in brain tumor
In brain tumors, although some features of the BBB are retained in brain tumor vasculature, some important characteristics of normal BBB are profoundly altered, which in turn significantly change the dispositions of anticancer drugs therein. Several key assembly proteins of tight junctions in variety primary brain tumor capillary endothelial cells were either downregulated or lost, including Zona Occludens-1 [47], Claudin-1, Claudin-5 and Occludin [48]. Thus, the structure of tight junctions at the BBB in brain tumors is compromised leading to a disrupted and “leaky” BBB. The disrupted tumor BBB may benefit anticancer drug penetration in regions of the tumor where the BBB is disrupted; however other areas, particularly at the outer rim of the tumor may have a more normal BBB that is still a challenge to achieve effective delivery [49,50]. Just as there are regional differences in membrane permeability, regional differences in tumor blood flow could effect drug delivery for drugs that are normally highly permeable to the BBB, and can be categorized as blood flow-limited transport. Another variable that can influence tumor uptake of drugs and macromolecules is interstitial fluid pressure, which when elevated due to a leaky tumor vasculature could reduce penetration.. The state of the tumor vasculature can vary from abnormal to normal to inadequate and depends on tumor growth and the balance of proangiogenic and antiangiogenic factors [51]. Concomitant administration of angiogenesis inhibitors can effect the structure and function of the tumor vasculature, and under certain conditions cause a “normalization” that can be associated with improved BBB penetration [52,53]. The action of antiangiogenic-based chemotherapy on the tumor vasculature are complex, including both dose- and time-dependent effects, which will require further investigations to fully appreciate their pharmacodynamic properties.
The expression of the aforementioned ABC transporters may also be altered in regions of the BBB associated with brain tumor. For instance, the expression of MRP1 and MRP3 was up-regulated in the peritumor vascular endothelial cells [54]. Compared to normal brain vasculature, P-gp expression in the vasculature of gliomas was down-regulated [41]. Interestingly, after repeated doxorubicin (DOX) treatment, P-gp expression was increased both in glioblastoma and its vascular endothelial cells [55], suggesting involvement of P-gp in DOX chemoresistance. There does not seem to be a consistent pattern for ABC transporter expression and function in the vasculature of brain tumors, and draws into question the strategy of inhibiting drug efflux pumps at the BBB in brain tumors. In a preclinical model using gene-disrupted mice that lacked P-gp, paclitaxel distribution was increased about 1.7 fold in brain tumors compared to wild-type mice [56]. Since paclitaxel concentrations were indicative of the whole tumor, it could be anticipated that P-gp's role could be more pronounced in regions where the BBB is intact. Many more studies will be needed to detail the role of drug pumps at the BBB in the presence of intracerebral tumors.
4. Drug delivery to brain tumors
4.1. Non-invasive drug delivery strategies without BBB disruption
Currently, most chemotherapeutic agents targeting brain tumors are delivered by systemic administrations including intravenous and oral route. However, aforementioned limitations reduced the effectiveness of antitumor drugs administered systemically. The limited success in treating brain tumors are resulted from the tumor cell chemoresistance (natural or acquired), poor selectivity of the antitumor drugs and most importantly, the BBB. High-dose chemotherapy via intravenous route has been investigated to enhance CNS penetration of the antitumor drugs including etoposide and carboplatin, yet little improvement was achieved due to the associated systemic toxicities. Different non-invasive drug delivery strategies without disrupting the BBB are discussed herein.
4.1.1. Direct conjugation of antitumor drugs
To improve lipophilicity of antitumor drugs, a lipophilic drug is made from parent drug by attaching a lipophilic promoiety. Upon entering the brain parenchyma, the functional group can be cleaved off from the parent drug, thus, improving CNS delivery of the parent drug. Alternatively, parent drugs can be directly conjugated with efficient “vectors” (antibodies, peptides, protein carrier, viruses) to cross the BBB. The latter method has been investigated extensively recently. For example, despite high hydrophobicity of paclitaxel, its brain uptake is relatively low and this poor BBB permeability is partly due to active efflux by P-gp. Angiopep-2, a 19-aminoacid peptide, has been discovered as a new vector to target the low-density lipoprotein (LDL) receptor-related protein. Thus, a conjugate between paclitaxel and the peptide Angiopep-2, named ANG1005, has been investigated as a novel CNS drug delivery system [57]. ANG1005 can be transported across the BBB by receptor-mediated endocytosis. It has been shown that ANG1005 possessed better brain permeability and in vivo antitumoral activity compared to paxlitaxel in an orthotopic brain tumor model, partly due to its ability to bypasses the P-gp. ANG1005 phase I clinical trials are ongoing to test its efficacy against recurrent primary or metastatic brain tumors. Similarly, paclitaxel and adriamycin (ADR) were conjugated with the iron binding protein p97 (melanotransferrin), a protein closely related to transferrin (Tf) [58,59]. Brain penetration of p97-drug conjugates was approximately 10 fold higher than that of free drugs. Additionally, p97-ADR conjugates significantly prolonged the survival of intracranial gliomas bearing-animals, suggesting p97 may be in treating brain tumors.
Besides improved penetration, direct conjugation is also capable of enhancing parent drug's efficacy. TMZ-Bio shuttle, formed by covalent chemical conjugation of temozolomide (1) to a transmembrane transport peptide, has been reported with better in vitro activity against some glioma cell lines at reduced dose levels, suggesting its potentials in minimizing systemic side effects [60]. It should be noted that this antitumor drug does not contain simple conjugation reactions. A suitable conjugation of temozolomide has been accomplished by using cycloaddition reactions in which the dien-component is obtained coupling the amide group of the temozolomide with a tetrazine [60].
4.1.2. Co-administration of chemotherapeutic agents with inhibitors of efflux transporters
Active efflux of anticancer drugs by P-gp, BCRP and MRPs contributes to resistance of brain tumor drugs. Thus, the co-administration of chemotherapeutic agents with specific inhibitors of P-gp and other efflux transporters can be utilized to increase the BBB permeability of anticancer drugs. In preclinic models, improved CNS penetration has been shown for paclitaxel, docetaxel and imatinib [17,61]. Problems with first-generation P-gp inhibitors, such as verapamil and cyclosporine A, include i) low binding affinities and unacceptable toxicity resulting from high dose regimen, ii) concomitant inhibition of drug-metabolizing cytochrome P450 3A (CYP3A) enzymes. Clinical application of second-generation P-gp inhibitors (e.g., the cyclosporine A analog valspodar 10, which possessed stronger P-gp inhibition and lower toxic effects, however, are also hindered by their interaction with CYP3A. Recently, newer-generation specific P-gp inhibitors, including elacridar 11, zosuquidar 12 and tariquidar 13 (Figure 1), without inhibiting CYP3A have been shown an improved side effects profile [62].
Additionally, inhibition of BCRP may be of clinical interest in light of its similar distribution and overlapping substrate profile of P-pg [62]. Well known BCRP inhibitors include elacridar 11 and the proton pump inhibitor pantoprazole. It should be noted that the selection of inhibitors co-administering with an anticancer drug is determined by the substrate profile of the chemotherapeutic agents and drug-drug interactions.
4.1.3. Available strategies to achieve targeted therapy
In brain tumor cells, over-expressed receptors or receptors not found in normal brain tissues constitute good candidates of targeted therapy, which requires specificity and high selectivity. In recent years, several potential cellular targets have been identified and characterized. For example, the over-expressed peripheral benzodiazepine receptors (PBRs), located between inner and outer mitochondrial membranes, in brain tumors may be an interesting intracellular target in light of their role in apoptosis [63]. Indeed, various ligands for PBR have shown pro-apoptotic activity [64]. PK 11195, a PBR ligand and isochinolincarboxamide derivative, has been shown to facilitate apoptosis in rat C6 glioma cells [63]. In an orthotopic glioma model, PK11195-gemcitabine (GEM) conjugate exhibited better brain and brain tumor penetration as well as a 2-fold enhancement in brain tumor selectivity compared to GEM alone [65]. It is also worth noting that the expression of PBR in astrocytomas positively correlates with the grade of malignancy of the tumor, as well as with proliferative and apoptotic indices, and negatively correlates with survival in a group of 130 patients [66], suggesting that PBR expression maybe useful in astrocytoma PET imaging or pathological diagnosis of astrocytomas [67].
Several signaling pathways, specifically, those involving growth factor receptors (EGFR), PI3K, mTOR and Ras/Raf/MAPK pathways, have been shown to play important roles in tumor cell growth and metastasis [4]. Chemotherapy targeting these signaling pathways has gained more and more attentions in recent years.
Over-expressed EGFR has been shown in up to 60% of GBM, which is the hallmark for primary glioblastomas [9] Thus, EGFR inhibitors as a novel targeted chemotherapy has been evaluated in both preclinic and clinic settings. Despite positive preclinical results, the first generation of EGFR inhibitors (e.g., gefitinib 2 and erlotinib 4) has not yielded satisfactory results in malignant gliomas, partly due to a shortened EGFR (i.e. EGFR vIII) expressed in 50 to 70% of EGFR-overexpressing gliomas [68]. EGFRvIII has been demonstrated to resist to the EGFR inhibitors 2 and 4 in vitro. In addition, intranasal administration of perillyl alcohol (POH), an inhibitor for nucleotide-binding protein Ras, led to regression of gliomas in a phaseI/II clinical trials [69].
Furthermore, an altered PI3K pathway is often observed in human cancers including glioblastoma. A negative correlation between PI3K pathway activation and apoptosis has been established in a clinical trial of primary gliomas, suggesting beneficial effects of inhibiting PI3K pathway [70]. Indeed, LY294002, an inhibitor targeting PI3K pathway, synergistically enhances both death receptor-and chemotherapy-induced apoptosis in glioblastoma cells [70].
In light of disappointing results from clinical trials using monoagent of first generation targeted therapy in malignant gliomas, multi-targeting drugs and combination drugs as novel targeted therapies are under investigation [71]. Combining mTOR inhibitors such as temsirolimus 7 and the EGFR inhibitors have shown partial responses. Bortezomib 8 and the VEGF inhibitor bevacizumab 9 are currently being evaluated clinically against recurrent malignant gliomas, and similar efficacy compared with temozolomide has been demonstrated [71]. In addition, combination of 9 and a cytotoxic agent or an EGFR inhibitor (e.g. irinotecan) might be effective in treating progressive recurrent malignant brain tumors with acceptable safety [72,73]. Ongoing combination targeted therapies include erlotinib 4 and temsirolimus 7 (EGFR and mTOR inhibition), erlotinib 4 and bevacizumab 9 (EGFR and VEGF inhibition) or temozolomide 1 [68].
More recently, immunotherapy, ribozymes and RNA interference (RNAi) as novel targeted therapy are also under investigation [74,77].
4.2 Invasive drug delivery strategies
4.2.1. Intra-arterial delivery with BBB disruption
In comparison to intra-venous route, intra-arterial (IA) administration increases drugs' systemic concentration by eliminating first pass metabolism. However, IA administration alone does not improve clinical outcomes in brain tumor patients partly due to the BBB. Transient osmotic BBB disruption (BBBD) followed by IA chemotherapy has been shown to increase drug CNS concentration while preserving neurocognitive functions and minimizing systemic toxicity [78].
Typically, the transient disruption is achieved by delivering pre-warmed 25% mannitol via internal carotid artery (or the vertebral artery) at a predetermined flow rate (3-12 ml/s), thereafter, the IA chemotherapeutic agent is infused for a short duration (e.g. 10 min) and tumor response is assessed by computed tomography or magnetic resonance imaging (MRI). In addition, localized BBB disruption can also result from ultrasound that, in some cases, has been shown to be well tolerated and without any evident tissue damages [79]. Overall, despite some side effects (e.g. ischemia) associated with catheterization procedure [16], transient BBBD in conjunction with IA chemotherapy is considered promising, particularly for some types of tumors (e.g., germ cell tumors) [80]. Currently, it is limited to a selected institutions because of the complexity and potentially serious complications of this procedure.
In this context, it should be noted that the conventional radiation therapy used to treat primary and metastatic brain cancer also increases BBB permeability [81], which contritutes to current standard treatment (i.e. temozolomide and radiotherapy) for newly diagnosed glioma patients [27].
4.2.2. Convection-enhanced delivery
To overcome poor brain distribution following drug infusion, convection-enhanced delivery (CED) was developed by Bobo et al., namely, directly infusing anticancer agent via a catheter located within or around a tumor under hydrostatic pressure [82]. It is demonstrated that the distribution of therapeutic agents with high molecular weight is enhanced by high flow micro-infusion via a local catheter in an animal model [82]. CED is a promising approach for the delivery of various agents including conjugates, monoclonal antibodies, antisense oligonucleotides or viral vectors [14]. In a phase III trial in patients with recurrent malignant gliomas, interleukin-13 conjugated to cintredekin besudotox (PE38QQR) delivered by CED slightly increases the median survival time [25]. CED has also been applied in antibody-mediated therapies and immunotherapies with acceptable toxicities yet highly variable efficacy. Additional hurdles of CED include limited distribution area, the requirement for surgery, high infusion rates and difficulty in real-time monitoring. Recent imaging techniques, such as fluorodeoxyglucose-positron emission tomography (FDG-PET), MRI and single photo emission computed tomography (SPECT), may be useful in real-time monitoring.
4.2.3. Implanted therapies
Intracerebral implantation of chemotherapeutic agents containing polymeric matrix or reservoir is another highly invasive drug delivery strategy, which is well established and commercially available. Characteristics of these implants, including rate-controlling mechanisms, degree of biodegradability, shapes and sizes, differ. Gliadel® is a polyanhydride biodegradable polymer wafer containing BCNU (carmustine) and approved by FDA since 1996 for recurrent high-grade gliomas, which has been shown a 2 months survival increment in patients with both newly diagnosed and recurrent malignant gliomas [83]. However, its clinical outcome is considered modest partly due to poor diffusion of the drug in the brain parenchyma and reported complications [83], thus, its application is limited. Implantations containing different chemotherapeutic agents (e.g. paclitaxel and cis-platin) or combining Gliadel® with other chemotherapeutic agents have been investigated clinically [84,85]. Gliadel® in combination with temozolomide for patients with recurrent high-grade gliomas has been found well tolerated and safe [86].
In the context of the implanted therapies, it should be also mentioned the use of “osmotic mini-pumps” (i.e., osmotic pumps) that allow a local and continuous drug delivery for a significant period of time. This strategy has been used successfully for the treatment of experimental tumors [87,88].
4.3. Nanoparticulate carriers as non-invasive delivery systems to brain tumors
Non-invasive delivery systems employing nanoparticulate carriers represent another valuable approach for enhancing therapeutic agents permeability across the BBB. Recent evidence [12] suggests that the physiologic upper limit of pore size in the BBB of malignant glioma microvasculature is approximately 12 nm. It follows that nanoparticles smaller than 12 nm with long blood half-lives would be able to cross effectively the BBB of malignant glioma microvasculature. The use of nanosystems (colloidal carriers) mainly focuses on liposomes and polymeric nanoparticles while other systems including solid lipid nanoparticles, polymeric micelles and dendrimers are also studied. Following intravenous administration, the colloidal systems can extravasate into brain tumor but to a less extent of normal brain tissue because of the disrupted BBB of brain tumors vessels [89], which leads to a more selective drug delivery into brain tumors. This passive targeting of nanoparticles in brain with disrupted BBB is known as “Enhanced Permeability and Retention (EPR)” effect which plays a critical role in drug delivery to solid tumors. Particles such as liposomes, which typically range between 50 to 150 nm, would remain within the microvasculature and small chemotherapy drugs would diffuse across the liposome membrane and then across the pores with the BBB of malignant gliomas.
An important requirement for using nanocarriers via systemic route is their ability to circulate in the bloodstream for a prolonged period of time. However, after intravenous administration, they often interact with the reticuloendothelial system (RES), leading to a rapid removal from systemic circulation [90]. This process mainly depends on particle size, charge and surface properties of the nanocarrier [91]. To minimize the interactions with the RES, poly (ethylene glycol) (PEG) coating or direct chemical linking of PEG to the particle surface extends plasma residence times. However, PEGylated carriers are not easily transported across the BBB resulting to their low affinity for brain tissue. Nevertheless, the nanosystems may still be useful tools for non-invasive CNS drug delivery if they are substrates of active-tranporting systems including carrier-mediated transport, receptor-mediated endocytosis and adsorptive-endocytosis [13]. Another advantage of this approach is that imaging agents can be encapsulated within the particle along with the anticancer drug allowing non invasive monitoring of drug delivery to brain tumors [92]. Colloidal systems, such as liposomes and nanoparticles, have shown promising features as drug carriers to target brain tumors after intravenous administration, and this technology is currently in early preclinical development phase.
4.3.1 Liposomes
Liposomes have historically been used as carrier systems for the delivery of therapeutic agents because of their easy preparation, good biocompatibility, low toxicity and commercial availability. Conventional liposomes are rapidly cleared from circulation by macrophages of the RES, which limits their developability as drug delivery systems. Extended circulation time can be accomplished either by decreasing the particle size (<100 nm) or by liposome-surface modification with PEG (stealth liposomes). To specifically target PEGylated liposomes to the brain, they can be additionally modified with monoclonal antibodies against glial fibrillary acidic proteins, transferrin receptors (OX-26), or human insulin receptors [93].
For example, to effectively deliver the anticancer drug 5-fluorouracil (5-FU), known to poorly penetrate the brain via a systemic route, into the brain, transferrin was conjugated to the surface of liposomes. Transferrin conjugated liposomes were prepared by coupling the -NH2 groups present on the surface of stearylamine containing liposomes with the -COOH groups of transferring, and the biodistribution of free 5-FU, non-coupled and coupled liposomes bearing 5-FU were determined following a single intravenous injection in rats [94]. An average of 10-fold increment of drug uptake in the brain was observed after the liposomal delivery of 5-FU, while the transferrin-coupled liposomes caused a 17-fold enhancement in the brain uptake of 5-FU, suggesting involvement of transferrin receptors on the BBB possibly through a receptor-mediated endocytosis process [94].
To achieve tumor-specific delivery of sodium borocaptate (Na2 10B12H11SH, BSH) to malignant glioma, an application of boron neutron capture therapy (BNCT), transferrin-conjugated PEGylated liposomes have been proposed [95]. BCNT is based on the nuclear reactions between 10B and thermal neutrons to give high linear energy transfer alpha particles (4He) and lithium-7 (7Li) nuclei (10B + 1n → 7Li + 4He). The resulting lithium ions and alpha particles are high linear energy transfer particles with strong biological effects. Their small distribution in tissue (5-9 mm) reduces non-specific radiation damages. Despite that, selective delivery of a sufficient number of 10B atoms to tumor cells is also important [96].
The 10B concentrations in U87D human glioma cells from three boron delivery systems (bare BSH, PEG-BSH, and transferrin-conjugated PEGylated liposomes, TF-PEG-BSH) were determined in vitro and in vivo by using inductively coupled plasma-atomic emission spectrometry (ICP-AES).. 10B delivery in tumor tissue by TF-PEG-BSH was highly selective and efficient among the three systems evaluated. Moreover, the survival rate in tumor-bearing mice after BNCT was best in the TF-PEG-BSH group, suggesting that TF-PEG-BSH is a potent Boron delivery system for BNCT due to its efficacy and selectivity [95].
Modified liposomes have also been used for enhancing gene delivery to brain tumors. Torchilin and coworkers investigated the potential of trans-activating transcriptional peptide (TATp)-modified liposomes to enhance the delivery of a gene encoding the green fluorescent protein (pEGFP-N1), to intracranial human brain tumor U-87 MG cells in nude mice. TATp-liposomes demonstrated an enhanced delivery of pEGFP-N1 in vivo with better selectivity compared to plasmid-loaded liposomes [97]. Thus, TATp-liposomes is a promising delivery system for transferring genes to human brain tumors in vivo.
4.3.2. Nanoparticles
Nanoparticles (NPs) are solid colloidal particles made of polymeric materials with sizes ranging from 1-1000 nm. NP includes both nanocapsules, a core-shell structure (a reservoir system), and nanospheres (a matrix system). NPs are used as a carrier system in which the drug is dissolved, entrapped, encapsulated, adsorbed or chemically linked to the surface. In addition, NPs are advantageous because of its high drug-loading capacity and protection against chemical and enzymatic degradation. Among the biodegradable polymers, poly(lactic acid-co-glycolic acid) (PLGA) is most used being FDA approved for delivery purpose and easily processed into nanoparticles having up to size of 200 nm in diameter. Similar to liposomes, NPs are rapidly cleared from the blood following intravenous administration. To minimize interactions with the RES, NPs need to be small (<100 nm). The biodistribution of NPs have been shown to be altered, specifically, with better uptake in endothelial cells, by coating NPs with hydrophilic surfactants or by covalently linking PEG- (PEGylation) or polyethylene oxide-chains on their surface.
An interesting application of NPs has been reported by Chertok et al., [98,92] who explored the possibility of using magnetic NPs, composed of a magnetic (e.g. iron oxide/magnetite) core and a biocompatible polymeric shell (e.g. dextran, starch), to target brain tumors. Magnetic NPs (12 mg Fe/kg) were injected in 9L-gliosarcomas bearing rats under a magnetic field. MR images were acquired prior to administration of NPs and immediately after at 1 h intervals for 4 h. Image analysis revealed that magnetic targeting induced a 5-fold increase in the total glioma over non-targeted tumors and a 3.6-fold enhancement in the target selectivity index (e.g., NPs accumulation in glioma over the normal brain). In addition, thermotherapy using magnetic NPs (i.e., magnetic fluid hyperthermia) has been investigated [99]. In this study, magnetic fluids were directly injected into tumors and subsequently heated in an alternating magnetic field, which enables precise heating of almost every part of the body. In vivo, it has been documented with good overall tolerability in a number of cancers including GBM [99].
Tsutsui and coworkers [100] examined the effect of bionanocapsules (BNCs) on drug delivery to brain tumors. These BNCs are composed of the surface antigen of hepatitis B virus and various components such as chemical compounds, protein, genes and small interference RNA (siRNA). To selectively target brain tumors, BNCs were conjugated with anti-human EGFR antibody that recognizes EGFRvIII known to overexpress in a variety of human malignancies of epithelial origin, particularly in gliomas. Indeed, the BNCs were both efficiently and selectively delivered to glioma cells in Gli36 glioma cell lines (expressing EGFRvIII but not wild-type EGFR) and Gli35 tumor bearing rats, indicating another promising brain tumor-targeting drug delivery system.
Schneider et al., [101] employed polybutyl cyanoacrylate NPs for the combined delivery of a vaccine and an antisense nucleotide to brain tumors. The rationale for this combination was that activating the immune systems by an active specific immunization with Newcastle-Disease-Virus infected tumor cells and blocking the transforming-growth-factor (TGF)-β production by TGF-β antisense oligonucleotides could be beneficial for brain tumors therapy. The polybutyl cyanoacrylate NPs in the study were coated with polysorbate 80 that facilitates the BBB penetration. It has been demonstrated that animals treated with the NPs survived longer than untreated controls with reduced TGF-β-levels and increased rates of activated CD25+ T-lymphocytes. Thus, this combined vaccination/gene therapy approach may offer a novel, “double-punch” attack to crack the immune defence of the very aggressive glioblastoma.
Solid lipid nanoparticles (SLNs) have also been reported for delivering drugs to the CNS. SLNs are dispersions of solid lipids stabilized with emulsifier or emulsifier/co-emulsifier complex in water. Solid lipids employed to prepare SLNs include widely used food lipids and commonly used emulsifiers including poloxamers, polysorbates and bile salts. Like liposomes and NPs, the biodistribution of SLNs can be manipulated by modifying the surface physico-chemical properties of SLNs to improve specificity of tissue delivery.
In recent years, the potential use of SLNs for brain drug delivering has been widely explored, and an interesting review on this topic has been published [102]. Specifically, brain delivery of antitumor drugs, including camptothecin, doxorubicin and paclitaxel, incorporated into SLNs and PEGylated SLNs were studied [103]. Significantly higher drug concentrations were detected in the brain when the antitumor drugs were encapsulated and delivered in SLNs, suggesting that SLNs may be capable of overcoming the BBB. In comparison with surfactant coated polymeric NPs (specifically useful in bypassing BBB), SLNs are advantageous in several counts including low intrinsic cytotoxicity, physical stability, protection of labile drugs from degradation, controlled release, and easy preparation. Interestingly, the very low cytotoxicity of SLNs and biodegradability of lipids used in their preparation makes them very attractive candidates for brain delivery and particularly for the treatment of brain tumors [104]. The efficacy of SLNs as carriers of different types of antineoplastic agents (such as doxorubicin, paclitaxel and the prodrug Cholesteryl butyrate) in brain tumor therapy has been reported in an experimental rat brain glioma model. It was demonstrated that doxorubicin prepared in SLNs achieved 12- (after 30 min) to 50- (after 24 hours) folds higher intratumoural concentrations compared to free solutions. In addition, in the contralateral healthy hemisphere in which BBB was not disrupted, doxorubicin-SLNs achieved subtherapeutic concentrations, while the free drug did not reach significant levels. Furthermore, i.v. administration of paclitaxel incorporated in SLNs to normal rabbits produced drug concentrations in brain tissue ten-folds higher than paclitaxel control solutions. These results strongly suggested that SLNs are able to successfully deliver cytotoxic drugs into the brain and to induce effective anti-tumoral response.
4.3.3. Polymeric micelles and dendrimers
Polymeric micelles are formed spontaneously in aqueous solutions of amphiphilic block copolymers and have core-shell architecture. Self-assembly occurs when the copolymer concentration reaches a threshold value known as the critical micelle concentration (CMC). The size of polymeric micelles usually varies from ca. 10 to 100 nm. The core is composed of hydrophobic polymer blocks [e.g., poly(propylene glycol) (PPG), poly(D,L-lactide), poly(caprolactone), etc.] and a shell of hydrophilic polymer blocks (e.g., PEG). Of particular interest are Pluronic block copolymers that contain two hydrophilic PEG and one hydrophobic PPG blocks (PEG-PPG-PEG). They were shown to cross the membranes of cultured brain microvessel endothelial cells and to inhibit P-gp [105]. Inhibition of drug efflux transporters by Pluronic block copolymers enhanced transport of a wide range of therapeutic agents across biological membranes including BBB. Pluronic based micellar delivery systems might represent novel and promising strategy for the treatment of brain cancers. Musacchio et al., [106] prepared surface PEG-PE micelles loaded with paclitaxel (PCL) and surface-modified with a PBR ligand (imidazopyridine derivative), the possible synergistic anticancer effects were examined. The cytotoxic effects of such micelles were studied against the LN 18 human glioblastoma cell line. The PCL-loaded PBR-targeted micelles showed a significantly enhanced toxicity due to the synergistic effect of the PBR-ligand with PCL. PBR-targeted nanopreparations loaded with anticancer drugs should be considered as potential promising antitumor nanomedicines.
Dendrimers are highly branched polymer molecules formed by a central core to which the branches are attached, the shell of the branches surrounding the core, and the surface formed by the branches termini. They have a size comparable with that of polymeric micelles or nanoparticles of small dimensions and can be smaller than 12 nm [12]. Dhanikula et al., [107] synthesized polyether-copolyester (PEPE) dendrimers loaded with methotrexate (MTX) and conjugated to D-glucosamine. Glucose conjugation to the dendrimers confers not only enhanced delivery across the BBB but also tumor-targeting specificity through facilitative glucose metabolism by the glucose transporters (GLUT) in the tumors. The antitumor activity of these MTX loaded dendrimers was evaluated against glioma cells and avascular human glioma tumor spheroids. More glucosylated dendrimers were found to be endocytosed than nonglucosylated dendrimers in both the cell lines. IC50 of MTX after administerding in dendrimers was lower than that of the free MTX, suggesting that PEPE dendrimers increased its potency.
5. Conclusions
In recent years, tremendous efforts have been made to develop efficient delivery strategies against brain tumors, which include both invasive and non-invasive strategies. However, only modest improvement was achieved in terms of prognosis and median survival of patients. Despite this, some useful improvements are undergoing investigations. For example, temozolomide, alone or in combination with other alkylating agents, is progressively gaining a prominent role in treatment protocols of brain tumors, owing to its good BBB penetration and low toxicity. Good BBB permeability and hence accumulation in the CNS is a critical feature for the successful antitumor therapy. In recognition of important roles of the BBB in brain tumor chemotherapy, and a better understanding of transport mechanisms and their modulators therein, it is possible to overcome the limitations presented by traditional chemotherapy. The research in this field is now focused on the development of non-invasive, more specific and targeted strategies that exploit the knowledges of the pathogenesis of brain cancers. Tumor growth critically depends on the formation of new blood vessels, thus, inhibition of angiogenesis pathways constitutes an attractive strategy for targeted therapy, which has been investigated with single or combined agents. Further improvements result from a better understanding of tumor biology and pathways involved therein. Another promising and non-invasive tool for the delivery of therapeutic drugs to brain tumors employs drug-loaded nanocarrier systems that take advantage of the disrupted BBB at tumor sites with disorganized vasculature and leakier capillaries to achieve selective tumor delivery. The modification of the nanocarrier surface properties improves the uptake by the endothelial cells. In addition, to selective deliver chemodrugs in brain tumors, magnetic NPs is of great interest with the possibility to monitor and quantify the process by MR imaging. Furthermore, SLNs have been found to be advantageous in delivering chemodrugs due to their good BBB permeability, low intrinsic cytotoxicity and biodegradability of lipids used in their preparation. In another dimension of the delivery strategies, the invasive strategies, local delivery of chemotherapeutic agents to brain tumors by CED improves drug distribution compared to other strategies only driven by diffusion, however, this technique is also confounded by the notable side effects.
Overall, despite several issues need to be addressed, targeted therapy and particulate systems are promising strategies that are worth investigating further for efficient CNS delivery of chemodrugs.
Expert Opinion
Although improvement in the prognosis of patients with brain cancer has been a major hurdle, the studies reviewed herein suggest that a new scenario is emerging for the management of patients with malignant brain tumours. In fact, it has been recognized that a shift is ongoing from the traditional cytotoxic chemotherapy toward targeted therapies. The development of new targeted chemical compounds as well as non-invasive targeted strategies are gaining more and more attention. Targeted cancer therapeutics is an ever-expanding field, including the use of monoclonal antibodies, gene therapy, stem cell-based techniques, and a large portfolio of low molecular weight drugs that serve as receptor tyrosine kinase inhibitors. Differential expression pattern of drug targets in tumor cells versus normal tissue contributes to high specificity and reduced toxicity of targeted chemotherapy. Surface-modifed nanocarriers with a low level of RES uptake lead to an increase in the systemic residence time, and offer a means to enable drug access to the tumor compartment. In light of nanocarriers' capability of bypassing the BBB and other defensive mechanisms, they seem promising in translational research, however, many important issues need to be addressed before all these new advances can be applied into clinical practice. The BBB is often disrupted within primary brain tumors; however there is considerable heterogeneity with the advancing edges of the tumor possessing a more intact BBB. This partial disruption of the BBB can be used as a selective advantage to deliver greater amounts of drug or delivery system to the tumor relative to normal brain. Nonetheless, limited delivery at sites where the BBB is intact continues to be a formidable challenge, and means to overcome should be considered in the development of new therapeutics for brain tumors. As new treatment approaches are advanced it will be important to critically evaluate drug concentrations within the brain tumor and normal brain. Preclinical models in rodents allow for discrete tissue sampling and PK model development that can be taken advantage to predict drug delivery performance in patients where samples are sparse. In addition, continued development and availability of noninvasive techniques, including PET and magnetic resonance spectroscopy, to assess drug distribution and BBB permeability is viewed as an invaluable direction, and can aid in the integration of preclinical and clinical investigations. [108].
The development of rational targeted therapy is predicated upon knowledge of the molecular biology of the brain tumor, and thus, continuing efforts to link genotype-phenotype characteristics to drug therapy will be critical. In this regard, relating tumor-based pharmacokinetic/pharmacodynamic models to genotype and pharmacogenomic variants offers a means to select patients for active therapies and individualize their drug doses. Given that the chemotherapy of brain tumors involves a combination of drugs, both targeted and cytotoxic agents, an increased emphasis should be placed on the development of predictive models to fully understand how drugs interact and design personalized drug treatment strategies.
Acknowledgments
Declaration of interest This work was supported by grants from Università degli Studi di Bari “Fondi d'Ateneo 2008” to G Trapani and in part by NIH grants [CA072937 and CA127963] awarded to JM Gallo.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Newton HB. Primary brain tumors: review of etiology, diagnosis and treatment. Am. Fam. Physician. 1994;49:787–797. [PubMed] [Google Scholar]
- 2.Davis FG, McCarthy BJ. Current epidemiological trends and surveillance issues in brain tumors. Expert Rev. Anticancer Ther. 2001;1:395–401. doi: 10.1586/14737140.1.3.395. [DOI] [PubMed] [Google Scholar]
- 3.Kleihues P, Burger PC, Scheithauer BW. International Histological Classification of Tumours. World Health Organization; Geneva, Switzerland: 1995. Histological typing of tumors of the central nervous system. [Google Scholar]
- 4••.Shai RM, Reichardt JKV, Chen TC. Pharmacogenomics of brain cancer and personalized medicine in malignant gliomas. Future Oncol. 2008;4:525–534. doi: 10.2217/14796694.4.4.525. [DOI] [PubMed] [Google Scholar]; This paper highlights the importance of pharmacogenomic approaches for more personalized chemotherapy
- 5.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- 6.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol (Berl) 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 7.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–23. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 8.Brandes AA, Fiorentino MV. The role of chemoterapy in recurrent malignant gliomas: An overview. Cancer Invest. 1996;14:551–559. doi: 10.3109/07357909609076900. [DOI] [PubMed] [Google Scholar]
- 9.Omuro AM, Faivre S, Raymond E. Lessons learned in the development of targeted therapy for malignant gliomas. Mol Cancer Ther. 2007;6:1909–1919. doi: 10.1158/1535-7163.MCT-07-0047. [DOI] [PubMed] [Google Scholar]
- 10.Groothuis DR. The blood-brain and blood-tumor barriers: a review of strategies for increasing drug delivery. Neuro-Oncology. 2000;2:45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huynh GH, Deen DF, Szoka FC., jr Barriers to carrier mediated drug and gene delivery to brain tumors. J. Control. Release. 2006;110:236–259. doi: 10.1016/j.jconrel.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 12.Sarin H, Kanevsky AS, Wu H, et al. Effective transvascular delivery of nanoparticles across the blood-brain tumor barrier into malignant glioma cells. J Transl Med. 2008;6:80. doi: 10.1186/1479-5876-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provenzale JM, Mukundan S, Dewhirst M. The role of blood-brain barrier permeability in brain tumor imaging and therapeutics. Am. J. Roentgenol. 2005;185:763–767. doi: 10.2214/ajr.185.3.01850763. [DOI] [PubMed] [Google Scholar]
- 14•.Beduneau A, Saulnier P, Benoit J-P. Active targeting of brain tumors using nanocarriers. Biomaterials. 2007;28:4947–4967. doi: 10.1016/j.biomaterials.2007.06.011. [DOI] [PubMed] [Google Scholar]; Review on the main targeted colloidal systems used for drug delivery to the brain by active targeting.
- 15.Mathieu D, Fortin D. Chemotherapy and delivery in the treatment of primary brain tumors. Current Clinical Pharmacol. 2007;2:197–211. doi: 10.2174/157488407781668767. [DOI] [PubMed] [Google Scholar]
- 16.Newton HB. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors Part 2:PI3K/Akt/PTEN, mTOR, SHH/PTCH and angiogenesis. Expert Rev. Anticancer Ther. 2004;4:105–128. doi: 10.1586/14737140.4.1.105. [DOI] [PubMed] [Google Scholar]
- 17•.Blakeley J. Drug delivery to brain tumors. Current Neurology and Neuroscience Reports. 2008;8:235–241. doi: 10.1007/s11910-008-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review on the main drug delivery systems to the brain.
- 18.Juillerat-Jeanneret L. The targeted delivery of cancer drugs across the blood-brain barrier: chemical modification of drugs or drug-nanoparticles? Drug Discov. Today. 2008;13:1099–1106. doi: 10.1016/j.drudis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Muldoon LL. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J. Clin. Oncol. 2008;25:2295–2305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 20.Huncharek M, Muscat J. Treatment of recurrent high grade astrocytoma; results of a systematic review of 1,415 patients. Anticancer Res. 1998;18:1303–1312. [PubMed] [Google Scholar]
- 21.Medical Research Council Brain Tumour Working Party Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytomas. a Medical Research Council Trial. J Clin Oncol. 2001;19:509–518. doi: 10.1200/JCO.2001.19.2.509. [DOI] [PubMed] [Google Scholar]
- 22.Baker SD, Wirth M, Statkevich P. Absorption, metabolism and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin. Cancer. Res. 1999;5:309–317. [PubMed] [Google Scholar]
- 23.Bower M, Newlands ES, Bleehen NM, et al. Multicentre CRC phase II trial of temozolomide in recurrent or progressive highgrade glioma. Cancer Chemother Pharmacol. 1997;40:484–488. doi: 10.1007/s002800050691. [DOI] [PubMed] [Google Scholar]
- 24.Stupp R, Mason W, van de Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 25.Yung WKA, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 26. http:www.cancer.gov/search/ViewClinicalTrials.
- 27.Osoba D, Brada M, Yung WKA, et al. Health-related quality of life in patients with anaplastic astrocytoma during treatment with temozolomide. Eur J Cancer. 2000;36:1788–1795. doi: 10.1016/s0959-8049(00)00165-9. [DOI] [PubMed] [Google Scholar]
- 28.Stupp R, Hegi ME, Gilbert MR, et al. Chemioradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 29.Abbott NJ, Ronnback L, Hansson E, et al. Astrocyte-endothelial interactions at the blood brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 30.Lai CH, Kuo KH. The critical component to establish in vitro BBB model: Pericyte. Brain Res Rev. 2005;50(2):258–65. doi: 10.1016/j.brainresrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34(1):207–17. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin VA, Patlak CS, Landahl HD, et al. Heuristic modeling of drug delivery to malignant brain tumors. J Pharmacokinet Biopharm. 1980;8(3):257–96. doi: 10.1007/BF01059646. [DOI] [PubMed] [Google Scholar]
- 33.Di L, Kerns EH, Bezar IF, et al. Comparison of blood-brain barrier permeability assays: in situ brain perfusion, MDR1-MDCKII and PAMPA-BBB. J Pharm Sci. 2009;98(6):1980–91. doi: 10.1002/jps.21580. [DOI] [PubMed] [Google Scholar]
- 34.Pardridge WM. Blood-brain barrier biology and methodology. J Neurovirol. 1999;5(6):556–69. doi: 10.3109/13550289909021285. [DOI] [PubMed] [Google Scholar]
- 35.Gaillard PJ, Visser CC, de Boer AG, et al. Targeted delivery across the blood-brain barrier. Expert Opin Drug Deliv. 2005;2(2):299–309. doi: 10.1517/17425247.2.2.299. [DOI] [PubMed] [Google Scholar]
- 36.Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3(2):90–105. 51. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 37.Hediger MA, Romero MF, Peng JP, et al. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004;447(5):465–8. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, Sadee W. Membrane transporters and channels in chemoresistance and - sensitivity of tumor cells. Cancer Lett. 2006;239(2):168–82. doi: 10.1016/j.canlet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 39.Ito K, Suzuki H, Horie T, et al. Apical/basolateral surface expression of drug transporters and its role in vectorial drug transport. Pharm Res. 2005;22(10):1559–77. doi: 10.1007/s11095-005-6810-2. [DOI] [PubMed] [Google Scholar]
- 40.Demeule M, Regina A, Jodoin J, et al. Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vascul Pharmacol. 2002;38(6):339–48. doi: 10.1016/s1537-1891(02)00201-x. [DOI] [PubMed] [Google Scholar]
- 41.Becker I, Becker KF, Meyermann R, et al. The multidrug-resistance gene MDR1 is expressed in human glial tumors. Acta Neuropathol. 1991;82(6):516–9. doi: 10.1007/BF00293387. [DOI] [PubMed] [Google Scholar]
- 42.Gallo JM, Li S, Guo P, et al. The effect of P-glycoprotein on paclitaxel brain and brain tumor distribution in mice. Cancer Res. 2003;63(16):5114–7. [PubMed] [Google Scholar]
- 43.Sikic BI, Fisher GA, Lum BL, et al. Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein. Cancer Chemother Pharmacol. 1997;40(Suppl):S13–9. doi: 10.1007/s002800051055. [DOI] [PubMed] [Google Scholar]
- 44.Kemper EM, Boogerd W, Thuis I, et al. Modulation of the blood-brain barrier in oncology: therapeutic opportunities for the treatment of brain tumours? Cancer Treat Rev. 2004;30(5):415–23. doi: 10.1016/j.ctrv.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22(47):7537–52. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 46.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat Rev Neurosci. 2005;6(8):591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 47.Sawada T, Kato Y, Kobayashi M, et al. Immunohistochemical study of tight junction-related protein in neovasculature in astrocytic tumor. Brain Tumor Pathol. 2000;17(1):1–6. doi: 10.1007/BF02478911. [DOI] [PubMed] [Google Scholar]
- 48.Liebner S, Fischmann A, Rascher G, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100(3):323–31. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- 49.Levin VA. Pharmacokinetics and central nervous system chemotherapy. Mcgraw-Hill; New York: 1987. [Google Scholar]
- 50.de Vries NA, Beijnen JH, Boogerd W, et al. Blood-brain barrier and chemotherapeutic treatment of brain tumors. Expert Rev Neurother. 2006;6(8):1199–209. doi: 10.1586/14737175.6.8.1199. [DOI] [PubMed] [Google Scholar]
- 51.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–22. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 52.Zhou O, Gallo JM. Differential effect of sunitinib on the distribution of temozolomide in an orthotopic glioma model. Neuro Oncol. 2009;11(3):301–310. doi: 10.1215/15228517-2008-088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou Q, Guo P, Gallo JM. Impact of angiogenesis inhibition by sunitinib on tumor distribution of temozolomide. Clin Cancer Res. 2008;14(5):1540–9. doi: 10.1158/1078-0432.CCR-07-4544. [DOI] [PubMed] [Google Scholar]
- 54.Haga S, Hinoshita E, Ikezaki K, et al. Involvement of the multidrug resistance protein 3 in drug sensitivity and its expression in human glioma. Jpn J Cancer Res. 2001;92(2):211–9. doi: 10.1111/j.1349-7006.2001.tb01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rittierodt M, Harada K. Repetitive doxorubicin treatment of glioblastoma enhances the PGP expression--a special role for endothelial cells. Exp Toxicol Pathol. 2003;55(1):39–44. doi: 10.1078/0940-2993-00287. [DOI] [PubMed] [Google Scholar]
- 56.Gallo JM, Li S, Guo P, et al. The effect of P-glicoprotein on paclitaxel brain and brain tumor distribution in mice. Cancer Res. 2003;63(16):5114–5117. [PubMed] [Google Scholar]
- 57.Regina A, Demeule M, Che C, et al. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br J Pharmacol. 2008;155:185–197. doi: 10.1038/bjp.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res. 2007;24:1759–1771. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karkan D, Pfeifer C, Vitalis TZ, et al. A unique carrier for delivery of therapeutic compounds beyond the blood-brain barrier. PLoS ONE. 2008;3(6):e2469. doi: 10.1371/journal.pone.0002469. doi:10.1371/journal.pone.0002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waldeck W, Wiessler M, Eheman V, et al. TMZ-Bioshuttle - a reformulated temozolomide. Int. J Med Sci. 2008;5:273–284. doi: 10.7150/ijms.5.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bihorel S, Camenisch G, Lemaire M, et al. Modulation of the brain distribution of imatinib and its metabolites in mice by valspodar, zosuquidar and elacridar. Pharm Res. 2007;24:1720–1728. doi: 10.1007/s11095-007-9278-4. [DOI] [PubMed] [Google Scholar]
- 62.Breedveld P, Beijnen JH, Schellens JHM. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. TRENDS in Pharmacological Sciences. 2006;27:17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 63.Pilkington GJ, Parker K, Murray SA. Approaches to mitochondrially mediated cancer therapy. Semin. Cancer Ther. 2008;18:226–235. doi: 10.1016/j.semcancer.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Decaudin D. Peripheral benzodiazepine receptor and its clinical targeting. Anticancer Drugs. 2004;15:737–45. doi: 10.1097/00001813-200409000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Guo P, Ma J, Li S, et al. Targeted delivery of a peripheral benzodiazepine receptor ligand-gemcitabine conjugate to brain tumors in a xenograft model. Cancer Chemother Pharmacol. 2001;48:169–176. doi: 10.1007/s002800100284. [DOI] [PubMed] [Google Scholar]
- 66.Vlodavsky E, Soustiel JF. Immunohistochemical expression of peripheral benzodiazepine receptors in human astrocytomas and its correlation with grade of malignancy, proliferation, apoptosis and serviva. J Neurooncol. 2007;81:1–7. doi: 10.1007/s11060-006-9199-9. [DOI] [PubMed] [Google Scholar]
- 67.Sekimata K, Hatano K, Ogawa M, et al. Radiosynthesis and in vivo evaluation of N [11C]methylated imidazopyridineacetamides as PET tracers for peripheral benzodiazepine receptors. Nucl Med Biol. 2008;35:305–314. doi: 10.1016/j.nucmedbio.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 68••.Omuro A. Exploring multi-targeting strategies for the treatment of gliomas. Curr Opin Investig Drugs. 2008;9:1287–1295. [PubMed] [Google Scholar]; This paper provides basic data on the pathways involved in the oncogenic process and useful for targeted therapy.
- 69.da Fonseca CO, Linden R, Futuro D, et al. Ras pathway activation in gliomas: a strategic target for intranasal administration of perillyl alcohol. Arch Immunol Ther Exp. 2008;56:267–276. doi: 10.1007/s00005-008-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Opel D, Westhoff M-A, Bender A, et al. Phosphatidylinositol 3-kinase inhibition broadly sensitizes glioblastoma cells to death receptor- and drug-induced apoptosis. Cancer Res. 2008;68:6271–80. doi: 10.1158/0008-5472.CAN-07-6769. [DOI] [PubMed] [Google Scholar]
- 71.Nappe A. Drug Discovery and Development of Innovative Therapeutics -IBC's 13th Annual World Congress. Approaches to cancer therapy. IDrugs; 2008. pp. 705–709. [PubMed] [Google Scholar]
- 72•.Dietrich J, Norden AD, Wen PY. Emerging antiangiogenic treatments for gliomas - efficacy and safety issues. Curr Opin Neurol. 2008;21:736–44. doi: 10.1097/WCO.0b013e3283131370. [DOI] [PubMed] [Google Scholar]; This paper reviews recent data on angiogenic inhibitors in malignant gliomas
- 73.Poulsen HS, Grunnet K, Sorensen M, et al. Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol. 2009;48:52–58. doi: 10.1080/02841860802537924. [DOI] [PubMed] [Google Scholar]
- 74.Sampson JH, Archer GE, Mitchell DA, et al. Tumor specific immunotherapy targeting the EGFRvIII mutation in patients with malignant glioma. Semin Immunol. 2008;20:267–275. doi: 10.1016/j.smim.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mintz A, Gibo DM, MandhanKumar AB, et al. Protein- and DNA-based active immunotherapy targeting interleukin-13 receptor alpha. Cancer Biother Radiopharm. 2008;23:581–589. doi: 10.1089/cbr.2008.0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Halatsch ME, Schmidt U, Behnke-Mursch J, et al. Epidermal growth factor receptor inhibition for the treatment of glioblastoma multiforme and other malignant brain tumours. Cancer Treat Rev. 2006;32:74–89. doi: 10.1016/j.ctrv.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 77.Bao S, Wu Q, Li Z, et al. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 2008;68:6043–6048. doi: 10.1158/0008-5472.CAN-08-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neuwelt EA. Mechanisms of disease: the blood brain barrier. Neurosurgery. 2004;54:131–140. doi: 10.1227/01.neu.0000097715.11966.8e. [DOI] [PubMed] [Google Scholar]
- 79.Vykhodtseva N, McDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics. 2008;48:279–296. doi: 10.1016/j.ultras.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jahnke K, Kraemer DF, Knight KR, et al. Intraarterial chemotherapy and osmotic blood-brain barrier disruption for patients with embryonal and germ cell tumors of the central nervous system. Cancer. 2008;112:581–588. doi: 10.1002/cncr.23221. [DOI] [PubMed] [Google Scholar]
- 81.Cao Y, Tsien CI, Shen Z, et al. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol. 2005;23:4127–4136. doi: 10.1200/JCO.2005.07.144. [DOI] [PubMed] [Google Scholar]
- 82.Bobo RH, Laske DW, Akbasak A, et al. Convenction-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vukelja SJ, Anthony SP, Arseneau JC, et al. Phase 1 study of escalating-dose OncoGel (ReGel/paclitaxel) depot injection, a controlled-release formulation of paclitaxel, for local management of superficial solid tumor lesions. Anticancer Drugs. 2007;18:283–289. doi: 10.1097/CAD.0b013e328011a51d. [DOI] [PubMed] [Google Scholar]
- 85.Sheleg SV, Korotkevich EA, Zhavrid EA, et al. Local chemotherapy with cisplatin-depot for glioblastoma multiforme. J Neurooncol. 2002;60:53–59. doi: 10.1023/a:1020288015457. [DOI] [PubMed] [Google Scholar]
- 86.Gururangan S, Cokor L, Rich JN, et al. Phase I study of Gliadel wafer plus temozolomide in adults with recurrent supratentorial high-grade gliomas. Neuro-oncol. 2001;3:246–250. doi: 10.1093/neuonc/3.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barth RF, Yang W, Wu G, et al. Thymidine kinase 1 as a molecular target for boron neutron capture therapy of brain tumors. Proc. Natl. Acad. Sci. USA. 2008;105:17493–17497. doi: 10.1073/pnas.0809569105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rousseau J, Barth RF, Moeschberger M, et al. Efficacy of intracerebral delivery of carboplatin in combination with photon irradiation for treatment of F98 glioma-bearing rats. Int. J. Radiat Oncol, Biol Phys. 2009;73:530–536. doi: 10.1016/j.ijrobp.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 89.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 90.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 91.Ogawara K, Furumoto K, Takakura Y, et al. Surface hydrophobicity of particles is not necessarily the most important determinant in their in vivo disposition after intravenous administration in rats. J Control Release. 2001;77:191–198. doi: 10.1016/s0168-3659(01)00468-0. [DOI] [PubMed] [Google Scholar]
- 92.Chertok B, Moffat BA, David AE, et al. Iron oxide nanoparticles as a drug delivery vehicle for MRI monitored magnetic targeting of brain tumors. Biomaterials. 2008;29:487–496. doi: 10.1016/j.biomaterials.2007.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pardridge W. Vector-mediated drug delivery to the brain. Adv Drug Deliv Rev. 1999;36:299–321. doi: 10.1016/s0169-409x(98)00087-8. [DOI] [PubMed] [Google Scholar]
- 94.Soni V, Kohli D, Jain SK. Transferrin-conjugated liposomal system for improved delivery of 5- fluorouracil to brain. J Drug Target. 2008;16:73–78. doi: 10.1080/10611860701725381. [DOI] [PubMed] [Google Scholar]
- 95.Doi A, Kawabata S, Iida K, et al. Tumor-specific targeting of sodium borocaptate (BSH) to malignant glioma by transferrin-PEG liposomes: a modality for boron neutron capture therapy. J Neurooncol. 2008;87:287–294. doi: 10.1007/s11060-008-9522-8. [DOI] [PubMed] [Google Scholar]
- 96.Yanagie H, Ogata A, Sugiyama H, et al. Application of drug delivery system to boron neutron capture therapy for cancer. Expert Opin Drug Deliv. 2008;5:427–443. doi: 10.1517/17425247.5.4.427. [DOI] [PubMed] [Google Scholar]
- 97.Gupta B, Levchenko TS, Torchilin VP. TAT Peptide-modified liposomes provide enhanced gene delivery intracranial human brain tumor xenografts in nude mice. Oncol Res. 2007;16:351–359. doi: 10.3727/000000006783980946. [DOI] [PubMed] [Google Scholar]
- 98.Chertok B, David AE, Huang Y, et al. Glioma selectivity of magnetically targeted nanoparticles: A role of abnormal tumor hydrodynamics. J Control Release. 2007;122:315–323. doi: 10.1016/j.jconrel.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Landeghem FKH, Maier-Hauff K, Jordan A, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30:52–57. doi: 10.1016/j.biomaterials.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 100.Tsutsui Y, Tomizawa K, Nagita M, et al. Development of bionanocapsules targeting brain tumors. J Control Release. 2007;122:159–164. doi: 10.1016/j.jconrel.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 101.Schneider T, Becker A, Ringe K, et al. Brain tumor therapy by combined vaccination and antisense oligonucleotide delivery with nanoparticles. J Neuroimmunol. 2008;195:21–27. doi: 10.1016/j.jneuroim.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 102.Blasi P, Giovagnoli S, Schoubben A, et al. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007;59:454–477. doi: 10.1016/j.addr.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 103.Wong HL, Bendayan R, Rauth AM, et al. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev. 2007;59:491–504. doi: 10.1016/j.addr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 104.Brioschi A, Zenga F, Zara GP, et al. Solid lipid nanoparticles: could they help to improve the efficacy of pharmacologic treatments for brain tumors? Neurol Res. 2007;29:324–330. doi: 10.1179/016164107X187017. [DOI] [PubMed] [Google Scholar]
- 105.Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130:98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Musacchio T, Laquintana V, Latrofa A, et al. PEG-PE micelles loaded with paclitaxel and surface-modified by a PBR-ligand: synergistic anticancer effect. Mol Pharm. 2009;6:468–479. doi: 10.1021/mp800158c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dhanikula RS, Argaw A, Bouchard J-F, et al. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: enhanced efficacy and intratumoral transport capability. Mol Pharm. 2008;5:105–116. doi: 10.1021/mp700086j. [DOI] [PubMed] [Google Scholar]
- 108.Zhou Q, Gallo JM. In vivo microdialysis for PK and PD studies of anticancer drugs. AAPS J. 2005;7:E659–E667. doi: 10.1208/aapsj070366. [DOI] [PMC free article] [PubMed] [Google Scholar]