Abstract
Objective
Perioperative stroke and periventricular leukomalacia have been reported to occur commonly in infants with congenital heart disease. We aimed to determine the incidence and type of brain injury in infants undergoing two-ventricle repair in infancy and to determine risk factors associated with such injury.
Methods
Forty-eight infants enrolled in a trial comparing two different hematocrits during surgical repair of congenital heart disease underwent brain MRI scans and neurodevelopmental testing at one year of age.
Results
Eighteen (38%) of our subjects had tiny foci of hemosiderin by susceptibility imaging, without evidence of abnormalities in corresponding regions on conventional MRI sequences. Subjects who had foci of hemosiderin had a significantly lower Psychomotor Developmental Index at one year of age (79.6 ± 16.5, mean ± SD) compared with subjects who did not have these foci (89.5 ± 15.3; p=0.04). Older age at surgery and diagnostic group were significantly associated with presence of hemosiderin foci. Only one subject had a small stroke (2%) and two had periventricular leukomalacia (4%).
Conclusions
Foci of hemosiderin without radiologic evidence of ischemic brain injury are an abnormality associated with adverse neurodevelopmental outcome not previously described in MRI studies of children with surgically repaired congenital heart disease. The association of hemosiderin foci with older age at surgery and cardiac diagnosis and not risk factors associated with brain injury in previous studies suggests that the etiology and pathogenesis of this abnormality is different from ischemic brain lesions reported previously.
Keywords: Congenital heart disease, Cardiac surgery, Brain MRI, Neurodevelopmental outcome, Brain injury
Introduction
Despite advances in the care of children with congenital heart disease (CHD), neurodevelopmental impairments remain common in this population (1–9). Deficits may occur in cognition and behavior, as well as in motor domains (5–8). However, the relationship of these neurodevelopmental impairments with specific neuropathologic abnormalities remains unclear. One recent neuropathological study of children who died days to months following early surgical repair of CHD showed that diffuse white matter gliosis and periventricular leukomalacia (PVL) were the dominant lesions (10). In MRI studies of children surviving CHD, several groups have reported a relatively high incidence of PVL and focal stroke (11–14). However, no reports have demonstrated a direct correlation between PVL or stroke and subsequent neurodevelopmental deficits.
By contrast, numerous studies have delineated perioperative factors that may contribute to the pathogenesis of brain injury that likely underlies later neurodevelopmental deficits (1,3,5,6,9,15–19). For example, previous reports have shown that balloon atrial septostomy (BAS), lower Apgar score at 5 minutes, lower intraoperative cerebral oxygenation saturation and lower blood pressure in the postoperative period were risk factors associated with radiologically apparent brain injury, specifically PVL and stroke (12–14). A previous trial conducted at our institution showed a significant effect of hematocrit during bypass surgery on neurologic and cardiac outcome (16). In that study, a higher hematocrit was associated with improved cardiac and neurologic outcome (16). In a subsequent randomized trial comparing two higher hematocrits (35% vs. 25%), the treatment groups were similar with respect to neurologic outcomes measured by brain MRI and neurodevelopmental evaluation at one year of age (20).
In a substudy of the latter hematocrit trial, we sought to determine the incidence of radiologically apparent brain injury by brain MRI at one year of age; the perioperative factors associated with brain injury; and the association of brain injury with one-year neurodevelopmental outcomes. We hypothesized that BAS, hypotension, and longer bypass, circulatory arrest or cross-clamp times would be associated with a higher incidence of radiologically apparent brain injury, and that brain injury found by MRI scan would be associated with worse neurodevelopmental outcome.
Methods
We enrolled infants < 9 months of age requiring surgical repair of one of the three following congenital heart defects: (1) D-transposition of the great arteries (TGA) with intact ventricular septum or ventricular septal defect; (2) tetralogy of Fallot (TOF) with or without pulmonary atresia or truncus arteriosus; and (3) ventricular septal defect (VSD) or complete common atrioventricular canal defect. Infants were excluded if they had birth weight < 2.3 kg, recognizable phenotypic syndrome of congenital anomalies, extracardiac anomalies of greater than minor severity, previous cardiac surgery, or associated cardiovascular anomalies requiring aortic arch reconstruction or additional open surgical procedures before the planned developmental follow-up. Infants were randomized to a hematocrit strategy of either 25% or 35%, with stratification by surgeon and by diagnostic group. A detailed description of the study design, anesthesia/perfusion methods and study outcomes has been previously reported (20). We obtained written informed consent in all cases. The study was approved by the Institutional Review Board at Children’s Hospital Boston. We collected relevant clinical variables regarding the subjects’ pre-, intra-and postoperative course, and one-year neurologic and developmental evaluations as described previously. Specifically, the Mental and Psychomotor Development Indices (MDI and PDI) were obtained for each subject at one year of age using the Bayley Scales of Infant Development II (BSID-II).
We obtained brain MRI data at one year of age in a subset of subjects enrolled in the trial. We obtained sagittal localizer, a standard clinical axial fast spin echo (FSE) T2-weighted sequence (TE 84, TR 4000, flip angle 90), and an axial multiplanar gradient recalled (MPGR) acquisition susceptibility weighted sequence (TE 40, TR 600, flip angle 30) for the detection of prior hemorrhage. Three-dimensional SPoiled Gradient Recalled (3D SPGR, with TE 5, TR 35) and dual echo FSE sequences (TE 50 & effective TE 150, TR 5000) were acquired in the coronal plane. We obtained axial diffusion tensor imaging data with six diffusion directions and b factors of 5 and 750 s/mm2 (21). Finally, we obtained single voxel proton magnetic resonance spectroscopy using a point resolved spectroscopy sequence (PRESS) technique (TR 1500 ms, TE 144 ms) with the voxel placed in the left parieto-occipital cerebrum (largely white matter), lateral and superior to the trigone of the lateral ventricle.
All the MRI sequences were evaluated for evidence of acquired or developmental abnormalities by a single pediatric neuroradiologist (RLR) who was unaware of the cardiac diagnosis, medical history or perioperative course of the subjects. The images were evaluated prospectively in a standardized fashion, and abnormalities were recorded using a form developed specifically for this trial. The images were examined for the presence of focal or multifocal acquired abnormalities such as focal stroke, hemorrhage, atrophy or mineralization, and diffuse abnormalities including ventriculomegaly, delayed myelination or diffuse T2 prolongation indicative of white matter gliosis. Imaging was also reviewed for the presence of developmental abnormalities including major anomalies such as schizencephaly or agenesis of the corpus callosum, or minor developmental abnormalities such as a persistent cavum septum pellucidum, Chiari I malformation or arachnoid cyst.
Categorical perioperative factors and MRI abnormalities were compared across the three cardiac diagnostic groups using Fisher’s exact test. Continuous perioperative factors were compared across diagnostic groups using Kruskal-Wallis tests. Developmental outcomes were compared across diagnostic groups using analysis of variance. The relationships between perioperative characteristics reported in Table 1 and MRI abnormalities were investigated using logistic regression. The relationship between MRI abnormalities and developmental outcome measures (i.e., MDI and PDI) was investigated using t-tests and linear regression, adjusting for diagnostic group. Statistical analyses were performed using SAS Version 9.1 (SAS Institute Inc., Cary, NC) and statistical significance was set at the 0.05 level.
Table 1.
Characteristics of 48 subjects undergoing infant heart surgery who had magnetic resonance imaging at one year, according to diagnosis group.
| Variable | ALL (N=48) | TGA (N=19) | TOF (N=20) | VSD (N=9) | P valuea |
|---|---|---|---|---|---|
| Preoperative Characteristics | |||||
| Birth weight, mean ± SD (kg) | 3.4±0.5 | 3.6±0.6 | 3.3±0.5 | 3.4±0.5 | .32 |
| Gestational age, mean ± SD (wk) | 39.2±1.3 | 39.0±1.2 | 39.3±1.1 | 39.2±1.8 | .56 |
| Apgar score at 5 min, mean ± SD | 8.5±1.2 | 8.2±1.7 | 8.7±0.7 | 8.9±0.3 | .24 |
| Sex, % male | 58 | 63 | 50 | 67 | .64 |
| Race, % nonwhite | 21 | 16 | 15 | 44 | .19 |
| Age at surgery, median (range) (days) | 41 (2–264) | 6 (2–13) | 63 (2–198) | 156 (39–264) | <.001 |
| Intubated prior to surgeryb, % | 10 | 21 | 5 | 0 | .19 |
| Abnormal neurologic exam, n/total (%) | 17/36 (47) | 8/15 (53) | 5/13 (38) | 4/8 (50) | .70 |
| Operative characteristics, mean ± SD | |||||
| Cross clamp time (min) | 64±26 | 90±21 | 49±10 | 44±11 | <.001 |
| Total support time (min) | 103±35 | 140±25 | 82±11 | 72±13 | <.001 |
| Total bypass time (min) | 96±30 | 124±26 | 80±12 | 72±13 | <.001 |
| Low-flow bypass time (min) | 48±26 | 70±24 | 33±16 | 33±16 | <.001 |
| Duration of circulatory arrest (min) | 7±11 | 16±11 | 3±7 | 0±0 | <.001 |
| Hematocrit at onset of low-flow bypass (%) | 28.5±5.3 | 28.0±5.2 | 28.0±5.4 | 30.4±5.9 | .47 |
| Nadir temperature (°C) | 20.0±5.6 | 15.2±1.4 | 21.7±5.4 | 26.3±1.7 | <.001 |
| Lowest pCO2 (mm Hg) | 32.6±5.1 | 32.6±5.1 | 32.5±5.0 | 33.0±5.9 | .98 |
| Highest pCO2 (mm Hg) | 76.0±15.0 | 86.0±12.4 | 71.0±14.5 | 65.9±8.6 | <.001 |
| Lowest pO2 (mm Hg) | 90.3±80.4 | 47.8±13.1 | 103.1±95.9 | 151.7±82.4 | <.001 |
| Postoperative variables | |||||
| Lactate 60 min after bypass, mean ± SD | 2.7±1.5 | 3.9±1.5 | 2.1±0.9 | 1.7±0.7 | <.001 |
| PRISM III Score, mean ± SD | |||||
| 12 h Postoperative | 5.5±3.3 | 6.2±2.2 | 6.2±3.8 | 2.3±1.8 | .001 |
| 24 h Postoperative | 7.4±3.8 | 8.1±2.5 | 8.5±4.5 | 3.7±2.2 | .002 |
| Lowest PaCO2, mean ± SD | 33.6±4.5 | 31.0±3.9 | 34.1±4.1 | 37.5±3.7 | .002 |
| Lowest pO2, mean ± SD | 75.9±23.9 | 88.4±23.2 | 63.8±20.9 | 78.0±19.8 | .003 |
| Days intubated, median (interquartile range) | 1.7 (1.1–2.9) | 2.0 (1.2–3.6) | 2.0 (1.2–2.9) | 1.0 (0.8–1.1) | .002 |
| Follow-up Data at One Year | |||||
| Abnormal neurologic exam, n/total (%) | 28/48 (58) | 7/19 (37) | 14/20 (70) | 7/9 (78) | .06 |
| Psychomotor Developmental Index, mean ± SD | 85.8±16.3 | 84.8±14.9 | 86.5±19.7 | 86.3±11.9 | .95 |
| Mental Developmental Index, mean ± SD | 94.7±11.3 | 96.6±10.2 | 93.9±13.3 | 92.3±8.5 | .61 |
P values are for differences between diagnostic groups. For dichotomous variables, P values were determined by exact tests. For Psychomotor Developmental Index and Mental Developmental Index, P values were determined by analysis of variance. For all other continuous variables, P values were determined by Kruskal-Wallis tests.
Excludes patients who were transiently intubated prior to surgery.
TGA, D-transposition of the great arteries. TOF, Tetralogy of Fallot. VSD, ventral septal defect.
Results
MRI scans were obtained in 48 of 106 subjects who returned for one-year follow up evaluations (n=25 and n=23 in the 25% and 35% hematocrit groups, respectively). The mean age at MRI was 1.2 years (SD = 0.2). In the remaining subjects, MRI scans were not obtained due to parental refusal, usually related to the need for general anesthesia (55 subjects, 52%), cancellations by the anesthesiologist due to medical concerns (2, 2%), and no MRI machine available (1, 1%). Among the subjects who returned for the one-year evaluation, those who underwent brain MRI, compared to those who did not, were similar with respect to scores on the BSID-II and neurologic examination.
Clinical characteristics of subjects
Clinical perioperative characteristics of subjects who underwent a brain MRI scan, according to diagnostic group, are shown in Table 1. Males outnumbered females in the TGA (12 vs. 7) and VSD (6 vs. 3) groups, but the cardiac diagnostic groups were similar with respect to birth weight, gestational age at birth, Apgar scores and hematocrit at onset of low-flow bypass (Table 1). Diagnostic group was highly associated with age at surgery. Subjects with TGA underwent neonatal surgical repair, whereas subjects with TOF and VSD generally underwent surgery at two to five months of age. Of the 19 subjects with TGA, 17 had BAS prior to surgery (3 BAS performed in the catheterization lab and 14 performed at the bedside), while 2 had no BAS. Only 2 subjects received a bolus of heparin prior to the BAS. No subject underwent a postoperative catheterization prior to the MRI scan and neurodevelopmental testing at one year of age.
The diagnostic groups differed in most intraoperative and postoperative variables (Table 1). Infants in the TGA group had the longest operations, greatest use of total circulatory arrest, lowest minimum temperature, and greatest postoperative morbidity as indicated by highest lactate 60 minutes after bypass, 12 and 24 hour PRISM III scores, and number of days of endotracheal intubation. Conversely, infants in the VSD group had the lowest intraoperative and postoperative complexity. No subjects had postoperative hypotension, defined as a systolic blood pressure < 40 mmHg in a neonate or < 45 mmHg in an infant > 30 days old.
The percentage of children with abnormal neurologic examinations at age one year tended to differ among the three diagnostic groups (p = 0.06, Table 1). Despite the greater perioperative morbidity of children with TGA, their prevalence of abnormal neurologic exam (37%) was lower than that of infants in the TOF and VSD groups (70 and 78% respectively). The diagnostic groups did not differ significantly in their scores on the PDI or MDI of the BSID-II.
MRI data
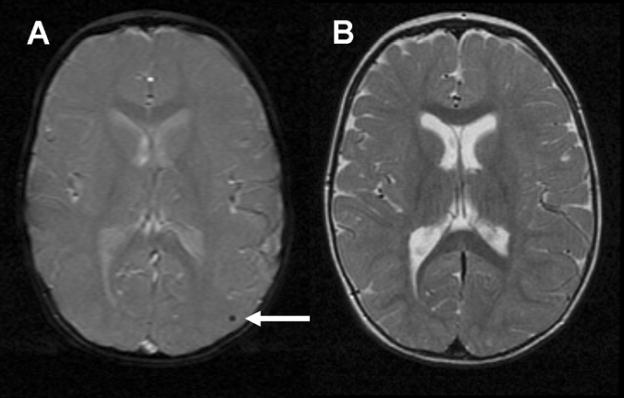
Qualitative analysis of the image data showed no evidence of severe brain injury or major brain malformations (Table 2). We found more acquired or developmental abnormalities in subjects with VSD (7 abnormal vs. 2 normal), compared with the TOF (11 vs. 9) or TGA (5 vs. 14) groups (p = 0.03). The majority of the acquired abnormalities were tiny foci of signal abnormality on the MPGR sequence consistent with small foci of hemosiderin in 18 subjects (38%, Fig 1). The foci were found throughout the cerebral cortex and white matter, basal ganglia and cerebellum, without predilection for any particular brain region or any specific pattern of distribution. Nine subjects had a single focus of hemosiderin, and the other nine subjects had two or more such foci. No signal abnormality was found in the region of these foci on any of the corresponding T1w or T2w images (Fig 1).
Table 2.
Qualitative evaluation of magnetic resonance imaging outcomes according to diagnosis group.
| Variable | ALL (N=48) | TGA (N=19) | TOF (N=20) | VSD (N=9) | P valuea |
|---|---|---|---|---|---|
| Age at MRI, mean ± SD (yr) | 1.2±0.2 | 1.1±0.1 | 1.2±0.2 | 1.3±0.3 | .10 |
| No. with abnormality (%) | |||||
| Any acquired or developmental abnormalities | 23 (48) | 5 (26) | 11 (55) | 7 (78) | .03 |
| Focal or multifocal abnormalities | 19 (40) | 4 (21) | 9 (45) | 6 (67) | .06 |
| Focal infarction or atrophy | 1 (2) | 1 (5) | 0 | 0 | .58 |
| Brain mineralization/hemosiderin | 18 (38) | 3 (16) | 9 (45) | 6 (67) | .03 |
| Diffuse abnormalities | 3 (6) | 1 (5) | 1 (5) | 1 (11) | .79 |
| Delayed myelination | 1 (2) | 0 | 0 | 1 (11) | .19 |
| Ventriculomegaly | 1 (2) | 1 (5) | 0 | 0 | .58 |
| T2 hyperintensities/gliosis/periventricular leukomalacia | 2 (4) | 0 | 1 (5) | 1 (11) | .49 |
| Developmental abnormalities | 4 (8) | 0 | 3 (15) | 1 (11) | .23 |
| Major malformation | 0 | 0 | 0 | 0 | 1.0 |
| Minor malformation | 4 (8) | 0 | 3 (15) | 1 (11) | .23 |
P values are for differences among diagnostic groups. For age at MRI, the P value was determined by a Kruskal-Wallis test. For dichotomous variables, P values were determined by exact tests.
TGA, D-transposition of the great arteries. TOF, Tetralogy of Fallot. VSD, ventral septal defect.
Figure 1.
Focus of hemosiderin in left parieto-occipital cortex (arrow) seen on axial MPGR sequence (A) without corresponding abnormality on axial T2w FSE sequence (B)
In addition to the hemorrhagic foci, several other abnormalities were present. One female subject with TGA had a small area of focal encephalomalacia in the left insular region consistent with a small focal stroke (Fig 2), but focal stroke was not seen in any other subjects (incidence of 2%). The subject with focal stroke underwent a preoperative BAS in the catheterization lab without heparin; thus, the incidence of focal stroke among infants with TGA undergoing BAS and surgical repair was 1/17 (6%). PVL was found only in two subjects (4%) – one female subject with VSD and one male subject with TOF (Fig 3). The subject with VSD and PVL was judged to have globally delayed myelination (Fig 3A and 3B). One female subject with TGA had ventriculomegaly, but did not have evidence of any white matter or other parenchymal abnormality. We detected minor developmental abnormalities in four subjects, three of whom had a Chiari I malformation and one had prominent perivascular spaces in the periatrial white matter. We found no major developmental abnormalities in our subjects.
Figure 2.
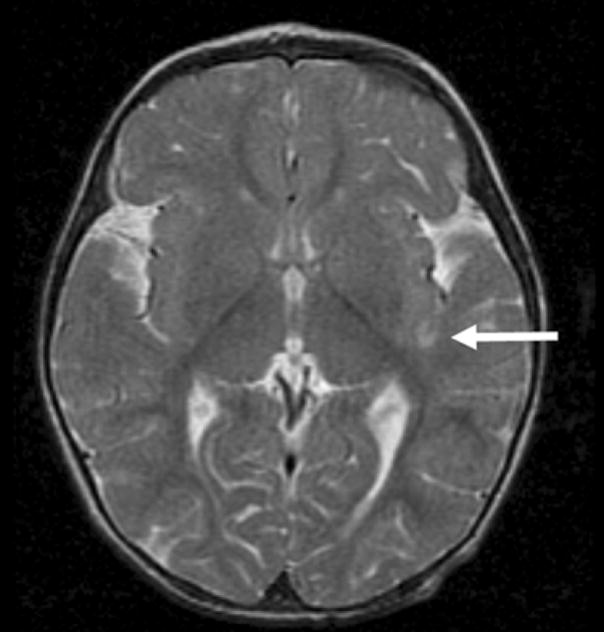
Small area of focal encephalomalacia seen as T2 hyperintensity in left insular region (arrow), consistent with old focal stroke
Figure 3.
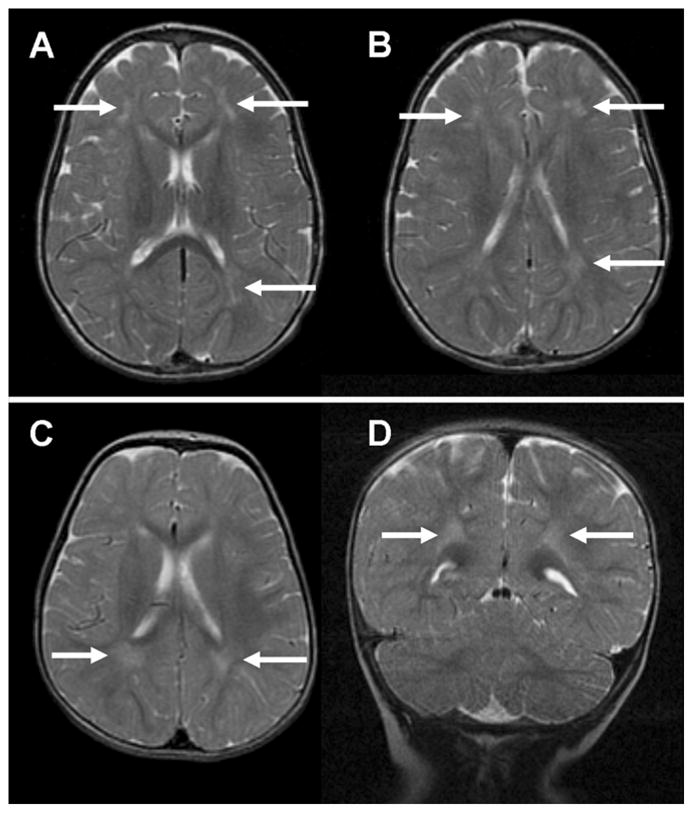
PVL in a subject with VSD (3A & B) and in a subject with TOF (3C & D) demonstrated by T2w imaging as patchy areas of T2 hyperintensity in the cerebral white matter dorsolateral to the frontal horns and occipital horns of the lateral ventricles (arrows)
Relationship between MRI abnormalities and neurodevelopmental outcome
Subjects with foci of hemosiderin, compared with those without such foci, had a 10-point lower mean PDI score (79.6 ± 16.5 vs. 89.5 ± 15.3; t-test p = 0.04), but were similar with respect to MDI scores (Fig 4). The association of hemosiderin with lower mean PDI score remained statistically significant in linear regression when adjusted for cardiac diagnostic group (p = 0.02). Although no other MRI abnormalities were associated with developmental outcome, our power to detect differences was low given the low frequency of each of the other abnormalities, e.g., only two cases of PVL.
Figure 4.
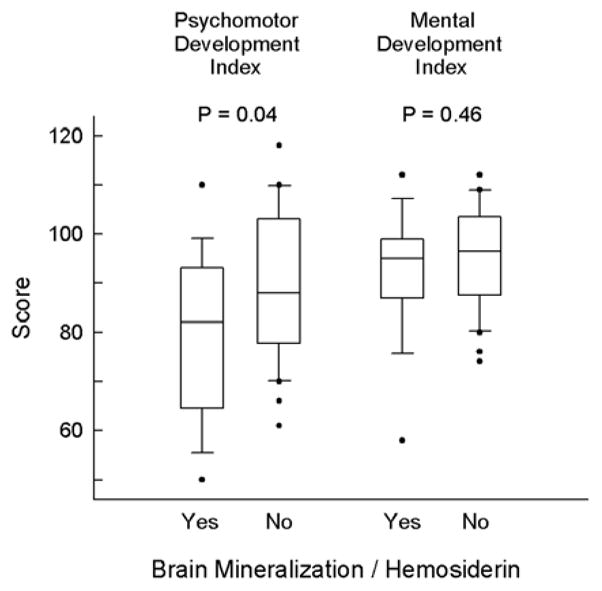
Boxplot of Psychomotor (PDI) and Mental (MDI) Development Index scores for subjects with and without foci of hemosiderin. The limits of the box indicate the 25th and 75th percentiles and the median is marked by the line within the box. The whiskers indicate the 10th and 90th percentiles and the observations outside of this range are shown as solid circles.
Relationship between perioperative characteristics and MRI abnormalities
We explored whether perioperative variables were associated with the presence of foci of hemosiderin. Eleven subjects received antifibrinolytic medications, including aprotinin (8 subjects), tranexamic acid (2 subjects), and aminocaproic acid (1 subject). There were no significant associations between antifibrinolytics administered (individually or combined) and the presence of hemosiderin (p = 1.0 for all). Cardiac diagnosis was the main characteristic associated with the presence of foci of hemosiderin. These foci were found in only 3 of 19 subjects with TGA (16%), but in 9 of 20 subjects with TOF (45%) and 6 of 9 subjects with VSD (67%; p = 0.03 comparing the three groups). Second, we found that older age at surgery, both as a continuous variable and as a categorical variable (> 30 days vs. ≤ 30 days), was associated with a higher rate of hemosiderin. Specifically, hemosiderin was detected in 15 of 26 subjects (58%) whose age at surgery was > 30 days vs. 3 of 22 subjects (14%) whose age at surgery was ≤ 30 days (Fisher’s exact test, p = 0.003). Because age at surgery was closely related to cardiac diagnosis, it was not possible to determine whether cardiac diagnosis (including conduct of surgical repair) or age at surgery was the main factor associated with hemosiderin. We performed logistic regression within each cardiac diagnosis group with age at surgery as a continuous or dichotomized (≤/> 30 days) predictor and did not find any statistically significant correlations, although the small numbers within each diagnosis group limited the power of these analyses. In additional univariate analyses, we found that higher nadir of intraoperative temperature was associated with presence of hemosiderin (p = 0.003) as was higher nadir of postoperative PaCO2 (p = 0.008). However, when the relationship between hemosiderin and either lowest intraoperative temperature or lowest postoperative PaCO2 was adjusted for age at surgery, the associations were no longer statistically significant. The strong association among variables including lowest intraoperative temperature and postoperative PaCO2, cardiac diagnosis, and age at surgery, limited our ability to determine which factors were explanatory. No other perioperative clinical variables, such as abnormal findings on pre-operative neurologic exam, were significantly associated with the presence of hemosiderin. Finally, the number of subjects with focal stroke (n = 1) and PVL (n = 2) was inadequate to analyze which perioperative variables were associated with these brain lesions.
Discussion
This study demonstrates a new finding of foci of signal loss on the susceptibility-weighted MRI sequence in a population of children undergoing two-ventricle repair in infancy. Specifically, 38% of our subjects had tiny foci of magnetic susceptibility artifact thought to be hemosiderin scattered throughout the cerebral and cerebellar parenchyma. This finding has not been reported in previous MRI studies of infants undergoing surgical repair of CHD in which a susceptibility weighted sequence was performed (11,12,22). The presence of these foci was associated with significantly lower PDI score at one year of age in our subjects, suggesting that this is a clinically important finding.
The neuropathology associated with the foci of susceptibility artifact seen in our patients is currently unknown. Susceptibility-weighted imaging is a gradient-echo MR technique that is sensitive to local perturbations in the magnetic field due to the products of prior hemorrhage, some forms of mineralization or air. The foci of susceptibility artifact found in our subjects are likely to represent hemosiderin rather than calcium, because calcium deposition is usually a consequence of focal injury such as ischemia or inflammation. We found no focal signal abnormality in the corresponding regions on T1w or T2w scans to suggest that these foci were associated with focal ischemia or inflammation (e.g., Fig 1), nor were these foci in brain regions typically affected by ischemic injury in CHD patients described in prior neuropathologic or MRI studies (10–14,23). None of the lesions reported in a previous neuropathological study from our institution would be expected to have this type of MRI appearance (10). However, this discrepancy could be due in part to differences in the management of patients with CHD, since the neuropathology study was conducted on patients whose cardiac surgery was performed in the period 1985–1993. One potential etiology for these foci is emboli, possibly related to catheterization, surgical procedures or bypass support during surgery. Since we did not perform MRI scans prior to surgical repair, we could not determine whether these foci were present preoperatively. We did not find any association between presence of these foci and administration of antifibrinolytics, thus hematologic management did not appear to be a risk factor. Given that these foci were tiny, any associated parenchymal abnormalities might be better elucidated by employing higher resolution conventional and susceptibility-weighted imaging sequences on a high field (e.g. 3T) MRI scanner.
Despite the lack of associated overt parenchymal abnormalities by conventional MRI sequences, the presence of these foci of presumed hemosiderin was associated with significantly lower PDI score at one year of age. Their association with developmental outcome supports the notion that these foci may be a marker of brain injury not detected by conventional T1w and T2w MRI sequences. We therefore examined our data to determine if the presence of hemosiderin was associated with perioperative factors known to be associated with brain injury, such as younger age at surgical repair. PVL is found more commonly in children who are repaired as newborns (54%) than as infants (4%) (12), and PVL and stroke often occur in the newborn period (13,14). We hypothesized that younger age at surgical repair might render immature small cerebral blood vessels more prone to rupture, producing foci of hemosiderin detected by MRI. However, hemosiderin foci were associated with older age at surgery in our subjects, suggesting a greater vulnerability to a different type of brain injury in older infants. A recent study reported that older age at surgery is associated with lower IQ score at age of school entry (24), although this relationship may be confounded by other clinical variables associated both with older age at surgery and later neurologic impairments. We found no association of hemosiderin foci with BAS, oxygenation or intraoperative bypass times - risk factors associated with a higher risk of brain injury in previous studies (12–14). Thus it appears that these foci are a marker of a type of brain injury with different etiologic risk factors than the white matter injury or stroke reported in prior studies of children with CHD.
The low incidence of overt ischemic injury such as focal stroke or PVL in our population was an interesting finding. Previous studies have reported that 41–67% of infants with CHD of all types had ischemic brain injury such as focal stroke and/or PVL by brain MRI (11–14). In contrast, we found only one subject with stroke (2%) and two subjects with PVL (4%) by MRI among our 48 subjects. It is possible that subtle ischemic injury may not be detected by brain MRI at one year of age, as opposed to the preoperative and early postoperative timing of scans performed in prior studies (11–14,25). However, focal stroke of moderate size as reported in previous studies (13,14) should be readily apparent as areas of encephalomalacia by brain MRI at one year of age. Moreover, the one subject in our series found to have a stroke had a very small area of focal encephalomalacia, demonstrating the sensitivity of MRI to detect an old small stroke at one year of age. It is not known whether the mild PVL reported by other groups would have the same appearance by one-year MRI as the PVL in our two subjects, or whether it would be inapparent by MRI at this later age. A previous report suggested that CHD patients show resolution of PVL at 4–6 months (11). However, this observation may be due in part to the relative insensitivity of MRI to detect mild PVL at 4–6 months of age, as suggested by the authors (11). For infants born prematurely, we have found that MRI scans performed at either term age or at one year of age or older are best for detection of PVL, since the white matter is of uniform signal intensity - either largely unmyelinated or myelinated at these ages (respectively). This background of uniform signal intensity in the white matter facilitates detection of focal areas of signal abnormality. At 4–6 months of age, the cerebral white matter is partially myelinated (26), rendering detection of abnormalities more difficult against a background of white matter with intermediate signal intensity. However, the lack of preoperative or early postoperative imaging in our study limits direct comparison of the incidence and severity of white matter injury with previous reports.
Because the incidence of stroke and PVL were low in our study, we could not determine which perioperative clinical factors were associated with these lesions. In particular, we did not find a strong association between BAS and either stroke or PVL. Only 1 of our 17 subjects who underwent BAS had a stroke (incidence of 6% of those with BAS, or 5% of all TGA subjects) and none of our subjects with TGA had PVL, compared with a previous report of PVL and stroke combined occurring in 58% of TGA patients who underwent BAS (13). These discrepant findings raise the question of whether focal stroke and PVL in TGA patients following BAS may be related to institutional differences in detection or interpretation of PVL by MRI or to differences in management. The strokes reported in prior studies of children with CHD appear to be embolic (11,13,14). In our series, only two subjects who underwent BAS received heparin; differences between institutions in the occurrence of stroke in TGA subjects thus are unlikely to be related to use of prophylactic anticoagulation during BAS.
In addition to ischemic injury, previous studies have reported frequent germinal matrix/intraventricular hemorrhage (GMH/IVH) or choroid plexus hemorrhage, subdural hemorrhage and (rarely) parenchymal hemorrhage occurring in infants with CHD, in both the preoperative and postoperative periods (11,14,22). We acquired a susceptibility-weighted sequence (MPGR) specifically to detect evidence of prior hemorrhage or hemorrhagic stroke, but found neither in our cohort. Evidence of old germinal matrix-intraventricular and parenchymal hemorrhage should be apparent by MPGR sequence one year later. Discrepancies in our findings from those in previous reports may reflect differences in management or in the population of CHD subjects in our study. The lack of specific description of the MRI sequences performed in previous studies precluded us from determining whether differences in detection of hemosiderin foci were related to variation in the acquisition parameters of the susceptibility-weighted sequences (11,12,22).
Our manuscript should be viewed in light of its limitations. Because we did not perform preoperative scans, we could not determine definitively whether hemosiderin foci were present prior to either catheterization or surgical procedures. Thus we could not determine whether these foci were related to interventions, or whether they might have been present in the preoperative period. In addition, findings in our sample of infants who underwent a single two-ventricle surgical repair at a single institution may not be generalizable to the broader population of children with CHD.
In summary, our data demonstrate a low incidence of focal stroke or PVL, but a new finding of tiny foci of hemosiderin associated with worse developmental outcome at age one year among children who underwent two-ventricle repair in infancy. These data support the notion that neurodevelopmental impairments in children surviving with surgically repaired CHD are due only in part to easily detected neurologic lesions such as stroke or PVL. Instead, we found that radiologically subtle brain injury was associated with neurodevelopmental impairments in our series, and that this lesion was associated with older age at surgery. This type of brain injury may be better detected with more sensitive or quantitative approaches to MRI data acquisition and analysis and the use of serial imaging studies to determine the timing of injury, as demonstrated in a study employing serial diffusion tensor imaging (27). The development of a susceptibility-weighted imaging technique that employs a high-spatial resolution 3D gradient MR technique with phase post-processing (28) may provide an even more sensitive method for detecting small foci of hemosiderin than the conventional 2D, long TE, T2-weighted gradient recalled echo sequence (with no additional image processing) used in this study. A standardized approach to serial imaging across institutions may help elucidate institutional differences in the incidence, type and causes of brain injury in children with CHD.
Acknowledgments
Supported by grants HL 063411 and RR 02172 from the National Institutes of Health.
Abbreviations
- CHD
congenital heart disease
- PVL
periventricular leukomalacia
- BAS
balloon atrial septostomy
- TGA
D-transposition of the great arteries
- TOF
tetralogy of Fallot
- VSD
ventricular septal defect
- MDI
Mental Development Index
- PDI
Psychomotor Development Index
- BSID-II
Bayley Scales of Infant Development II
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Registered with clinicaltrials.gov (#) NCT00006183
References
- 1.Bellinger D, Jonas R, Rappaport L, Wypij D, Wernovsky G, Kuban K, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. New Eng J Med. 1995;332:549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 2.Miller G, Tesman J, Ramer J, Baylen B, Myers J. Outcome after open-heart surgery in infants and children. J Child Neurol. 1996;11:49–53. doi: 10.1177/088307389601100112. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger DC, Wypij D, Kuban KC, Rappaport LA, Hickey PR, Wernovsky G, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100(5):526–32. doi: 10.1161/01.cir.100.5.526. [DOI] [PubMed] [Google Scholar]
- 4.Bellinger DC, Bernstein JH, Kirkwood MW, Rappaport LA, Newburger JW. Visual-spatial skills in children after open-heart surgery. J Dev Behav Pediatr. 2003;24(3):169–79. doi: 10.1097/00004703-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Bellinger DC, Wypij D, duDuplessis AJ, Rappaport LA, Jonas RA, Wernovsky G, et al. Neurodevelopmental status at eight years in children with dextro-transposition of the great arteries: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126(5):1385–96. doi: 10.1016/s0022-5223(03)00711-6. [DOI] [PubMed] [Google Scholar]
- 6.Karl TR, Hall S, Ford G, Kelly EA, Brizard CP, Mee RB, et al. Arterial switch with full-flow cardiopulmonary bypass and limited circulatory arrest: neurodevelopmental outcome. J Thorac Cardiovasc Surg. 2004;127(1):213–22. doi: 10.1016/j.jtcvs.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Wernovsky G, Shillingford AJ, Gaynor JW. Central nervous system outcomes in children with complex congenital heart disease. Curr Opin Cardiol. 2005;20(2):94–9. doi: 10.1097/01.hco.0000153451.68212.68. [DOI] [PubMed] [Google Scholar]
- 8.Majnemer A, Limperopoulos C, Shevell M, Rosenblatt B, Rohlicek C, Tchervenkov C. Long-term neuromotor outcome at school entry of infants with congenital heart defects requiring open-heart surgery. J Pediatr. 2006;148(1):72–7. doi: 10.1016/j.jpeds.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 9.Hsia TY, Gruber PJ. Factors influencing neurologic outcome after neonatal cardiopulmonary bypass: what we can and cannot control. Ann Thorac Surg. 2006;81(6):S2381–8. doi: 10.1016/j.athoracsur.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 10.Kinney HC, Panigrahy A, Newburger JW, Jonas RA, Sleeper LA. Hypoxic-ischemic brain injury in infants with congenital heart disease dying after cardiac surgery. Acta Neuropathol (Berl) 2005;110(6):563–78. doi: 10.1007/s00401-005-1077-6. [DOI] [PubMed] [Google Scholar]
- 11.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, et al. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106(12 Suppl 1):I109–14. [PubMed] [Google Scholar]
- 12.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, et al. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2004;127(3):692–704. doi: 10.1016/j.jtcvs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 13.McQuillen PS, Hamrick SE, Perez MJ, Barkovich AJ, Glidden DV, Karl TR, et al. Balloon atrial septostomy is associated with preoperative stroke in neonates with transposition of the great arteries. Circulation. 2006;113(2):280–5. doi: 10.1161/CIRCULATIONAHA.105.566752. [DOI] [PubMed] [Google Scholar]
- 14.McQuillen PS, Barkovich AJ, Hamrick SE, Perez M, Ward P, Glidden DV, et al. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38(2 Suppl):736–41. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- 15.du Plessis AJ, Jonas RA, Wypij D, Hickey PR, Riviello J, Wessel DL, et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114(6):991–1000. doi: 10.1016/S0022-5223(97)70013-8. discussion 1000–1. [DOI] [PubMed] [Google Scholar]
- 16.Jonas RA, Wypij D, Roth SJ, Bellinger DC, Visconti KJ, du Plessis AJ, et al. The influence of hemodilution on outcome after hypothermic cardiopulmonary bypass: results of a randomized trial in infants. J Thorac Cardiovasc Surg. 2003;126(6):1765–74. doi: 10.1016/j.jtcvs.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Newburger JW, Wypij D, Bellinger DC, du Plessis AJ, Kuban KC, Rappaport LA, et al. Length of stay after infant heart surgery is related to cognitive outcome at age 8 years. J Pediatr. 2003;143(1):67–73. doi: 10.1016/S0022-3476(03)00183-5. [DOI] [PubMed] [Google Scholar]
- 18.Wypij D, Newburger JW, Rappaport LA, duPlessis AJ, Jonas RA, Wernovsky G, et al. The effect of duration of deep hypothermic circulatory arrest in infant heart surgery on late neurodevelopment: the Boston Circulatory Arrest Trial. J Thorac Cardiovasc Surg. 2003;126(5):1397–403. doi: 10.1016/s0022-5223(03)00940-1. [DOI] [PubMed] [Google Scholar]
- 19.Tabbutt S, Nord AS, Jarvik GP, Bernbaum J, Wernovsky G, Gerdes M, et al. Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatrics. 2008;121(3):476–83. doi: 10.1542/peds.2007-1282. [DOI] [PubMed] [Google Scholar]
- 20.Newburger JW, Jonas RA, Soul J, Kussman BD, Bellinger DC, Laussen PC, et al. Randomized trial of hematocrit 25% versus 35% during hypothermic cardiopulmonary bypass in infant heart surgery. J Thorac Cardiovasc Surg. 2008;135(2):347–54. 354, e1–4. doi: 10.1016/j.jtcvs.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Maier SE, Gudbjartsson H, Patz S, Hsu L, Lovblad KO, Edelman RR, et al. Line scan diffusion imaging: characterization in healthy subjects and stroke patients. AJR Am J Roentgenol. 1998;171(1):85–93. doi: 10.2214/ajr.171.1.9648769. [DOI] [PubMed] [Google Scholar]
- 22.Tavani F, Zimmerman RA, Clancy RR, Licht DJ, Mahle WT. Incidental intracranial hemorrhage after uncomplicated birth: MRI before and after neonatal heart surgery. Neuroradiology. 2003;45(4):253–8. doi: 10.1007/s00234-003-0946-8. [DOI] [PubMed] [Google Scholar]
- 23.Glauser T, Rorke L, Weinberg P, Clancy R. Acquired neuropathologic lesions associated with the hypoplastic left heart syndrome. Pediatrics. 1990;85(6):991–1000. [PubMed] [Google Scholar]
- 24.Majnemer A, Limperopoulos C, Shevell M, Rohlicek C, Rosenblatt B, Tchervenkov C. Developmental and functional outcomes at school entry in children with congenital heart defects. J Pediatr. 2008;153(1):55–60. doi: 10.1016/j.jpeds.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, et al. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007;357(19):1928–38. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 26.Barkovich AJ, Kjos BO, Jackson DE, Jr, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166(1 Pt 1):173–80. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- 27.Partridge SC, Vigneron DB, Charlton NN, Berman JI, Henry RG, Mukherjee P, et al. Pyramidal tract maturation after brain injury in newborns with heart disease. Ann Neurol. 2006;59(4):640–51. doi: 10.1002/ana.20772. [DOI] [PubMed] [Google Scholar]
- 28.Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI) Magn Reson Med. 2004;52(3):612–8. doi: 10.1002/mrm.20198. [DOI] [PubMed] [Google Scholar]