Abstract
Cited2 is an important transcriptional cofactor involved in multiple organ development. Gene profile analysis has identified Cited2 as one of the transcription factors expressed at high levels in adult mouse cornea. To address the function of Cited2 in corneal morphogenesis, we deleted Cited2 in surface ectoderm derived ocular structures including cornea by crossing Cited2-floxed mice with Le-Cre transgenic mice. Cited2flox/flox;Le-Cre+ eyes invariably displayed corneal opacity and developed spontaneous corneal neovascularization at older age. Fewer layers of corneal epithelial cells and the absence of cytokeratin 12 (K12) expression featured Cited2 deficient postnatal and adult eyes. Cited2 deficient cornea exhibited impaired healing in response to corneal epithelial debridement by manifesting abnormal histology, lack of K12 expression and corneal neovascularization. Moreover, mechanistic studies suggest that Cited2 may play a role in corneal morphogenesis in part through modulating the expression of Pax6 and Klf4. Collectively, these findings demonstrate a novel function of Cited2 in postnatal corneal morphogenesis and maintenance. Our study will help better understand the molecular mechanisms involved in corneal biology, and more importantly, it may provide a valuable animal model for testing therapeutics in the treatment of corneal disorders, especially blindness as a result of corneal epithelial cell deficiency.
Keywords: Cited2, Corneal epithelial cell, K12 expression, Wound healing
Introduction
Localized at the most anterior aspect of the eye, the transparent cornea is not only a major refractive component of the vision system, but also functions as the primary protective barrier against environmental insults. Defects in its development, maturation, infection or trauma can result in visual impairment. Corneal opacification, neovascularization, fibrosis and improper wound healing as a result of disease or injury have been recognized as the second-leading cause of blindness worldwide (Whitcher et al., 2001). Therefore, preservation of the integrity and transparency of the cornea is an essential component for vision. To achieve this, it is important to understand the molecular mechanisms responsible for the maintenance and restoration of corneal integrity in response to physiological and pathological injury, which in turn will facilitate the development of therapeutic strategies fighting the blindness caused by corneal dysfunction.
The cornea is composed of three major cellular layers: an outer epithelium, a central stroma and an inner monolayered endothelium. In mouse, the eyes open around postnatal day 14 and cells in the one- to two-cell-layered epithelium proliferate and differentiate to form a four- to five-cell-layered corneal epithelium by 21 days after birth. The mature corneal epithelium contains five to eight cell layers and the maturation process is achieved by 6 to 8 weeks after birth (Hay, 1979; Zieske, 2004). The superbasal corneal epithelial cells slough off on a regular basis and are replenished by corneal epithelial stem cells (Thoft and Friend, 1983; Collinson et al., 2002). The program of proliferation and differentiation is under meticulous control in the adult mouse, which is essential for corneal homeostasis as it allows the most superficial corneal epithelial cells to be continuously replenished by oligopotent stem cells distributed throughout the ocular surface (Majo et al., 2008).
The differentiation mechanisms responsible for replenishing corneal epithelial cells are believed to be associated with changes in gene expression. SAGE (Serial Analysis of Gene Expression) identified Cited2 (CBP/p300-interacting transactivators with glutamic acid (E) and aspartic acid (D)-rich tail 2) as one of the transcriptional regulators with higher expression in adult mature cornea versus postnatal day 9 mouse cornea (Norman et al., 2004), suggesting the potential involvement of Cited2 in adult corneal maturation and maintenance. Cited2 is a member of a newly identified family of transcriptional modulators (Sun et al., 1998; Shioda et al., 1997; Bhattacharya et al., 1999). It is a nuclear protein that binds directly with high affinity to the first cysteine-histidine-rich (CH1) region of the transcriptional cofactors p300 and CBP. Cited2 physically interacts with several nuclear receptors and transcriptional factors, such as peroxisome proliferator-activated receptor (PPAR) (Tien et al., 2004), LIM domain-containing transcription factor Lhx2 (Glenn and Maurer, 1999), AP-2 transcription factors (Bamforth et al., 2001), SMAD2/3 (Chou et al., 2006), HNF4 α (Qu et al., 2007) and WT1 (Val et al., 2007). Cited2 also functions as a negative regulator of hypoxia inducible factor (HIF)-1 mediated signaling by competing with HIF-1α for binding to CBP/p300 (Bhattacharya et al., 1999). Cited2 is induced by many biological stimuli such as cytokines, serum and LPS in different cell types (Sun et al., 1998). Overexpression of Cited2 in Rat1 cells results in loss of cell contact inhibition, anchorage-independent growth and tumor formation in nude mice, suggesting that Cited2 is a transforming gene (Sun et al., 1998). These in vitro studies underscore the potential roles of Cited2 in various biological processes. Targeted deletion of Cited2 is embryonic lethal with embryos manifesting developmental defects in multiple organs (Bamforth et al., 2001; Bamforth et al., 2004; Barbera et al., 2002; Buaas et al., 2009; Chen et al., 2007; Qu et al., 2007; Val et al., 2007; Weninger et al., 2005; Withington et al., 2006; Xu et al., 2008; Yin et al., 2002). It is worth noting that Cited2 is essential for eye development since Cited2 deficient eyes display lens stalk formation, hyaloid hypercellularity and aberrant hyaloid vasculature during embryonic development and postnatal life (Chen et al., 2008). In the present study, we have observed corneal opacity, thinning of corneal epithelium, loss of differentiation marker K12 expression in the corneal epithelium, impaired corneal epithelial wound healing and spontaneous corneal neovascularization in Cited2 deficient eyes. Therefore, we have identified Cited2 as a novel molecule involved in postnatal corneal epithelial cell differentiation and maintenance.
Materials and Methods
Mouse lines
Both Cited2flox/flox (Preis et al., 2006) and Le-Cre transgenic (Ashery-Padan et al., 2000) mice lines were maintained on the C57BL/6 background. Cited2flox/flox mice were bred with Le-Cre transgenic mice to generate Cited2flox/flox;Le-Cre+ and Cited2flox/flox;Le-Cre− mice.
Histology
Enucleated eye balls were fixed in 10% formalin, dehydrated, embedded in paraffin and processed with 7μm transverse sectioning. Histology of the eyes was examined by light microscopy after hematoxylin and eosin (H&E) staining.
Immunohistochemistry
Heads from embryonic day 17.5 (E17.5) embryos were fixed in 4% paraformaldehyde, equilibrated in 30% sucrose, embedded in O.C.T. and processed with 10μm cryosectioning. Enucleated eye balls from postnatal and adult mice were fixed in 10% formalin and processed for paraffin embedding. Paraffin-embedded sections were deparaffinized first before processed for immunohistochemistry. Cited2 immunostaining was performed using primary antibody against Cited2 (sc25375, Santa Cruz) and Alexa Fluor 488-conjugated anti-rabbit secondary antibody (A11008, Invitrogen). K12 immunostaining was performed using antibody against K12 (sc17101, Santa Cruz) in conjunction with ABC kit (PK-4005, Vector Laboratory) and antibody staining was developed with 3, 3′-diaminobenzidine (Sigma). α-smooth muscle actin (α-SMA) immunostaining was performed with antibodies against α-SMA (A5228, Sigma) and antibody staining was visualized with Alexa Fluor 594-conjugated anti-mouse secondary antibody (A11005, Invitrogen).
Real-time PCR
Corneas were dissected from enucleated eyes of mice at 6 weeks old. Total RNA was extracted using TRIzol (Invitrogen) and reverse transcribed using Superscript II reverse transcriptase. Real-time PCR was performed to measure the mRNA expression levels of Pax6 (Chen et al., 2008) and Klf4 using the following primers: Klf4 forward 5′-ccaccaggactacccctaca-3′: Klf4 reverse:5′-ggggacttgtgactgcatct-3′.
Wound healing assay
Mice at 6 weeks of age were anesthetized before performing the corneal epithelial debridement. The central corneal epithelium (2 mm in diameter) was demarcated with a 2 mm trephine and subsequently removed using an AlgerbrushII (Alger Co.) tipped with a 0.5 mm burr under a dissecting stereomicroscope. To prevent ocular surface desiccation, eyes were kept moisturized with methylcellulose until recumbent. The mice were sacrificed 7 days after the wounding and the eyes were enucleated and fixed in 4% paraformaldehyde for further histological and immunohistochemical examination.
Luciferase assay
HCE (Human Corneal Epithelial), NMuMG (Normal Murine Mammary Gland Epithelial) and HEK293 (Human Embryonic Kidney) cells were seeded in 24-well plates for transfection of 0.15ng pRLSV40, 40ng of 1Kb human Klf4 promoter-containing firefly luciferase reporter, various amounts of Cited2 expression plasmid and control plasmid so that the total amount of DNA was 0.4μg/well. 20ng of various truncated mouse Klf4 promoter-containing firefly luciferase reporter constructs (Chen et al., 2002) were also tested in a similar manner. Fugene6 transfection reagent (Roche) was used for the transfection. Firefly and Renilla luciferase activities were measured 24 hours after transfection using Dual-luciferase assay reagents (Promega) on luminometer. Relative luciferase activity was calculated by dividing firefly luciferase activity by Renilla luciferase activity.
Results
Le-Cre mediated conditional deletion of Cited2, a gene highly expressed in adult cornea epithelial cells, results in corneal opacity
The expression of Cited2 in adult mouse cornea was first demonstrated by SAGE analysis (Norman et al., 2004). To verify the protein expression of Cited2 in adult cornea epithelial cells, immunostaining was performed and revealed Cited2 nuclear localization mainly in the majority of basal corneal epithelial cells and some of the supra-basal cells (Figure 1a, 1c). To explore the function of Cited2 in corneal epithelial cells, conditional deletion of Cited2 in the surface ectoderm derived tissues including cornea was performed by crossing Le-Cre transgenic mice (Ashery-Padan et al., 2000) with Cited2flox/flox mice (Preis et al., 2006) since targeted deletion of Cited2 results in embryonic lethality. Le-Cre is expressed in the surface ectoderm from embryonic day (E) 9.5 and in surface ectoderm-derived structures including the developing lens, cornea, conjunctiva and skin of the eye lids. As expected, Cited2 nuclear localization was not detected in corneal epithelial cells of Cited2flox/flox;Le-Cre mice, indicating efficient deletion of floxed Cited2 alleles mediated by Le-Cre transgene (Figure 1b). The ocular effect of Le-Cre mediated conditional deletion of Cited2 was notable due to the external feature of small eyes (Chen et al., 2008). Macroscopic examination further showed that, in contrast to the normal transparent and avascular cornea in Cited2flox/flox controls examined at ages up to 8-month old (Figure 1d), opaque cornea was characteristic of all the Cited2flox/flox;LeCre+ mice (Figure 1e). In addition, by 8-month of age, spontaneous corneal neovascularization was invariably detected in Cited2flox/flox;LeCre+ mice (Figure 1e).
Figure 1. Le-Cre mediated deletion of Cited2 results in opaque cornea and age-dependent spontaneous corneal neovascularization.
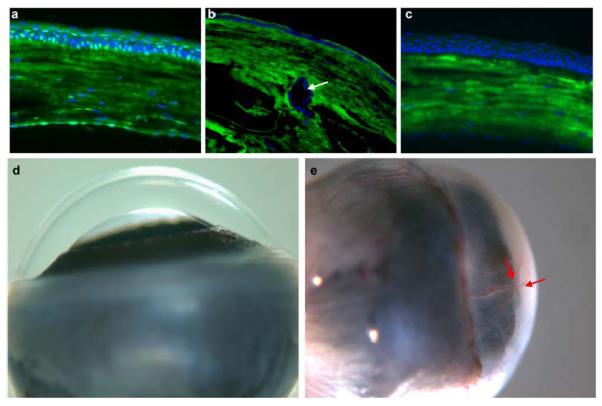
Eyeballs were enucleated from wild type and Cited2flox/flox;Le-Cre+ mice at 6 weeks of age and cryosections were prepared for immunostaining using rabbit anti-Cited2 primary antibody and Alexa Fluor 488 anti-rabbit secondary antibody (Green). Nuclei counterstain was performed using DAPI (Blue). Merged images showed Cited2 immunoreactivity in wild type corneal epithelial cells (a). Cited2 expression was barely detected in corneal epithelial cells of Cited2flox/flox;Le-Cre+ mice (b). Arrow in b indicates the abnormally formed lens stalk in Cited2flox/flox;Le-Cre+ eyes (Chen et al., 2008). Negative control for immunostaining was performed without the primary antibody (c). The immunofluorescence background in cornea detected in (a)-(c) is mainly due to the nonspecific binding of the secondary antibody. Macroscopic examination revealed that in contrast to the transparent and avascular cornea in Cited2flox/flox controls (d), Cited2flox/flox;Le-Cre+ eyes showed opaque cornea and prominent corneal neovascularization at 8 month of age (red arrows in e).
Le-Cre mediated conditional deletion of Cited2 results in defective corneal morphogenesis
The cornea of Cited2flox/flox;Le-Cre+ mice is opaque and the corneal epithelium is fragile compared to the Cited2flox/flox control (data not shown). We further examined the histological features of Cited2 deficient eyes. Histological examination revealed that the corneal epithelium was composed of six to seven cell layers in Cited2flox/flox controls at 6 weeks of age (Figure 2a), whereas thinner corneal epithelium with two to three layers of corneal epithelial cells was invariably detected in age-matched Cited2flox/flox;Le-Cre+ eyes (Figure 2b). Moreover, blood vessels were detected in the corneal stroma in Cited2flox/flox;LeCre+ mice at 8 months of age (Supplemental Figure 1), consistent with the macroscopic observation mentioned above (Figure 1c). These data indicate that Le-Cre mediated conditional deletion of Cited2 results in abnormal corneal morphogenesis in adult eyes. The impaired corneal epithelial function was further supported by the absence of basement membrane in Cited2flox/flox;Le-Cre+ eyes (Figure 2d) compared to the Cited2flox/flox controls (Figure 2c).
Figure 2. Le-Cre mediated deletion of Cited2 results in abnormal corneal morphology and increased corneal epithelial proliferation.
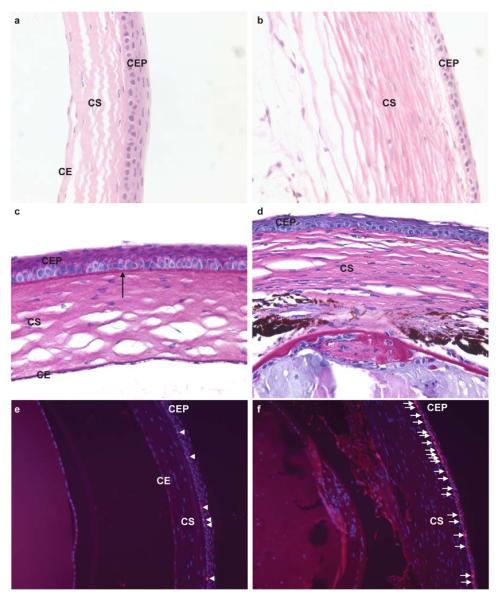
Histological examination was performed by the H&E staining of eye sections collected from 6.5-week old mice. Compared to the normal morphology of the Cited2flox/flox control cornea (a), thinner corneal epithelium and fewer corneal epithelial cells were observed in Cited2flox/flox;Le-Cre+ cornea (b). PAS staining was also performed in conjunction with H&E staining and revealed intact basement membrane in Cited2flox/flox control cornea (arrow in c), but not in Cited2flox/flox;Le-Cre+ cornea (d). Ki-67 immunostaining (red color) and DAPI counterstain (blue color) further showed increased Ki-67 positive cells (pink color merged from Ki-67 and DAPI staining) in Cited2flox/flox;Le-Cre+ corneal epithelium (arrows in f) compared to the few Ki-67 positive corneal basal cells detected in Cited2flox/flox control (arrowheads in e) (CEP: corneal epithelium; CS: corneal stroma; CE: corneal endothelium).
To test whether thinner corneal epithelium occurs as a result of decreased proliferation of corneal epithelial cells, we further examined the expression of Ki-67, a molecular marker for cellular proliferation. Interestingly, an increased number of Ki-67 positive cells was detected in Cited2flox/flox;Le-Cre+ corneal epithelium (Figure 2f) compared to that from the Cited2flox/flox controls (Figure 2e). This result indicates that decreased cell proliferation is not likely to be responsible for fewer corneal epithelial layers and thinner corneal epithelium in Cited2 deficient eyes. However, excessive sloughing-off as a result of corneal epithelial fragility due to Cited2 deficiency may increase the proliferation of corneal epithelial cells as a compensatory mechanism.
Cited2 deficient corneal epithelium lacks expression of K12 in postnatal life
The histological abnormalities suggest defective corneal epithelial differentiation in Cited2 deficient cornea. K12 is a tissue specific keratin expressed specifically in cornea epithelium and its expression is differentiation-dependent (Kurpakus et al., 1994; Liu et al., 1999; Tanifuji-Terai et al., 2006). Loss of K12 has been shown to be responsible for corneal fragility (Kao et al., 1996). K12 expression was therefore examined by immunohistochemistry at different developmental stages in Cited2flox/flox;Le-Cre+ eyes with Cited2flox/flox littermates included as normal controls. At E17.5, K12 expression was readily detected in both Cited2flox/flox littermate control (Figure 3a) and Cited2flox/flox;Le-Cre+ corneal epithelium (Figure 3b). However, at postnatal day 14, K12 expression was detected in the corneal epithelium in Cited2flox/flox littermate controls (Figure 3c) but not in the corneal epithelium of Cited2flox/flox;Le-Cre+ mice (Figure 3d). In 6.5-week old mice, abundant expression of K12 was detected in Cited2flox/flox littermate control (Figure 3e) but not in Cited2flox/flox;Le-Cre+ corneal epithelium (Figure 3f). Our data indicate that corneal epithelial K12 expression is affected by Cited2 deficiency in postnatal life but not during embryonic development. This indicates that Cited2 deficiency may be associated with defective differentiation of the corneal epithelium in postnatal life rather than in the development of the corneal epithelium in the embryo.
Figure 3. K12 expression is not detected in Cited2flox/flox;Le-Cre+ cornea at postnatal life.
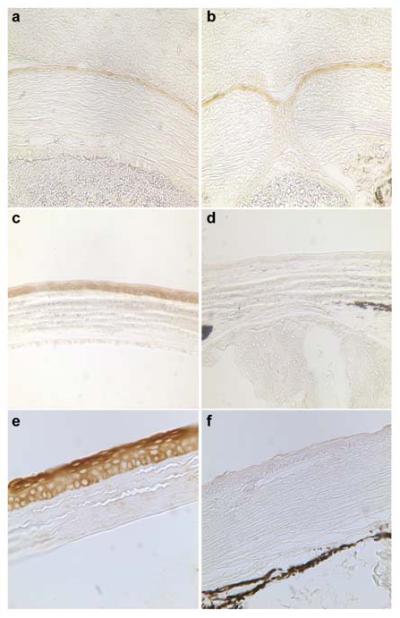
Immunohistochemical examination of K12 expression in corneal epithelium was performed on the eye sections. At E17.5, K12 expression was readily detected in both Cited2flox/flox controls (brown color in a) and Cited2flox/flox;Le-Cre+ corneal epithelium (brown color in b). In P14 mice, K12 expression showed organized and uniform expression pattern in corneal epithelial cells in Cited2flox/flox control mice (brown color in c); however, K12 expression was barely detected in Cited2flox/flox;Le-Cre+ mouse cornea (d). In sharp contrast to the abundant K12 expression in Cited2flox/flox controls (brown color in e), K12 expression was barely detected in the corneal epithelium in Cited2flox/flox;Le-Cre+ mice at 6.5 weeks of age (f).
Aberrant corneal epithelial wound healing response in Cited2 deficient eyes
To further address the role of Cited2 in adult corneal maintenance, a corneal epithelial wound healing model was employed. A 2-mm diameter circular corneal epithelial wound was created in Cited2flox/flox;Le-Cre+ mice and Cited2flox/flox littermate controls at 6 weeks of age. Corneal wound healing was assessed macroscopically and followed by histological examination 1 week after wounding. Cited2flox/flox control cornea healed normally as the corneas displayed transparent and avascular features macroscopically (Figure 4a). However, the Cited2flox/flox;Le-Cre+ cornea healed with severe opacification and neovascularization (Figure 4b). The onset of corneal neovascularization was observed 48 hours after wounding (data not shown) in Cited2flox/flox;Le-Cre+ eyes, which was never observed in Cited2flox/flox littermate control eyes. Fluorescein injection to visualize the vasculature was implemented to verify corneal vascularization in Cited2flox/flox;Le-Cre+ eyes. In Cited2flox/flox control eyes, the fluorescein injection revealed normal limbal vasculature and the cornea itself is fluorescein negative (Figure 4c). Consistent with the macroscopic observation under bright field, fluorescein positive vasculature was detected in Cited2flox/flox;Le-Cre+ cornea (Figure 4d). Further histological analysis by H&E staining on eye sections revealed vessels containing red blood cells in Cited2flox/flox;Le-Cre+ cornea (Figure 4f) compared to the normal histological feature in Cited2flox/flox control cornea (Figure 4e). It is also notable that neutrophils were present in Cited2flox/flox;Le-Cre+ cornea (Figure 4f), suggestive of an inflammatory response. The aberrant neovascularization in the Cited2flox/flox;Le-Cre+ cornea was further visualized by CD31 (Figure 4g) and α-SMA (Figure 4h) immunostaining, which detects vascular endothelial cells and pericytes/myofibroblasts, respectively. Furthermore, K12 expression was not detected in Cited2flox/flox;Le-Cre+ corneas (Figure 4j), whereas it was readily detected in Cited2flox/flox controls (Figure 4i). These results indicate that wound healing response is significantly impaired in Cited2 deficient cornea and further suggest that Cited2 plays an important role in corneal epithelial differentiation and maintenance in postnatal life.
Figure 4. Impaired corneal epithelial wound healing response in Cited2 deficient eyes.
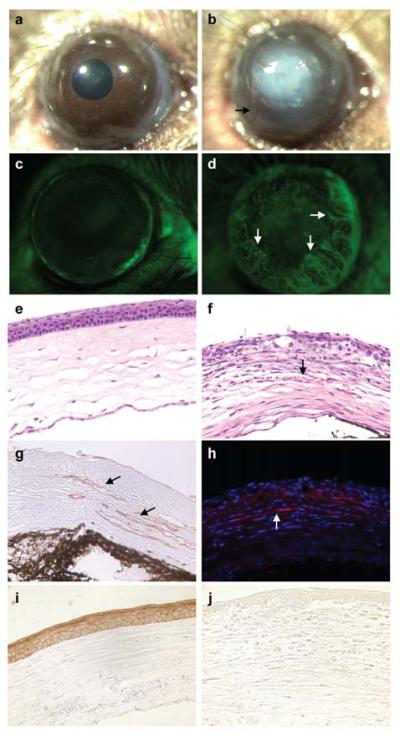
Corneal epithelial debridement was created in the eyes of 6-week old Cited2flox/flox littermate controls and Cited2flox/flox;Le-Cre+ mice. One week after wounding, macroscopic examination showed severe corneal opacification (b) and aberrant corneal neovascularization (arrow in b) in Cited2flox/flox;Le-Cre+ eyes, which is in distinct contrast to the well healed cornea in Cited2flox/flox controls (a). Fluorescein injection was also performed and showed that only the limbus region was fluorescein positive in Cited2flox/flox controls, whereas the Cited2flox/flox;Le-Cre+ eyes showed fluorescein positive vasculature in the cornea (arrows in d). Further histological examination after H&E staining on eye sections revealed vessels containing red blood cells in Cited2flox/flox;Le-Cre+ corneal stroma (arrow in f) compared to the avascular feature of Cited2flox/flox controls (e). The corneal neovascularization in Cited2flox/flox;Le-Cre+ eyes was further confirmed by CD31 immunostaining (g, brown color; arrows) and α-SMA (h, red for α-SMA and blue for DAPI; arrow) immunostaining. K12 immunostaining was performed and abundant K12 expression was detected in healed corneal epithelium in Cited2flox/flox controls (i, brown color); however, K12 expression was barely detected in Cited2flox/flox;Le-Cre+ corneas (j).
Decreased expression of Pax6 and Klf4 in Cited2 deficient cornea
The defective corneal epithelial morphogenesis exhibited by Cited2flox/flox;Le-Cre+ eyes is similar to the corneal phenotypes in mice deficient for Pax6 (Collinson et al., 2004; Davis et al., 2003) or with conditional deletion of Klf4 (Swamynathan et al., 2007). Pax6 deficient eyes display decreased thickness of the corneal epithelium consisting of fewer layers of corneal epithelial cells and significantly decreased expression of K12. The Klf4 deficient cornea is composed of fewer layers of corneal epithelial cells and K12 expression is undetectable in Klf4 deficient corneal epithelium. It has also been reported that both Pax6 and Klf4 directly regulate K12 expression (Swamynathan et al., 2007; Davis et al., 2003; Li et al., 2008; Shiraishi et al., 1998). In addition, we have shown that Cited2 controls Pax6 expression in developing lens, as decreased Pax6 mRNA expression was observed in Cited2 deficient embryonic lens and abnormally formed lens stalk was corrected by increasing Pax6 expression in Cited2 deficient eyes (Chen et al., 2008). Thus, it is likely Pax6 could mediate Cited2 function in the cornea. To achieve a quantitative comparison of Pax6 expression in the cornea, we assessed mRNA expression of Pax6 in Cited2flox/flox;Le-Cre+ corneas and their wild type littermate controls using quantitative real-time PCR. Pax6 mRNA level in Cited2flox/flox;Le-Cre+ corneas was decreased about 2.5-fold compared to that from Cited2flox/flox controls (Figure 5a), which suggests that Cited2 could function in part through Pax6 during corneal morphogenesis. Interestingly, in addition to decreased Pax6 expression, Klf4 mRNA expression level was also significantly decreased about 5-fold in Cited2flox/flox;Le-Cre+ corneas compared to that of Cited2flox/flox controls (Figure 5b). We further performed a luciferase reporter assay to test the hypothesis that Cited2 is required for Klf4 expression in corneal epithelial cells. HCE cells were transfected with a luciferase reporter plasmid containing the Klf4 promoter and different amounts of Cited2 expressing plasmid. Klf4 promoter activity was enhanced in response to Cited2 overexpression in a dose-dependent manner compared to the control without Cited2 overexpression (Figure 5c). The effect of Cited2 overexpression on increasing Klf4 reporter activity was reproducibly observed in two other epithelial cell lines, NmuMG and HEK 293 (Figure 5c). To further dissect the transcriptional regulation of Klf4 promoter by Cited2, a series of truncated mouse Klf4 promoter constructs (Chen et al., 2002) were tested for the response to Cited2 expression in HCE cells. As shown in Figure 5d, Cited2 overexpression consistently increased the full-length mouse Klf4 promoter activity. The region responsible for the most positive response of mouse Klf4 promoter to Cited2 expression was narrowed down to −1190 to −716 upstream of transcriptional starting site. Sequence analysis of this region revealed numerous transcriptional factor-binding sites, including AP-2α previously shown to be associated with Cited2’s function during cardiac development (Bamforth et al., 2001). Since AP-2α is implicated in corneal epithelial cell functions (Dwivedi et al., 2005), the effect of AP-2α on the Klf4 promoter activity also examined. As shown in Figure 5e, AP-2α did not activate the Klf4 promoter. Instead, overexpression of AP-2α resulted in decreased Klf4 promoter activity, suggesting more complicated positive and negative regulatory mechanisms are involved. Nonetheless, our data suggest that Cited2 is required for Klf4 expression and Cited2 deficiency results in decreased Klf4 expression, which is in part responsible for the defective corneal morphogenesis in Cited2flox/flox;Le-Cre+ eyes.
Figure 5. Cited2 deficiency results in decreased expression of Pax6 and Klf4 in mouse cornea.
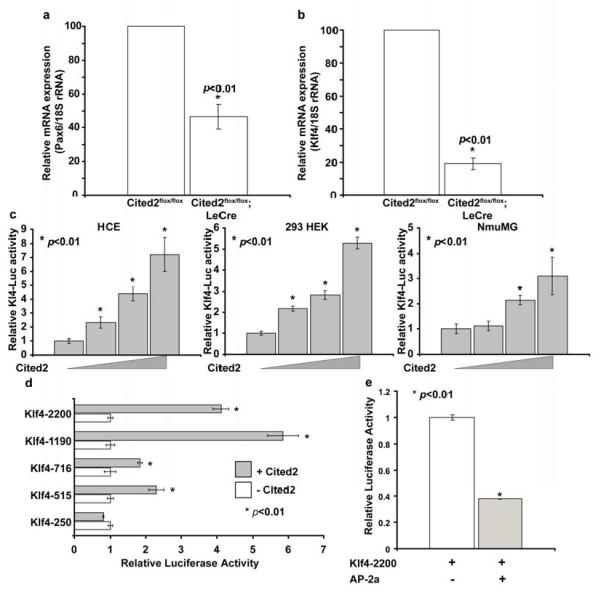
Pax6 and Klf4 mRNA expression was quantitatively examined in corneas from mice at 6 weeks of age by Real-time PCR. Around 50% reduction of Pax6 mRNA expression was detected in Cited2flox/flox;Le-Cre+ corneas (n=3) as compared to Cited2flox/flox littermate controls (n=3) (a). In the meantime, Klf4 mRNA expression in Cited2flox/flox;Le-Cre+ corneas (n=3) decreased to about 20% of that in Cited2flox/flox littermate controls (n=3) (b). Effect of Cited2 on Klf4 transcription was measured in vitro by examining the transcriptional activation of luciferase reporter containing 1Kb Klf4 promoter (Klf4-Luc) in cells without or with Cited2 overexpression. Human corneal epithelial (HCE) cells were transfected with Klf4-Luc reporter (40ng) without or with Cited2 expression plasmid (from 20ng to 400ng). Cited2 overexpression in HCE cells significantly increased the activity of Klf4-Luc reporter in a dose-dependent manner (c). Similar results were obtained from two other epithelial cell lines, HEK 293 and NumMG (c). The effect of Cited2 on Klf4 transcription was further dissected by measuring the response of various truncated mouse Klf4 promoter in the absence and presence of Cited2 overexpression. HEC cells were transfected with 20ng of truncated Klf4 promoter constructs and 200ng of Cited2 expression plasmid (d). The effect of AP-2α on the Klaf promoter was also analyzed in a similar strategy and showed significantly decreased reporter activity in response to AP-2α expression (e).
Discussion
The present study demonstrates that Le-Cre mediated selective deletion of Cited2 results in an array of corneal epithelial pathologies, including abnormal corneal epithelial differentiation marked by fewer layers of corneal epithelial cells and lack of K12 expression, impaired corneal epithelial wound healing response and spontaneous corneal neovascularization in older mice, and decreased expression of Pax6 and Klf4. This indicates for the first time that Cited2 plays a critical role in postnatal corneal maturation, maintenance and repair.
We have previously shown that Cited2 is ubiquitously expressed in the lens, cornea and retina of a developing eye. Cited2 deficiency results in persistent lens stalk formation and aberrant hyaloid vascular formation/regression, revealing a novel function of Cited2 in eye development (Chen et al., 2008). Interestingly, an independent SAGE study identified Cited2 as one of the most abundantly expressed transcription factors in the adult cornea (Norman et al., 2004). However, the potential role of Cited2 in adult corneal maturation and maintenance was hindered as targeted deletion of Cited2 results in embryonic lethality (Yin et al., 2002). In the present report, the Le-Cre mouse line was used to mediate ablation of floxed-Cited2 in surface ectoderm derived ocular tissues including the cornea (Ashery-Padan et al., 2000). Interestingly, Cited2flox/flox;Le-Cre+ eyes display opaque cornea, age-dependent spontaneous corneal neovascularization, decreased thickness of corneal epithelium composed of fewer layers of corneal epithelial cells, absence of K12 expression in postnatal life and impaired corneal wound healing response. Reduction in the thickness of the corneal epithelium may result from defective differentiation of corneal epithelial cells, which is supported by the lack of K12 expression in the Cited2 deficient cornea in postnatal life. This might result in increased proliferation of corneal epithelial cells to compensate for the excessive loss of corneal epithelial cells, a similar finding reported by others in Pax6 deficient cornea (Ramaesh et al., 2005). It is also possible that Cited2 deficiency may skew the balance between cellular proliferation and differentiation. Unchecked proliferation may lead to defective differentiation in Cited2 deficient corneal epithelium. These possibilities require further studies for their validation.
K12 is specifically expressed in the corneal epithelium in a differentiation-dependent manner. Similar to other keratins, K12 is required for the formation of cytoskeletal intermediate filaments and thus the maintenance of corneal epithelial integrity. Targeted deletion of K12 results in fewer cellular layers in corneal epithelium and corneal fragility (Kao et al., 1996; Kurpakus et al., 1994; Liu et al., 1999; Tanifuji-Terai et al., 2006). Similarly, Cited2flox/flox;Le-Cre+ corneas displayed decreased thickness in corneal epithelium. The lack of K12 expression in Cited2 deficient corneal epithelial cells suggests that corneal epithelial cell differentiation might be impaired as a result of Cited2 deficiency. This notion was further supported by the observation that Cited2flox/flox;Le-Cre+ corneas could not heal normally compared to Cited2flox/flox corneas. K12 expression was never detected in wounded adult Cited2flox/flox;Le-Cre+ cornea although K12 was uniformly and abundantly expressed in the healed corneas from Cited2flox/flox control mice. Collectively, these results provide evidence that defective corneal epithelial cell differentiation may occur as a result of Cited2 deficiency. It is also worth noting that diminished K12 expression was only observed in postnatal corneas with its expression being readily detected in embryonic corneas in Cited2 deficient eyes. This suggests that Cited2 may be specifically required for adult corneal maintenance, consistent with the finding that Cited2 expression is upregulated in adult corneas (Norman et al., 2004). Cited2 deficient corneas manifested impaired healing in response to corneal epithelial wounding by displaying neutrophil infiltration and neovascularization, whereas K12 deficient cornea did not show defects in corneal epithelial wound healing, suggesting that lack of K12 expression in the corneal epithelial cells is not likely to be responsible for impaired wound healing response in Cited2 deficient eyes. The absence of K12 expression could be directly due to the loss of Cited2, with Cited2 required for K12 expression, or indirectly due to a defect in differentiation. We have observed increased K12 luciferase reporter activity in response to Cited2 expression in a dose dependent manner (Supplemental Figure 2); however, the mechanism through which Cited2 regulates K12 expression remains to be further investigated.
We have previously shown that Cited2 regulates Pax6 expression in the developing mouse lens (Chen et al., 2008). Pax6 is a highly expressed transcription factor in the ocular components including cornea and it is required for cornea morphogenesis through regulating K12 expression, limbal stem cell activity and corneal epithelial cell migration and adhesion (Davis et al., 2003; Ramaesh et al., 2005; Shiraishi et al., 1998). Pax6+/− mice share common features with Cited2flox/flox;Le-Cre+ mice such as fewer layers of corneal epithelial cells. K12 expression was detected in some of the central corneal epithelial cells, and peripheral corneal epithelial cells showed much weaker K12 expression in Pax6+/− mice. However, K12 expression was invariably undetectable in Cited2 deficient corneas postnatally, suggesting that Pax6 might be partially involved in Cited2 mediated functions in corneal epithelial cells. This hypothesis is supported by about 2.5-fold reduction in Pax6 expression in Cited2 deficient corneas (Fig. 5a). We have previously shown that Cited2 coactivates MMP9 expression (Chou et al., 2006). MMP9 is expressed at the migrating epithelial front in the cornea during reepithelialization and coordinates multiple events in corneal epithelial regeneration. Loss of MMP9 is associated with increased inflammatory response during corneal reepithelialization (Mohan et al., 2002). Interestingly, Pax6 controls the expression of MMP9 during this process (Sivak et al., 2000; Sivak et al., 2004). Therefore, Cited2 might be required for the expression of both Pax6 and MMP9 and thereby facilitate corneal repair.
Significantly decreased expression of Klf4 was also detected in the Cited2 deficient cornea. Since Klf4 is an essential factor for corneal epithelial cell differentiation as it regulates K12 expression (Swamynathan et al., 2007), a Cited2 expression plasmid was cotransfected with a luciferase reporter plasmid containing the Klf4 promoter into human corneal epithelial cells to determine if Cited2 enhances Klf4 transcription. The enhanced activity of the Klf4 promoter with increasing amounts of Cited2 (Figure 5) indicates that Cited2 may be required for Klf4 expression. Various truncated forms of the Klf4 promoter were further analyzed to identify regions on the Klf4 promoter that could be responsible for Cited2 action. The analysis identified −1190 to −716, which contains putative binding sites for AP-2α, to be most responsive to Cited2 overexpression. Although we found that AP-2α significantly inhibits Klf4 promoter activity, as was reported by others (Pfisterer et al., 2002), our study suggests multiple positive and negative transcription factors may participate in Cited2-mediated Klf4 expression. Further studies are required to dissect the transcription factor/cofactor network involved in controlling Klf4 expression.
The lack of K12 expression in Cited2 deficient corneal epithelial cells in the absence and presence of wound healing could also suggest that the corneal epithelial stem cell population is dependent upon Cited2. Cornea epithelial cells are established in embryos and undergo dynamic loss and replenishment in response to physiological wounding under the control of corneal epithelial stem cells (Collinson et al., 2002; Majo et al., 2008; Sun and Lavker, 2004). Interestingly, accumulated evidence has indicated that Cited2 is a transcriptional modulator involved in stem cell functions. Cited2 is highly expressed in the enriched hematopoietic stem cells (HSCs) and is required for the activity of HSCs (Chen et al., 2007). Cited2 has also been shown to be involved in embryonic stem cells maintenance (Pritsker et al., 2006). Pax6 deficiency is associated with dysfunction of limbal stem cells in a cell-autonomous manner (Collinson et al., 2004), which is also indicative of a potential role of Cited2 in corneal stem cells given that Cited2 regulates Pax6 expression. Therefore, we can not exclude the possibility that Cited2 deficiency may result in impaired corneal epithelial stem cell function, which could be responsible for impaired healing in response to physiological and pathological wounding, leading to defective corneal morphogenesis and maintenance (Dua et al., 1994; Huang and Tseng, 1991; Ma et al., 2006; Sun and Lavker, 2004; Wilson et al., 2001; Wolosin et al., 2000). Although the role of lens in corneal epithelial specification has not been clearly demonstrated, the lens indeed influences the normal development of neural crest cell-derived corneal components such as corneal endothelium and stroma (Beebe and Coats, 2000). In light of the role of Cited2 in lens development (Chen et al., 2008), the deleterious influence of defective lens morphogenesis on corneal phenotypes should also be considered and further addressed.
Collectively, the present study reveals novel functions of Cited2 in corneal morphogenesis, maintenance and wound healing through mechanisms involving Pax6 and Klf4. Therefore, our study not only provides a new mouse model for studying relevant corneal disorders, but also adds new knowledge to our understanding of the molecular mechanisms underlying adult corneal biology, which will facilitate therapeutic development targeting corneal disorders.
Supplementary Material
Supplemental Figure 1 Histological validation of corneal neovascularization in Cited2 deficient eyes at older age. Macroscopic revelation of spontaneous corneal neovascularization was further validated by H&E staining of corneal cross-section from Cited2flox/flox;Le-Cre+ mice at 8 months of age. Blood vessels were evident in the cornea (arrows).
Supplemental Figure 2 Cited2 overexpression enhances the K12 promoter activity. Effect of Cited2 on K12 gene transcription was tested by luciferase reporter assay. Mouse K12 -1027/-33-bp promoter was amplified using primers: antisense 5′-GATCAAGCTTACCGCAGTGCTGGTATGCCAGAAG-3′ and sense 5′-GATCGCTAGCCATCTCTCTGACCCCTGGATAAA-3′ primers. The promoter fragment was cloned upstream of the luciferase reporter gene in pGL3 Basic vector digested with NheI and HindIII to generate 995-bp K12-Luc reporter construct. HEK293 cells were transfected with K12-Luc reporter plasmid (50ng) without or with Cited2 expression plasmid (from 6.25ng to 75ng). Cited2 overexpression in HEK293 cells significantly increased the activity of K12-Luc reporter in a dose-dependent manner.
Acknowledgement
We thank Andrew Jarrell for help with mouse genotyping, Yong-qiu Doughman and Catherine Doller for tissue sectioning and Yan Sun for providing HEC cells.
This work was supported by National Institute of Health grants R01HL075436 (Y.C.Y.), HL75427 (M.K.J.). S.L.D. is an NHMRC Senior Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamforth SD, Braganca J, Eloranta JJ, Murdoch JN, Marques FI, Kranc KR, Farza H, Henderson DJ, Hurst HC, Bhattacharya S. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat Genet. 2001;29:469–474. doi: 10.1038/ng768. [DOI] [PubMed] [Google Scholar]
- Bamforth SD, Braganca J, Farthing CR, Schneider JE, Broadbent C, Michell AC, Clarke K, Neubauer S, Norris D, Brown NA, Anderson RH, Bhattacharya S. Cited2 controls left-right patterning and heart development through a Nodal-Pitx2c pathway. Nat Genet. 2004;36:1189–1196. doi: 10.1038/ng1446. [DOI] [PubMed] [Google Scholar]
- Barbera JP, Rodriguez TA, Greene ND, Weninger WJ, Simeone A, Copp AJ, Beddington RS, Dunwoodie S. Folic acid prevents exencephaly in Cited2 deficient mice. Hum. Mol. Genet. 2002;11:283–293. doi: 10.1093/hmg/11.3.283. [DOI] [PubMed] [Google Scholar]
- Beebe DC, Coats JM. The Lens Organizes the Anterior Segment: Specification of Neural Crest Cell Differentiation in the Avian Eye. Developmental Biology. 2000;220:424–431. doi: 10.1006/dbio.2000.9638. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev. 1999;13:64–75. doi: 10.1101/gad.13.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas FW, Val P, Swain A. The transcription co-factor CITED2 functions during sex determination and early gonad development. Hum. Mol Genet. 2009 doi: 10.1093/hmg/ddp237. in press. [DOI] [PubMed] [Google Scholar]
- Chen Y, Haviernik P, Bunting KD, Yang YC. Cited2 is required for normal hematopoiesis in the murine fetal liver. Blood. 2007;110:2889–2898. doi: 10.1182/blood-2007-01-066316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Doughman Y.q., Gu S, Jarrell A, Aota S.i., Cvekl A, Watanabe M, Dunwoodie SL, Johnson RS, van Heyningen V, Kleinjan DA, Beebe DC, Yang YC. Cited2 is required for the proper formation of the hyaloid vasculature and for lens morphogenesis. Development. 2008;135:2939–2948. doi: 10.1242/dev.021097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Shie JL, Tseng CC. STAT1 Is Required for IFN-[gamma]-Mediated Gut-Enriched Krnppel-like Factor Expression. Experimental Cell Research. 2002;281:19–27. doi: 10.1006/excr.2002.5633. [DOI] [PubMed] [Google Scholar]
- Chou YT, Wang H, Chen Y, Danielpour D, Yang YC. Cited2 modulates TGF-beta-mediated upregulation of MMP9. Oncogene. 2006;25:5547–5560. doi: 10.1038/sj.onc.1209552. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Morris L, Reid AI, Ramaesh T, Keighren MA, Flockhart JH, Hill RE, Tan SS, Ramaesh K, Dhillon B, West JD. Clonal analysis of patterns of growth, stem cell activity, and cell movement during the development and maintenance of the murine corneal epithelium. Dev. Dyn. 2002;224:432–440. doi: 10.1002/dvdy.10124. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Chanas SA, Hill RE, West JD. Corneal Development, Limbal Stem Cell Function, and Corneal Epithelial Cell Migration in the Pax6+/− Mouse. Invest. Ophthalmol. Vis. Sci. 2004;45:1101–1108. doi: 10.1167/iovs.03-1118. [DOI] [PubMed] [Google Scholar]
- Davis J, Duncan MK, Robison WG, Jr., Piatigorsky J. Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J Cell Sci. 2003;116:2157–2167. doi: 10.1242/jcs.00441. [DOI] [PubMed] [Google Scholar]
- Dwivedi DJ, Pontoriero GF, Ashery-Padan R, Sullivan S, Williams T, West-Mays JA. Targeted Deletion of AP-2{alpha} Leads to Disruption in Corneal Epithelial Cell Integrity and Defects in the Corneal Stroma. Invest. Ophthalmol. Vis. Sci. 2005;46:3623–3630. doi: 10.1167/iovs.05-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED. Development of the vertebrate cornea. Int. Rev. Cytol. 1979;63:263–322. doi: 10.1016/s0074-7696(08)61760-x. 263-322. [DOI] [PubMed] [Google Scholar]
- Kao WW, Liu CY, Converse RL, Shiraishi A, Kao CW, Ishizaki M, Doetschman T, Duffy J. Keratin 12-deficient mice have fragile corneal epithelia. Invest Ophthalmol Vis. Sci. 1996;37:2572–2584. [PubMed] [Google Scholar]
- Kurpakus MA, Maniaci MT, Esco M. Expression of keratins K12, K4 and K14 during development of ocular surface epithelium. Curr. Eye Res. 1994;13:805–814. doi: 10.3109/02713689409025135. [DOI] [PubMed] [Google Scholar]
- Li W, Chen YT, Hayashida Y, Blanco G, Kheirkah A, He H, Chen SY, Liu CY, Tseng SC. Down-regulation of Pax6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J Pathol. 2008;214:114–122. doi: 10.1002/path.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Kao WW, Wilson SE. Corneal epithelium-specific mouse keratin K12 promoter. Exp Eye Res. 1999;68:295–301. doi: 10.1006/exer.1998.0593. [DOI] [PubMed] [Google Scholar]
- Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature. 2008;456:250–254. doi: 10.1038/nature07406. [DOI] [PubMed] [Google Scholar]
- Mohan R, Chintala SK, Jung JC, Villar WVL, McCabe F, Russo LA, Lee Y, McCarthy BE, Wollenberg KR, Jester JV, Wang M, Welgus HG, Shipley JM, Senior RM, Fini ME. Matrix Metalloproteinase Gelatinase B (MMP-9) Coordinates and Effects Epithelial Regeneration. J. Biol. Chem. 2002;277:2065–2072. doi: 10.1074/jbc.M107611200. [DOI] [PubMed] [Google Scholar]
- Norman B, Davis J, Piatigorsky J. Postnatal gene expression in the normal mouse cornea by SAGE. Invest Ophthalmol Vis. Sci. 2004;45:429–440. doi: 10.1167/iovs.03-0449. [DOI] [PubMed] [Google Scholar]
- Pfisterer P, Ehlermann J, Hegen M, Schorle H. A Subtractive Gene Expression Screen Suggests a Role of Transcription Factor AP-2alpha in Control of Proliferation and Differentiation. J. Biol. Chem. 2002;277:6637–6644. doi: 10.1074/jbc.M108578200. [DOI] [PubMed] [Google Scholar]
- Preis JI, Wise N, Solloway MJ, Harvey RP, Sparrow DB, Dunwoodie SL. Generation of conditional Cited2 null alleles. Genesis. 2006;44:579–583. doi: 10.1002/dvg.20251. [DOI] [PubMed] [Google Scholar]
- Pritsker M, Ford NR, Jenq HT, Lemischka IR. Genomewide gain-of-function genetic screen identifies functionally active genes in mouse embryonic stem cells. PNAS. 2006;103:6946–6951. doi: 10.1073/pnas.0509861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaesh T, Ramaesh K, Martin CJ, Chanas SA, Dhillon B, West JD. Developmental and cellular factors underlying corneal epithelial dysgenesis in the Pax6+/− mouse model of aniridia. Exp Eye Res. 2005;81:224–235. doi: 10.1016/j.exer.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Shioda T, Fenner MH, Isselbacher KJ. MSG1 and its related protein MRG1 share a transcription activating domain. Gene. 1997;19(204):235–241. doi: 10.1016/s0378-1119(97)00551-9. [DOI] [PubMed] [Google Scholar]
- Shiraishi A, Converse RL, Liu CY, Zhou F, Kao CW, Kao WW. Identification of the cornea-specific keratin 12 promoter by in vivo particle-mediated gene transfer. Invest. Ophthalmol. Vis. Sci. 1998;39:2554–2561. [PubMed] [Google Scholar]
- Sivak JM, Mohan R, Rinehart WB, Xu PX, Maas RL, Fini ME. Pax-6 expression and activity are induced in the reepithelializing cornea and control activity of the transcriptional promoter for matrix metalloproteinase gelatinase B. Dev. Biol. 2000;222:41–54. doi: 10.1006/dbio.2000.9694. [DOI] [PubMed] [Google Scholar]
- Sivak JM, West-Mays JA, Yee A, Williams T, Fini ME. Transcription Factors Pax6 and AP-2{alpha} Interact To Coordinate Corneal Epithelial Repair by Controlling Expression of Matrix Metalloproteinase Gelatinase B. Mol. Cell. Biol. 2004;24:245–257. doi: 10.1128/MCB.24.1.245-257.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HB, Zhu YX, Yin T, Sledge G, Yang YC. MRG1, the product of a melanocyte-specific gene related gene, is a cytokine-inducible transcription factor with transformation activity. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13555–13560. doi: 10.1073/pnas.95.23.13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun TT, Lavker RM. Corneal Epithelial Stem Cells: Past, Present, and Future. J Investig Dermatol Symp Proc. 2004;9:202–207. doi: 10.1111/j.1087-0024.2004.09311.x. [DOI] [PubMed] [Google Scholar]
- Swamynathan SK, Katz JP, Kaestner KH, Ashery-Padan R, Crawford MA, Piatigorsky J. Conditional Deletion of the Mouse Klf4 Gene Results in Corneal Epithelial Fragility, Stromal Edema, and Loss of Conjunctival Goblet Cells. Mol. Cell. Biol. 2007;27:182–194. doi: 10.1128/MCB.00846-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanifuji-Terai N, Terai K, Hayashi Y, Chikama T.i., Kao WWY. Expression of Keratin 12 and Maturation of Corneal Epithelium during Development and Postnatal Growth. Invest. Ophthalmol. Vis. Sci. 2006;47:545–551. doi: 10.1167/iovs.05-1182. [DOI] [PubMed] [Google Scholar]
- Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest. Ophthalmol. Vis. Sci. 1983;24:1442–1443. [PubMed] [Google Scholar]
- Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134:2349–2358. doi: 10.1242/dev.004390. [DOI] [PubMed] [Google Scholar]
- Weninger WJ, Floro KL, Bennett MB, Withington SL, Preis JI, Barbera JP, Mohun TJ, Dunwoodie SL. Cited2 is required both for heart morphogenesis and establishment of the left-right axis in mouse development. Development. 2005;132:1337–1348. doi: 10.1242/dev.01696. [DOI] [PubMed] [Google Scholar]
- Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull. World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- Withington SL, Scott AN, Saunders DN, Lopes FK, Preis JI, Michalicek J, Maclean K, Sparrow DB, Barbera JP, Dunwoodie SL. Loss of Cited2 affects trophoblast formation and vascularization of the mouse placenta. Dev. Biol. 2006;294:67–82. doi: 10.1016/j.ydbio.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Xu B, Qu X, Gu S, Doughman YQ, Watanabe M, Dunwoodie SL, Yang YC. Cited2 is required for fetal lung maturation. Dev. Biol. 2008;317:95–105. doi: 10.1016/j.ydbio.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Haynie J, Yang X, Han B, Kiatchoosakun S, Restivo J, Yuan S, Prabhakar NR, Herrup K, Conlon RA, Hoit BD, Watanabe M, Yang YC. The essential role of Cited2, a negative regulator for HIF-1alpha, in heart development and neurulation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10488–10493. doi: 10.1073/pnas.162371799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD. Corneal development associated with eyelid opening. Int. J Dev. Biol. 2004;48:903–911. doi: 10.1387/ijdb.041860jz. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Histological validation of corneal neovascularization in Cited2 deficient eyes at older age. Macroscopic revelation of spontaneous corneal neovascularization was further validated by H&E staining of corneal cross-section from Cited2flox/flox;Le-Cre+ mice at 8 months of age. Blood vessels were evident in the cornea (arrows).
Supplemental Figure 2 Cited2 overexpression enhances the K12 promoter activity. Effect of Cited2 on K12 gene transcription was tested by luciferase reporter assay. Mouse K12 -1027/-33-bp promoter was amplified using primers: antisense 5′-GATCAAGCTTACCGCAGTGCTGGTATGCCAGAAG-3′ and sense 5′-GATCGCTAGCCATCTCTCTGACCCCTGGATAAA-3′ primers. The promoter fragment was cloned upstream of the luciferase reporter gene in pGL3 Basic vector digested with NheI and HindIII to generate 995-bp K12-Luc reporter construct. HEK293 cells were transfected with K12-Luc reporter plasmid (50ng) without or with Cited2 expression plasmid (from 6.25ng to 75ng). Cited2 overexpression in HEK293 cells significantly increased the activity of K12-Luc reporter in a dose-dependent manner.


