Abstract
Transgenic over expression of apolipoprotein A-I (ApoA-I) the major structural apolipoprotein of HDL appears to convey the most consistent and strongest anti atherogenic effect observed in animal models so far. We tested the hypothesis that ApoA-I mediates its cardio protective effects additionally through ApoA-I induced differentiation of bone marrow derived progenitor cells in vitro. This study demonstrates that lineage negative bone marrow cells (lin−BMCs) alter and differentiate in response to free ApoA-I. We find that lin−BMCs in culture treated with recombinant free ApoA-I at a concentration of 0.4µM are twice as large in size and have altered cell morphology compared to untreated cells; untreated cells retain the original spheroid morphology. Further, the total number of CD31 positive cells in the ApoA-I treated population consistently increased by two fold. This phenotype was significantly reduced in untreated cells and points towards a novel ApoA-I dependent differentiation. A protein lacking its best lipid-binding region (ApoA-IΔ10) did not stimulate any changes in the lin−BMCs cells indicating that ApoA-I may mediate its effects by regulating cholesterol efflux. The increased CD31 correlates with an increased ability of the lin−BMCs to adhere to both fibronectin and Mouse Brain Endothelial Cells. Our results provide the first evidence that exogenous free ApoA-I has the capacity to change the characteristics of progenitor cell populations and suggests a novel mechanism by which HDL may mediate its cardiovascular benefits.
Keywords: ApoA-I, bone marrow cells (BMCs), CD31, lineage minus, vascular progenitor cell, adhesion
Introduction
High Density Lipoprotein (HDL) and the principal structural protein of HDL, apolipoprotein (Apo) A-I, are known to protect against the development of atherosclerosis [1], [2]. The exact mechanism is still unknown but is believed to involve reverse cholesterol transport [3], [4]. Previous studies have shown that the transgenic overexpression of ApoA-I in liver substantially reduces progression of atherosclerosis in mice and rabbits [1], [5]. In addition somatic gene transfer of ApoA-I to liver using adenoviral vectors reduces progression of atherosclerosis [6].
The majority of the vascular progenitor cell population including the hematopoietic stem cell (HSC) and mesenchymal stem cell (MSC) populations reside within the adult bone marrow (BM), the potential source of vascular progenitor cells that contributes to arterial remodeling in models of post angioplasty restenosis and hyperlipidemia induced atherosclerosis [7]. The progenitor cells continuously migrate from the BM to the blood and back, in a way such that they are always available in circulation [8]. Vascular progenitors are thought to home to tissues in response to local release of proinflammatory cytokines and chemokines because stem cells administered during bone marrow transplantation in humans incorporate into atherosclerotic arteries [9] [10].
Consistent with the proposed ideas of the role of progenitor cells and vascular repair, there exists a strong inverse correlation between the number of circulating progenitor cells and progression of cardiovascular disease [10]. We have shown previously that injection of unfractionated bone marrow cells isolated from age matched wild type young but not old apoE−/− donor mice substantially reduced atherosclerosis [11]. Further FACS studies revealed that CD31+ cells were significantly diminished in BM from 6-month-old ApoE−/− mice (3.79±2.02% gated cells, n=5) compared with 1-month-old ApoE−/− mice (7.03±2.81% gated cells, n=5) and WT mice (6.36±1.02% gated cells, n=5) [11]. The loss of vascular progenitor cells in BM obtained from older ApoE−/− mice may explain, at least in part, the loss of antiatherosclerotic effect of the older ApoE−/− BM cells. An alternative/additive hypothesis to the could be the inability to repair vascular damage due to the diminished numbers of CD31 cells in BM obtained from older ApoE−/− mice. Interestingly, age-associated changes in the serum level, mRNA levels and mobilization of ApoA-I and A-IV have been described previously [12, 13]. These studies [12, 13], have suggested that the age associated changes in ApoAI and ApoAIV could possibly lead to increased incidence of age related diseases including cardiovascular disorders.
We tested the hypothesis that ApoA-I induces differentiation of lin− BMCs. We found that exposure to ApoA-I led to an increase in CD31 expression in lin− BMCs which correlated with an increased rate of attachment of the cultured ApoA-I treated BMCs to fibronectin and to endothelial cells under motion.
Materials and Methods
Animal care
Wild type Mus musculus of the B6 strain background (Jackson Laboratories, Bar Harbor, MA) were fed a standard chow diet and handled according to Duke University animal care and use regulations. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Bone marrow cell purification and culture
Bone marrow cells were isolated from tibia and femora were dissected from mice aged 4–6 weeks. Lineage Minus BMCs (lin− BMC)were prepared by depletion of lineage positive cells from whole bone marrow by affinity chromatography using anti-biotin MicroBeads (Miltenyi Biotech #130-090-858) according to manufacturer’s directions. Briefly, whole bone marrow was incubated in a cocktail of biotinylated antibodies against a panel of the lineage antigens (CD5, CD45R (B220), CD11b, Gr-1 (Ly-6G/C), 7-4, and Ter-119) and lineage positive cells removed using magnetically labeled anti-Biotin MicroBeads. Characterization of the lineage minus cells using flow cytometry revealed that >95% of the lineage minus cells are CD34- Sca1-. Cells were cultured on coverslips and dishes were pretreated with 30 µg/ml fibronectin at 37°C for 30 minutes in Dulbecco’s MEM with 20% FBS supplemented with purified apolipoproteins or buffer control and cultured at 37°C for 1–7 days.
ApoA1 cloning, expression and purification
Full length mouse ApoA-I(mApoA-I), human ApoA-IV(hApoA-IV) and human ApoA-IΔ10 (hApoAIΔ10) were cloned into pET vectors and purified using a bacterial expression system and affinity chromatography; the epitope tag was released by proteolysis as developed previously [14]. hApoA-I from blood plasma was a kind gift of Sean Davidson. All proteins were greater than 99% pure by SDS gel electrophoresis and confirmed to be biologically active as judged by reconstitution into HDL particles (data not shown). All bacterially expressed recombinant proteins were depleted of bacterial endotoxin by passing protein solution twice through Polymixin B columns (Cambrex, Allentown, NJ) and then residual endotoxin levels were estimated using a chromogenic assay based on Limulus Amoebocyte Lysis (LAL, Cambrex, Allentown, NJ ) sensitive in a range of 0.01 to 0.1 ng. Protein samples containing <0.001 ng/µg were used for culture experiments.
Immunofluorescence
Cells were processed for immunofluorescence using standard methods. Briefly, adherent cells were fixed in freshly prepared 4% paraformaldehyde in PBS for 10 min. at 25°C, washed twice in PBS, and blocked in 1% BSA in PBS for 30 min. at 37°C. Cells were incubated with primary antibody diluted into 1% BSA in PBS for 1 hour at 37°C, washed four-six times in PBS, incubated in secondary antibody diluted in 1% BSA in PBS for 30 min. at 25°C, washed 4 times with PBS, once briefly with dH2O, and mounted with mounting media containing 1.5 µg/ml DAPI. Secondary antibody alone was used as a control. Primary antibodies CD31, CD11b and CD44 were obtained from BD Biosciences (catalog # 553708, 553307 and 553130 respectively). Secondary AlexaFluor conjugates were obtained from Invitrogen, catalog # A-11007. Cells were imaged by DIC and fluorescence using a Nikon TE2000E inverted fluorescence microscope and a Hamamatsu ORCA cooled CCD digital camera. Captured digital images were analyzed using Metamorph v6.2 (Molecular Devices).
Adhesion assays
Lin− BMCs were cultured on fibronectin with either hApoA-I or buffer alone, harvested on day 5 using a brief rinse of ice cold 10mM cold EDTA, washed in PBS, resuspended in PBS and counted in a haemocytometer. For adhesion assays to fibronectin, culture dishes inscribed with a 10 mm grid were pretreated with 1 µg/ml fibronectin for 30 min. at 37°C. MBEC’s were a kind gift from Garrett Muramota and Nelson Chao at Duke University Medical Center and were prepared as described previously [15]. For adhesion to MBEC’s, cells were plated and grown to 80–90% confluence. Typically between 1 × 104 and 1 × 106 lin− BMCs were added in each experiment. Dishes were rotated on a shaker placed at room temperature at a fixed speed of 50 rpm/min. The number of cells attached in PBS was counted using a light microscope at specific time intervals. For CD31 blocking experiments, harvested ApoA-I or buffer treated lin− BMCs were harvested, washed, and incubated for 30 min. on ice with goat polyclonal anti-PECAM1 before adding to either fibronectin-coated dishes or dishes confluent with MBEC’s. Cells were washed with PBS and adhesion assay proceeded as described above. BMCs attached were counted for only 30 min. in the adhesion assays because MBEC cultures began to show evidence of cell death by 45 min.
Results
ApoA-I induces an increase in cell size and a morphological change in cultured lin− BMC’s
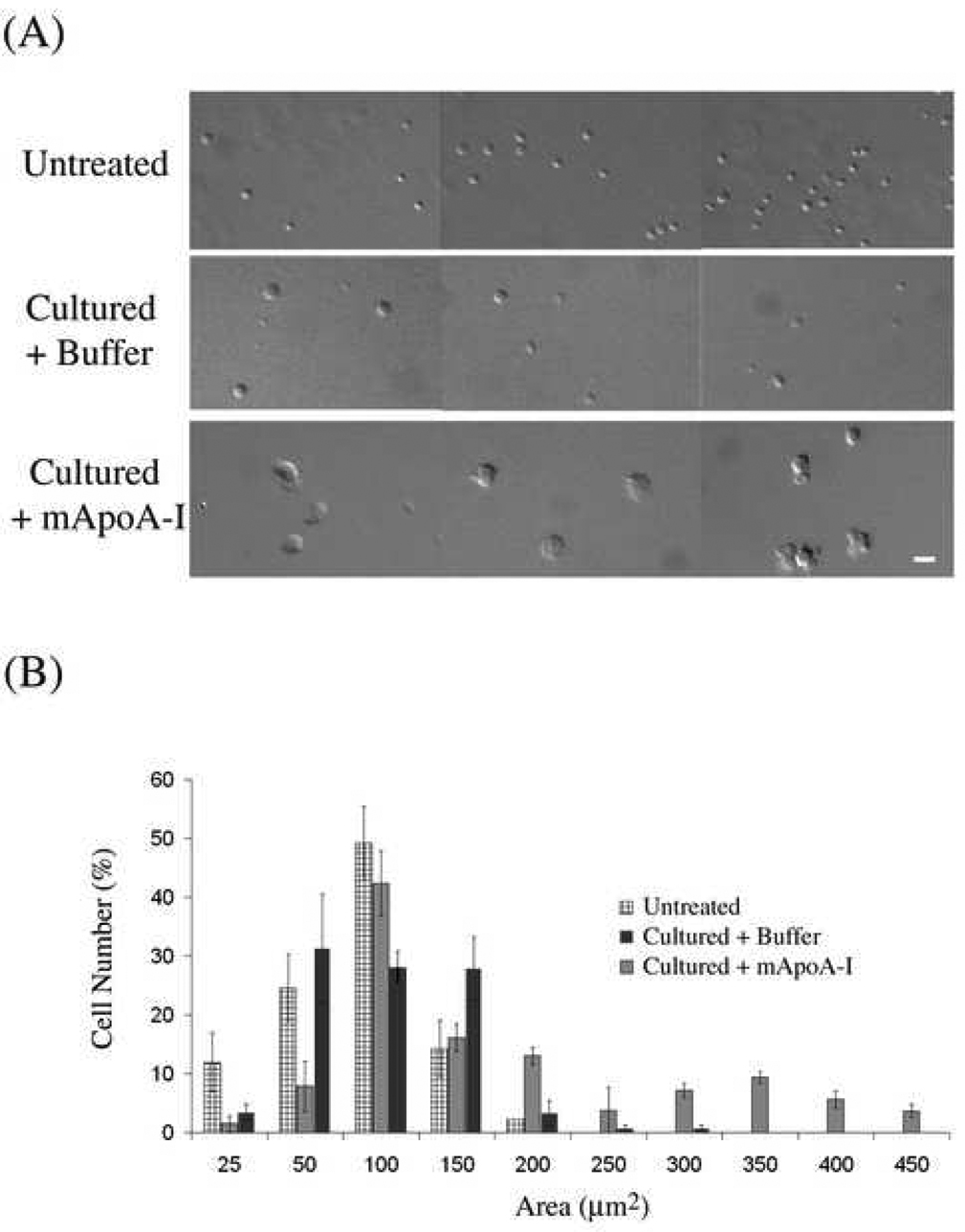
To determine a putative role of ApoA-I on differentiation of lin− BMCs in vitro, exogenous murine ApoA-I (mApoA-I, 10µg/ml) or buffer alone was added to cultures of lin− BMCs and morphological changes observed by DIC microscopy. 1–5×104 purified lin− BMCs were seeded onto fibronectin coated coverslips in DMEM and 20% FCS at 37°C and photographed daily for 7 days. We found that mApoA-I treatment induced cell to grow and to flatten and exhibit multiple cell protrusions, sometimes as many as three per cell (Figure 1). In contrast, cells treated with buffer only flattened, and on average maintained a spherical shape (Figure 1). Cells began to grow after approximately three days in culture with maximal changes between 5 to 7 days. To quantify changes in a population of treated cells, single cells were analyzed individually in DIC micrographs of three groups: freshly isolated cells, cultured cells treated with ApoA-I, and cultured cells treated with buffer only by using a cell size analysis function of Metamorph v6.2. We found that 49% of untreated cells had a cell size between 50–100µm2. Upon culture 80% of cells had a cell size distributed between 25–150 µm2 with less than 2% being 300µm2. In contrast, cells treated with ApoA-I exhibited 18% of cells larger than 300µm2 (Figure 1B).
Figure 1. Treatment of BMCs in culture with bacterially expressed mouse ApoA-I increases cell size and alters morphology relative to controls.
Lineage minus BMCs were cultured for 5 days in DMEM with 20% serum supplemented with either 10 µg/ml mouse ApoA-I or with buffer alone (A) Representative DIC images of freshly isolated and purified BMCs or BMCs cultured for 5 days that were treated either with mouse ApoA-I or buffer control. Bar = 21µm. (B). Histogram depicting a distribution of cell areas binned in 50 µm segments beginning at the lowest to the highest area observed in the three treatment groups shown in (A). Cell area measurements were calculated in Metamorph (Universal Imaging) v6.2 from DIC micrographs and expressed as µm 2. Only unambiguous single cells were included in cell numbers, and greater than 200 cells were counted per treatment group.
Cell surface vascular lineage progenitor markers change in response to culture and in response to ApoA-I
To examine if the alteration in cell size and changes in cell morphology upon exposure to ApoA-I were associated with changes in cell surface markers, cell surface antigens CD44, VEGFR2, CD11b and CD31/PECAM1 were scored by immunofluorescence (Table 1). CD31 is a marker for the hematopoietic lineage, cardiomyocyte progenitors and also is present on many mature cells such as platelets, endothelial cells and leukocytes. We found that CD31 was present on 13% of both freshly isolated cells and cells in adherent culture treated with PBS. Strikingly, 45% of cells in adherent culture with ApoA-I (10 µg/ml) treatment for 5 days scored positive for CD31, a 3-fold increase over the control cells (14% buffer control versus 45% ApoA-I treated, Table 1 and Figure 2). Other key markers of differentiation were altered in response to adherent culture rather than ApoA-I treatment. Bone marrow derived lin− BMCs prior to culture have 63% of cells expressing VEGFR2 (a receptor for VEGF found on cells of hematopoietic lineage and mature endothelial cells) and 58% of cells expressing the mesenchymal marker CD44 (a cell adhesion molecule that mediates attachment and rolling of leukocytes along endothelium). Upon culture there is a statistically similar reduction in both buffer treated and ApoA-I treated cells (Buffer −51% and ApoA-I 46%, Table 1.). Similarly CD44 levels remain unaltered after culture in both control and ApoA-I treated cells. CD11b/Mac1 (a monocyte/macrophage and neutrophil adhesion molecule) is depleted from lin- BMC’s, however upon culture there is an increase in CD11b cells in the population, with both buffer and ApoA-I treated cells having between 13% (ApoA-I treated) and 16% (Buffer control) cells expressing CD11b. Hence culture alone can contribute to increases in CD11b expressing cells.
Table 1. Effect of ApoA-I on expression of cell surface markers on lin- BMCs in culture.
Lin-BMC’s were isolated, cultured and immunofluorescence performed as described in Methods for the respective markers indicated. % cells with a positive signal for a given antigen with respect to the total number scored is presented. 200 cells were counted for each antigen.
| Marker | Percentage of cells expressing the marker# | ||
|---|---|---|---|
| Fresh (Before culture) |
Buffer control (After culture) |
ApoA-I treated (After culture) |
|
| CD31 | 13 ± 6 | 14 *± 4 | 45 *± 5 |
| CD11b | <1 | 16 ± 2 | 13 ± 4 |
| CD44 | 58 ± 9 | 53# ± 6 | 46# ± 3 |
| CD45 | 8 ± 2 | 13 ± 3 | 15 ± 3 |
| VEGFR2 | 63 ± 2 | 51 ± 2 | 46 ± 5 |
Average of three experiments, Mean ±SD.
values are statistically significant from each other, p<0.0001 , # values are not statistically significant from each other.
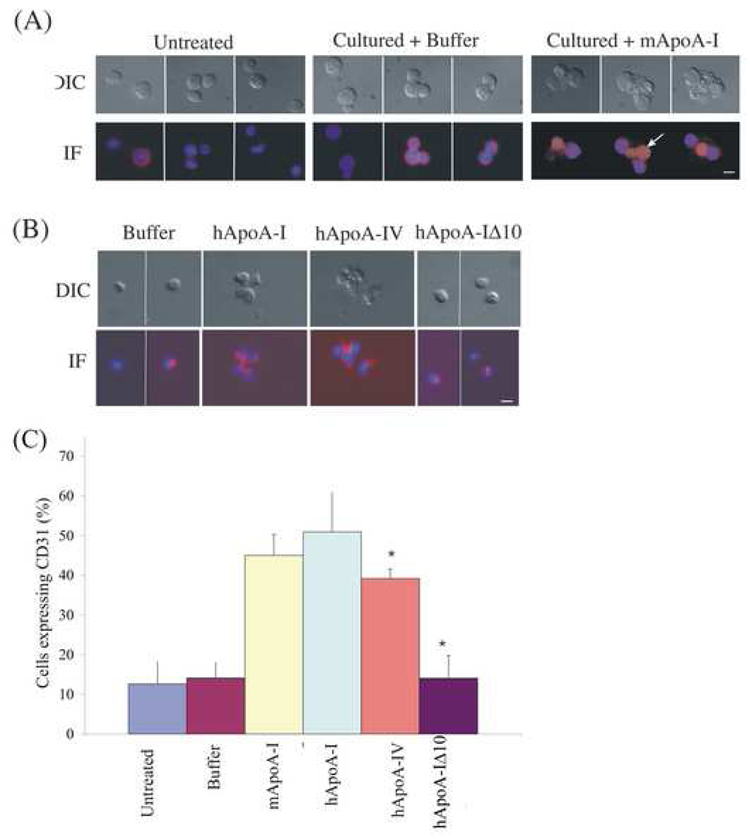
Figure 2. ApoA-I induced cell surface expression of CD31 on lineage minus BMCs in culture requires the lipid binding domain.
(A). Representative DIC and immunofluorescence digital image overlay of DAPI (nucleus) and Rhodamine (CD31) images of either freshly isolated, buffer control cells in culture and mApoA-I treated cells taken after 5 days in culture. Freshly isolated cells are round with very little CD31. CD31 expression pattern in buffer control tends to be at the surface of the cells in contrast CD31 expression in ApoA-I treated cells tends to be at cell-cell junctions and in cytoplasmic protrusion of the cell as indicated with the solid white arrow. Bar = 10 µm.
(B). Representative images of the morphological changes (DIC) and CD31 localization pattern as described in (A) for the human proteins human ApoA-I, human ApoA-IV or ApoA-IΔ10 observed. Bar=20µm.
(C). Histogram of CD31 expression in cells treated with either mouse ApoA-I, human ApoA-I, human ApoA-IV or ApoA-IΔ10. All proteins were added at the same concentration of 10µg/ml and cells imaged after 5 days. Average values of 3 independent experiments are represented with error bare represent SEM values from the three experiments. * ApoA-IV is significantly different from hApoA-I, and untreated control cells p<0.0001 and ApoA-IΔ10 is significantly different from hApoA-I, p<0.0001.
To determine if the localization of CD31 in the lin− BMC’s was altered upon treatment with ApoA-I we immunostained cells with anti-CD31 antibody and find that the distribution pattern of CD31 in ApoA-I treated cells is distinct from the control population (Figure 2A). CD31 was largely restricted to the cytoplasm of closely juxtaposed cells (Figure 2A) and also appeared to be localized to large cytoplasmic protrusions in single cells (Figure 2A). This phenotype was reduced in untreated cells. Hence ApoA-I appears to specifically increase the number of cells positive for CD31 in cultured lin− BMCs and the CD31 signal is most often observed in dramatic cell protrusions that are a signature of the ApoA-I induced morphological changes.
Effect of mApoA-I is mimicked by hApoA-I and ApoA-IV and requires a lipid binding domain
ApoA-I stimulates reverse cholesterol transport, the process by which free cholesterol is removed from tissues and transferred by HDL to the liver for excretion in the bile [16], [17]. ATP-binding cassette protein A1 (ABCA1) mediates cholesterol removal to nascent ApoA-I containing HDL particles, which then mature and are taken up by the hepatocyte HDL receptor, scavenger receptor, class B, type 1 (SR-BI) [16], [17]. We prepared purified mouse and human ApoA-I and two specificity controls: an engineered deletion in alpha helix 10 of human ApoA1 that results in loss of function ABCA1-mediated cholesterol uptake that is negative for cholesterol efflux, and human ApoA-IV, a protein highly related to ApoA-I in structure and function, which also stimulates cholesterol efflux from cells via SR-B1 and is positive for cholesterol efflux [16], [17]. To examine if the changes observed in the lin− BMCs upon ApoA-I treatment were a functional consequence of ApoA-I’s role in lipid binding and cholesterol efflux, the different human proteins ApoA-I, ApoA-IV or ApoA-IΔ10 were assayed for ability to induce morphological changes and CD31 expression in lin− BMCs in culture. We found that human proteins ApoA-I and ApoA-IV, exhibit similar morphological changes in cultured lin− BMC’s as those observed with mApoA-I (Figure 2) and 50% of cells express CD31 upon exposure to hApoA-I closely mimicking the mApoA-I phenotype. Interestingly, in hApoA-IV treated cells 39% scored positive for CD31, significantly less than the 50% that scored positive in hApoA-I treated cells (Figure 2C),). In contrast, ApoA-IΔ10 treatment failed to induce any morphological changes observed in treatments with ApoA-I or an increase in the number of cells expressing CD31 (Figure 2B and 2C). Hence, hApoA-I closely mimick mApoA-I, hApoA-IV exhibits a slightly lowered but significant increase in CD31 whereas hApoA-IΔ10 failed to stimulate morphological changes or increase in CD31 + cells in cultured lin− BMC’s.
Elevated CD31 correlates with an increase in adherence of the lin− BMC’s
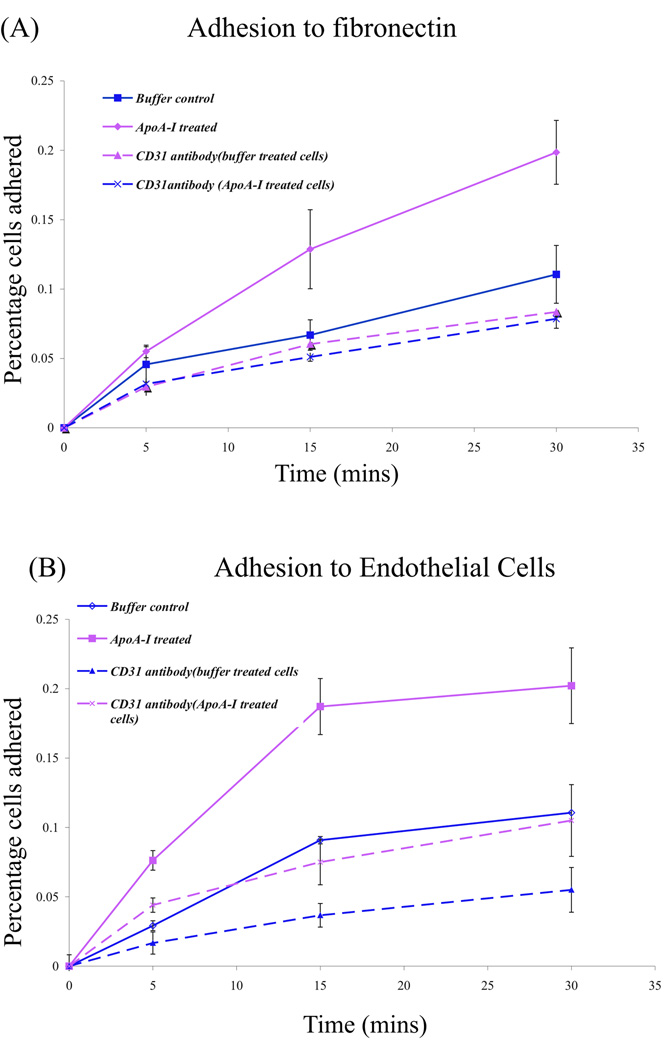
Since ApoA-I treatment results in increased expression of CD31, we tested the ability of the lin− BMC’s subsequent to ApoA-I treatment to attach to either fibronectin or to a layer of confluent MBEC’s. For both experiments, cultured cells either treated with ApoA-I or buffer were removed from dishes using 10mM EDTA (Methods) and added to fibronectin dishes precoated with 1µg/ml fibronectin. We found that cells cultured with ApoA-I attach better to fibronectin with time as compared to control cells (Buffer +serum) (Figure 3A). The percentage of cells attached after 15 minutes was 0.06% for buffer treated cells in contrast to 0.13% cells attached upon ApoA-I treatment. There was also a 2 fold increase in the rate of attachment of ApoA-I treated cells relative to buffer treated cells.
Figure 3. Treatment with ApoA-I increases attachment to fibronectin and to endothelial cells.
(A) Graph showing the percent of the total number of BMCs plated that attached to fibronectin coated coverslips over time under rotational force. Equal number of cultured cells (1 × 104−1 × 105) treated with buffer control or with ApoA-I for 5 days were collected and then exposed to CD31 antibody or buffer alone before seeding onto fibronectin coated coverslips rotating at room temperature. The numbers of cells attached were counted at the time points indicated and expressed as a percentage of the total number of BMCs seeded. The average of two experiments is presented. (B) Mouse brain endothelial cells were a kind gift from Garrett Muramoto and Nelson Chao and were grown to confluence and used in the adhesion assays as in (A). For experiment conducted in the absence of CD31 antibody, error bars represent values obtained from two independent trials. For experiment in the presence of CD31 antibody, representative experiment is presented with error bars from six multiple values.
Next we tested the effect of ApoA-I on attachment to a confluent layer of mouse brain endothelial cells (MBEC’s) as substrate. Lin− BMC’s were cultured as described previously, harvested, washed with PBS and added to the endothelial cells and adhesion was measured as described (Methods) for 30 min in PBS at room temperature. Cell morphology did not alter in the 30 min duration of the experiment. We find a similar increase in adhesion of cells treated with ApoA-I to the endothelial substrate layer (Figure 3B). The percentage cells attached after 15 minutes was 0.09% for buffer treated cells and 0.18% upon ApoA-I treatment.
To determine if the increase in the number of BMCs attached was dependent on cell surface CD31, cultured ApoA-I or buffer treated BMCs were harvested as above and incubated with a polyclonal antibody to CD31 antibody for 30 min at 37°C, rinsed and then added to either fibronectin coated dishes or to dishes of confluent MBEC’s. Antibody treatment resulted in a dramatic reduction in the number of ApoA-I treated BMCs adhered (Figure 3A) resulting in a reduction in adhesion whereas adhesion of buffer treated BMCs was unaffected (Figure 3). Pretreatment of ApoA-I treated BMCs with antibody to CD31 also reduced the number of BMCs attached to endothelial cells and had little effect on BMCs treated with buffer along (Figure 3B).
Hence, increased number of CD31 positive cells in lin− BMCs induced by treatment with ApoA-I correlates with an increased ability of cells to attach to either fibronectin or to endothelial cells in vitro.
Discussion
The aim of this study was to study the impact of free ApoA-I directly on bone marrow derived cells to get possible insights into the beneficial cardioprotective function of ApoA-I. Our study demonstrates that free ApoA-I has an important direct effect on lineage minus bone marrow derived cells that increases adhesion of the lineage minus cells to endothelial cells. The importance of CD31 was confirmed by using a blocking antibody for CD31, which significantly decreased both adhesion of the lineage minus bone marrow cells to fibronectin and endothelial cells in vitro. These data provide a possible mechanism by which progenitor and other bone marrow derived cells could be retained at sites of angiogenesis thereby suggesting a novel alternative mechanism by which ApoA-I may mediate its cardiovascular benefits.
Our observations of CD31 localization are consistent with CD31’s role in mediating cell-cell adhesions and interactions [18] involved in interactive events taking place during thrombosis, wound healing, and angiogenesis [19]. CD31/PECAM1 has been considered to play a primary role in transmigration and a minor role in attachment [20] however CD31 has been shown both invitro and invivo to being capable of inducing and amplifying adhesion signaling via integrins and thereby affecting attachment to fibronectin[21, 22]. Hence it would be interesting to examine the effect of increasing CD31 in vivo by ApoA-I treatment on atherosclerosis in addition to possible role of CD31 in inducing integrin mediated adhesion in bone marrow progenitor cells.
The outcome of ApoA-I-mediated cholesterol efflux is a lipidated form of ApoA-I consistent with our observation that a protein lacking its best lipid-binding region (ApoA-IΔ10) did not stimulate any changes in the bone marrow cells. There currently is very little information on the expression profile of ABCA1 and ABCG1 in an endothelial progenitor cell population . However based on the fact that ABCG1 does not promote cholesterol efflux to lipid free ApoAI and ApoAIV we expect the contribution of ABCG1 to be minimum and suggests that cholesterol efflux most likely via the ABCA1 transporter may cause alteration in cellular morphology and phenotype. Interaction of ApoA-I with the ABCA1 transporter has been shown to be required for particle formation and proper functioning of ApoA-I [23]. The response of cells to ApoA-IV suggests that the observed morphological changes and increase in cells expressing CD31 are not specific to only ApoA-I but can also be triggered by other apolipoproteins that contain multiple amphipathic helical domains, hence, implicating ABCAI-mediated cellular binding of free apolipoproteins and lipid efflux [24]Alternatively, interaction of apolipoproteins with ABCA1-expressing cells has been shown to activate protein tyrosine kinases such as JAK2, which have been shown to in turn activate processes that enhances lipid removal from cells. [25]. ApoA-IV shares many features with ApoA-I and can also exist both in the lipid free state and in association with HDL [26]. About 2%–5% of ApoA-I in human plasma is present in lipid free form or associated with lipid poor particles. Similar levels have been reported for ApoA-IV.
Overexpression of ApoA-I and its variants have been shown to have multiple beneficial effects including protection against atherosclerosis [1], systemic inflammation and organ damage [27], and in the protection against myocardial ischemia/reperfusion injury [28]. In addition HDL containing ApoA-I mimetic peptides have been shown to have anti-inflammatory and cardioprotective effects [29] as well. Many fundamental questions regarding the mechanisms of ApoA-I function remain unanswered. In order to maximize the homing of bone marrow derived repair proficient progenitor cells to damaged endothelium or ischemic tissues, we need to elucidate the key molecules that take part in these physiological or pathologic processes.
Acknowledgements
This study was funded by an unrestricted gift provided by "The Jenny Zoline Foundation" and NIH grant AG023073 to Pascal J Goldschmidt-Clermont, and by grant number HL62542 to Sean Davidson. We thank Garrett G. Muramoto and Nelson J. Chao for providing us with the Mouse Brain Endothelial cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- 2.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorenzi I, von Eckardstein A, Cavelier C, Radosavljevic S, Rohrer L. Apolipoprotein A-I but not high-density lipoproteins are internalised by RAW macrophages: roles of ATP-binding cassette transporter A1 and scavenger receptor BI. J Mol Med. 2007 doi: 10.1007/s00109-007-0267-1. [DOI] [PubMed] [Google Scholar]
- 4.Weibel GL, Alexander ET, Joshi MR, Rader DJ, Lund-Katz S, Phillips MC, Rothblat GH. Wild-type ApoA-I and the Milano variant have similar abilities to stimulate cellular lipid mobilization and efflux. Arterioscler Thromb Vasc Biol. 2007;27:2022–2029. doi: 10.1161/ATVBAHA.107.148403. [DOI] [PubMed] [Google Scholar]
- 5.Duverger N, Kruth H, Emmanuel F, Caillaud JM, Viglietta C, Castro G, Tailleux A, Fievet C, Fruchart JC, Houdebine LM, Denefle P. Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein A-I-transgenic rabbits. Circulation. 1996;94:713–717. doi: 10.1161/01.cir.94.4.713. [DOI] [PubMed] [Google Scholar]
- 6.Belalcazar LM, Merched A, Carr B, Oka K, Chen KH, Pastore L, Beaudet A, Chan L. Long-term stable expression of human apolipoprotein A-I mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation. 2003;107:2726–2732. doi: 10.1161/01.CIR.0000066913.69844.B2. [DOI] [PubMed] [Google Scholar]
- 7.Sata M, Saiura A, Kunisato A, Tojo A, Okada S, Tokuhisa T, Hirai H, Makuuchi M, Hirata Y, Nagai R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–409. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 8.Goodman JW. Stem cells circulating in the blood. Rev Eur Etud Clin Biol. 1970;15:149–150. [PubMed] [Google Scholar]
- 9.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 10.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 11.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 12.Araki S, Goto S. Age-associated changes in the serum level of apolipoproteins A-I and A-IV and the gene expression as revealed by fasting and refeeding in mice. Exp Gerontol. 2003;38:499–506. doi: 10.1016/s0531-5565(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 13.Araki S, Okazaki M, Goto S. Impaired lipid metabolism in aged mice as revealed by fasting-induced expression of apolipoprotein mRNAs in the liver and changes in serum lipids. Gerontology. 2004;50:206–215. doi: 10.1159/000078349. [DOI] [PubMed] [Google Scholar]
- 14.Panagotopulos SE, Horace EM, Maiorano JN, Davidson WS. Apolipoprotein A-I adopts a belt-like orientation in reconstituted high density lipoproteins. J Biol Chem. 2001;276:42965–42970. doi: 10.1074/jbc.M106462200. [DOI] [PubMed] [Google Scholar]
- 15.Chute JP, Muramoto GG, Salter AB, Meadows SK, Rickman DW, Chen B, Himburg HA, Chao NJ. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood. 2007;109:2365–2372. doi: 10.1182/blood-2006-05-022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman MJ, Assmann G, Fruchart JC, Shepherd J, Sirtori C. Raising high-density lipoprotein cholesterol with reduction of cardiovascular risk: the role of nicotinic acid--a position paper developed by the European Consensus Panel on HDL-C. Curr Med Res Opin. 2004;20:1253–1268. doi: 10.1185/030079904125004402. [DOI] [PubMed] [Google Scholar]
- 17.Linsel-Nitschke P, Tall AR. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat Rev Drug Discov. 2005;4:193–205. doi: 10.1038/nrd1658. [DOI] [PubMed] [Google Scholar]
- 18.Newman PJ, Newman DK. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arterioscler Thromb Vasc Biol. 2003;23:953–964. doi: 10.1161/01.ATV.0000071347.69358.D9. [DOI] [PubMed] [Google Scholar]
- 19.Albeda FW, van der Meer J, Vellenga E. Vascular proliferation as an unusual cause of hemorrhagic diathesis in myelofibrosis. Am J Clin Pathol. 1991;95:564–566. doi: 10.1093/ajcp/95.4.564. [DOI] [PubMed] [Google Scholar]
- 20.Price DT, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med. 1999;107:85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 21.Buttiglieri S, Pasqui D, Migliori M, Johnstone H, Affrossman S, Sereni L, Wratten ML, Barbucci R, Tetta C, Camussi G. Endothelization and adherence of leucocytes to nanostructured surfaces. Biomaterials. 2003;24:2731–2738. doi: 10.1016/s0142-9612(03)00088-7. [DOI] [PubMed] [Google Scholar]
- 22.Reedquist KA, Ross E, Koop EA, Wolthuis RM, Zwartkruis FJ, van Kooyk Y, Salmon M, Buckley CD, Bos JL. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol. 2000;148:1151–1158. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet. 1999;22:336–345. doi: 10.1038/11905. [DOI] [PubMed] [Google Scholar]
- 24.Remaley AT, Stonik JA, Demosky SJ, Neufeld EB, Bocharov AV, Vishnyakova TG, Eggerman TL, Patterson AP, Duverger NJ, Santamarina-Fojo S, Brewer HB., Jr Apolipoprotein specificity for lipid efflux by the human ABCAI transporter. Biochem Biophys Res Commun. 2001;280:818–823. doi: 10.1006/bbrc.2000.4219. [DOI] [PubMed] [Google Scholar]
- 25.Tang C, Vaughan AM, Oram JF. Janus kinase 2 modulates the apolipoprotein interactions with ABCA1 required for removing cellular cholesterol. J Biol Chem. 2004;279:7622–7628. doi: 10.1074/jbc.M312571200. [DOI] [PubMed] [Google Scholar]
- 26.Green PH, Glickman RM, Riley JW, Quinet E. Human apolipoprotein A-IV. Intestinal origin and distribution in plasma. J Clin Invest. 1980;65:911–919. doi: 10.1172/JCI109745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Dong JB, Wu MP. Human ApoA-I overexpression diminishes LPS-induced systemic inflammation and multiple organ damage in mice. Eur J Pharmacol. 2008 doi: 10.1016/j.ejphar.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 28.Calabresi L, Rossoni G, Gomaraschi M, Sisto F, Berti F, Franceschini G. High-density lipoproteins protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac tumor necrosis factor-alpha content and enhancing prostaglandin release. Circ Res. 2003;92:330–337. doi: 10.1161/01.res.0000054201.60308.1a. [DOI] [PubMed] [Google Scholar]
- 29.Gomaraschi M, Calabresi L, Rossoni G, Iametti S, Franceschini G, Stonik JA, Remaley AT. Anti-inflammatory and cardioprotective activities of synthetic high-density lipoprotein containing apolipoprotein A-I mimetic peptides. J Pharmacol Exp Ther. 2008;324:776–783. doi: 10.1124/jpet.107.129411. [DOI] [PubMed] [Google Scholar]