Abstract
Studies investigating response reversal consistently implicate regions of medial and lateral prefrontal cortex when reinforcement contingencies change. However, it is unclear from these studies how these regions give rise to the individual components of response reversal, such as reinforcement value encoding, response inhibition, and response change. Here we report a novel instrumental learning task designed to determine whether regions implicated in processing reversal errors are uniquely involved in this process, or whether they play a more general role in representing response competition, reinforcement value, or punishment value in the absence of demands for response change. In line with previous findings, reversal errors activated orbitofrontal cortex, dorsomedial prefrontal cortex, ventrolateral prefrontal cortex, caudate, and dorsolateral prefrontal cortex. These regions also showed increased activity to errors in the absence of contingency changes. In addition, ventrolateral PFC, caudate, and dorsolateral PFC each exhibited increased activity following correct reversals. Activity in these regions was not significantly modulated by changes in reinforcement value that were not sufficient to make an alternative response advantageous. These data do not support punishment-processing or prepotent reponse inhibition accounts of ventrolateral prefrontal cortex function. Instead, they support recent conceptualizations of ventrolateral prefrontal cortex function that implicate this region in resolving response competition by manipulating the representation of either motor response options, or object features. These data also suggest that dorsolateral prefrontal cortex plays a role in reversal learning, probably through top down attentional control of object or reinforcement features when task demands increase.
Keywords: Response reversal, affective shift, response competition, ventrolateral prefrontal cortex, decision-making
Introduction
Regions of medial (BA 10), ventrolateral, and dorsomedial prefrontal cortex (PFC) have been implicated in altering behaviour when the reward value of available response options change [1-4]. This phenomenon, referred to as “response reversal,” likely consists of functionally distinct components such as stimulus value representation, response inhibition, response selection, and response initiation. However, precisely how the separate regions of PFC map on to these potentially distinct functions in the context of reversal learning remains unclear. The current paper presents the results of a novel fMRI task designed to dissociate some of these components of response reversal and decision-making.
Commonly, a functional division is made between medial orbitofrontal cortex (mOFC) and lateral regions of PFC based on valence processing. For example, mOFC is thought to represent reward value [5-7]. Alternatively, mOFC may serve a decision-making or response selection function [8], perhaps using expected reinforcement (emotional) information to guide responding and stimulus choice [9]. The functional role that ventrolateral PFC plays in decision-making remains unclear. Data exist in support of several theoretical cognitive functions including punishment processing [10, 11], response change [1], biasing attention to categories [12], and response-inhibition [13-15]. It has also been suggested that ventrolateral PFC supports response reversal by representing object-motor features and interacting with the caudate to facilitate the resolution of motor response conflict [9, 16].
Neuroimaging studies involving response reversal also show activity in dorsolateral regions of PFC [2, 10]; however, the functional significance of this activity has been given less consideration. Its role is particularly unclear as human and non-human lesion studies strongly suggest that dorsolateral PFC is not necessary for response reversal [17, 18]. In marmosets, a region of lateral prefrontal cortex (BA 9), has been implicated in attentional shifts [18]. Adjacent regions of lateral prefrontal cortex (BA 46) have been implicated in attentional selection or set-shifting in human imaging studies [19, 20]. It has been proposed that lateral PFC supports this function by augmenting the representation of relevant stimulus features at the expense of competing ones [21]. An alternative conceptualization has been proposed by Hampshire and Owens [12] who suggest that this region provides “a higher level role in attentional control, involving the coordination of search behaviour for active solution derivation” (pg. 1687). Taken together, these models suggest a more generalized role for dorsolateral PFC in reversal learning. Both attentional selection and search coordination accounts of DLPFC predict that this region should show enhanced activity whenever task demands increase, or contingencies have changed.
In this study, participants engaged in a novel stimulus-response instrumental learning task in which they decided whether to sell or keep stocks (stimuli) based on their current market (reinforcement) value. Importantly, the task was designed to assess instrumental learning and not investment behaviour; it did not seek to mimic real world stock market trading. After an initial stimulus-response acquisition phase, a reinforcement shift phase followed in which the value of the correct response changed for 80% of stocks. Half of these stocks underwent a change in value such that the established advantageous response (keeping the stock for later sale) became disadvantageous (providing incentive for response change). For the remainder, the value of the advantageous response changed, but the change was not sufficient to make response change optimal.
Following previous investigations of response reversal, reversal errors (previously rewarding responses that became punished) were contrasted with correct responses to unchanged stimuli. This contrast identified neural regions previously implicated in response reversal. Regions of interest (ROIs) based on this contrast were used to examine our experimental questions: First, are regions of ventrolateral PFC that respond to reversal errors also active to other errors; i.e., acquisition errors or non-reversal errors (errors to stimuli that do not warrant a response change during the reinforcement shift phase)? If ventrolateral PFC's role is to inhibit prepotent responses, then this region should show significantly greater activity during reversal errors than acquisition or non-reversal errors. Second, are regions of ventrolateral PFC only significantly responsive in the context of punishment information? If ventrolateral PFC represents punishment information then it should only show significant activation following punished responses. However, if ventrolateral PFC serves a broader function, such as in performing processes involved in resolving response conflict, then we should observe significant activity in this region even during correct reversal trials (i.e., when perhaps conflict, but certainly not punishment information are present). Third, are regions implicated in response reversal also involved in detecting reinforcement level changes associated with responses even if these do not motivate response change? Fourth, what is the role of dorsolateral PFC in response reversal?
Methods
Participants
Thirteen right-handed healthy volunteers (4 females and 9 males) took part in the study (aged 22 to 38 years; mean 29.15; standard deviation 6.76). All participants underwent a medical exam performed by a physician, were free of psychotropic medication, and were screened to exclude those with a history of psychiatric or neurological illness. Prior to proceeding to the fMRI scanner, all participants completed an abbreviated practice version of the task consisting of 24 trials to insure that they understood the objectives of the task and were proficient in their responding.
fMRI data acquisition
Subjects were scanned during task performance using a 1.5 Tesla GE Signa scanner. Functional images were acquired with a gradient echo planar imaging (EPI) sequence (repetition time = 2500ms, echo time = 40ms, 64 × 64 matrix, flip angle 90°, FOV 24cm). Coverage was obtained with 29 axial slices (thickness, 4-mm; in-plane resolution, 3.75 × 3.75 mm). A high resolution anatomical scan (three-dimensional Spoiled GRASS; repetition time = 8.1ms, echo time = 3.2ms; field of view = 24cm; flip angle = 20°; 124 axial slices; thickness = 1.0 mm; 256 × 256 matrix) in register with the EPI dataset was obtained covering the whole brain.
The Stock Market Task
The Stock Market Task is a novel task designed to engage the participant in decision-making under conditions of changing response values and demands. In it, participants decide to either sell or retain “stocks.” Although the task was placed within an artificial “stock exchange” context, it was designed to investigate dissociable elements of decision-making rather than to simulate stock-market investment behaviour. Each participant underwent a practice task outside of the scanner before being positioned in the scanner to complete eight different versions of the task, with different stock stimuli and values. Each stimulus (“stock”) was a black and white drawing from Snodgrass and Vanderwart's [22] standard images. Individual stimuli were assigned to one of three reinforcement schedules: response reversal, value change, or a control condition (each described in detail below). On each trial, the participant was presented with a stimulus (“stock”), and had the option to either sell the stock immediately or to retain the stock for a period before selling it. Following each response, participants were told how much money they received. Each trial consisted of a fixation point (250 ms), a response option screen (4500ms), a blank screen (500ms), and a feedback screen (4000ms). Participants could only respond during the presentation of the response-option screen.
At the start of each run, participants underwent an “acquisition phase,” which consisted of 15 trials (3 blocks of each of the five stimuli which were presented once per block). The starting values of the stimuli differed between runs to prevent subjects from anticipating the reinforcement contingency. The best response option during each acquisition phase was always to retain the stock before selling it, rather than to sell it immediately. On each trial after making their response, subjects were informed the value of their response, and the value of the forgone response. Thus, subjects gained experience with both the sell and retain values of a stimulus on every single exposure. During blocks 1, 2, and 3, subjects encountered each stimulus three times (once per block). This allowed them the opportunity to learn the correct response and value of each stimulus. At the end of the acquisition phase, a “reinforcement shift phase” began without warning. Within each run, the reinforcement shift phase consisted of 25 trials (5 blocks of each of the 5 stimuli, which were presented once per block). During the reinforcement shift phase, the dollar value associated with immediately selling the stock remained the same as it was during the acquisition phase. However, the value of retaining the stock for later sale was systematically altered for four of the five stimulus types. For 2 of the 5 stimuli presented in each run (the reversal stimuli), the value of keeping the stock changed and the participant's best response changed; it became best to immediately sell the stock. All errors to these stimuli were referred to as “reversal errors,” and all correct selections referred to as “correct reversals.” For 2 other stimuli, the value of keeping the stock changed, but the participant's best response was still to keep the stock (“value change” stimuli). For a fifth “control” stimulus, neither the value of keeping or selling the stock changed. Errors to value change or control stimuli were considered “non-reversal errors.” Thus, at the start of the fourth block, subjects had to reverse their previous response for 2 stimuli, and retain the same response for 3 others.
Figure 1 provides a sample of the response option and feedback screen, as well as examples of correct and incorrect responses for a reversal stimulus during acquisition and reinforcement shift. Table 1 is a contingency table depicting in greater detail the reinforcement values (2 reversal, 2 value change, and 1 control stimuli) that were presented in each run (the start values changed between each run, but the format was the same). During the reinforcement shift change, only the value of the keep stock response changed. Note, the difference in value for the keep stock response between phases was held constant across stimulus types within a run ($150 in this run).
Figure 1.
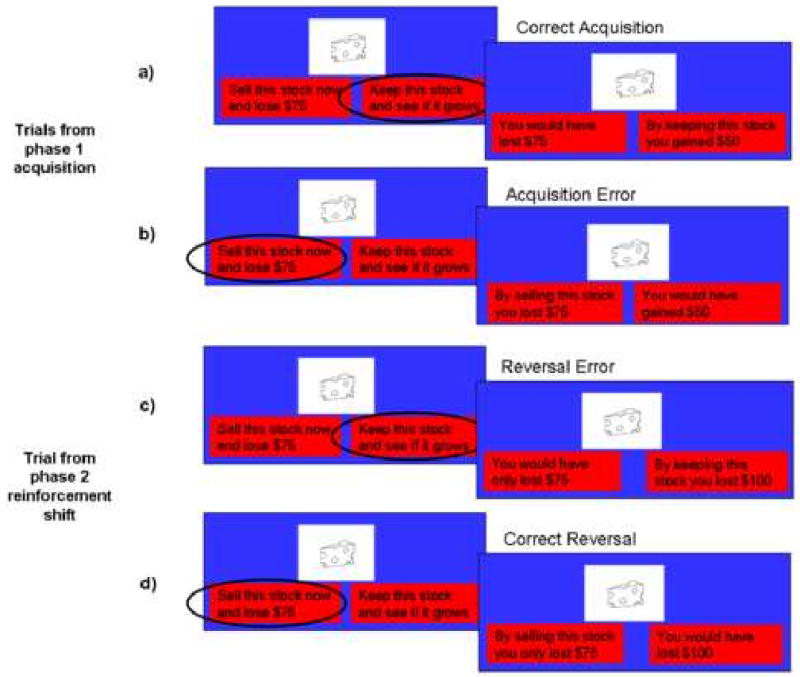
The stock market task. One distinct stimulus type is shown. The “cheese” stock undergoes a change in value that makes response change advantageous (depicted in a-d). Each row represents a separate sample trial. a) Correct acquisition trial. Left: The response option screen shows the stock (cheese), and the current market value of the stock “Sell this stock now and lose $75.” Next to the current market value is the alternate response option: “Keep this stock and see if it grows.” In this case, the participant elected to keep the stock. Right: The feedback screen. Here the subject gained $50 instead of losing $75 as indicated by the updated red boxes, which show the value of the response just made (“By keeping this stock, you gained $50”), as well as the value of the forgone response (“You would have lost $75”). b) Acquisition error. Left: The subject chooses to sell the stock now and lose $75. Right: The feedback screen indicates that selling the stock cost the subject $75 whereas they would have gained $50 had they kept the stock. c) Reversal error. This sample depicts a stimulus that changes in valence of reinforcement and best response. Left: The subject chooses to keep the stock. However, this stimulus has undergone a reversal. Right: The feedback screen indicates that while selling the stock still costs the subject $75, keeping the stock now costs even more ($100). So in the reinforcement shift phase, this stimulus underwent a change of valence and best response (the value of keeping the stock went from a gain of $50 to a loss of $100 and the best response changed from keeping the stock to selling it). d) Correct reversal. Left: The subject now chooses to sell the stock. Right: The feedback screen reflects that the subject received a loss of only $75 by selling the stock versus the $100 loss that would have resulted from keeping it.
Table 1.
Sample reinforcement table
| Phase 1: Acquisition | Phase 2: Reinforcement Shift | |||
|---|---|---|---|---|
| Sell Now | Keep Stock | Sell Now | Keep Stock | |
| Reversal Stimuli | ||||
| Valence change | - $75 | $50 | - $75 | - $100 |
| No valence change | - $100 | - $50 | - $100 | - $200 |
| Value Change Stimuli | ||||
| Valence change | - $100 | - $50 | - $100 | $100 |
| No Valence change | - $250 | - $200 | - $250 | - $50 |
| Control Stimulus | ||||
| No change | - $100 | - $50 | - $100 | -$50 |
Across all conditions on all trials, one response was always superior to the other (one response always yielded either a greater gain or a smaller loss than the alternative response). A response was scored an error if it yielded a smaller gain or a greater loss than the alternative. Participants were able to identify whether they had made an error at feedback as the reinforcements for both responses (retain and sell) were presented. In each task version, the participants received a new set of five stimuli (“stocks”). The reinforcements associated with the five different types of stimuli varied across runs so that subjects could not predict the stimuli's reinforcement contingency on the basis of the starting value. In total, there were 120 acquisition (24 for each of the 5 conditions) and 200 “reinforcement shift phase” trials (40 for each of the 5 conditions). The participants were given the scenario that they were a successful trader controlling stock for a number of clients.
fMRI analysis
Data were analyzed within the framework of the general linear model using the Analysis of Functional Neuroimages program (AFNI) [23]. Motion correction was performed by registering all volumes in the EPI dataset to a volume collected shortly before the high resolution anatomical dataset was acquired. EPI datasets were spatially smoothed (isotropic 6mm Gaussian kernel) and converted into percent signal change from baseline.
A total of 13 regressors were convolved with a gamma-variate hemodynamic response function to account for the slow hemodynamic response [24]. This hemodynamic response was modeled to the feedback stimuli. The first five regressors represented the correct responses to each of the 5 stimulus types presented during the acquisition phase (see Table 1). The second five regressors represented the correct responses to each of the same 5 stimulus types presented during the reinforcement shift phase. The five stimulus types were: (i) reversal valence change: where the magnitude, valence, and response associated with these stimuli all changed during the reinforcement shift phase; (ii) reversal no valence change: where the magnitude and appropriate response change during the reinforcement shift phase but the valence of reinforcement remains the same; (iii) valence change: where the value and valence both change during the reinforcement shift phase, but the correct response remains the same; (iv) no valence change: where the magnitude but not the valence or correct response change during the reinforcement shift phase; (v) control: where neither the value, valence, nor response change. The remaining 3 regressors comprised the 3 different error types that were possible: (vi) acquisition errors: errors during the acquisition phase; (vii) reversal errors: errors to either of the reversal stimuli during the reinforcement shift phase; (viii) non-reversal errors: errors to either of the valence change stimuli or the control stimuli during the reinforcement shift phase.
The regressors were combined to form our seven conditions of interest: (1) correct acquisition: all correct responses during the acquisition phase; (2) acquisition errors: errors during the acquisition phase; (3) correct response reversal: correct responses to the reversal stimuli during the reinforcement shift phase; (4) correct value change: correct responses to the value change stimuli during the reinforcement shift phase; (5) correct control: correct responses to the control stimulus during the reinforcement shift phase; (6) response reversal errors: incorrect responses to the reversal stimuli during the reinforcement shift phase; (7) non-reversal errors: incorrect responses to the value change and control stimuli in the reinforcement shift phase.
In line with previous work [10], the BOLD response to reversal errors following reinforcement shift was contrasted with activity to correct responses during the reinforcement shift phase. All clusters of activity showing a significant BOLD response (p < 0.001) were used to form our functionally defined ROIs. Average percent signal change was measured within each ROI for each regressor.
For each ROI, 5 planned paired t-tests were conducted. The first of these was constructed to determine whether regions active during reversal errors, particularly ventrolateral prefrontal cortex, are uniquely or preferentially activated to reversal errors as opposed to errors when a prepotent response is not yet established (i.e., acquisition or non-reversal errors). These tests included: (1) non-reversal errors versus the control condition; (2) acquisition errors versus correct responses during acquisition; and (3) response reversal errors versus acquisition errors. The next t-test, (4) correct reversals versus the control condition, addressed the question of whether regions implicated in processing reversal errors are specifically involved in processing punishment, or whether they also play a role during correct reversals. Finally, to address the question of whether regions implicated in response reversal are also involved in detecting changes in the value of responses that are not relevant for response change, the contrast (5) value change versus the control condition was performed. These tests were conducted as a stringent examination of the hypothesis that activity in regions implicated in reversal learning are also implicated in other aspects of decision-making including updating the value and appropriate motor response associated with a set of options.
Results
Behavioural Results
The proportion of errors was calculated for both the acquisition and reinforcement shift phases. Repeated-measures ANOVA was conducted on the proportion of correct selections revealed a significant main effect of condition (F(3,36) = 61.41; p < 0.001). As predicted, participants made significantly more errors for stimuli that reversed their contingency relative to any other condition (in each case, p < 0.001).
fMRI Results
Response reversal errors versus control condition
The contrast identified regions showing significantly greater activation to feedback following response reversal errors (responses that were previously correct, but were now incorrect following a change in reinforcement value) versus activation following a correct response to the control condition during the reversal phase of the task (p < 0.001). This contrast yielded significant activity in left middle frontal gyrus (BA 10), left and right caudate, left ventrolateral PFC (BA 47), dorsomedial PFC (BA 6/8), and right dorsolateral PFC (BA 8). This activation is summarized in Table 2.
Table 2.
Regions showing significantly greater activity to reversal errors than the control condition
| Region | L/R | BA | x | y | z | Volume |
|---|---|---|---|---|---|---|
| Ventrolateral PFC | L | 47 | -47 | 22 | -1 | 3489 |
| Dorsomedial PFC | R | 6 | 5 | 27 | 60 | 3684 |
| Dorsolateral PFC | R | 8 | 49 | 18 | 51 | 668 |
| Middle frontal gyrus | L | 10 | -25 | 58 | 28 | 337 |
| Caudate | L | - | -6 | 1 | 13 | 2016 |
| Caudate | R | - | 13 | 6 | 16 | 1288 |
| Thalamus | R | - | 2 | -22 | 5 | 396 |
(x, y, and z coordinates are MNI and refer to the location of the voxel with maximum signal intensity; p < 0.01, corrected for multiple comparisons).
Our functionally-defined ROIs were derived from these significant clusters of activity. The average percent signal change within each of these ROIs for each regressors was calculated in AFNI using “3dmaskave” [23]. This yielded an average percent signal change in each of our predefined conditions. Figure 2 depicts the area of activation and associated percent signal change for each condition within the dorsomedial PFC, ventrolateral PFC, and caudate. Figure 3 depicts the area of activation and associated percent signal change across conditions within dorsolateral prefrontal cortex. Planned paired t-tests were conducted on the percent-signal change for each of the ROIs as described above. These t-tests were designed to address our primary experimental questions:
Figure 2.
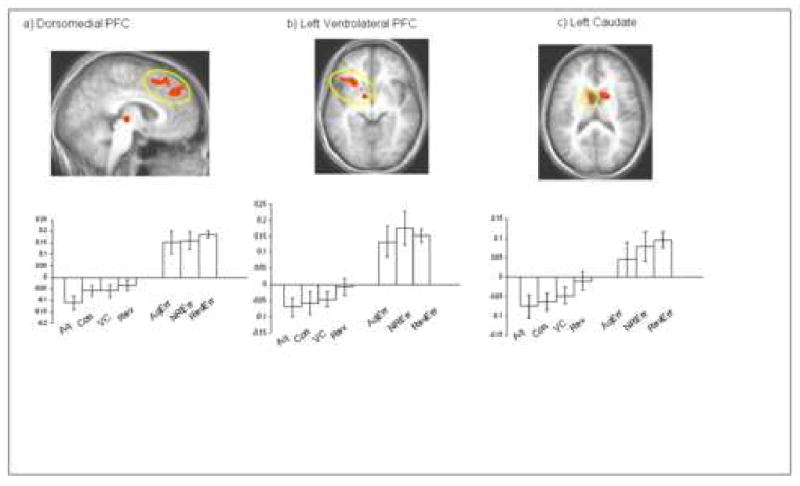
Images depicting significant activation in dorsomedial PFC, ventrolateral PFC, and caudate elicited by the contrast response reversal errors versus the control condition (p < 0.001, and p < 0.01 corrected). The conditions are abbreviated as follows: correct acquisition (Aq), correct control (Con), correct value change (VC), correct reversals (Rev), acquisition errors (AqErr), non-reversal errors (NRErr) and reversal errors (RevErr).
Figure 3.
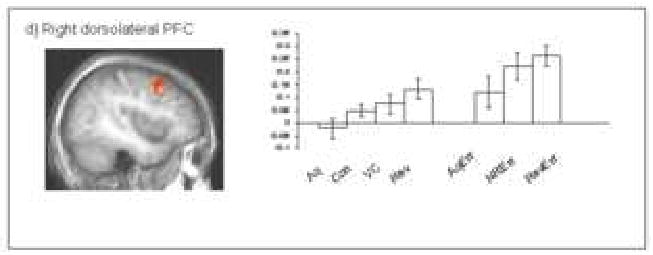
The graph depict the percent signal change across conditions within the region of dorsolateral prefrontal cortex shown.
-
(1) Are regions of ventrolateral PFC that respond to reversal errors also active to other errors?
If ventrolateral PFC's role is to inhibit prepotent responses, then this region should show significantly greater activity during reversal errors than acquisition or non-reversal errors. However, ventrolateral PFC did not show significantly greater activity to reversal errors relative to acquisition errors (p > 0.1), or to non-reversal errors (p > 0.1). In contrast, paired t-tests revealed greater activity in ventrolateral prefrontal cortex to acquisition errors relative to correct acquisition responses (p < 0.005), and to non-reversal errors relative to the control condition (p < 0.005).
Interestingly, our other functional ROIs of interest (dorsomedial PFC, bilateral caudate, and right dorsolateral PFC) all failed to show significantly greater activity to reversal errors relative to acquisition errors (p > 0.1 in all cases). However, all showed significantly greater activity to acquisition errors relative to correct acquisition responses and to non-reversal errors relative to the control condition (dorsomedial PFC (p < 0.001; p < 0.01), and bilateral caudate (p < 0.05; p < 0.005)).
-
(2) Are regions of ventrolateral PFC only significantly responsive in the context of punishment information?
If ventrolateral PFC represents punishment information then it should only show significant activation following errors. The data did not support this prediction; ventrolateral PFC showed significantly greater activity during correct reversals relative to the control condition (p < 0.05). This functional pattern was also observed bilaterally within the caudate ROI (p < 0.05), but not in dorsomedial PFC (p > 0.05).
-
(3) Are regions implicated in response reversal also involved in detecting reinforcement level changes associated with behaviour even if these do not motivate response change?
Planned comparisons were conducted to determine whether the regions implicated in response reversal were also activated by changes in the value of reinforcement that were not sufficient to motivate behavioural change. For each ROI, a paired t-test was conducted to compare activity to these changes of value versus the control condition (no change in reinforcement values). None of the regions sensitive to response reversal errors (ventrolateral PFC, middle frontal gyrus, dorsomedial PFC, dorsolateral PFC and bilateral caudate) showed significantly greater activity to value changes relative to the control condition (p >0.1).
-
What is the role of dorsolateral PFC in response reversal?
Significant dorsolateral PFC activity was evident during reversal errors. Unlike other regions implicated in reversal error processing, however, dorsolateral prefrontal cortex showed greater activity to response reversal errors relative to acquisition errors (p < 0.05). Like ventrolateral prefrontal cortex, this activity was significantly greater during acquisition errors relative to correct responses during acquisition (p = 0.05), and non-reversal errors versus the control condition (p < 0.01). As with ventrolateral PFC and caudate, greater activity was observed during correct reversals relative to the control condition (p < 0.05). Activity within dorsolateral PFC to changes in value not sufficient to motivate response change versus the control condition was not significant (p > 0.1).
Discussion
The current study included a decision-making task that engaged regions associated with response reversal and examined their functional characteristics across changing levels of reinforcement and response change demands. Significant activation to response reversal errors were seen in ventrolateral, dorsomedial and dorsolateral PFC as well as caudate. Notable BOLD responses were also seen in these functionally defined regions to non-reversal errors, and acquisition errors. In addition, ventrolateral and dorsolateral PFC and caudate showed enhanced activity to correct responses following a reversal of contingency.
Previous studies of reversal learning have implicated lateral regions of orbitofrontal or ventrolateral PFC in representing punishment [2, 6], response change following punishment [1], or the suppression of previously rewarded responses [5, 13, 14]. If the ventrolateral PFC supports response reversal by inhibiting representations of acquired response options (reducing perseverative responding in particular), greater activity in this region should be observed to response reversal errors over errors during acquisition (before a prepotent response is acquired). However, in the present study, the same region of ventrolateral PFC active to response reversal errors, also showed increased activity to acquisition errors (i.e., to never-rewarded stimulus-response mappings; Question 1). Moreover, ventrolateral PFC showed no greater activation to reversal errors than acquisition errors. In short, the current data are inconsistent with the suggestion that ventrolateral PFC serve to inhibit prepotent responses.
Alternatively, if the ventrolateral PFC encodes punishment or response change information, then it should not show significant activation for correct reversals relative to the control condition. However, this was seen in the current study (see Question 2). A compatible finding has recently been reported in a study of reversal learning that used a “probabilistic” reinforcement contingency. In probabilistic tasks, correct responses are rewarded most, but not all of the time, and incorrect responses are punished most, but not all of the time (e.g., 70% of correct responses are rewarded). Interestingly, this study found significantly increased ventrolateral PFC BOLD responding to both acquisition errors, and rewarded errors (probabilistic errors) during acquisition (i.e., even when no punishment information was received) [16]. Other decision-making studies have also observed increased activity to reward in lateral inferior frontal cortex [2, 25], and caudate [26, 27]. On the basis of these data, these regions cannot be considered to simply represent punishment.
An alternative functional model of ventrolateral PFC suggests that it supports response selection by increasing the salience of alternative motor response option representations through interactions with the striatum [9, 16]. Studies consistently implicate caudate in operant responding [28-31]. It is suggested that while the caudate represents motor responses necessary for simple operant behaviour, object/ motor features are also represented within ventrolateral PFC to allow for more flexible control over motor responding [9, 16]. According to the model, functional connectivity between ventrolateral PFC and caudate increases or decreases the probability that a given motor response will be selected on a subsequent trial. The model therefore predicts enhanced ventrolateral prefrontal and caudate activity whenever motor response competition is increased. Response competition would increase during response inhibition, but also to error feedback, false feedback, or a reversal in reinforcement contingencies. As predicted by this model, we observed increased activity in the same regions of ventrolateral PFC and caudate that were activated to reversal errors, during both non-reversal errors and correct reversals.
The current study differs from most previous studies of reversal learning in its use of multiple, concurrent discriminations rather than simple object discriminations. Most studies of reversal learning require reversals of a single pair of objects [1-3]. In our study, as in more complex forms of decision-making, subjects were required to make multiple stimulus discriminations and to change their responses for only some of these discriminations in the reinforcement shift phase (for 2 out of the 5 discriminations). As a consequence, they likely attended closely to each stimulus to determine which response to change, and which to maintain. This feature may account for the activity in ventrolateral PFC observed in situations where it was not previously found (e.g., during correct reversals). Here, as in a previous reversal learning task involving multiple stimulus discriminations [4], we suggest that the increased task demands involve conflict between different motor response representations. However, a recent study that also involved more complex object discriminations (using semi-transparent overlapping compound stimuli), specifically implicated VLPFC in attentional shifts, and a region of lateral OFC in response reversal [12]. We did not observe significant lateral OFC activity in our study. However, activity in this ventral region of prefrontal cortex is particularly susceptible to signal drop-out. Given that our scanning parameters were not specifically optimized for this region, this may account for why were unable to detect signal at these coordinates.
Dorsolateral PFC activity has also been reported in previous reversal learning studies [3, 10, 32-34]. Its functional significance has been given less attention, but recent decision-making studies suggest that dorsolateral PFC may play a higher-order executive role involving attention [2], or that it coordinates search behaviour [12]. One potential explanation for the role of dorsolateral PFC to decision-making involves theories of attentional, or “cognitive” control. Enhanced dorsolateral PFC activity is thought to result in increased attentional control of task-relevant stimulus features [21, 35, 36]. In the present study, the area of dorsolateral PFC active during reversal errors also showed increased activity during non-reversal errors and correct reversals. Unexpectedly, and unlike other regions of interest, dorsolateral PFC showed significantly greater activity to reversal errors than acquisition errors.
In the context of the current study, we suggest that increased conflict is likely to emerge whenever a significant increase in errors occurs, or whenever new reinforcement information becomes behaviourally relevant (as occurred after a change in contingency in phase 2). In such circumstances, increased attention might facilitate the detection of alternative cues to guide successful responding. The extent to which this enhanced attention facilitates performance will likely be related to task demands (e.g., number of potentially relevant stimulus features). Accordingly, lesions of the dorsolateral PFC may not impair simple object reversals when demands on attention are relatively minor [17, 18, 37]. However, relatively intact dorsolateral PFC functioning might be more important for decision-making or reversal learning tasks with multiple stimuli or stimulus properties, as is the case of the Iowa Gambling task [38], and reversal learning studies involving multiple objects [16] or stimulus dimensions[12].
Conclusion
In the present study, significant activation to response reversal errors was seen within dorsomedial PFC, ventrolateral PFC, caudate, and dorsolateral PFC. Each of these regions also showed increased responding to non-reversal errors, suggesting that their involvement is not restricted to situations in which errors occur to a previously rewarded response. Importantly, regions of ventrolateral PFC activated by reversal errors also showed significantly increased responding to correct reversals. These data suggest that conceptualizations of ventrolateral PFC function as representing punishment or inhibiting a prepotent response are too constrained. These findings are, however, compatible with suggestions that VLPFC shifts representations of motor responses [9] or object features [12] to guide responding. We also observed significant activity in dorsolateral PFC following errors. This finding is in line with recent suggestions that dorsolateral PFC supports response selection through mechanisms associated with increased attention to object/reinforcement features when response conflict increases [39], or to processes involved in solution search [12]. These data begin to show how functions associated with response reversal may be parsed and supported by dissociable regions of PFC. Further investigations will be required to determine, with greater specificity, the function and relative importance of these regions of PFC to more complex forms of decision-making.
Acknowledgments
This research was conducted at the National Institutes of Health in Bethesda Maryland and was supported by the Intramural Research Program of the NIH: NIMH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22(11):4563–7. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remijnse PL, Nielen MM, Uylings HB, Veltman DJ. Neural correlates of a reversal learning task with an affectively neutral baseline: an event-related fMRI study. Neuroimage. 2005;26(2):609–18. doi: 10.1016/j.neuroimage.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 3.O'Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23(21):7931–9. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budhani S, Marsh AA, Pine DS, Blair RJ. Neural correlates of response reversal: considering acquisition. Neuroimage. 2007;34(4):1754–1765. doi: 10.1016/j.neuroimage.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 5.Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10(3):308–17. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- 6.O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85(3):1315–21. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- 7.Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 8.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 9.Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55(1):198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 10.O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 11.Zald DH, Hagen MC, Pardo JV. Neural correlates of tasting concentrated quinine and sugar solutions. J Neurophysiol. 2002;87(2):1068–75. doi: 10.1152/jn.00358.2001. [DOI] [PubMed] [Google Scholar]
- 12.Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cereb Cortex. 2006;16(12):1679–89. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- 13.Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, Thomas KM, Noll DC. Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Hum Brain Mapp. 2001;13(1):26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iversen SD, Mishkin M. Perseverative interference in monkeys following selective lesions of the inferior prefrontal convexity. Exp Brain Res. 1970;11(4):376–86. doi: 10.1007/BF00237911. [DOI] [PubMed] [Google Scholar]
- 15.Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12(2):100–9. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budhani S, Marsh AA, Pine DS, Blair RJ. Neural correlates of response reversal: Considering acquisition. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 17.Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126(Pt 8):1830–7. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- 18.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 19.Rowe JB, Passingham RE. Working memory for location and time: activity in prefrontal area 46 relates to selection rather than maintenance in memory. Neuroimage. 2001;14(1 Pt 1):77–86. doi: 10.1006/nimg.2001.0784. [DOI] [PubMed] [Google Scholar]
- 20.Kondo H, Osaka N, Osaka M. Cooperation of the anterior cingulate cortex and dorsolateral prefrontal cortex for attention shifting. Neuroimage. 2004;23(2):670–9. doi: 10.1016/j.neuroimage.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 22.Snoddgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 23.Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 24.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 25.Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23(1):303–7. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–4. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 27.Richmond BJ, Liu Z, Shidara M. Neuroscience. Predicting future rewards. Science. 2003;301(5630):179–80. doi: 10.1126/science.1087383. [DOI] [PubMed] [Google Scholar]
- 28.Divac I, Rosvold HE, Szwarcbart MK. Behavioral effects of selective ablation of the caudate nucleus. J Comp Physiol Psychol. 1967;63(2):184–190. doi: 10.1037/h0024348. [DOI] [PubMed] [Google Scholar]
- 29.Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41(2):281–92. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 30.Kawagoe R, Takikawa Y, Hikosaka O. Reward-predicting activity of dopamine and caudate neurons--a possible mechanism of motivational control of saccadic eye movement. J Neurophysiol. 2004;91(2):1013–24. doi: 10.1152/jn.00721.2003. [DOI] [PubMed] [Google Scholar]
- 31.Takikawa Y, Kawagoe R, Hikosaka O. Reward-dependent spatial selectivity of anticipatory activity in monkey caudate neurons. J Neurophysiol. 2002;87(1):508–15. doi: 10.1152/jn.00288.2001. [DOI] [PubMed] [Google Scholar]
- 32.Nagahama Y, Okada T, Katsumi Y, Hayashi T, Yamauchi H, Oyanagi C, Konishi J, Fukuyama H, Shibasaki H. Dissociable mechanisms of attentional control within the human prefrontal cortex. Cereb Cortex. 2001;11(1):85–92. doi: 10.1093/cercor/11.1.85. [DOI] [PubMed] [Google Scholar]
- 33.Kringelbach ML, Rolls ET. Neural correlates of rapid reversal learning in a simple model of human social interaction. Neuroimage. 2003;20(2):1371–83. doi: 10.1016/S1053-8119(03)00393-8. [DOI] [PubMed] [Google Scholar]
- 34.Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12(1):142–62. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- 35.Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–6. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 36.Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17(4):1820–9. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- 37.Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57(12):1518–24. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb Cortex. 2005;15(1):58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- 39.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8(12):539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]


