Abstract
Abused solvents have effects similar to those of abused depressant drugs. This experiment evaluated the time course of the discriminative stimulus effects of toluene and 1,1,1-trichloroethane (TRI). Mice were trained to discriminate between i.p. injections of ethanol (EtOH;1.25 g/kg) and saline in a two-lever operant task in which responding was under the control of a fixed-ratio 20 schedule. After 20-min inhalation exposures to toluene (500–6000 ppm) or TRI (1,000–12,000 ppm), stimulus generalization was examined at 0, 5, 10, 20, and 40 min post-exposure. Ethanol doses ≥ 0.25 g/kg produced increases in EtOH-lever responding with full substitution occurring immediately after testing for doses between 1.25 and 2.5 g/kg. Toluene and TRI produced increased EtOH-lever responding at 0–10 min post exposure with some EtOH-lever responding occurring up to 20-min post exposure. Response rates were not decreased for any concentration of toluene or TRI immediately following inhalant exposure but several concentrations elevated rates from 5–40 min post exposure. These results confirm and extend previous studies and show these solvents produce similar effects in EtOH-lever responding but with potency differences. The time-dependent differences in EtOH-lever responding suggest that as solvents are cleared from the body, the EtOH-like subjective effects also fade.
Keywords: Inhalant abuse; solvents; operant behavior; drug discrimination; toluene; 1,1,1-trichloroethane
INTRODUCTION
The mind-altering effects of inhaled volatile substances have been known for many years. Toluene and 1,1,1-trichlorethane (TRI), along with a wide variety of other volatile compounds, are components in many commercially abused products (airplane glue, paint thinners, gasoline, etc.) and are believed to be responsible for their abuse potential (Balster, 1987). While inhaling solvents has been popular for a number of years, surprisingly little is known about the nature of their behavioral effects. A prominent hypothesis is that the acute intoxication produced by volatile inhalants may be similar to the intoxication produced by classic central nervous system (CNS) depressants such as the barbiturates and ethanol (EtOH). Support for this hypothesis has come from investigations in which various volatile compounds (e.g., toluene; TRI) were each shown to produce a profile of pharmacological and behavioral effects similar to that of pentobarbital and EtOH (Bowen et al., 2006). For example, these inhalants impair coordinated motor performance (Moser and Balster, 1985a), produce reversible drug-like effects on operant response rates (Bowen, 2006; Bowen and Balster, 1997a, b, 2006; Glowa, 1985, 1981; Moser and Balster, 1985a, 1986), and cross-dependence (Evans and Balster, 1993). Further, these effects are intensified when inhalants are combined with EtOH (Johnstone et al., 1975; Woolverton and Balster, 1981).
The cellular mechanisms by which abused inhalants produce these effects are only beginning to be understood. Several electrophysiological studies have demonstrated that toluene, as well as other solvents, have effects on several ligand-gated ion channels. Like ethanol, toluene appears to be a noncompetitive NMDA receptor antagonist and several NMDA receptor subtypes (NR1 and NR2B) have been identified as being the most sensitive to toluene’s inhibitory effects (Bale et al., 2005; Cruz et al., 1998). Antagonism of the NMDA-receptor is not unique to toluene and has also been indicated for other solvents, such as benzene, xylene, TCE, ethylbenzene and propylbenzene (Cruz et al., 2000; Raines et al., 2004). In addition, a recent study has shown that prolonged exposure to toluene increases NMDA receptor levels in the brain which is similar to reports for prolonged exposure to ethanol in animals and humans (Williams et al., 2005). Other targets for solvent effects have been recently studied. Toluene, trichloroethylene and 1,1,1-trichloroethane have also been shown to enhance the function of two inhibitory ligand-gated ion channels: GABAA and glycine (Beckstead et al., 2000). Toluene has also been shown to block recombinant and native cardiac sodium channels, an effect that is consistent with the arrhythmogenic properties attributed to toluene (Cruz et al., 2003). Finally and not surprisingly, toluene, like other drugs of abuse, is known to modulate dopamine transmission, an action that may be related to its rewarding effects (Gerasimov et al., 2002; Riegel and French, 1999; Riegel et al., 2007). A recent paper reviews the most important findings in this area during the last decade of animal research (Bowen et al., 2006).
If the behavioral and pharmacological effects of solvents are similar to those of abused depressant drugs, it would be expected that the subjective effects produced by solvent intoxication would be similar to those produced by the CNS depressant drugs. The discriminative stimulus effects of drugs in animals are accepted models of subjective effects in humans (Schuster et al., 1981) and these procedures are used widely to investigate the neuropharmacological effects produced by a number of abused drugs (Samele et al., 1991; Stolerman et al., 1995). While the use of drug discrimination procedures for measuring drug effects are well established, this methodology has been used only sparingly in the investigations of abused vapors. The few studies that have investigated the discriminative stimulus properties of inhalants have shown that some volatile inhalants have effects similar to those of a number of CNS depressant drugs, including EtOH (Balster et al., 1997; Rees et al., 1987). In addition to abused solvents, volatile anesthetic gases have also been shown to produce EtOH-like discriminative stimulus effects (Bowen and Balster, 1997b). The discriminative stimulus properties these compounds share with EtOH suggest that these inhalants may share some of EtOH’s pharmacological properties.
While some of the other behavioral effects of solvent exposure, such as the motor effects (Moser and Balster, 1985b) appear to be time-dependent, few studies have investigated the time course of drug discriminations with solvents. Therefore, the purpose of the present study was to evaluate the time course of the behavioral effects of abused inhalants using a drug discrimination procedure. Stimulus generalization tests were conducted 0, 5, 10, 20 and 40 min after 20 min vapor exposures to toluene and TRI in mice trained to discriminate i.p. injections of EtOH (1.25 g/kg) from saline under a two-lever fixed ratio (FR20) schedule of milk reinforcement. It was hypothesized that exposures to toluene or TRI would produce concentration-related increases in EtOH-lever responding that would decrease in a time-dependent fashion.
METHOD
Subjects
Ten experimentally naïve male mice (CFW, Charles River Co., Wilmington, MA) were housed individually in standard mouse cages (18 × 29 × 13 cm) with wood chip bedding and steel-wire tops. Animals were kept in a room with controlled temperature (22–24°C) on a 12-hr light/dark cycle. Mice were brought into the laboratory and tested during the light cycle (0700–1200 hrs). Mice were maintained on a restricted diet by post-session feeding of rodent chow (Rodent Laboratory Chow, Ralston-Purina C., St. Louis, MO) in amounts sufficient to maintain weights at 85% of free-feeding weights. Water was available ad libitum in the home cage. All animal procedures had prior approval by the Wayne State University Institutional Animal Care and Use Committee (IACUC) and were in accordance with the NIH “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academy Press 1996; NIH publication No. 85-23, revised 1996).
Apparatus
Behavioral training and testing were conducted in ten computer-interfaced operant conditioning chambers (Med Associates, St. Albans, VT) which have been previously described (Bowen, 2006). Briefly, test chambers consisted of floors containing parallel stainless steel rods and walls constructed of aluminum with a single Plexiglas wall containing a door. Each of the chambers was enclosed in a cubicle which attenuated external light and sound. Every chamber was equipped with two low-force response levers (Med Associates, St. Albans, VT) which extended 0.8 cm into the chamber. Located above each of the levers were LED stimulus lamps. A recessed food trough was located midway between the levers into which a liquid dipper would deliver 0.02 ml of sweetened-condensed milk (one part sugar, one part condensed milk, and two parts water by volume). The illumination of a houselight located in the top rear of the chamber signaled that the session was in progress.
Inhalation Exposure
Vapor exposures were conducted in a static exposure system consisting of sealed 36-l cylindrical glass jars with acrylic lids (identical to the system detailed in (Bowen, 2006)). The lids had injection ports, a fan and a stainless steel mesh box holding filter paper. During exposures, a single mouse was placed onto a grid floor 20 cm from the bottom of the exposure chamber. For air-only exposures, the lid was sealed (with nothing injected onto the filter paper) and the fan turned on. For toluene or TRI exposures, the lid was sealed and a calculated amount of solvent was injected onto filter paper suspended below the sealed lid. The amount of liquid needed to produce a given vapor concentration was calculated using the ideal gas law equation simplified for room temperature (Nelson, 1971). The fan was then turned on which volatilized and distributed the solvent within the exposure chamber. Vapor concentrations were confirmed by single wavelength-monitoring infrared spectrometry (Miran 1A, Foxboro Analytical). Mean concentrations of toluene and TRI were within 3% of nominal ~2.5 min after the solvent was added and remained within 2% of the nominal concentrations throughout the 20-min exposures. Levels of waste gases (i.e., water vapor and CO2) had been previously monitored during pilot studies and changes during 15-min sessions were negligible. After the exposure, mice were removed immediately and placed in their operant conditioning chambers for behavioral testing/training.
Discrimination Training
The testing protocol employed for the EtOH discrimination in the present study was similar to conditions used in previous drug discrimination studies in EtOH-trained mice (Bowen and Balster, 1997b). Initially, mice were trained to press one lever following administration of ethanol (EtOH; 1.25 g/kg) and to press another lever after injection with saline, each according to a fixed-ratio 1 (FR-1) schedule of milk reinforcement. During each day of discrimination training responses on only one of the two levers delivered reinforcement. The position of the reinforced (correct) lever was determined by the type of injection the mouse received on a given day. Each response on the incorrect lever reset the ratio requirement on the correct lever. The daily injections for each mouse were administered in a double alternation sequence of drug and saline. During acquisition of the discrimination, mice were injected and placed in their home cages 20 min prior to the start of the experimental session. Daily (Monday–Friday) session length was 20 min.
Once mice were responding reliably on the injection-appropriate levers, the number of responses required for delivery of milk reinforcement was gradually increased from a FR1 to FR20. Test sessions with the injections used for training were conducted on Tuesdays and Fridays. Completion of the response requirement on either lever during the two-min probe test sessions produced a milk reinforcer. These initial test sessions were followed immediately by an 18-min training session during which only correct responses were reinforced. Acquisition of the discrimination was defined as the successful completion of four consecutive test sessions (two EtOH and two saline). Success was defined as both completing the first FR and responding at least 85% on the correct lever throughout the session. After successful acquisition training, mice were adapted to the inhalation procedure by placing them in the inhalation chamber with “air only” exposure immediately following injection for the 20-min pre-session injection interval. This exposure procedure was continued during training sessions throughout the study.
Generalization Testing
Following the acquisition of discrimination between 1.25 g/kg EtOH and saline and the adaptation period, generalization testing was begun. Discrimination tests were performed on Tuesdays and Fridays contingent on the subject completing the first FR on the correct response lever and having over 85% correct-lever responding over the entire training session preceding the test day. To preserve the discrimination, mice continued to be trained on the double alternation sequence of EtOH and saline training sessions (with air-only exposure during pre-session interval) on non-test days (Mondays, Wednesdays and Thursdays). During test sessions with different doses of the training drug and control test sessions with vehicle and the training drug, mice were injected with the appropriate dose of drug, placed into the inhalation chamber and exposed to air-only for the pre-session injection interval (20 min). During test sessions with inhalants, mice were first injected with saline and then placed in the inhalation chamber for 20-min exposures to the appropriate concentration of the inhalant (or air). For all test sessions, mice were rapidly removed from the exposure chambers and placed into the operant chambers within 30 sec of termination of the exposure. The ensuing operant test session began 0, 5, 10, 20 or 40 min after exposure and lasted two-min, during which responding on either lever was reinforced. Following the two-min test, the subjects were returned to their home cages.
Initial discrimination tests were conducted with several doses of EtOH in the order shown (0.125, 0.25, 0.5. 1.25, 1.50, 2.0, 2.5 g/kg), its vehicle (water), and saline given 20 min pre-session. Substitution tests were conducted in the order shown with the vapors TRI (1,000, 2,000, 4,000, 8,000, 10,000, 12,000 ppm) first followed by toluene (500, 1,000, 2,000, 4,000, 6,000 ppm). Control test sessions with saline and the training dose of EtOH (plus air exposure) were performed before and after each concentration- or dose-effect curve determination.
Data Analysis
The ability of the tested concentrations of EtOH, toluene or TRI to substitute for the training dose of EtOH was measured as the percentage of responses emitted on the EtOH -appropriate lever during each of the 2-min test trials. For test components that resulted in the suppression of an individual subject’s response rate to less than 3 responses/min (less than 6 responses total), the corresponding percentage of EtOH-lever responding for that subject for that test was excluded from determination of the mean percentage EtOH-lever responding, whereas response rate averages were determined using all test data.
Complete substitution was defined as at least 80% responding on the EtOH-appropriate lever with mean EtOH-lever responding >20% but <80% being defined as partial substitution. Mean EtOH-lever responding <20% was considered to be evidence of no substitution for EtOH. Response rates (responses/sec) were recorded during each test session. Averages of all the animals for each of the five time trials are presented. For statistical analyses, response rates from each trial were averaged to arrive at total response rate for each dose/concentration tested. These data were analyzed separately for each test substance utilizing repeated-measures ANOVAs and Bonferroni post hoc comparisons (α < 0.05) for differences from the saline/air control. For analyses across different time points/trials tested, the response rates from each of the five trials were averaged to arrive at total session response rates for each of the five FR trials. These data were analyzed separately for each test substance utilizing two-way (concentration × trial) repeated-measures ANOVAs and Bonferroni post hoc comparisons (α < 0.05).
Chemicals
The test agents, toluene (T-324, Fisher Scientific Co., Fairlawn, NJ) and TRI (T391, Fisher Scientific Co., Fairlawn, NJ) were purchased commercially (purity > 99.5%). Ethanol was diluted with sterile water to a 10% EtOH solution (100 mg/ml) and injections were given i.p. in a volume of 10 ml/kg.
RESULTS
Ethanol Exposures
All mice were successful in acquiring the discrimination between 1.25 g/kg EtOH and saline in ≤ 60 sessions. The results of the subsequent generalization tests over a range of EtOH doses are shown in Figure 1. As expected, mice responded for milk almost exclusively on the EtOH lever when they were given an injection of the training dose of EtOH (1.25 g/kg) and on the saline lever when injected with saline. Response rates for saline control tests were 1.86 ± 0.24 responses/second. Tests with other doses of EtOH produced dose-dependent substitution for the EtOH training dose with >90% EtOH-lever selection being demonstrated at the training dose of EtOH (1.25 g/kg). Full substitution also occurred at the next highest dose tested (2.0 g/kg) but not the lower doses. A significant main effect of EtOH exposure was seen for response rates, F(8,64)=6.51, p<0.001. Post hoc analyses revealed that the 2.5 g/kg severely depressed response rates compared to saline control levels (p<0.001).
Figure 1.
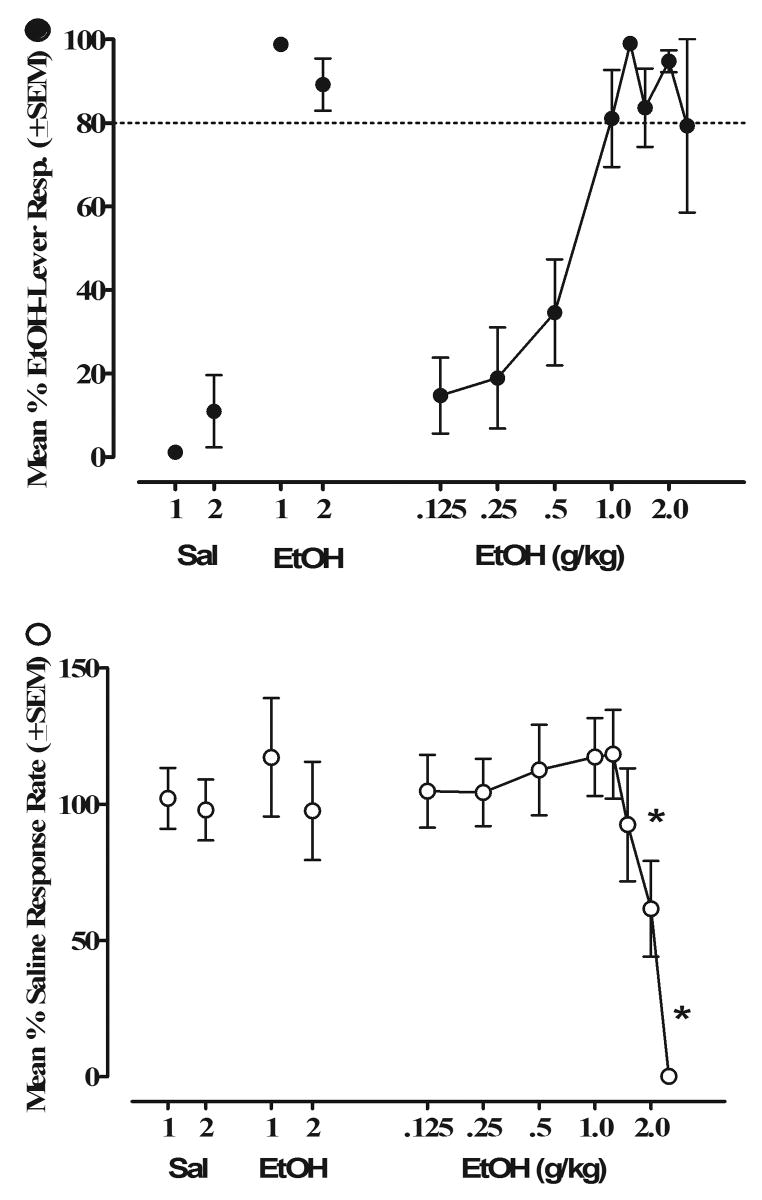
Dose-effect curves for EtOH in mice trained to discriminate 1.25 g/kg EtOH from saline (n=10). Percentage of drug-lever responding (mean ± S.E.M.) is shown in the top panel; response rates as responses per second (mean ± S.E.M.) are shown in the bottom panel. Control data for EtOH and saline represent the drug or saline-test session which occurred immediately before (1) or after (2) drug testing. *Significantly different from saline/air (p < 0.05).
Toluene Exposures
The results of substitution testing with toluene are shown in Fig. 2. As seen in the top panel of Fig 2, tests with toluene 0–5 min after exposure resulted in more responding on the EtOH lever at all concentrations tested as compared to the “air-only” exposure. Exposure to 6000-ppm toluene produced the highest EtOH-lever responding (89%) immediately after exposure (time point “0”), with eight of the ten mice completing the FR20 requirement on either lever during this first trial. As shown in the top panel of Fig. 2, 5 min after exposure the highest concentration of toluene was still producing 85% EtOH-lever responding without significant decreases in response rates. However, when animals were tested 10 min after exposure, this concentration of toluene resulted in only 43% EtOH-lever responding which decreased to 0% EtOH-lever responding 40 min after exposure. The toluene concentration of 4000 ppm initially produced ~84% EtOH-lever responding immediately after exposure (time point “0”) with the degree of substitution very similar to that of 6000 ppm of toluene across all time points tested. The lower concentrations of toluene tested (500–2000 ppm) demonstrated a maximum of 47% EtOH-lever responding immediately after exposure, which continued to decrease across trials reaching 0% between 20–40 min after exposure. As can be seen in the bottom panel of Fig. 2, the increases in EtOH-lever responding after each toluene exposure were associated with alterations in response rates. Significant main effects were observed for Toluene Concentration F(5,35) = 3.92, p<0.01) and Time (F(4,28) = 21.09, p<0.001), with the 1000–4000 concentrations of toluene increasing response rates as compared to saline/air controls (Figure 2, bottom panels). A significant Toluene Concentration × Time interaction (F(20,140) = 3.87, p<0.001) indicated that response rates increased as time from exposure increased. Post hoc analyses revealed that 1000 ppm increased response rates compared to saline/air control levels at 0, 5, and 40 min after exposure (p<0.05) with similar increases observed for the 2000 ppm.concentration at 5 and 10 min after exposure (p<0.05). As compared to saline/air control levels, 4000 ppm of toluene increased rates at 10, 20 and 40 min after exposure with 6000 ppm increasing rates above saline/air at 20 min post exposure (p<0.05).
Figure 2.
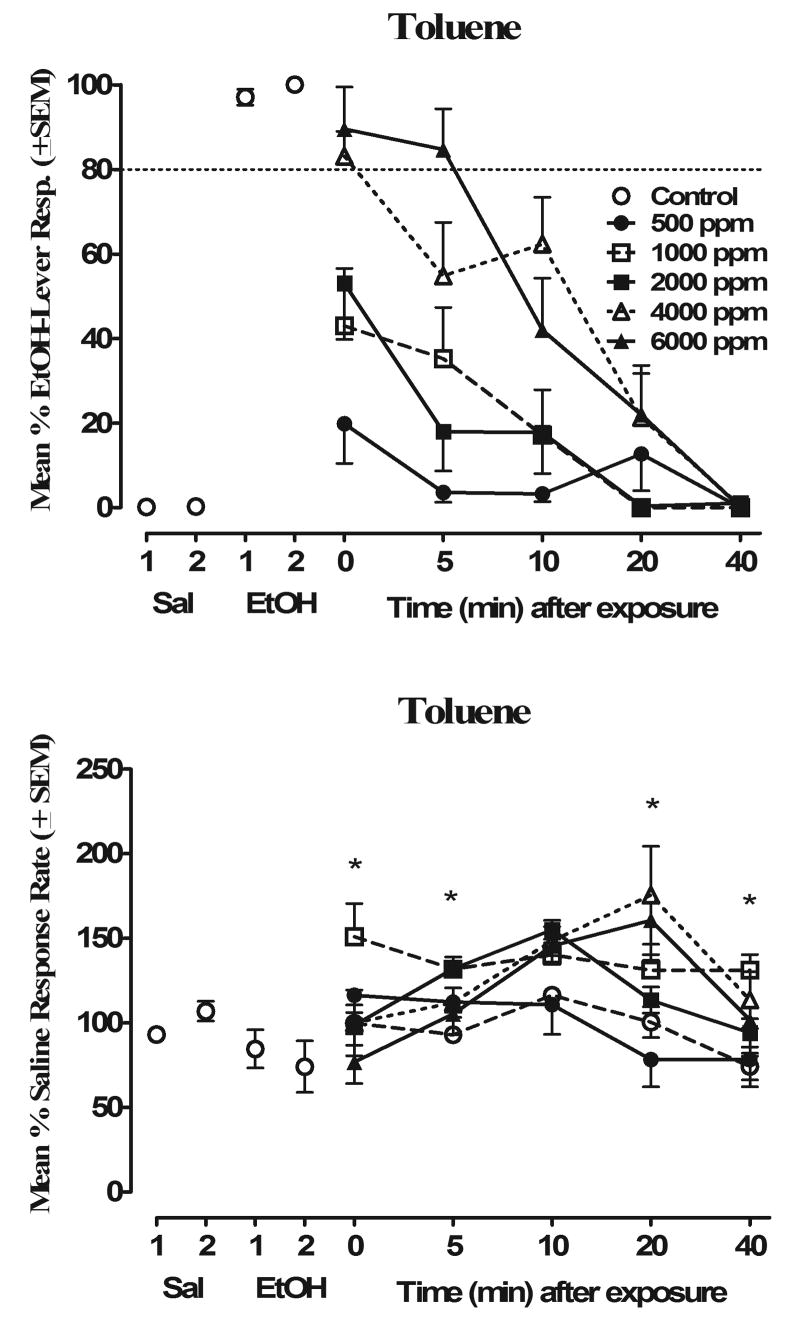
Concentration- and time-effect curves for toluene in mice trained to discriminate 1.25 g/kg EtOH from saline (n=10). Percentage of drug-lever responding (mean ± S.E.M.) is shown in the top panel; response rates as responses per second (mean ± S.E.M.) are shown in the bottom panel. Control data for EtOH and saline represent the drug or saline-test session which occurred immediately before (1) or after (2) toluene testing. *Significantly different from saline/air (p < 0.05).
TRI Exposures
Exposure to TRI also resulted in concentration-related increases in EtOH-lever responding (Fig. 3) but at higher concentrations than were observed for toluene. As seen in the top panel of Fig. 3, tests with TRI 0–5 min after exposure resulted in increases in responding on the EtOH lever at all concentrations tested as compared to the “air-only” exposure. The highest concentrations of TRI (10,000 and 12,000 ppm) produced an average of 89% and 81% EtOH-lever responding respectively with seven of the ten mice that responded showing full generalization at these concentrations immediately after exposure. Beginning 10 min after exposure, these concentrations of TRI resulted in approximately 52% EtOH-lever responding which dropped to ~20% by 40 min after exposure. The lower TRI concentrations of 8000 to 2000 ppm initially produced ~65-45% EtOH-lever responding immediately after exposure (time point “0”) with the degree of substitution dropping to the point of zero by 40 min after exposure. As can be seen in the bottom panel of Fig. 3, the increases in EtOH-lever responding after each TRI exposure were associated with alterations in response rates. No significant main effects were observed for TRI Concentration (p>0.33) or Time (p>0.41). However, a significant TRI Concentration × Time interaction (F(24,216) = 5.61, p<0.001) was observed. Post hoc analyses revealed that the three highest concentrations (8000–12,000 ppm) increased response rates as compared to saline/air controls when assessed 40 min after exposure (p<0.05).
Figure 3.
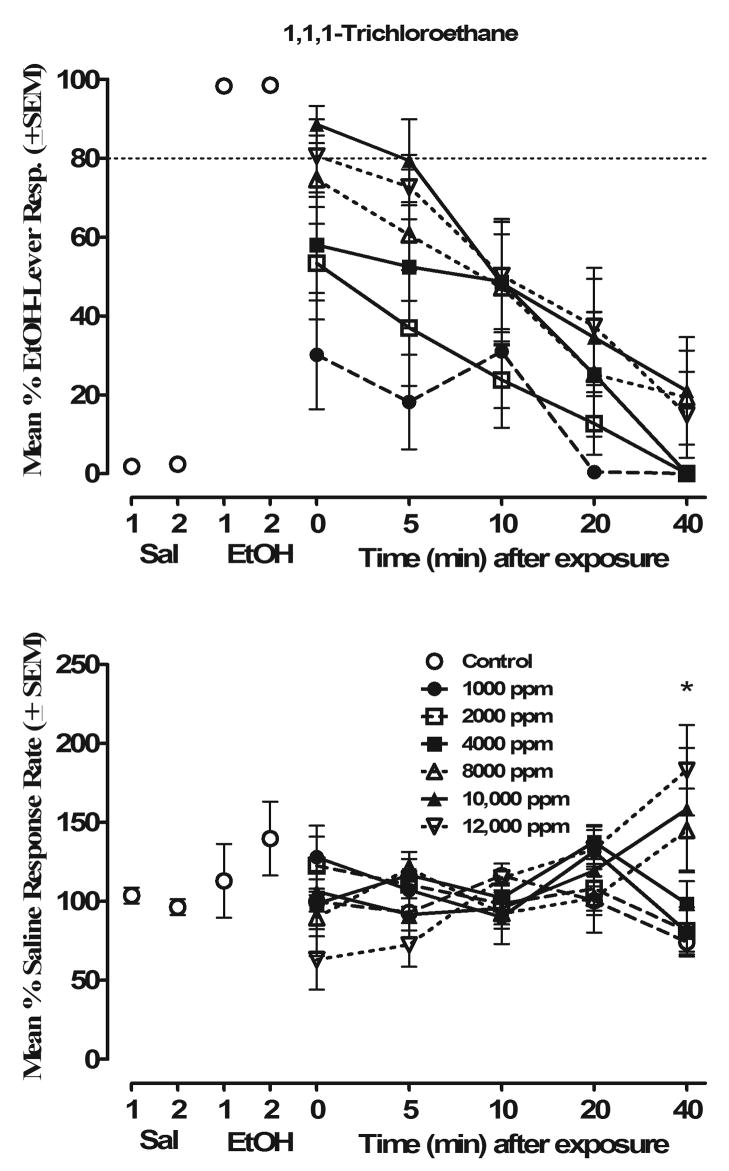
Concentration- and time-effect curves for TRI in mice trained to discriminate 1.25 g/kg EtOH from saline (n=10). Percentage of drug-lever responding (mean ± S.E.M.) is shown in the top panel; response rates as responses per second (mean ± S.E.M.) are shown in the bottom panel. Control data for EtOH and saline represent the drug or saline-test session which occurred immediately before (1) or after (2) TRI testing. *Significantly different from saline/air (p < 0.05).
DISCUSSION
Drug discrimination procedures in animals are valuable for studying the behavioral and pharmacological effects of drugs, in particular for investigating drugs of abuse. However, these procedures have only been used sparingly to investigate the discriminative stimulus properties of various volatile organic solvents so relatively little is known about their subjective effects, in general, or the time course of those effects. In the present investigation, we used an inhalation exposure system in mice to demonstrate that learned operant behaviors sensitive to ethanol (EtOH) are also altered when the mice are exposed to graded concentrations of organic solvent vapors of toluene and TRI, and that these effects on operant responding follow a time course that further demonstrates support for the hypothesis that these inhalants act like the CNS depressant EtOH.
The first finding was that toluene and TRI produced qualitatively similar acute behavioral effects in mice as EtOH. The results clearly demonstrate that inhalation exposure to each of these vapors produced concentration-dependent generalization to EtOH immediately after exposure. In addition, both toluene and TRI produced greater than 85% EtOH-lever responding at one or more concentrations in nearly all of the animals tested. While toluene produced a maximum mean of 85% EtOH-lever responding at any single concentration, all mice tested showed full substitution for EtOH at one or more concentrations. For inhaled TRI, the largest EtOH-lever responding occurred at the 12,000 ppm concentration with all but one of the mice responding greater than 85% on the EtOH lever. These stimulus generalization test results are consistent with previous research showing EtOH-like discriminative stimulus effects in mice with these two volatile inhalants as well as other abused inhalants (Balster et al., 1997; Bowen and Balster, 1997b; Rees et al., 1987).
The second finding was that examination of these effects at later time points clearly demonstrates that for both of these vapors the levels of EtOH substitution decreased in an orderly fashion as the time after exposure increased suggesting that as these solvents are removed from the body, the EtOH-like subjective effects fade. It is plausible that the fading of the subjective effects in these two studies are correlated with clearance of these solvents from the body/brain. However, this relationship remains to be determined. With the concentration-dependent changes in subjective effects, there were also changes in response rates. For the lower concentrations, there was a tendency for increased response rate activity and this increased activity was sustained throughout post-exposure testing. As concentrations became higher, response rate activity was not initially increased immediately after exposure but began to increase as the time after exposure progressed. While it is not clear what produces the response rate decreasing and increasing effects of volatile solvents, it is possible that they occur as a consequence of either effects on the CNS or from sensory effects resulting from their irritation or strong odors. Evidence against the latter (i.e., sensory effects) comes from Kjellstrand et al. (Kjellstrand et al., 1985) and our lab (unpublished results) who have shown that exposure to strongly scented colognes has no effect on motor activity, suggesting that the solvents effects on motor activity are not likely due to their odoriferous properties.
The third finding was that although both of the volatile solvents substituted for EtOH, there were differences in their potency for exhibiting EtOH-lever selection. In general, toluene was the more potent of the two solvents tested with the highest level of EtOH substitution produced at the 4,000 and 6,000 ppm concentrations of toluene with only a moderate reduction in response rate at the 6,000 ppm concentration when tested immediately after exposure. In contrast, the same concentrations of TRI produced much lower levels of substitution for EtOH when tested immediately after exposure. The largest substitutions for TRI did not occur until TRI concentrations were ≥10,000 ppm with a slight non-significant decrease in response rates observed at the highest concentration of 12,000 ppm immediately after exposure. The evidence that the organic solvents substitute behaviorally for EtOH gives further support that the solvents share with EtOH comparable effects on inhibitory and/or excitatory amino acid neurotransmission as important cellular mechanisms for those behavioral effects (Grant, 1994; Hoffman et al., 1989; Lovinger et al., 1990; Mehta and Ticku, 1988). Drug discrimination studies have shown that multiple mechanisms are probably responsible for the discriminative stimulus effects of EtOH, with GABAergic and NMDA antagonist actions being important (Balster et al., 1992; Grant, 1994; Shelton and Balster, 1994). Previous studies comparing the discriminative stimulus effects of EtOH to those of solvent and anesthetic vapors in mice have shown that both toluene and TRI produce significant EtOH-like discriminative stimulus effects (Rees et al., 1987), as do several the anesthetic inhalants (e.g., desflurane, isoflurane & diethyl ether; (Bowen and Balster, 1997b; Rees et al., 1987)). Inhaled toluene has also been shown to produce a concentration-related partial substitution for phencyclidine (PCP) in a drug-discrimination assay (Bowen et al., 1999) suggesting that toluene’s mechanism of action may entail antagonism of the NMDA receptor and/or GABA receptor stimulation, although Shelton and colleagues have demonstrated that toluene does not substitute for the selective NMDA receptor antagonist dizocilpine (Shelton and Balster, 2004). While this relationship remains to be determined, there is a possibility that dual GABA/NMDA mechanisms of action contribute to the discriminative stimulus effects of volatile solvents and anesthetics.
While the published investigations describing the behavioral effects of solvents are limited, some comparisons can be made between previous and present reports. In general, the present results support the hypothesis that similarities exist between certain volatile chemicals and those of CNS depressants. The overlapping discriminative stimulus properties, particularly the time course results, demonstrated in the present investigation between toluene and TRI and the classic CNS depressant EtOH offer another example of the shared pharmacological and behavioral properties emerging for these and similar volatile compounds (see (Bowen et al., 2006; Evans and Balster, 1991) for review). For example, these inhalants have been shown to disrupt locomotor activity (Hinman, 1987; Kjellstrand et al., 1985; Woolverton and Balster, 1981) and produce effects on schedule-controlled activity that are similar to that of depressants (Bowen and Balster, 1998a, b; Moser and Balster, 1981, 1986). The biphasic effect of these vapors on response rates and the effective concentration range are analogous to what have been reported previously for both toluene and chlorinated hydrocarbons (Hinman, 1987; Kjellstrand et al., 1985; Woolverton and Balster, 1981). For example, toluene increases spontaneous locomotor activity in mice at low to intermediate concentrations (560–1780 ppm) and decreases activity at higher concentrations (≥3000 ppm; (Kjellstrand et al., 1985; Wood and Colotla, 1990). A comparable profile of effects has been observed with TRI (Bowen and Balster, 1996). In operant investigations, one-hour exposures to low levels of toluene (150 ppm) have been shown to increase responding in a multiple FR-FI schedule of reinforcement (Geller et al., 1979) with similar increases in response rates being reported for toluene under a DRL component in rats trained to respond under a multiple FR-DRL schedule (Colotla et al., 1979). Other studies, however, demonstrated that toluene exposure can decrease response rates, similar to trial one in the present study. For example, Bowen and Balster (Bowen and Balster, 1998a) reported that 30-min exposures of 100 ppm to 6,000 ppm toluene decreased response rates at concentrations ≥4,000 ppm in a multiple FR20/FI 3-min schedule. Other studies have shown that combining depressant drugs with exposures to volatile anesthetics with depressant-like effects has mutually accentuating effects (Woolverton and Balster, 1981) and cross physical dependence with pentobarbital and EtOH has been demonstrated for TRI (Evans and Balster, 1993). While the cellular mechanisms for the behavioral effects of inhaled anesthetics are not well understood, the similarities that exist between these compounds and those of EtOH and other classic CNS depressants may provide an essential link suggesting that studies on the cellular actions of EtOH may be very relevant to anesthetic and solvent effects as well.
In summary, sub-anesthetic concentrations of toluene and TRI produced concentration-related increases in EtOH-lever responding without pronounced depressant effects on response rates. The present behavioral results suggest that the subjective effects of solvents may be similar to the intoxication produced by classic CNS depressants. These results also imply that procedural differences, specifically the amount of time allowed to lapse between solvent exposure and behavioral testing, may significantly influence results. Additionally, it could be postulated that as solvents are cleared from the body/brain, the EtOH-like subjective effects also fade and that drug discrimination procedures can be useful for determining these detailed time-course changes. Additional studies using abuse exposures to both high and low concentrations of these solvents to investigate these time course effects are warranted. Further, the results add support to the theory that the solvents may share mechanisms of action with EtOH and other CNS depressants, although it is not yet clear which cellular mechanisms are responsible. Finally, any future behavioral studies would benefit greatly from investigations into solvent-blood concentrations.
Acknowledgments
This research was supported in part by a grant from the National Institute on Drug Abuse (R01-DA15095). I thank Dr. John H. Hannigan for editing an early version of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bale AS, Tu Y, Carpenter-Hyland EP, Chandler LJ, Woodward JJ. Alterations in glutamatergic and gabaergic ion channel activity in hippocampal neurons following exposure to the abused inhalant toluene. Neuroscience. 2005;130:197–206. doi: 10.1016/j.neuroscience.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 2.Balster RL. Abuse potential evaluation of inhalants. Drug Alcohol Depend. 1987;19:7–15. doi: 10.1016/0376-8716(87)90082-2. [DOI] [PubMed] [Google Scholar]
- 3.Balster RL, Bowen SE, Evans EB, Tokarz ME. Evaluation of the acute behavioral effects and abuse potential of a C8-C9 isoparaffin solvent. Drug Alcohol Depend. 1997;46:125–135. doi: 10.1016/s0376-8716(97)00055-0. [DOI] [PubMed] [Google Scholar]
- 4.Balster RL, Grech DM, Bobelis DJ. Drug discrimination analysis of ethanol as an N-methyl-D-aspartate receptor antagonist. Eur J Pharmacol. 1992;222:39–42. doi: 10.1016/0014-2999(92)90460-l. [DOI] [PubMed] [Google Scholar]
- 5.Beckstead MJ, Weiner JL, Eger EI, 2nd, Gong DH, Mihic SJ. Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol. 2000;57:1199–1205. [PubMed] [Google Scholar]
- 6.Bowen SE. Increases in amphetamine-like discriminative stimulus effects of the abused inhalant toluene in mice. Psychopharmacology. 2006;186:517–524. doi: 10.1007/s00213-006-0381-8. [DOI] [PubMed] [Google Scholar]
- 7.Bowen SE, Balster RL. A comparison of the acute behavioral effects of inhaled amyl, ethyl, and butyl acetate in mice. Fundam Appl Toxicol. 1997a;35:189–196. doi: 10.1006/faat.1996.2278. [DOI] [PubMed] [Google Scholar]
- 8.Bowen SE, Balster RL. Desflurane, enflurane, isoflurane and ether produce ethanol-like discriminative stimulus effects in mice. Pharmacol Biochem Behav. 1997b;57:191–198. doi: 10.1016/s0091-3057(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 9.Bowen SE, Balster RL. A direct comparison of inhalant effects on locomotor activity and schedule-controlled behavior in mice. Exp Clin Psychopharmacol. 1998a;6:235–247. doi: 10.1037//1064-1297.6.3.235. [DOI] [PubMed] [Google Scholar]
- 10.Bowen SE, Balster RL. Effects of inhaled 1,1,1-trichloroethane on locomotor activity in mice. Neurotoxicol Teratol. 1996;18:77–81. doi: 10.1016/0892-0362(95)02024-1. [DOI] [PubMed] [Google Scholar]
- 11.Bowen SE, Balster RL. The effects of inhaled isoparaffins on locomotor activity and operant performance in mice. Pharmacol Biochem Behav. 1998b;61:271–280. doi: 10.1016/s0091-3057(98)00108-7. [DOI] [PubMed] [Google Scholar]
- 12.Bowen SE, Balster RL. Tolerance and sensitization to inhaled 1,1,1-trichloroethane in mice: results from open-field behavior and a functional observational battery. Psychopharmacology. 2006;185:405–415. doi: 10.1007/s00213-006-0335-1. [DOI] [PubMed] [Google Scholar]
- 13.Bowen SE, Batis JC, Paez-Martinez N, Cruz SL. The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol Teratol. 2006;28:636–647. doi: 10.1016/j.ntt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Bowen SE, Wiley JL, Jones HE, Balster RL. Phencyclidine- and diazepam-like discriminative stimulus effects of inhalants in mice. Exp Clin Psychopharmacol. 1999;7:28–37. doi: 10.1037//1064-1297.7.1.28. [DOI] [PubMed] [Google Scholar]
- 15.Colotla VA, Bautista S, Lorenzana-Jimenez M, Rodriguez R. Effects of solvents on schedule-controlled behavior. Neurobehav Toxicol. 1979;1(Suppl 1):113–118. [PubMed] [Google Scholar]
- 16.Cruz SL, Balster RL, Woodward JJ. Effects of volatile solvents on recombinant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Br J Pharmacol. 2000;131:1303–1308. doi: 10.1038/sj.bjp.0703666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz SL, Mirshahi T, Thomas B, Balster RL, Woodward JJ. Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1998;286:334–340. [PubMed] [Google Scholar]
- 18.Cruz SL, Orta-Salazar G, Gauthereau MY, Millan-Perez Pena L, Salinas-Stefanon EM. Inhibition of cardiac sodium currents by toluene exposure. Br J Pharmacol. 2003;140:653–660. doi: 10.1038/sj.bjp.0705481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans EB, Balster RL. CNS depressant effects of volatile organic solvents. Neurosci Biobehav Rev. 1991;15:233–241. doi: 10.1016/s0149-7634(05)80003-x. [DOI] [PubMed] [Google Scholar]
- 20.Evans EB, Balster RL. Inhaled 1,1,1-trichloroethane-produced physical dependence in mice: effects of drugs and vapors on withdrawal. J Pharmacol Exp Ther. 1993;264:726–733. [PubMed] [Google Scholar]
- 21.Geller I, Hartmann RJ, Randle SR, Gause EM. Effects of acetone and toluene vapors on multiple schedule performance of rats. Pharmacol Biochem Behav. 1979;11:395–399. doi: 10.1016/0091-3057(79)90114-x. [DOI] [PubMed] [Google Scholar]
- 22.Gerasimov MR, Schiffer WK, Marstellar D, Ferrieri R, Alexoff D, Dewey SL. Toluene inhalation produces regionally specific changes in extracellular dopamine. Drug Alcohol Depend. 2002;65:243–251. doi: 10.1016/s0376-8716(01)00166-1. [DOI] [PubMed] [Google Scholar]
- 23.Glowa JR. Behavioral effects of volatile organic solvents. In: Seiden LSaBRL., editor. Behavioral pharmacology: the current status. New York: Alan R. Liss; 1985. pp. 537–552. [Google Scholar]
- 24.Glowa JR. Some effects of sub-acute exposure to toluene on schedule-controlled behavior. Neurobehav Toxicol Teratol. 1981;3:463–465. [PubMed] [Google Scholar]
- 25.Grant KA. Emerging neurochemical concepts in the actions of ethanol at ligand-gated ion channels. Behav Pharmacol. 1994;5:383–404. doi: 10.1097/00008877-199408000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Hinman DJ. Biphasic dose-response relationship for effects of toluene inhalation on locomotor activity. Pharmacol Biochem Behav. 1987;26:65–69. doi: 10.1016/0091-3057(87)90535-1. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman PL, Rabe CS, Moses F, Tabakoff B. N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J Neurochem. 1989;52:1937–1940. doi: 10.1111/j.1471-4159.1989.tb07280.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnstone RE, Kulp RA, Smith TC. Effects of acute and chronic ethanol administration on isoflurane requirement in mice. Anesth Analg. 1975;54:277–281. doi: 10.1213/00000539-197505000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Kjellstrand P, Holmquist B, Jonsson I, Romare S, Mansson L. Effects of organic solvents on motor activity in mice. Toxicology. 1985;35:35–46. doi: 10.1016/0300-483x(85)90130-1. [DOI] [PubMed] [Google Scholar]
- 30.Lovinger DM, White G, Weight FF. Ethanol inhibition of neuronal glutamate receptor function. Ann Med. 1990;22:247–252. doi: 10.3109/07853899009148935. [DOI] [PubMed] [Google Scholar]
- 31.Mehta AK, Ticku MK. Ethanol potentiation of GABAergic transmission in cultured spinal cord neurons involves gamma-aminobutyric acidA-gated chloride channels. J Pharmacol Exp Ther. 1988;246:558–564. [PubMed] [Google Scholar]
- 32.Moser VC, Balster RL. Acute motor and lethal effects of inhaled toluene, 1,1,1-trichloroethane, halothane, and ethanol in mice: effects of exposure duration. Toxicol Appl Pharmacol. 1985a;77:285–291. doi: 10.1016/0041-008x(85)90328-x. [DOI] [PubMed] [Google Scholar]
- 33.Moser VC, Balster RL. The effects of acute and repeated toluene exposure on operant behavior in mice. Neurobehav Toxicol Teratol. 1981;3:471–475. [PubMed] [Google Scholar]
- 34.Moser VC, Balster RL. The effects of inhaled toluene, halothane, 1,1,1-trichloroethane, and ethanol on fixed-interval responding in mice. Neurobehav Toxicol Teratol. 1986;8:525–531. [PubMed] [Google Scholar]
- 35.Moser VC, Balster RL. Effects of toluene, halothane and ethanol vapor on fixed-ratio performance in mice. Pharmacol Biochem Behav. 1985b;22:797–802. doi: 10.1016/0091-3057(85)90530-1. [DOI] [PubMed] [Google Scholar]
- 36.Nelson GO. Principles and Techniques. Ann Arbor: Ann Arbor Science Publishers; 1971. Controlled Test Atmospheres. [Google Scholar]
- 37.Raines DE, Gioia F, Claycomb RJ, Stevens RJ. The N-methyl-D-aspartate receptor inhibitory potencies of aromatic inhaled drugs of abuse: evidence for modulation by cation-pi interactions. J Pharmacol Exp Ther. 2004;311:14–21. doi: 10.1124/jpet.104.069930. [DOI] [PubMed] [Google Scholar]
- 38.Rees DC, Knisely JS, Breen TJ, Balster RL. Toluene, halothane, 1,1,1-trichloroethane and oxazepam produce ethanol-like discriminative stimulus effects in mice. J Pharmacol Exp Ther. 1987;243:931–937. [PubMed] [Google Scholar]
- 39.Riegel AC, French ED. An electrophysiological analysis of rat ventral tegmental dopamine neuronal activity during acute toluene exposure. Pharmacology & toxicology. 1999;85:37–43. doi: 10.1111/j.1600-0773.1999.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 40.Riegel AC, Zapata A, Shippenberg TS, French ED. The Abused Inhalant Toluene Increases Dopamine Release in the Nucleus Accumbens by Directly Stimulating Ventral Tegmental Area Neurons. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301273. [DOI] [PubMed] [Google Scholar]
- 41.Samele C, Shine PJ, Stolerman IP. A bibliography of drug discrimination research, 1989–1991. Behav Pharmacol. 1991;3:171–192. [PubMed] [Google Scholar]
- 42.Schuster CR, Fischman MW, Johanson CE. Internal stimulus control and subjective effects of drugs. In: Grabowski J, Walsh JM, editors. Behavioral Pharmacology of Human Drug Dependence. Washington, D.C: Government Printing Office; 1981. pp. 116–129. National Institute on Drug Abuse Monograph No. 37. [PubMed] [Google Scholar]
- 43.Shelton KL, Balster RL. Effects of abused inhalants and GABA-positive modulators in dizocilpine discriminating inbred mice. Pharm Biochem Behav. 2004;79:219–228. doi: 10.1016/j.pbb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Shelton KL, Balster RL. Ethanol drug discrimination in rats: substitution with GABA agonists and NMDA antagonists. Behav Pharmacol. 1994;5:441–451. [PubMed] [Google Scholar]
- 45.Stolerman IP, Samele C, Kamien JB, Mariathasan EA, Hague DS. A bibliography of drug discrimination research, 1992–1994. Behav Pharmacol. 1995;6:643–668. [PubMed] [Google Scholar]
- 46.Williams JM, Stafford D, Steketee JD. Effects of repeated inhalation of toluene on ionotropic GABA A and glutamate receptor subunit levels in rat brain. Neurochem Int. 2005;46:1–10. doi: 10.1016/j.neuint.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Wood RW, Colotla VA. Biphasic changes in mouse motor activity during exposure to toluene. Fundam Appl Toxicol. 1990;14:6–14. doi: 10.1016/0272-0590(90)90226-a. [DOI] [PubMed] [Google Scholar]
- 48.Woolverton WL, Balster RL. Behavioral and lethal effects of combinations of oral ethanol and inhaled 1,1,1-trichloroethane in mice. Toxicol Appl Pharmacol. 1981;59:1–7. doi: 10.1016/0041-008x(81)90446-4. [DOI] [PubMed] [Google Scholar]


