Abstract
NK cells use NKG2D receptor to recognize ‘induced-self’. In apparent violation of the ‘missing-self’ hypothesis, NK cells stimulated through NKG2D can lyse target cells despite normal expression levels of MHC class I molecules. Although, ‘overriding’ of the inhibitory by the activating signals had been postulated the precise role of inhibitory Ly49 receptors on NKG2D-mediated activation has only started emerging. We propose that NKG2D-mediated activation is a function of ‘altering the balance’ in the signaling strength between the activating NKG2D and inhibiting Ly49 receptors. Balance in the signaling strength depends on the expression levels of activating ligands on the target cells. Qualitative and quantitative variations of MHC class I molecules expressed on the target cells also plays a major role in determining this ‘altered-balance’. Consequently, the nature of Ly49 receptors expressed on specific NK subsets determines the level of NKG2D-mediated NK cell activation. These observations provide a firm basis of ‘altered-balance’ in NK signaling and describe an active interplay between inhibitory Ly49 and activating NKG2D receptors.
Keywords: Natural killer cells, NKG2D, H60, Ly49, Altered-balance
1. Introduction
Natural killer (NK) cells are an important and necessary effector population of the innate immunity [1]. NK cells are derived from the bone marrow and play vital roles in the clearance of virally infected or malignant tumor cells [2]. NK cells also have the ability to regulate the adoptive immunity through the generation of a variety of cytokines and chemokines [3–5] NK cells have also been shown to play key roles in bone marrow transplant rejections [6–10], maternal–fetal interactions, viral/bacterial infections [11,12], autoimmune and inflammatory diseases [13]. For the purpose of functional analyses, NK cells have long been studied as a classical example of an effector lymphocyte capable of utilizing both activating and inhibitory receptors. This review specifically discusses the effect of inhibitory Ly49 on the activating NKG2D receptor. I propose that the signalling balance between these two types of receptors forms the molecular cues for the NK cells to mediate select effector function(s).
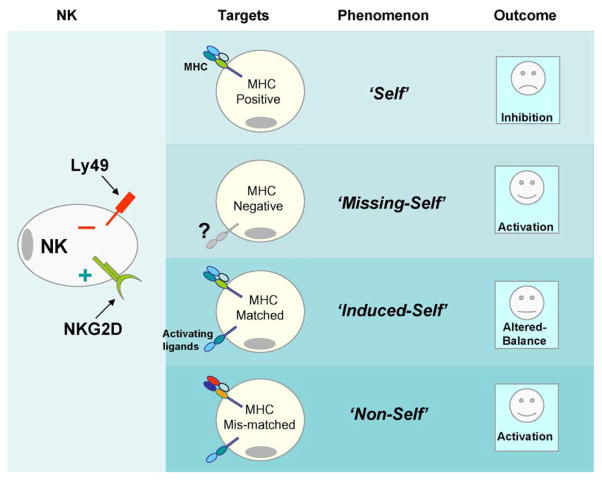
Each NK cell is known to express one or more inhibitory receptors, which interact with a specific MHC class I molecules on the target cells [14]. Interaction of MHC class I with the Ly49 receptors prevent the activation of NK cells and thereby the lysis of the target cell. Thus, NK cells utilize the Ly49 receptors to differentiate ‘self’ from ‘missing-self’ (Fig. 1). The ‘missing-self’ hypothesis proposes that NK cells lyse target cells that have lost expression of MHC class I molecules, i.e., that are missing self [15]. Thus, in an unchallenged steady-state, murine NK cells discriminate ‘self’ cells from those that are ‘missing-self’ through an array of Ly49 receptors, which inhibit lysis upon recognition of MHC class I molecules on target cells [16–18]. Loss of MHC class I molecules on target cells relieves the NK cell of Ly49-mediated inhibition, thus allowing the NK cells to mediate cytotoxicity [19]. Inhibition mediated through Ly49 receptors are thought to globally affect NK cell functions. However, the possibility that inhibitory Ly49 function as regulatory receptors, dictating specific effector functions have not been explored.
Fig. 1.
Different mechanisms through which NK cells can be activated. Normal cells express optimal levels of MHC class I. Interaction of MHC class I to Ly49 receptors leads to the induction of negative signals through recruitment and activation of phosphatases. These negative signals are known to actively down-regulate the effector functions of NK cells. Each NK cell is known to express one or more inhibitory Ly49 receptors, which interact with a specific MHC class I molecules on the target cells. Thus, expression of optimal levels of MHC class I defines ‘self’ recognition by NK cells. However, a reduction in the MHC class I due to viral infection or tumor transformation leads to the activation of NK cells. Thus, NK cells utilize inhibitory Ly49 receptors to differentiate ‘self’ from ‘missing-self’. On the other hand, target cells can also express inducible ligands such as H60, which are recognized by activating NKG2D receptor. This phenomenon is defined as ‘induced-self’ and concomitant activation through NKG2D and inhibition through Ly49 receptor results in ‘altered-balance’. Strength of NKG2D-mediated stimulation depends on the balance between activating and inhibitory signals. Another clinically relevant phenomenon is the ‘non-self’, where target and NK cells are derived from different individuals. Since each subset of NK are defined by the type of Ly49 or KIR receptor they express, it is possible to formulate NK-based cellular immunotherapy by miss-matching MHC and inhibitory receptor combinations to prevent potential inhibition and thereby successful activation of NK cells.
Our recent analyses indicate that the NKG2D receptor is also subject to the regulation of inhibitory Ly49 receptors [20]. In particular, the levels of H60 and the nature of MHC class I molecules on the target cells directly influenced the magnitude of NKG2D-mediated cytotoxicity. Successful engagement of specific inhibitory Ly49 receptor to their cognate MHC class I molecules determined the extent of inhibition on NKG2D-mediated activation. Furthermore, the level of inflammatory cytokines such as IFN-γ and GM-CSF generated by NK cells through NKG2D-mediated activation is also regulated by the Ly49 receptor. Independent studies by Carbone et al., demonstrates that the ability of the human NK cells to recognize and clear multiple myeloma cells through NKG2D receptor depends not only on the expression of activating ligands such as MIC-A but also level of MHC class I expression [21].
Thus, it appears that the exclusive ‘missing-self’ hypothesis proposed by Karre et al. [22] almost thirty years ago holds also true in the context of ‘induced-self’. In this review, I discuss the role of specific interplay between inhibitory Ly49 and activating NKG2D receptors and its role in the regulation of NK cell-mediated effector functions. Studies directed towards unraveling the balance between inhibitory and activating receptors will provide us a critical understanding in the formulation of NK-based cellular immunotherapies directed to hematological malignancies.
2. ‘Self’ versus ‘missing-self’ versus ‘induced-self’
Over a period of time, distinct mechanisms have been proposed to explain NK cell activations, which appear to fall into multiple categories. First, NK cell activations occur due to the absence of inhibition. The ‘missing-self’ hypothesis proposes that NK cells lyse target cells that have lost the expression of MHC class I molecules (Fig. 1) [23–25]. Loss of MHC class I molecules on target cells relieves the NK cell of Ly49-mediated inhibition, thus allowing cytotoxicity [26].
The second immunological situation is the activation of NK cells due to absence of inhibition and presence of stimulatory receptors. A set of widely expressed activation receptors, NKp30, NKp44, NKp46, and 2B4, have been implicated in their ability to mediate NK cell stimulation which are otherwise ‘masked’ under the strong negative signaling from inhibitory receptor-MHC class I molecules interactions. This phenomenon of MHC class I obstruction to NK cell activation is referred as ‘target interference’ [27–30].
The third immunological concept that defines NK cell activation is the ‘induced-self’, where overriding of inhibitory receptors occur by the signals mediated through activating receptors. Under challenged conditions, target cells express a family of stress-inducible ligands, including ULBP [31,32], MICA/B in humans [33], and H60 [34,35], Rae-1 [36], and Mult-1 [37,38] in mice, which are recognized by the activating receptor, NKG2D [34,39–42]. Although, these findings demonstrated a non-requirement for the down-regulation MHC class I, the interplay between NKG2D-mediated activation and Ly49 receptor-mediated inhibition has only started emerging. Below we have a detailed description of these phenomena.
2.1. ‘Self’ versus ‘missing-self’
‘Missing self’ hypothesis was put forward by Karre and coworkers which formed the basis for how NK cell functions are regulated [43]. Expression levels of MHC class I on target cells defined ‘self’. In contrary, significant reduction in the expression of MHC class I on tumor or virally infected cells lead to activation of NK cells [44–46]. In this context, it is important to point that several families of inhibitory receptors have been defined to function on NK cells. They belong to immunoglobulin super-family such as KIR [47] molecules (human) or, C-type lectin [48–55] super-family (murine). Majority of the C-type lectins belong to Ly49 sub-family that recognize classical MHC class I molecules. There are 21 defined murine Ly49 genes out of which 18 of them are known to be transcribed [56–62]. The second set of lectin members are the CD94/NKG2 (A&B) that recognize non-classical MHC (HLA-E in human and Qa-1 in mouse) [63]. The last set of lectin receptors are collectively called as NK1.1 complex which are also known as NKR-P1 and interestingly their inhibitory ligands are encoded by the Clr gene family [64,65]. These C-type lectin-like molecules or structurally distinct killer immunoglobulins like inhibitory receptors (KIR) function through a well-defined cytoplasmic consensus amino acid sequence defined as immunoreceptor tyrosine-based inhibitory motif (ITIM) [66–71]. The prototype six amino acid ITIM sequence is (Ile/Val/Leu/Ser)-X-Tyr-X-X, where X denotes any amino acid [72].
2.2. ‘Target interference’
A wide array of NK-activating receptors have been characterized in humans, including KIR2DS, KIR3DS, NKp36, NKp44, NKp46 and p75/AIRM, collectively called ‘Natural Killer Cell Receptors’ (NCRs) [73–75]. Recent studies have also implicated some of the defined T cell co-stimulatory molecules such as CD2, CD28, 2B4 and LFA-1 also as activating receptors for NK cells [76–78]. When normal levels of MHC class I are expressed on the target cells, these receptors fail to activate NK cells due to the strong negative signals received by the NK cells through their inhibitory receptors. This phenomenon is defined as ‘target interference’.
2.3. ‘Self’ versus ‘Induced-self’
Recently, a novel paradigm defined as ‘induced-self’ has been introduced to explain NK cell activation mediated through the NKG2D receptor. Eloquent studies by Raulet and Lanier laboratories have demonstrated that interaction of NKG2D with its cognate ligands can override the negative signals provided by inhibitory receptors [34,35]. These findings while promoting a novel hypothesis ‘induced-self’ [34,35] mediated by NKG2D receptor also changed the long-held ‘self’ versus ‘missing-self’-NK cell paradigm [79–81]. Their studies equivocally argued that activation through NKG2D can lead to target cell lysis despite their normal expression levels of MHC class I molecules [82,83].
Although, the above phenomena have been proved in different systems, recent studies are pointing to a unified molecular mechanism as the common basis for NK cell activation. Based on others and our studies, we predict that NK cell activation is purely a function of balance between the activating and inhibitory stimuli. One exciting possibility is that this balance in the signaling strength could very well dictate the nature of NK-mediated effector functions in vivo.
3. Role of activating ligand density and NKG2D-mediated NK cell activation
Multiple ‘stress-inducible’ activating ligands belonging to a non-classical MHC class I family (H60, Rae1α-ε and Mult-1) have been defined to interact with NKG2D receptor on murine NK cells. The requirements for their optimal in vivo expression have yet to be determined. Specific cell types which have the ability to express these activating ligands are not well defined. Our preliminary studies demonstrate that some of these ligands can be expressed in T, B, macrophage and dendritic cells (Haiyan Chu and Malarkannan, unpublished observations). In view of the fact that the activating ligands are inducible, it is expected that depending on the type and potency of the stimuli, the expression levels of these ligands may vary. Along these lines, it is arguable that a normal but activated B or dendritic cell may express H60 or Rae1 during an immune response. Further, these cells may not necessarily down-regulate the expression levels of MHC class I molecules. Under these circumstances, it may not be obligatory for the NK cells to mediate cytotoxicity against these activated yet normal cells. Therefore, it is highly possible that the balance between the activating and the inhibitory signals may form the cues for the NK cells to perform chosen effector functions.
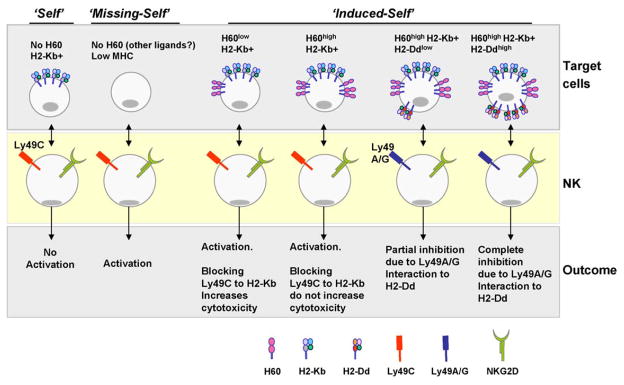
One of the ways to determine the level of negative inhibition from Ly49 receptors on NKG2D-mediated activation is varying the levels of activating ligands such as H60. To mimic different intensity of stress, we generated and used stable cell lines expressing varied levels of H60 [20]. As expected, the percentages of NK-mediated cytotoxicity were dependent of the level of H60 expression (Fig. 2). Interestingly, blocking the interaction of Ly49C to H2-Kb on a model tumor cell with moderate levels of H60 (similar to lipopolysaccharide stimulated splenocytes of BALB origin) augmented the level of NK-mediated cytotoxicity. This provided the first clue that the activation through NKG2D receptor was under the dynamic control of Ly49 receptors. Blocking Ly49C/I receptors with specific monoclonal antibodies or using purified, homogeneous Ly49C+/I+ or Ly49C−/I− NK subsets demonstrated that the expression levels of activating ligand (H60) on target cells play a major role in balancing the effect of inhibitory receptors (Fig. 2). Thus, at an optimal level of H60 expression, activation through NKG2D receptor is highly susceptible to Ly49C/I receptor-mediated inhibition. In contrast, when target cells made to express significantly higher levels of H60, blocking the interaction between Ly49C and H2-Kb had no effect. There were two key functional outcomes from these observations. They are: (a) NK cell activation by the physiological levels of H60 (such that of splenocytes) is subjected to strong negative regulation of inhibitory receptors, and (b), however, an over expression of H60 (pathological) can generate a stronger positive signal leading to a possible over-riding of inhibition by NKG2D-mediated activation.
Fig. 2.
Activation through NKG2D receptor is subjected to the negative inhibition of Ly49 receptors. Cells expressing optimal levels of MHC class I (‘Self’) and no activating ligands such as H60 were not lysed by the NK cells. We assume that in these combinations the NK cells are under active inhibition and any stimulatory ligands present on the target cells are unable to overcome the inhibition. Pathological conditions may lead to the down-regulation of MHC class I leading to the ‘missing-self’ recognition by the NK cells. Absence of inhibition leads to the activation. However, the activating ligand(s) involved in these specific combinations have not been defined in murine target cells. Target cells under stress express self ligands which has been defined as ‘induced-self’. Our studies demonstrate that the ‘induced-self’ is a component of ‘self’ and ‘missing-self’ recognition. Optimal expression of H60 along with normal expression of MHC on the target cells leads to the activation of NK cells. However, blocking the interaction of Ly49C to H2-Kb augments the ability of NK cells to mediate cytotoxicity. This indicates active inhibitory signals along with the NKG2D-mediated activating signals occurring concomitantly. Increasing the levels of H60 on the target cells resulted in a higher level of NKG2D-mediated cytotoxicity and blocking Ly49C to H2-Kb did not alter the outcomes. Interestingly, introduction of additional MHC class I molecule, H2-Dd, on target cells could significantly affect the activation mediated through NKG2D receptor. Thus, the functional outcome of NK cell activation depends on the balance between the activatory and inhibitory signals.
4. Nature of Ly49 receptors affects the strength of NKG2D-mediated activation
It is also important to discuss the inherent limitations with the tumor model discussed in the earlier section that was used by us and others. To our knowledge, expression of H60 protein is restricted to the BALB and 129J backgrounds and the transcription of its gene is undetectable in either C57BL/6 or B10 strains. The other major difference between BALB and C57BL/6 are their MHC complexes. Ly49 receptor acquisition and expression during the terminal maturation of NK cells are known to be strongly ‘calibrated’ by the ‘self’ MHC molecules. Recent studies provide further proof for an active process defined as ‘licensing’ that is mediated by ‘self’ MHC class I molecules [84,85]. This process governs both the functional maturation of NK cell subsets and prevention of autoimmune reactivity. However, the molecular mechanism by which a specific MHC complex impacts upon the terminal maturation of NK cells and thereby the strength of NKG2D-mediated activation is currently not well understood.
Antibody-mediated blocking experiments described above were mostly directed to Ly49C NK subset. Ly49C subset constitutes approximately 20–30% of the total NK population in both C57BL/6 and BALB strains. NKG2D-mediated activation of Ly49C+ subsets were under significantly stronger inhibition when the target cells expressed only a ‘physiologically’ comparable, moderate level of H60. This particular system dealt with a single Ly49 receptor and its interaction to its MHC ligand. However, tumor cells, particularly of human origin, compared to that of inbred mice may express more than two MHC class I molecules. Thus, tumor cells expressing multiple MHC may have the ability to mediate additional inhibitions through more KIR/Ly49 receptors. Therefore, to further offset the balance between activation and inhibition, additional MHC class I molecule, H2-Dd, were introduced into the model tumor cells (Fig. 2). Compared to Ly49C/I-mediated inhibition, expression of H2-Dd that serves as a ligand for inhibitory Ly49A/G receptors, on target cells, significantly reduced NKG2D-mediated NK cell cytotoxicity [20].
We conclude that the operation of ‘missing-self’ hypothesis in NKG2D-mediated ‘induced-self’ is convincingly evident due to the following reasons. In the case of H-2d+ strain-derived Ly49A/G+ NK cell subsets (BALB/c and B10.D2), the cytotoxicity through NKG2D receptor is significantly abolished when the target cells express normal levels of H2-Dd, thereby, establishing ‘self’. Even an augmented expression of H60 could not over ride the inhibition mediated through the Ly49A/G receptors. Therefore, ‘down-regulation’ of self-MHC is necessitated to establish ‘missing-self’ for the Ly49A/G+ NK cells to mediate the efficient lysis. Recognition of ‘self’ is also evident in NK cells from H-2b+ strain (C57BL/6), but the extent of inhibition is strongly subjected to the level of activating ligand due to the insufficient inhibition mediated by Ly49C/I receptors. Molecular basis for the affinity variations between distinct Ly49 receptors to their respective MHC class I molecules that dictate the strength of inhibition is started emerging [86]. These results also demonstrate specific NK subsets would tender select effector functions depending on the balance between positive and negative signals for a given target cell. Collectively, a successful immune surveillance by NK cells is determined not only by the interaction of NKG2D to the activating ligand H60 but also dependent on the scanning for specific MHC class I molecules on the target cells by the inhibitory Ly49 receptors. Thus, the quantitative and qualitative strength of Ly49-mediated inhibitory signals help determining the level of NKG2D-mediated effector functions.
Does the balance between signal strengths regulate distinct effector functions mediated by NK cells? Can the interplay between the activating (NKG2D) and the inhibitory (Ly49) signals form the environmental cues determining specific NK-mediated effector functions? In a more physiological context, what is the functional outcome of an NK cell recognizing an activated B cell expressing H60 and normal levels of MHC class I? Interaction of inhibitory Ly49 receptors to MHC class I molecules results in the recruitment of SH2 domain-containing phosphatases to immuno tyrosine-based inhibitory motifs [87,88]. Activated phosphatases are known to dephosphorylate membrane proximal signaling substrates [89]. However, the identities of many of these substrates are yet to be determined. Many of the membrane proximal signaling events occurring downstream of NKG2D receptor have been defined. However, only minimal information is currently available on the distant signaling events downstream of NKG2D receptor. Although, signaling requirements for cytotoxicity and cytokine generations through NKG2D receptor are understandably different, future studies are needed to distinguish ‘global’ versus ‘selective’ effects of the inhibitory signals. Together, we postulate that the ‘altered-balance’ will determine the functional outcome of NK cell activations in terms of cytotoxicity, cytokine or chemokine productions and other non-cytotoxic functions.
5. Concluding remarks
The magnitude of activation through NKG2D is a function of ‘altered-balance’ in the signaling strength between activating NKG2D and inhibitory Ly49 receptors. The magnitude of NKG2D-mediated cytotoxicity depends on the quantities of H60, MHC class I and the nature of MHC class I expressed on the target cells. Based on these observations, we put forward an integrative hypothesis in which a balance in the signaling strength may govern distinct effector functions of NK cells. To better understand ‘altered-balance’, a detailed future study involving the intracellular signaling molecules is needed in delineating the specific activation pathway(s) that are targeted by the Ly49-recruited phosphatases. Also, similar to that of the murine system described here, a parallel analysis is required for human NK cells. This can lead to successful formulation of cellular immunotherapy that will be based on the adoptive transfer of HLA-mismatched NK cells for leukemia ablation.
Acknowledgments
Work in the laboratory of the author is supported in part by American Cancer Society grants RSG-02-172-01-LIB; Roche Organ Transplant Research Foundation grant #111662730; and NIH grants U19 AI062627; NO1-HHSN26600500032C. Author thanks Dr Jack Gorski, Senior Investigator, Blood Research Institute for coining the terminology ‘Altered-Balance’.
Abbreviations
- NK
natural killer cells
- KIR
killer immunoglobulin-like receptor
- IFN-γ
interferon gamma
- GM-CSF
granulocyte-macrophage colony stimulating factor
References
- 1.Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55(3):221–8. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 2.Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Immunobiology of human NK cells. Transplant Proc. 2001;33(1–2):60–1. doi: 10.1016/s0041-1345(00)02790-1. [DOI] [PubMed] [Google Scholar]
- 3.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 4.Farag SS, VanDeusen JB, Fehniger TA, Caligiuri MA. Biology and clinical impact of human natural killer cells. Int J Hematol. 2003;78(1):7–17. doi: 10.1007/BF02983234. [DOI] [PubMed] [Google Scholar]
- 5.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2(2):123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 6.Farag SS, Fehniger TA, Becknell B, Blaser BW, Caligiuri MA. New directions in natural killer cell-based immunotherapy of human cancer. Expert Opin Biol Ther. 2003;3(2):237–50. doi: 10.1517/14712598.3.2.237. [DOI] [PubMed] [Google Scholar]
- 7.Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA. Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood. 2002;100(6):1935–47. doi: 10.1182/blood-2002-02-0350. [DOI] [PubMed] [Google Scholar]
- 8.Bennett M, D’Orazio T, Kumar V, Stenoien D, Blomer KC, Lindahl KF. Bone marrow cell transplants involving donors and hosts with haplotypes derived from spretus mice. Transplantation. 1995;59(10):1452–9. doi: 10.1097/00007890-199505270-00016. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Sentman CL, Kumar V, Bennett M. Murine marrow coexpressing H2-Dsp2 and H2-Db on host natural killer cell rejection. Transplantation. 1997;63(3):444–9. doi: 10.1097/00007890-199702150-00019. [DOI] [PubMed] [Google Scholar]
- 10.Davenport C, George T, Devora GA, et al. Facilitation of parental-strain marrow engraftment by T cells of neonatally-tolerant mice. Biol Blood Marrow Transplant. 1997;3(6):294–303. [PubMed] [Google Scholar]
- 11.Lodoen M, Ogasawara K, Hamerman JA, et al. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J Exp Med. 2003;197(10):1245–53. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahon CW, Zajac AJ, Jamieson AM, et al. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8(+) T cells. J Immunol. 2002;169(3):1444–52. doi: 10.4049/jimmunol.169.3.1444. [DOI] [PubMed] [Google Scholar]
- 13.Yokoyama WM, Scalzo AA. Natural killer cell activation receptors in innate immunity to infection. Microbes Infect. 2002;4(15):1513–21. doi: 10.1016/s1286-4579(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, George T, Devora GA, et al. Murine natural killer cells and hybrid resistance to hemopoietic cells in vivo. Methods Mol Biol. 2000;121:61–71. doi: 10.1385/1-59259-044-6:61. [DOI] [PubMed] [Google Scholar]
- 15.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 16.Held W, Cado D, Raulet DH. Transgenic expression of the Ly49A natural killer cell receptor confers class I major histocompatibility complex (MHC)-specific inhibition and prevents bone marrow allograft rejection. J Exp Med. 1996;184(5):2037–41. doi: 10.1084/jem.184.5.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu YY, George T, Dorfman JR, Roland J, Kumar V, Bennett M. The role of Ly49A and 5E6(Ly49C) molecules in hybrid resistance mediated by murine natural killer cells against normal T cell blasts. Immunity. 1996;4(1):67–76. doi: 10.1016/s1074-7613(00)80299-x. [DOI] [PubMed] [Google Scholar]
- 18.Hanke T, Takizawa H, McMahon CW, et al. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity. 1999;11(1):67–77. doi: 10.1016/s1074-7613(00)80082-5. [DOI] [PubMed] [Google Scholar]
- 19.Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55(3):221–8. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 20.Regunathan J, Chen Y, Wang D, Malarkannan S. NKG2D receptor-mediated NK cell function is regulated by inhibitory Ly49 receptors. Blood. 2005;105(1):233–40. doi: 10.1182/blood-2004-03-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbone E, Neri P, Mesuraca M, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105(1):251–8. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 22.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 23.Held W, Cado D, Raulet DH. Transgenic expression of the Ly49A natural killer cell receptor confers class I major histocompatibility complex (MHC)-specific inhibition and prevents bone marrow allograft rejection. J Exp Med. 1996;184(5):2037–41. doi: 10.1084/jem.184.5.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu YY, George T, Dorfman JR, Roland J, Kumar V, Bennett M. The role of Ly49A and 5E6(Ly49C) molecules in hybrid resistance mediated by murine natural killer cells against normal T cell blasts. Immunity. 1996;4(1):67–76. doi: 10.1016/s1074-7613(00)80299-x. [DOI] [PubMed] [Google Scholar]
- 25.Hanke T, Takizawa H, McMahon CW, et al. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity. 1999;11(1):67–77. doi: 10.1016/s1074-7613(00)80082-5. [DOI] [PubMed] [Google Scholar]
- 26.Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55(3):221–8. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu YY, George T, Dorfman JR, Roland J, Kumar V, Bennett M. The role of Ly49A and 5E6(Ly49C) molecules in hybrid resistance mediated by murine natural killer cells against normal T cell blasts. Immunity. 1996;4(1):67–76. doi: 10.1016/s1074-7613(00)80299-x. [DOI] [PubMed] [Google Scholar]
- 28.Kumar V, George T, Yu YY, Liu J, Bennett M. Role of murine NK cells and their receptors in hybrid resistance. Curr Opin Immunol. 1997;9(1):52–6. doi: 10.1016/s0952-7915(97)80158-6. [DOI] [PubMed] [Google Scholar]
- 29.George TC, Ortaldo JR, Lemieux S, Kumar V, Bennett M. Tolerance and alloreactivity of the Ly49D subset of murine NK cells. J Immunol. 1999;163(4):1859–67. [PubMed] [Google Scholar]
- 30.Sivakumar PV, George T, Puzanov IJ, et al. Hybrid resistance by mouse NK cells in vitro. Methods Mol Biol. 2000;121:73–9. doi: 10.1385/1-59259-044-6:73. [DOI] [PubMed] [Google Scholar]
- 31.Sutherland CL, Chalupny NJ, Cosman D. The UL16-binding proteins, a novel family of MHC class I-related ligands for NKG2D, activate natural killer cell functions. Immunol Rev. 2001;181:185–92. doi: 10.1034/j.1600-065x.2001.1810115.x. [DOI] [PubMed] [Google Scholar]
- 32.Cosman D, Mullberg J, Sutherland CL, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14(2):123–33. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 33.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci USA. 1996;93(22):12445–50. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cerwenka A, Bakker AB, McClanahan T, et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12(6):721–7. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- 35.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1(2):119–26. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 36.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413(6852):165–71. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diefenbach A, Hsia JK, Hsiung MY, Raulet DH. A novel ligand for the NKG2D receptor activates NK cells and macrophages and induces tumor immunity. Eur J Immunol. 2003;33(2):381–91. doi: 10.1002/immu.200310012. [DOI] [PubMed] [Google Scholar]
- 38.Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM. Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J Immunol. 2002;169(8):4079–83. doi: 10.4049/jimmunol.169.8.4079. [DOI] [PubMed] [Google Scholar]
- 39.Ho EL, Heusel JW, Brown MG, Matsumoto K, Scalzo AA, Yokoyama WM. Murine Nkg2d and Cd94 are clustered within the natural killer complex and are expressed independently in natural killer cells. Proc Natl Acad Sci USA. 1998;95(11):6320–5. doi: 10.1073/pnas.95.11.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J, Song Y, Bakker AB, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285(5428):730–2. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 41.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391(6668):703–7. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 42.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci USA. 2001;98(20):11521–6. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ljunggren HG, Sturmhofel K, Wolpert E, Hammerling GJ, Karre K. Transfection of beta 2-microglobulin restores IFN-mediated protection from natural killer cell lysis in YAC-1 lymphoma variants. J Immunol. 1990;145(1):380–6. [PubMed] [Google Scholar]
- 44.Ohlen C, Bastin J, Ljunggren HG, et al. Resistance to H-2-restricted but not to allo-H2-specific graft and cytotoxic T lymphocyte responses in lymphoma mutant. J Immunol. 1990;145(1):52–8. [PubMed] [Google Scholar]
- 45.Moretta L, Ciccone E, Moretta A, Hoglund P, Ohlen C, Karre K. Allorecognition by NK cells: nonself or no self? Immunol Today. 1992;13(8):300–6. doi: 10.1016/0167-5699(92)90042-6. [DOI] [PubMed] [Google Scholar]
- 46.Karre K. MHC gene control of the natural killer system at the level of the target and the host. Semin Cancer Biol. 1991;2(5):295–309. [PubMed] [Google Scholar]
- 47.Vyas YM, Mehta KM, Morgan M, et al. Spatial organization of signal transduction molecules in the NK cell immune synapses during MHC class I-regulated noncytolytic and cytolytic interactions. J Immunol. 2001;167(8):4358–67. doi: 10.4049/jimmunol.167.8.4358. [DOI] [PubMed] [Google Scholar]
- 48.Held W, Roland J, Raulet DH. Allelic exclusion of Ly49-family genes encoding class I MHC-specific receptors on NK cells. Nature. 1995;376(6538):355–8. doi: 10.1038/376355a0. [DOI] [PubMed] [Google Scholar]
- 49.Held W, Dorfman JR, Wu MF, Raulet DH. Major histocompatibility complex class I-dependent skewing of the natural killer cell Ly49 receptor repertoire. Eur J Immunol. 1996;26(10):2286–92. doi: 10.1002/eji.1830261003. [DOI] [PubMed] [Google Scholar]
- 50.Yu YY, George T, Dorfman JR, Roland J, Kumar V, Bennett M. The role of Ly49A and 5E6(Ly49C) molecules in hybrid resistance mediated by murine natural killer cells against normal T cell blasts. Immunity. 1996;4(1):67–76. doi: 10.1016/s1074-7613(00)80299-x. [DOI] [PubMed] [Google Scholar]
- 51.Held W, Raulet DH. Expression of the Ly49A gene in murine natural killer cell clones is predominantly but not exclusively mono-allelic. Eur J Immunol. 1997;27(11):2876–84. doi: 10.1002/eji.1830271120. [DOI] [PubMed] [Google Scholar]
- 52.Raulet DH, Held W, Correa I, Dorfman JR, Wu MF, Corral L. Specificity, tolerance and developmental regulation of natural killer cells defined by expression of class I-specific Ly49 receptors. Immunol Rev. 1997;155:41–52. doi: 10.1111/j.1600-065x.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 53.McQueen KL, Freeman JD, Takei F, Mager DL. Localization of five new Ly49 genes, including three closely related to Ly49c. Immunogenetics. 1998;48(3):174–83. doi: 10.1007/s002510050421. [DOI] [PubMed] [Google Scholar]
- 54.Kase A, Johansson MH, Olsson-Alheim MY, Karre K, Hoglund P. External and internal calibration of the MHC class I-specific receptor Ly49A on murine natural killer cells. J Immunol. 1998;161(11):6133–8. [PubMed] [Google Scholar]
- 55.Natarajan K, Boyd LF, Schuck P, Yokoyama WM, Eliat D, Margulies DH. Interaction of the NK cell inhibitory receptor Ly49A with H-2Dd: identification of a site distinct from the TCR site. Immunity. 1999;11(5):591–601. doi: 10.1016/s1074-7613(00)80134-x. [DOI] [PubMed] [Google Scholar]
- 56.Michaelsson J, Achour A, Rolle A, Karre K. MHC class I recognition by NK receptors in the Ly49 family is strongly influenced by the beta 2-microglobulin subunit. J Immunol. 2001;166(12):7327–34. doi: 10.4049/jimmunol.166.12.7327. [DOI] [PubMed] [Google Scholar]
- 57.Fahlen L, Lendahl U, Sentman CL. MHC class I-Ly49 interactions shape the Ly49 repertoire on murine NK cells. J Immunol. 2001;166(11):6585–92. doi: 10.4049/jimmunol.166.11.6585. [DOI] [PubMed] [Google Scholar]
- 58.Dimasi N, Sawicki MW, Reineck LA, et al. Crystal structure of the Ly49I natural killer cell receptor reveals variability in dimerization mode within the Ly49 family. J Mol Biol. 2002;320(3):573–85. doi: 10.1016/s0022-2836(02)00498-9. [DOI] [PubMed] [Google Scholar]
- 59.Wilhelm BT, Gagnier L, Mager DL. Sequence analysis of the ly49 cluster in C57BL/6 mice: a rapidly evolving multigene family in the immune system. Genomics. 2002;80(6):646–61. doi: 10.1006/geno.2002.7004. [DOI] [PubMed] [Google Scholar]
- 60.McQueen KL, Wilhelm BT, Harden KD, Mager DL. Evolution of NK receptors: a single Ly49 and multiple KIR genes in the cow. Eur J Immunol. 2002;32(3):810–7. doi: 10.1002/1521-4141(200203)32:3<810::AID-IMMU810>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 61.Natarajan K, Dimasi N, Wang J, Margulies DH, Mariuzza RA. MHC class I recognition by Ly49 natural killer cell receptors. Mol Immunol. 2002;38(14):1023–7. doi: 10.1016/s0161-5890(02)00031-7. [DOI] [PubMed] [Google Scholar]
- 62.Dam J, Guan R, Natarajan K, et al. Variable MHC class I engagement by Ly49 natural killer cell receptors demonstrated by the crystal structure of Ly49C bound to H-2K(b) Nat Immunol. 2003;4(12):1213–22. doi: 10.1038/ni1006. [DOI] [PubMed] [Google Scholar]
- 63.Takei F, McQueen KL, Maeda M, et al. Ly49 and CD94/NKG2: developmentally regulated expression and evolution. Immunol Rev. 2001;181:90–103. doi: 10.1034/j.1600-065x.2001.1810107.x. [DOI] [PubMed] [Google Scholar]
- 64.Iizuka K, Naidenko OV, Plougastel BF, Fremont DH, Yokoyama WM. Genetically linked C-type lectin-related ligands for the NKRP1 family of natural killer cell receptors. Nat Immunol. 2003;4(8):801–7. doi: 10.1038/ni954. [DOI] [PubMed] [Google Scholar]
- 65.Carlyle JR, Jamieson AM, Gasser S, Clingan CS, Arase H, Raulet DH. Missing self-recognition of Ocil/Clr-b by inhibitory NKR-P1 natural killer cell receptors. Proc Natl Acad Sci USA. 2004;101(10):3527–32. doi: 10.1073/pnas.0308304101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fahlen L, Lendahl U, Sentman CL. MHC class I-Ly49 interactions shape the Ly49 repertoire on murine NK cells. J Immunol. 2001;166(11):6585–92. doi: 10.4049/jimmunol.166.11.6585. [DOI] [PubMed] [Google Scholar]
- 67.Natarajan K, Dimasi N, Wang J, Margulies DH, Mariuzza RA. MHC class I recognition by Ly49 natural killer cell receptors. Mol Immunol. 2002;38(14):1023–7. doi: 10.1016/s0161-5890(02)00031-7. [DOI] [PubMed] [Google Scholar]
- 68.Scharenberg AM, Kinet JP. The emerging field of receptor-mediated inhibitory signaling: SHP or SHIP? Cell. 1996;87(6):961–4. doi: 10.1016/s0092-8674(00)81790-0. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura MC, Niemi EC, Fisher MJ, Shultz LD, Seaman WE, Ryan JC. Mouse Ly-49A interrupts early signaling events in natural killer cell cytotoxicity and functionally associates with the SHP-1 tyrosine phosphatase. J Exp Med. 1997;185(4):673–84. doi: 10.1084/jem.185.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mason LH, Gosselin P, Anderson SK, Fogler WE, Ortaldo JR, McVicar DW. Differential tyrosine phosphorylation of inhibitory versus activating Ly-49 receptor proteins and their recruitment of SHP-1 phosphatase. J Immunol. 1997;159(9):4187–96. [PubMed] [Google Scholar]
- 71.Yusa S, Catina TL, Campbell KS. SHP-1- and phosphotyrosine-independent inhibitory signaling by a killer cell Ig-like receptor cytoplasmic domain in human NK cells. J Immunol. 2002;168(10):5047–57. doi: 10.4049/jimmunol.168.10.5047. [DOI] [PubMed] [Google Scholar]
- 72.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290(5489):84–9. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 73.Moretta L, Bottino C, Pende D, Mingari MC, Biassoni R, Moretta A. Human natural killer cells: their origin, receptors and function. Eur J Immunol. 2002;32(5):1205–11. doi: 10.1002/1521-4141(200205)32:5<1205::AID-IMMU1205>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 74.Moretta L, Ferlazzo G, Mingari MC, Melioli G, Moretta A. Human natural killer cell function and their interactions with dendritic cells. Vaccine. 2003;21(Suppl 2):S38–42. doi: 10.1016/s0264-410x(03)00197-x. [DOI] [PubMed] [Google Scholar]
- 75.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23(2):255–9. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson JL, Charo J, Martin-Fontecha A, et al. NK cell triggering by the human costimulatory molecules CD80 and CD86. J Immunol. 1999;163(8):4207–12. [PubMed] [Google Scholar]
- 77.Watzl C, Stebbins CC, Long EO. NK cell inhibitory receptors prevent tyrosine phosphorylation of the activation receptor 2B4 (CD244) J Immunol. 2000;165(7):3545–8. doi: 10.4049/jimmunol.165.7.3545. [DOI] [PubMed] [Google Scholar]
- 78.Stuart G, Phillips JH, Lanier LL. The CD2-subset of the Ig superfamily of cell surface molecules: receptor-ligand pairs expressed by NK cells and other immune cells. Semin Immunol. 2000;12(2):149–57. doi: 10.1006/smim.2000.0217. [DOI] [PubMed] [Google Scholar]
- 79.Ljunggren HG, Karre K. Host resistance directed selectively against H-2-deficient lymphoma variants. Analysis of the mechanism. J Exp Med. 1985;162(6):1745–59. doi: 10.1084/jem.162.6.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ljunggren HG, Karre K. Experimental strategies and interpretations in the analysis of changes in MHC gene expression during tumour progression. Opposing influences of T cell and natural killer mediated resistance? J Immunogenet. 1986;13(2–3):141–51. doi: 10.1111/j.1744-313x.1986.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 81.Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55(3):221–8. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 82.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3(10):781–90. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 83.Lanier LL. On guard–activating NK cell receptors. Nat Immunol. 2001;2(1):23–7. doi: 10.1038/83130. [DOI] [PubMed] [Google Scholar]
- 84.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436(7051):709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 85.Yokoyama WM, Kim S. How do natural killer cells find self to achieve tolerance? Immunity. 2006;24(3):249–57. doi: 10.1016/j.immuni.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 86.Dam J, Guan R, Natarajan K, et al. Variable MHC class I engagement by Ly49 natural killer cell receptors demonstrated by the crystal structure of Ly49C bound to H-2K(b) Nat Immunol. 2003;4(12):1213–22. doi: 10.1038/ni1006. [DOI] [PubMed] [Google Scholar]
- 87.Nakamura MC, Niemi EC, Fisher MJ, Shultz LD, Seaman WE, Ryan JC. Mouse Ly-49A interrupts early signaling events in natural killer cell cytotoxicity and functionally associates with the SHP-1 tyrosine phosphatase. J Exp Med. 1997;185(4):673–84. doi: 10.1084/jem.185.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mason LH, Gosselin P, Anderson SK, Fogler WE, Ortaldo JR, McVicar DW. Differential tyrosine phosphorylation of inhibitory versus activating Ly-49 receptor proteins and their recruitment of SHP-1 phosphatase. J Immunol. 1997;159(9):4187–96. [PubMed] [Google Scholar]
- 89.Binstadt BA, Brumbaugh KM, Dick CJ, et al. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity. 1996;5(6):629–38. doi: 10.1016/s1074-7613(00)80276-9. [DOI] [PubMed] [Google Scholar]