Abstract
Background
SERCA2a deficiency is commonly seen in advanced heart failure (HF). This study is designed to investigate safety and biological effects of enzyme replacement using gene transfer in patients with advanced HF.
Methods and Results
A total of 9 patients with advanced HF (New York Heart Association [NYHA] Class III/IV, ejection fraction [EF] ≤30%, maximal oxygen uptake [VO2 max] <16 mL·kg·min, with maximal pharmacological and device therapy) received a single intracoronary infusion of AAV1/SER-CA2a in the open-label portion of this ongoing study. Doses administered ranged from 1.4 × 1011 to 3 × 1012 DNase resistant particles per patient. We present 6- to 12-month follow-up data for these patients. AAV1/SERCA2a demonstrated an acceptable safety profile in this advanced HF population. Of the 9 patients treated, several demonstrated improvements from baseline to month 6 across a number of parameters important in HF, including symptomatic (NYHA and Minnesota Living with Heart Failure Questionnaire, 5 patients), functional (6-minute walk test and VO2 max, 4 patients), biomarker (NT-ProBNP, 2 patients), and LV function/remodeling (EF and end-systolic volume, 5 patients). Of note, 2 patients who failed to improve had preexisting anti-AAV1 neutralizing antibodies.
Conclusions
Quantitative evidence of biological activity across a number of parameters important for assessing HF status could be detected in several patients without preexisting neutralizing antibodies in this open-label study, although the number of patients in each cohort is too small to conduct statistical analyses. These findings support the initiation of the Phase 2 double-blind, placebo-controlled portion of this study.
Keywords: Gene therapy, heart failure, SERCA2a, cardiovascular disease
Chronic heart failure (HF) is an increasingly important health problem. It is the leading medical cause of hospitalization and is expected to result in an estimated direct and indirect cost to the healthcare system in 2009 of $37.2 billion.1 Despite important therapeutic advances in pharmacologic and device therapies, the prognosis of patients with chronic HF remains poor. Nonpharmacologic therapies (such as heart transplantation and the use of implantable assist devices) are considered only in the later stages of the disease, and access to such therapies is restricted to a fraction of patients who need them.2,3 In this context, alternative approaches such as cell and gene therapy have attracted increased attention. Recent studies have demonstrated that gene therapy could be an effective option to treat the failing myocardium.4–9
One of the key abnormalities in both human and experimental models of HF is a defect in sarcoplasmic reticulum (SR) function, which in turn causes abnormal intracellular calcium ion handling. A large body of experimental evidence indicates that SERCA2a plays an important role in regulating the progression of dilated cardiomyopathy. SERCA2a activity is known to decline in late-stage HF, and SERCA2a protein and messenger RNA levels are decreased in cardiac tissue isolated from failing hearts of patients and animals with HF.6,10–14 Low SERCA2a levels have been shown to correlate with the abnormally high diastolic levels of cytosolic calcium and low systolic calcium released from the SR, which are typical of HF, as well as with poor clinical outcomes.15–17 Gene transfer is the therapeutic platform tested in this study to increase expression and/or function of this integral membrane protein by restoring SERCA2a activity in HF patients. There has been overwhelming evidence that unlike standard pharmacological inotropic agents, which increase cAMP, exacerbate cellular calcium overload, increase ventricular arrhythmias, aggravate energy wasting, and worsen survival, augmented inotropy resulting from increasing SERCA2a activity results in very different and salutary changes. These include a decrease in calcium overload, restoration of energetics, abrogation of ventricular arrhythmias, and improved survival.18,19
In preclinical HF models in rodents,20 pigs,18 and sheep,21 increasing the level of SERCA2a using recombinant AAV vectors was well tolerated and restoration of SERCA2a levels resulted in significant improvement in cardiac function and energetics, even when the underlying pathophysiology or insult (eg, mitral valve rupture or pacing induced heart failure) was not corrected. Based on these findings, this first-in-human Phase 1/2 Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) trial4 aims to restore levels of this key enzyme in HF patients via gene transfer of the SERCA2a cDNA by delivering a recombinant AAV (AAV1/SERCA2a) via percutaneous intra-coronary infusion.
A major challenge for recombinant viral based therapeutics is the presence of preexisting neutralizing antibodies (NAbs) against the viral capsid proteins. Preexisting anti-AAV1 NAbs result from prior natural exposure to AAV and have been shown to inhibit vector uptake in a number of studies.22–24 Therefore, before consideration for eligibility in the CUPID trial, a serum prescreening protocol was performed to assess baseline NAb status.
Methods
Study Overview
The CUPID trial is a multicenter, Phase 1/2 trial. The Phase 1 portion is an open-label, sequential dose escalation study. The Phase 2 portion is a randomized, double-blind, placebo-controlled, parallel-group, dose ranging, feasibility trial that compares the use of intra-coronary administered AAV1/SERCA2a at 2 or 3 dose levels with placebo. Detailed information about the study design rationale and protocol has been published elsewhere.4 Patient data available through mid-September 2008 were summarized for the purposes of this report.
In the Phase 1 portion of the study, AAV1/SERCA2a was administered as a single intracoronary infusion at doses of 1.4 × 1011, 6 × 1011, or 3 × 1012 DRP to three patients each in Cohorts 1, 2, and 3, respectively. All adverse events and serious adverse events (AEs and SAEs) were reviewed by a Data Monitoring Committee dedicated to this study. Cause of hospitalizations and deaths were adjudicated by an Endpoints Committee also dedicated to the study. We report here on the first 9 patients enrolled at 5 centers in the United States in the Phase 1 portion of this study, which includes eligible patients receiving optimal pharmacologic and device therapy for HF. The main objectives of the Phase 1/2 study are to evaluate the safety of a single intracoronary infusion of AAV1/SERCA2a in HF patients while also exploring the activity/efficacy to inform future studies.
Study Population
Key inclusion and exclusion criteria that were used for the patients described in this study have been previously described.4 Of note, patients were required to have New York Heart Association (NYHA) Class III/IV HF, a left ventricular ejection fraction (LVEF) ≤30%, a maximal oxygen uptake (VO2 max) ≤16 mL·kg·min, an implantable cardiac defibrillator, and be on stable (at least 30 days) optimal outpatient therapy for HF. Patient baseline characteristics are provided in Table 1. For the first 3 cohorts in the Phase 1 portion of the study, 15 patients were screened, of which 9 were enrolled and infused and 6 were screen failures. Of the 6 screen failures, 2 failed VO2 max criterion; 2 had intravenous inotropes, vasodilators, or diuretics within 30 days; 1 required percutaneous coronary intervention within 30 days; and 1 had significant stenosis.
Table 1.
Patient Screening/Baseline Characteristics
| Cohort 1 1.4 × 1011 DRP n = 3 |
Cohort 2 6 × 1011 DRP n = 3 |
Cohort 3 3 × 1012 DRP n = 3 |
Total n = 9 |
|
|---|---|---|---|---|
|
Demographics | ||||
| Age, y, mean (SD) | 53 (7.0) | 55 (4.6) | 47.7 (9.0) | 51.9 (7.0) |
| Male, n | 3 | 2 | 2 | 7 |
| Race, n | ||||
| Caucasian | 3 | 3 | 1 | 7 |
| African American | 0 | 0 | 2 | 2 |
|
| ||||
|
NYHA status, number (%) | ||||
| NYHA Class | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
|
| ||||
|
Cardiac, mean (SD) | ||||
| MLWHFQ (points) | 46.0 (31.8) | 46.3 (20.5) | 40.7 (20.2) | 44.3 (21.6) |
| 6-min walk (meters) | 376.7 (29.3) | 421.7 (129.3) | 386.3 (115.2) | 394.9 (90.2) |
| VO2 max (mL ·kg·min) | 14.6 (2.5) | 13.0 (2.8) | 14.9 (2.9) | 14.2 (2.5) |
| LVESV (mL) | 213.3 (44.9) | 252.0 (86.6) | 210.7 (47.4) | 225.3 (57.8) |
| LVEF (%) | 24.3 (6.0) | 20.3 (4.5) | 22 (3.6) | 22.2 (4.5) |
| NT-Pro BNP (pg/mL) | 1857 (1369) | 3084 (1954) | 10644 (17373) | 5195 (9688) |
|
| ||||
|
Medical history, number | ||||
| Coronary artery disease | 2 | 1 | 1 | 4 |
| Diabetes mellitus | 1 | 0 | 2 | 3 |
| Hypertension | 1 | 0 | 2 | 3 |
| Previous MI | 2 | 1 | 1 | 4 |
|
| ||||
|
Physical findings, mean (SD) | ||||
| Systolic blood pressure (mm Hg) | 105.3 (20) | 98.0 (5.3) | 105.3 (4.2) | 102.9 (11.2) |
| Diastolic blood pressure (mm Hg) | 69.3 (1.2) | 68.3 (10.4) | 79.0 (1.7) | 72.2 (7.4) |
| Body weight (kg) | 94.0 (17.7) | 85.0 (13.6) | 94.7 (21.1) | 91.2 (16.0) |
DRP, DNase resistant particles; NYHA, New York Heart Association; MLWHFQ, Minnesota Living with Heart Failure Questionnaire; VO2 max, maximal oxygen uptake; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
Product Source and Administration
AAV1/SERCA2a (tgABG12, MYDICAR) was manufactured by Targeted Genetics Corporation (Seattle, WA). Antegrade epicardial coronary artery infusion (without any vessel balloon occlusion) was chosen for the administration procedure in humans based on extensive delivery optimization and safety studies in large animals (pigs and sheep). Because AAV particle size is significantly smaller than adenovirus, 23 nm vs. 80 to 90 nm in diameter, respectively, AAV viral particles pass through the vessel wall and perfuse the underlying tissue.
In CUPID, percutaneous intracoronary delivery was accomplished using standard catheters (5 Fr or 6 Fr guide or diagnostic) and infused using the MEDRAD Mark V ProVis angiographic injection system, (Indianola, PA). Infusions occurred over a 10- minute period in a cardiac catheterization laboratory after angiography. Dominance was defined for each patient by the arterial system (left coronary, right coronary, or both) that gave rise to the posterior descending artery. For purposes of this study, codominant circulation was infused as right dominant. Standard catheter engagement technique with the coronary arteries was accomplished in the usual fashion with angiographic confirmation of good coaxial position and secure intubation to assure forward infusion antegrade into the coronary circulation. Approximately 60% of the general population are right dominant, 25% are co-dominant, and 15% are left dominant. Multiple infusion scenarios were allowed based on coronary vessel collateralization patterns, presence of occlusive disease, and anatomic variation, with the overall goal to provide diffuse, homogenous myocardial exposure to the drug. Generally, this involves delivering two-thirds of the dose to the anterolateral and one-third to the posterolateral myocardium, based on the coronary anatomy. See Table 2 for details of infusion technique by patient, based on individual anatomy.4 Administration of nitroglycerin was routinely performed at some sites as a preventive measure against vasospasm and additional preclinical work in pigs has since confirmed the enhanced uptake of AAV1/SERCA2a with nitroglycerin administration. Nitroglycerin use has therefore been standardized in all patients in the Phase 2 portion of the study. In the Phase 1 portion of the study various, doses of nitroglycerin were used before AAV1/SERCA2a infusion in 5 of 9 patients, as shown in Table 2.
Table 2.
Nitroglycerin Use and MYDICAR Infusion Technique by Patient
| MYDICAR Infusion (mL) |
Nitroglycerin before MYDICAR |
||||
|---|---|---|---|---|---|
| Cohort/Patient Identification | LCA | RCA | LCA IC (μg) |
RCA IC (μg) |
Other |
| Cohort 1/Pt# 1 | 30 | 30 | 0 | 150 | 0 |
| Cohort 1/Pt# 2 | 60 | 0 | 0 | 0 | 0 |
| Cohort 1/Pt# 3 | 40 | 20 | 0 | 0 | 20 μg/min IV for total of 2118 μg |
| Cohort 2/Pt# 1 | 40 | 19 | 0 | 0 | 20 μg/min IV for total of 691.3 μg + 0.4 mg PO |
| Cohort 2/Pt# 2 | 59 | 0 | 0 | 0 | 0 |
| Cohort 2/Pt# 3 | 38 | 18 | 0 | 0 | 0 |
| Cohort 3/Pt# 1 | 40 | 20 | 0 | 150 | 0 |
| Cohort 3/Pt# 2 | 40 | 21 | 150 | 150 | 0 |
| Cohort 3/Pt# 3 | 61 | 0 | 150 | 0 | 0 |
LCA, left coronary artery; RCA, right coronary artery; IC, intracoronary.
Evaluation of Study Endpoints
Safety monitoring was performed weekly for the first month, at weeks 5 and 6 and at months 2, 3, 6, 9, and 12. Enzyme-linked ImmunoSPOT (ELISPOT) assays for detecting cellular immune responses to AAV1 capsid proteins were conducted at baseline; weeks 2 and 4; and months 2, 3, and 9. After completion of the 12 months, patients receive a follow-up phone call every 6 months for an additional 2 years to elicit information about hospitalizations, new medical conditions, HF status, and long-term survival. Efficacy end points were assessed at various intervals during study visits at months 1, 2, 3, 6, 9, and 12.
The following core laboratories were used for the Phase 1 portion of this study: clinical chemistries were evaluated at Mayo Clinic; echocardiography was initially overread by University of California, San Diego, Medical Center Echocardiography Core Laboratory and subsequently by ICON Medical Imaging; cardio-pulmonary exercise testing was overread by the Cardiopulmonary Exercise Core Laboratory New York-Presbyterian Hospital Columbia University Medical Center; ELISPOT assays were performed by Cellular Technology Ltd, Cleveland, OH; and clinical end points were adjudicated by the Clinical Endpoints Center and Cardiac Imaging Core Lab at Brigham and Women’s Hospital, Boston, MA. The Data Monitoring Committee was composed of 2 HF specialists, an interventionalist, an immunologist, and a statistician.
Regarding measurement of therapeutic activity in early stages of clinical evaluation, there is neither a consensus opinion nor a universally accepted methodology for determining what constitutes clinically meaningful changes in HF studies. Most HF therapies are approved based on outcome studies conducted in very large populations; however, these end points are not feasible in early stages of clinical evaluation where much smaller populations are studied. Therefore, similar to other therapeutic areas such as rheumatology, where composite endpoints are used for drug evaluation (American College of Rheumatology Criteria), composite end points that correlate with clinical outcomes in HF were used. The following efficacy/biological activity parameters were measured: symptomatic (NYHA Classification and Minnesota Living with Heart Failure Questionnaire [MLWHFQ]), functional (6-minute walk test [6MWT] and VO2 max], biomarker (NT-proBNP) and LV function/remodeling (LVEF) and left ventricular end-systolic volume [LVESV]). To evaluate potential biological response at an individual level, results of large HF clinical trials were reviewed and expert opinions were collected. Thresholds of clinically meaningful changes were established based on the magnitude of changes associated with improvement or worsening in clinical outcomes (including mortality/morbidity) and parameter variability as follows: a 1-class change in NYHA classification,25 a 10-point change in total MLWHFQ score,26–29 a 50-meter change in the 6MWT,25–30 a 1.5-mL·kg·min change in VO2 max,25 a change in NT-proBNP of 35% or 300 pg/mL, whichever is greater,31–34 a change in LVESV of 20 mL or 10%, whichever is greater, and a change in LVEF of at least 5% (absolute).35
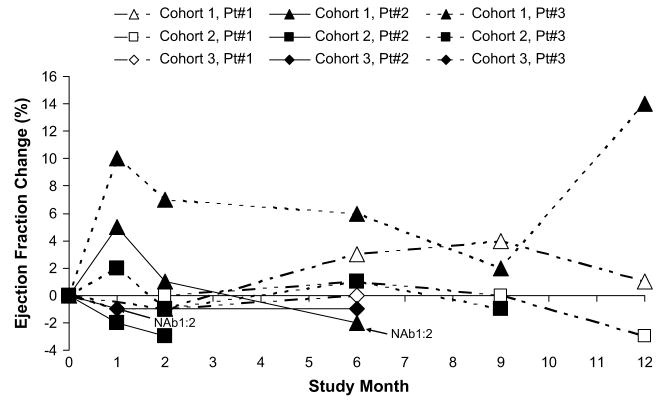
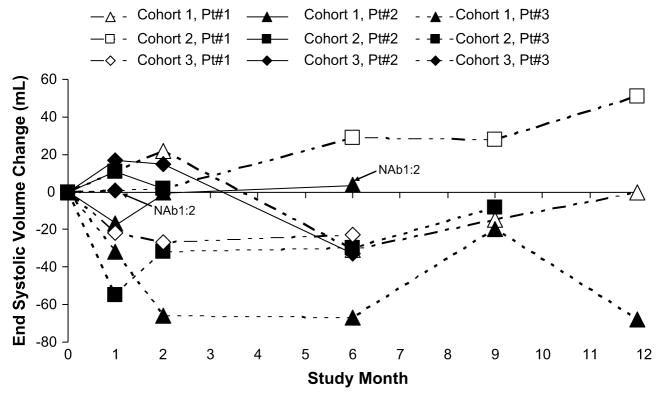
Echocardiograms were performed both with and without a contrast agent to assess response to therapy. For end point summarization, the selection of which echocardiogram to report was prespecified and based on image quality over time, comparability with baseline, and completeness. The data presented in Figures 1 and 2 are contrast-enhanced for 4 patients and noncontrast-enhanced for 5 patients.
Fig. 1.
Absolute change from baseline in ejection fraction over time.
Fig. 2.
Change from baseline in end systolic volume over time.
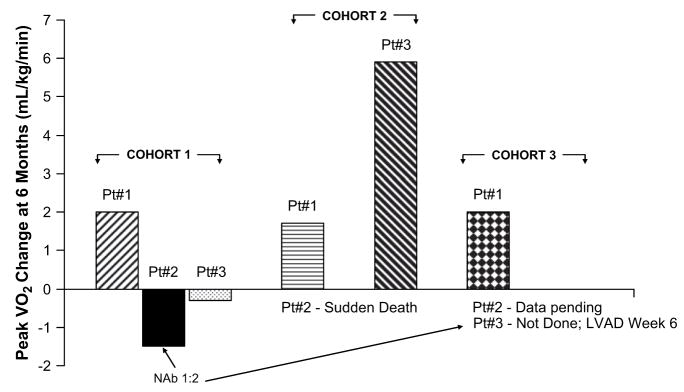
VO2 max is measured at baseline and at month 6 for all patients. Patient data available through mid-September 2008 were evaluated for the purposes of this report, and at that time, 6-month VO2 max results were not available for 3 patients. One patient required rescheduling because of an asthma exacerbation during the month 6 visit and 2 other patients were no longer on study at 6 months and therefore did not have the follow-up VO2 max measurement.
The study was approved by Institutional Review Boards and Institutional Biosafety Committees at each site, and written informed consent was obtained from all patients enrolled.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
The results include data from the Phase 1 portion of this ongoing study for Cohorts 1 and 2 (12-month follow-up), and Cohort 3 (6-month follow-up).
Safety
Overall review of study safety assessments to date has been unremarkable. No significant changes were noted after AAV1/SERCA2a administration in exams of major organ systems, and no consistent clinically meaningful changes in blood pressure, heart rate, or body temperature were observed. No trends or significant changes have been noted in blood chemistries, electrolytes, or liver and kidney function tests. No clinically significant changes or trends were noted in any components of the electrocardiogram including intervals, rhythm, or QRS morphology. In the 9 patients reported here, 37 AEs have been reported, 81% of which were mild or moderate in severity. The organ systems most frequently identified for these AEs were general (7 events) and metabolic (5 events) with the remainder distributed across the other organ systems. Adverse events are summarized in Table 3. SAEs were present in 5 of 9 of patients. One of these SAEs occurred before infusion or cardiac catheterization. None of these events was considered to be related to AAV1/SERCA2a. Events that were possibly related included 1 event of orthopnea that was possibly from fluid overload associated with the cardiac catheterization at the time of AAV1/SERCA2a administration, 2 events of increased fatigue, 1 of fever, and 1 of muscle spasms. In addition, events of influenza, nasopharyngitis, and herpes zoster could not be ruled out as related to AAV1/SERCA2a, although it seems unlikely. Other SAEs included hospitalizations for unstable angina and uncontrolled diabetes, administration of intravenous medications after observation of poor hemodynamics during right heart catheterization workup for transplant eligibility and decompensated HF.
Table 3.
Incidence and Severity of Adverse Events (n = 9)
| BODY SYSTEM | MILD | MODERATE | SEVERE | TOTAL | ||||
|---|---|---|---|---|---|---|---|---|
| Event | Rel | NR | Rel | NR | Rel | NR | Rel | NR |
|
CARDIAC DISORDERS | ||||||||
| Angina unstable | 1 | 1 | ||||||
| Cardiac failure | 1 | 1 | ||||||
| Cardiac failure congestive | 1 | 1 | ||||||
| Cardiogenic shock | 1 | 1 | ||||||
| Ventricular tachycardia | 1 | 1 | 1 | 1 | ||||
|
| ||||||||
|
GASTROINTESTINAL DISORDERS | ||||||||
| Abdominal distention | 1 | 1 | ||||||
|
| ||||||||
|
GENERAL | ||||||||
| Catheter site hemorrhage | 1 | 1 | ||||||
| Chest discomfort | 1 | 1 | ||||||
| Fatigue | 2 | 1 | 3 | |||||
| Pyrexia | 1 | 1 | ||||||
| Sudden death | 1 | 1 | ||||||
|
| ||||||||
|
INFECTIONS and INFESTATIONS | ||||||||
| Bronchitis | 1 | 1 | ||||||
| Herpes zoster | 1 | 1 | ||||||
| Influenza | 1 | 1 | ||||||
| Nasopharyngitis | 1 | 1 | ||||||
|
| ||||||||
|
INJURY, POISONING, and PROCEDURAL COMPLICATIONS | ||||||||
| Device lead damage | 1 | 1 | ||||||
|
| ||||||||
|
INVESTIGATIONS | ||||||||
| Blood CK increased | 1 | 1 | ||||||
| Weight increased | 1 | 1 | ||||||
|
| ||||||||
|
METABOLISM and NUTRITION DISORDERS | ||||||||
| Diabetes mellitus inadequate control | 1 | 1 | ||||||
| Gout | 1 | 1 | ||||||
| Hypokalemia | 2 | 2 | ||||||
| Hyponatremia | 1 | 1 | ||||||
|
| ||||||||
|
MUSCULOSKELETAL and CONNECTIVE TISSUE DISORDERS | ||||||||
| Muscle spasms | 1 | 1 | 1 | 1 | ||||
| Nervous system disorders | ||||||||
| Hypoesthesia | 1 | 1 | ||||||
|
| ||||||||
|
RENAL and URINARY DISORDERS | ||||||||
| Renal impairment | 2 | 2 | ||||||
|
| ||||||||
|
RESPIRATORY | ||||||||
| Asthma | 1 | 1 | ||||||
| Orthopnea | 1 | 1 | ||||||
| Throat tightness | 1 | 1 | ||||||
|
| ||||||||
|
SKIN | ||||||||
| Ecchymosis | 1 | 1 | ||||||
|
| ||||||||
|
VASCULAR DISORDERS | ||||||||
| Hematoma | 1 | 1 | ||||||
| Hypertension | 1 | 1 | ||||||
Related, definitely, probable, or possible as judged by the investigator; NR, unlikely or not related to investigational product as judged by the investigator; CK, creatine kinase.
Event tabulated once as worst severity or most related for the same event occurring multiple times within the same patient.
ELISPOT assays were used to monitor for potential cellular immune responses to AAV1 capsid proteins. There was a single event of a slight elevation above background in this assay at Weeks 4 and 6 in a patient in Cohort 3 (pt#1), which was temporally related to a concurrent viral infection (flu). This patient’s ELISPOT results returned to baseline by month 2. This single occurrence of an ELI-SPOT elevation above background (~90 spots/106 peripheral blood mononuclear cells) occurred without any clinical sequelae or elevations in clinical chemistry parameters.
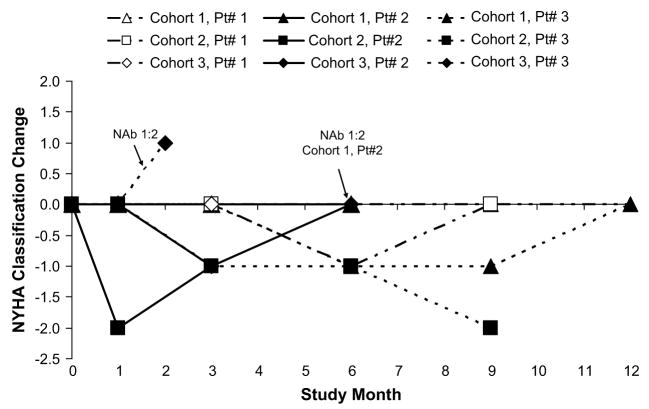
With more than 70 patient-months of follow-up, 8 of the 9 patients who received AAV1/SERCA2a are alive at this reporting. Despite symptomatic improvement (NYHA Class III to I/II), 1 patient with a history of cardiac arrest died of presumed sudden cardiac death 96 days after administration (Cohort 2, pt#2). Based on this patient’s advanced HF status at baseline (EF 16%; ESV 352 mL;VO2 max 10.2 mL·kg·min), lack of temporal relationship to study drug administration (>3 months), and prior episode of cardiac arrest, the death was determined by the investigator, in concurrence with the independent medical monitor and safety officer, to be neither unexpected nor related to the investigational product. Based on the inclusion criteria requirement, all patients enrolled, including this patient, had an implantable cardiac defibrillator; however, because of circumstances surrounding the reporting of the death to the investigator, the device was not able to be recovered for interrogation. Of 2 other patients, 1 failed to improve and received a transplant at month 8 (Cohort 1, pt#2), and the other continued to worsen and received a mechanical support device at week 6 (Cohort 3, pt#3). Of note, these last 2 patients had baseline NAb titers of 1:2. Quantitative polymerase chain reaction for AAV1/SERCA2a vector sequences was performed on cardiac tissue obtained from these 2 NAb positive patients during their open-chest procedures. Vector sequences were undetectable (limit of detection <20 copies vector DNA/μg total DNA).
Activity/Efficacy End Points
Six-month follow-up data on activity end points for patients in Phase 1, Cohorts 1, 2, and 3 are shown in Table 4. Changes from baseline considered clinically meaningful (both improvements and worsening) for this study (see Methods section) are in bold and underlined. Of the 9 patients treated, several demonstrated improvements from baseline to month 6 across efficacy/biological activity parameters important in HF, including symptomatic (5 patients), functional (4 patients), biomarker (2 patients), and LV function/remodeling (5 patients). One patient each showed clinically meaningful worsening in a functional parameter (VO2 max), biomarker (NT-ProBNP), and LVESV.
Table 4.
Change from Baseline to Month 6 in Key Efficacy/Activity Parameters
| MLWHFQ VO2 Max (mL·kg·min) 6MWT (m) NYHA Class NT-Pro BNP (pg/mL) ESV (mL) EF (%) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort/Patient Identification | BL NAb | Visit | Obs | Chg | Obs | Chg | Obs | Chg | Obs | Chg | Obs | %Chg | Obs | Chg | Obs | Chg |
| Cohort 1/Pt# 1 | <1:2 | BL | 54 | 17.3 | 408 | 3 | 315 | 162 | 30 | |||||||
| M6 | 39 | −15 | 19.3 | +2 | 549 | +141 | 3 | 0 | 326 | +3.5 | 131 | −31 | 33 | +3 | ||
| Cohort 1/Pt# 2 | 1:2 | BL | 73 | 12.3 | 372 | 3 | 2329 | 233 | 18 | |||||||
| M6 | 79 | +6 | 10.8 | −1.5 | 366 | +6 | 3 | 0 | 1640 | −29.6 | 237 | +4 | 16 | −2 | ||
| Cohort 1/Pt# 3 | <1:2 | BL | 11 | 14.3 | 350 | 3 | 2928 | 245 | 25 | |||||||
| M6 | 12 | +1 | 14.0 | −0.3 | 439 | +89 | 2 | −1 | 1161 | −60.4 | 178 | −67 | 31 | +6 | ||
| Cohort 2/Pt# 1 | <1:2 | BL | 34 | 13.0 | 466 | 3 | 1420 | 200 | 20 | |||||||
| M6 | 22 | −12 | 14.7 | +1.7 | 456 | −10 | 2 | −1 | 874 | −38.5 | 229 | +29 | 21 | +1 | ||
| Cohort 2/Pt# 2 | <1:2 | BL | 35 | 10.2 | 276 | 3 | 5236 | 352 | 16 | |||||||
| M6* | 22 | −13 | ND | NA | 276 | 0 | 2 | −1 | 6061 | +15.8 | 354 | +2 | 13 | −3 | ||
| Cohort 2/Pt# 3 | <1:2 | BL | 70 | 15.8 | 523 | 3 | 2596 | 204 | 25 | |||||||
| M6 | 45 | −25 | 21.7 | +5.9 | 627 | +104 | 2 | −1 | 2186 | −15.8 | 174 | −30 | 26 | +1 | ||
| Cohort 3/Pt# 1 | <1:2 | BL | 28 | 17.1 | 311 | 3 | 770 | 279 | 21 | |||||||
| M6 | 12 | −16 | 19.1 | +2 | 348 | +37 | 3 | 0 | 1203 | +56 | 256 | −23 | 21 | 0 | ||
| Cohort 3/Pt# 2 | <1:2 | BL | 30 | 16.0 | 519 | 3 | 458 | 275 | 22 | |||||||
| M6 | 47 | +17 | 3 | 0 | 602 | +31 | 242 | −33 | 21 | −1 | ||||||
| Cohort 3/Pt# 3 | 1:2 | BL | 64 | 11.6 | 329 | 3 | 30704 | 265 | 18 | |||||||
| M6† | ||||||||||||||||
BL, baseline or screening value; NAb, neutralizing antibody; MLWHFQ, Minnesota Living with Heart Failure Questionnaire; VO2 max, maximal oxygen uptake; 6MWT, 6-minute walk test; NYHA, New York Heart Association; ESV, end systolic volume; EF, ejection fraction; Obs, observed; Chg, change; M6, month 6; ND, not done; NA, not available.
Prespecified clinically meaningful changes per Table 2 are underlined. See Evaluation of Study End Points.
Data are available through Month 2 or 3 for Cohort 2/pt # 2 who died on Day 96; sudden death was assessed as unlikely related to investigational product. Month 6 data are last observations carried forward.
Data are not available for Month 6 for Cohort 3/pt # 3 who received mechanical assist device at Week 6.
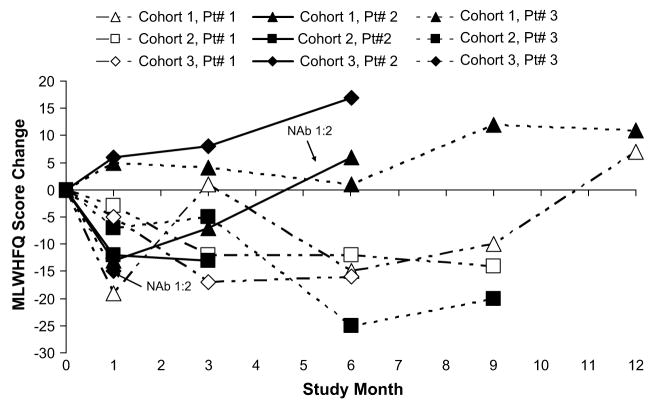
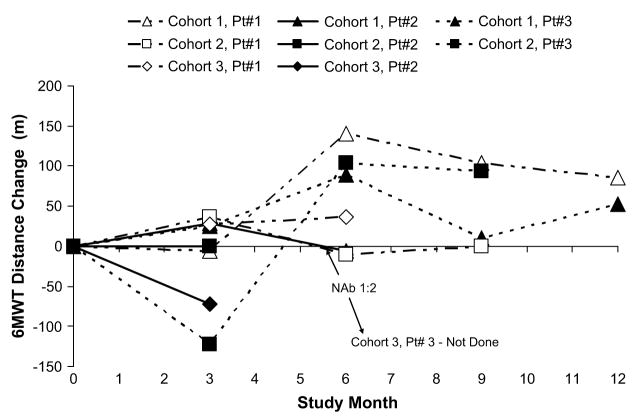
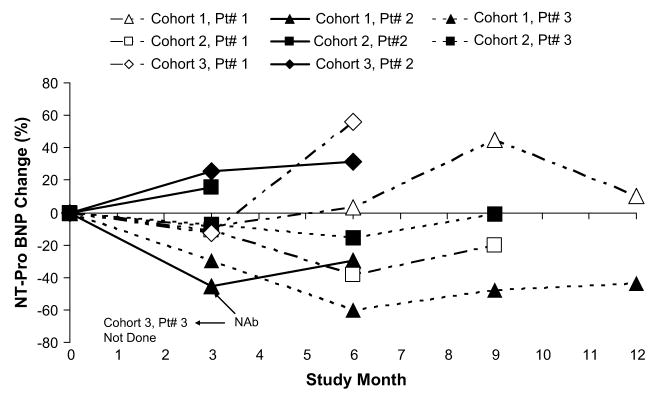
Change from baseline to month 6 in ESV, EF, NYHA status classification, VO2 max, 6MWT, NT-Pro BNP, and MLWHFQ are depicted graphically over time in Figures 1 through 7, respectively. Changes in HF medications during the study are summarized by patient in Table 5.
Fig. 7.
Change from baseline in Minnesota Living with Heart Failure Questionnaire (MLWHFQ) total score over time.
Table 5.
Change in Heart Failure Medications On-Study by Patient
| Cohort/Patient ID | Change |
|---|---|
| Cohort 2/Pt# 1 | β-blocker decreased at week 4 |
| Cohort 2/Pt# 2 | Diuretic increased on day 2 |
| Cohort 2/Pt# 3 | β-blocker decreased at week 6; diuretic decreased (week 7), increased (month 3) and then returned to baseline regimen (month 6) |
| Cohort 3/Pt# 1 | Diuretic decreased at week 2; aldosterone antagonist decreased at week 4 |
| Cohort 3/Pt# 2 | Diuretic decreased at week 5 and again at week 6 |
| Cohort 3/Pt# 3 | Diuretics increased at week 2; other antihypertensive agent added at week 5; β-blocker decreased at week 3 and then increased to baseline dose over the weeks before early termination; angiotensin-converting enzyme inhibitor switched at week 4 |
The 3 patients in Cohort 1 had no changes in HF medications.
Discussion
Despite pharmacological and device therapies, morbidity and mortality in HF is significant, and hence investigation of new therapeutic platforms such as cell and gene therapies is warranted. In this first in man study of gene transfer in HF, AAV1/SERCA2a appears to have an acceptable safety profile, given the high expected morbidity and mortality in the HF study population.34,36,37 These data are consistent with the safety profile established for other recombinant AAV vectors, which has been established in clinical studies in more than 500 patients. The basic safety aspects of recombinant AAV are summarized as follows: (1) they are derived from a nonreplicative and nonpathogenic human virus to which approximately 90% of the human population has been previously exposed; (2) they do not integrate into the chromosome, contain the regulatory elements needed to activate or modify expression of other genes, or contain the protein machinery needed to cause host chromosomal DNA breaks for integration; and, (3) as such, recombinant AAV vectors have been designated as nonintegrating by the EMEA Expert Committee on Medicinal Products Gene Therapy Expert Committee38 and the Food and Drug Administration.39 In target cells, they exist as nonintegrated episomal concatamers.40–42 In contrast, the history with adenoviral vectors demonstrates that they induce acute inflammation of infected tissues from activation of the innate immune system.43 AAV vectors are not associated with significant inflammation experimentally or clinically.
Although promising, these new therapeutic modalities have unique pharmacological attributes that need to be taken into consideration during early clinical investigation. For instance, after administration of AAV1/SERCA2a, restoration of SERCA2a enzyme levels will not occur immediately. Molecular studies with similar AAV1 vectors, especially with large transgenes such as SERCA2a, suggest that expression may have an onset of expression in 1 to 2 weeks with an initial peak around 1 month, followed by a brief decline, and eventually increase back to the 1 month values over ensuing months.44 This initial burst of expression around 1 month may be due to the generation of transcriptionally active but unstable, short-lived linear double-stranded DNA intermediates formed from the annealing of single-stranded AAV vector genomes of opposite polarity, followed by gradually increasing stable expression from circular concatameric, double-stranded AAV genomes.45 In the CUPID study, a pattern of change in EF from baseline in several patients resembles the kinetics of expression from other AAV-based therapeutics (Fig. 1).44 Regarding the expected longevity of expression, in the absence of cellular immune responses to the viral capsid proteins, transgene expression from AAV-based therapeutics after intramuscular administration in humans has been documented for >4 years.46
For all viral-based therapeutics, the presence of preexisting NAbs against the viral capsid proteins can block entry of the investigational agents into their target cells, and for agents administered through the vasculature, NAb status is an important consideration during patient selection.22–24 Based on preclinical studies with AAV1/SERCA2a, a pre-screening protocol was performed, and only patients with either low level or nonexistent NAbs (titer 1:2 or <1:2, respectively) were further screened and enrolled in the CUPID trial. After evaluation of the first 9 patients, a potential difference in HF progression was observed between patients with and without preexisting NAbs. Of the 2 NAb-positive patients (titer 1:2), 1 failed to improve and received a transplant at month 8, and the other continued to worsen and received a mechanical assist device at week 6. Further, in these 2 patients, AAV1/SERCA2a vector sequences were undetectable by qualitative polymerase chain reaction. Although these results as well as preclinical studies22–24 are suggestive of a neutralization effect of AAV1/SERCA2a by preexisting NAbs, the data are too preliminary to draw any conclusions. However, for the Phase 2 portion of the study, the protocol was modified to exclude all NAb positive patients. In the United States, ~60% of the HF population has qualifying NAb titers <1:2. If safety and efficacy of this therapy is established, future studies may employ methods such as plasmapheresis to reduce the impact of preexisting NAbs.
Some patients received intracoronary nitroglycerin prior to AAV1/SERCA2a infusion. Recent studies in minipigs demonstrate that prior intracoronary administration of nitroglycerin enhances delivery of AAV1/SERCA2a to regions of the myocardium furthest away from the infusion site (Krisztina Zsebo, PhD, Roger Hajjar, MD; unpublished data, 2008). Based on these results and findings that nitric oxide donors enhance myocardial viral entry,47 the protocol was modified to standardize bolus intracoronary nitroglycerin administration before AAV1/SERCA2a infusion.
Although this is a small Phase 1 open label dose escalation study, a number of end points that correlate with survival and hospitalizations in other HF clinical trials have been followed.24–34 Some improvement in these parameters was observed in all NAb-negative patients (7 patients), including symptomatic improvement (1 to 2 NYHA Class change) in the patient who died of presumed sudden cardiac death more than 3 months after administration. The threshold criteria for the study, summarized in the Methods section, include improvement in several parameters including symptomatic (NYHA and MLWHFQ, 5 patients), functional (6MWT and VO2 max, 4 patients), biomarker (NT-ProBNP, 2 patients), and LV function/remodeling (EF and ESV, 5 patients). A subset of patients (4 of 6 NAb negative patients) with 6-month data available showed improvement in VO2 max in the range of 1.7 to 5.9 mL·kg·min, with a median improvement of 1.9 mL·kg·min for all 6 patients (Fig. 4). VO2 max provides an indirect measure of cardiac reserve and in contrast to standard inotropic agents, an improvement after increase in SERCA2a activity may be predicted based on the mechano-energetic state of the heart.20 The differences can potentially be explained by the fact that the processes of excitation-contraction coupling and mitochondrial energetics are highly interrelated, and defects in excitation-contraction coupling directly translate into defects in mitochondrial energetics.48 In HF, energy reserves in the form of phosphocreatine are depleted from a deficiency of creatine kinase, whereas energy consumption is inefficient. In contrast to conventional inotropic agents that result in further energy wasting, increasing SERCA2a activity normalizes excitation-contraction coupling, restores energy reserves, and normalizes the oxygen cost of mechanical energy.20
Fig. 4.
Change from baseline to month 6 in maximal oxygen uptake (VO2 max).
Evaluation of biological activity end points in the Phase 1 portion of this trial has not furthered an understanding of dose response characteristics of AAV1/SERCA2a; however, the number of patients in each cohort is small, and prior administration of nitroglycerin and baseline NAbs varied within each dose cohort and may have contributed to variable levels of vector uptake within the myocardium. Moving forward in Phase 2, these parameters have been standardized (all patients will receive nitroglycerin administration prior to AAV1/SERCA2a infusion and patients with baseline NAbs are excluded). Treatment success will be evaluated based on safety, as well as trends in between-group and within individual patient comparisons for the prespecified efficacy/biological activity domains described here, as well as clinical outcome.
Conclusions
Early results of the Phase 1 open label portion of this first-in-human study of AAV1/SERCA2a in advanced HF showed no unexpected safety concerns. Biological activity was assessed across independent parameters important in evaluation of HF status. Although the number of patients in each cohort was small, quantitative evidence of biological activity could be detected in individual patients without preexisting NAbs. Further clinical evaluation is therefore warranted.
Fig. 3.
Change from baseline in New York Heart Association (NYHA) Class over time.
Fig. 5.
Change from baseline in 6-minute walk test over time.
Fig. 6.
Percent change from baseline in NT-Pro BNP over time.
Acknowledgments
The authors would like to thank Anthony N. DeMaria, MD, and Ajit B. Raisinghani, MD, for their evaluation of echocardiograms at the UCSD Medical Center Echocardiography Core Laboratory; Craig A. Thompson, MD, MMSc, for his contribution to the infusion protocol method; Jeffery J. Rudy for managing the study; Kim Wagner, MA, for manuscript preparation; and Alex Yaroshinsky, PhD, for data summaries and assessment of clinically meaningful changes in efficacy end points.
Funding for this study was provided by Celladon Corporation, La Jolla, CA. RJH is supported in part by grants from the National Institutes of Health: R01 HL078691, HL080498, HL 78731, HL083156, and a Leducq Transatlantic Network.
Footnotes
R.J. Hajjar: Celladon Corporation, ownership interest (includes stock and stock options, and rights in patents); D.M. Mancini, Celladon Corporation, consultant/advisory board; B. Greenberg, Celladon Corporation, consultant/advisory board; H. Dittrich, Celladon Corporation, ownership interest (includes stock options), consultant/advisory board; K. Borow, Celladon Corporation, significant, consultant/advisory board; K.M. Zsebo, Celladon Corporation, employment.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson T, Flegal K, et al. Heart Disease and stroke statistics–2009 update. A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation Epub. 2008 Dec 15;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Cleland J, Swedberg K, Poole-Wilson P. Successes and failures of current treatment of heart failure. Lancet. 1998;352(Suppl 1):SI19–28. doi: 10.1016/s0140-6736(98)90015-0. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein D, Oz M, Rose E. Implantable left ventricular assist devices. N Engl J Med. 1998;339:1522–33. doi: 10.1056/NEJM199811193392107. [DOI] [PubMed] [Google Scholar]
- 4.Hajjar R, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14:355–67. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 5.del Monte F, Hajjar RJ, Harding SE. Overwhelming evidence of the beneficial effects of SERCA gene transfer in heart failure. Circ Res. 2001;88:E66–7. doi: 10.1161/hh1101.092004. [DOI] [PubMed] [Google Scholar]
- 6.Hajjar R, Schmidt U, Matsui T, Guerrero J, Lee K, Gwathmey J, et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci. 1998;95:5251–6. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ly H, Kawase Y, Yoneyama R, Hajjar R. Gene therapy in the treatment of heart failure. Physiology. 2007;22:81–96. doi: 10.1152/physiol.00037.2006. [DOI] [PubMed] [Google Scholar]
- 8.Hajjar R, Samulski R. Heart failure: a silver bullet to treat heart failure. Gene Ther. 2006;13:997. doi: 10.1038/sj.gt.3302747. [DOI] [PubMed] [Google Scholar]
- 9.Periasamy M, Kalyanasundaram A. SERCA2a gene therapy for heart failure: ready for primetime? Mol Ther. 2008;16:1002–4. doi: 10.1038/mt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+–ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75:434–42. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 11.Schwinger R, Bohm M, Schmidt U, Karczewski P, Bavendiek U, Flesch M, et al. Unchanged protein levels of SERCA II and phospholamban but reduced calcium2+ uptake and calcium2+–-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation. 1995;92:3220–8. doi: 10.1161/01.cir.92.11.3220. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhri BB, del Monte F, Harding SE, Hajjar RJ. Gene transfer in cardiac myocytes. Surg Clin North Am. 2004;84:141–59. doi: 10.1016/S0039-6109(03)00209-3. [DOI] [PubMed] [Google Scholar]
- 13.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993;72:463–9. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- 14.de la Bastie D, Levitsky D, Rappaport L, Mercadier J, Marotte F, Wisnewsky C, et al. Function of the sarcoplasmic reticulum and expression of its Ca2+–ATPase gene in pressure overload-induced cardiac hypertrophy in the rat. Circ Res. 1990;66:554–64. doi: 10.1161/01.res.66.2.554. [DOI] [PubMed] [Google Scholar]
- 15.Gianni D, Chan J, Gwathmey JK, del Monte F, Hajjar RJ. SERCA2a in heart failure: role and therapeutic prospects. J Bioenerg Biomembr. 2005;37:375–80. doi: 10.1007/s10863-005-9474-z. [DOI] [PubMed] [Google Scholar]
- 16.Terracciano C, Hardy J, Birks E, Khaghani A, Banner N, Yacoub M. Clinical recovery from end-stage heart failure using left-ventricular assist device and pharmacological therapy correlates with increased sarcoplasmic reticulum calcium content but not with regression of cellular hypertrophy. Circulation. 2004;109:2263–5. doi: 10.1161/01.CIR.0000129233.51320.92. [DOI] [PubMed] [Google Scholar]
- 17.Stüdeli R, Jung S, Mohacsi P, Perruchoud S, Castiglioni P, Seiler C, et al. Diastolic dysfunction in human cardiac allografts is associated with reduced SERCA2a gene expression. Am J Transplant. 2006;6:775–82. doi: 10.1111/j.1600-6143.2006.01241.x. [DOI] [PubMed] [Google Scholar]
- 18.Kawase Y, Ly H, Prunier F, Lebeche D, Shi Y, Jin H, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51:1112–9. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 19.del Monte F, Williams E, Lebeche D, Schmidt U, Rosenzweig A, Gwathmey JK, et al. Improvement in survival and cardiac metabolism after gene transfer of sarcoplasmic reticulum Ca2+-ATPase in a rat model of heart failure. Circulation. 2001;104:1424–9. doi: 10.1161/hc3601.095574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly E, Liang L, et al. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol. 2007;42:852–61. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrne M, Power J, Preovolos A, Mariani J, Hajjar R, Kaye D. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008 Jul 24; doi: 10.1038/gt.2008.120. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Scallan C, Jiang H, Liu T, Patarroyo-White S, Sommer J, Zhou S, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–7. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 23.Moskalenko M, Chen L, van Roey M, Donahue B, Snyder R, McArthur J, et al. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol. 2000;74:1761–6. doi: 10.1128/jvi.74.4.1761-1766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wobus Ce H-DB, Girod A, Petersen G, Hallek M, Kleinschmidt JA. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. Blood. 2000;74:9281–93. doi: 10.1128/jvi.74.19.9281-9293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Marco T, Wolfel E, Feldman A, Lowes B, Higginbotham M, Ghali J, et al. Impact of cardiac resynchronization therapy on exercise performance, functional capacity, and quality of life in systolic heart failure with QRS prolongation: COMPANION trial sub-study. J Card Fail. 2008;14:9–18. doi: 10.1016/j.cardfail.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Wyrwich K, Nienaber N, Tierney W, Wolinsky F. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care. 1999;37:469–78. doi: 10.1097/00005650-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Wyrwich K, Tierney W, Wolinsky F. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52:861–73. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 28.Rector T. Overview of Minnesota Living With Heart Failure® Questionnaire. Available from: http://www.mlhfq.org/
- 29.Alla F, Briançon S, Guillemin F, Juillière Y, Mertès P, Villemot J, et al. Self-rating of quality of life provides additional prognostic information in heart failure. Insights into the EPICAL study. Eur J Heart Fail. 2002;4:337–43. doi: 10.1016/s1388-9842(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 30.Ingle L, Shelton R, Rigby A, Nabb S, Clark A, Cleland J. The reproducibility and sensitivity of the 6-min walk test in elderly patients with chronic heart failure. Eur Heart J. 2005;26:1742–51. doi: 10.1093/eurheartj/ehi259. [DOI] [PubMed] [Google Scholar]
- 31.Pereira-Barretto A, Oliveira Junior MTd, Strunz C, Del Carlo C, Scipioni A, Ramires J. Serum NT-proBNP levels are a prognostic predictor in patients with advanced heart failure. Arq Bras Cardiol. 2006;87:174–7. doi: 10.1590/s0066-782x2006001500016. [DOI] [PubMed] [Google Scholar]
- 32.Bruins S, Fokkema M, Römer J, Dejongste M, van der Dijs F, van den Ouweland J, et al. High intraindividual variation of B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with stable chronic heart failure. Clin Chem. 2004;50:2052–8. doi: 10.1373/clinchem.2004.038752. [DOI] [PubMed] [Google Scholar]
- 33.Clerico A, Carlo Zucchelli G, Pilo A, Passino C, Emdin M. Clinical relevance of biological variation: the lesson of brain natriuretic peptide (BNP) and NT-proBNP assay. Clin Chem Lab Med. 2006;44:366–78. doi: 10.1515/CCLM.2006.063. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann F, Packer M, Coats A, Fowler M, Krum H, Mohacsi P, et al. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation. 2004;110:1780–6. doi: 10.1161/01.CIR.0000143059.68996.A7. [DOI] [PubMed] [Google Scholar]
- 35.Drozdz J, Krzemińska-Pakula M, Plewka M, Ciesielczyk M, Kasprzak J. Prognostic value of low-dose dobutamine echocardiography in patients with idiopathic dilated cardiomyopathy. Chest. 2002;121:1216–22. doi: 10.1378/chest.121.4.1216. [DOI] [PubMed] [Google Scholar]
- 36.Grayburn P, Appleton C, DeMaria A, Greenberg B, Lowes B, Oh J, et al. Echocardiographic predictors of morbidity and mortality in patients with advanced heart failure. J Am Coll Cardiol. 2005;45:1064–71. doi: 10.1016/j.jacc.2004.12.069. [DOI] [PubMed] [Google Scholar]
- 37.Abraham W, Fisher W, Smith A, Delurgio D, Leon A, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 38.European Medicines Agency. Expert Committee on Medicinal Products Gene Therapy. [Accessed February 23, 2009];Report from the CPMP Gene Therapy Expert Group Meeting 26th–27th February 2004, EMEA/CPMP/1879/04/Final. Available at: http://www.emea.europa.eu/pdfs/human/genetherapy/187904en.pdf.
- 39. [Accessed February 23, 2009];FDA Guidance for Industry: Gene Therapy Clinical Trials - Observing Participants for Delayed Adverse Events. 2006 November; Available at: http://www.fda.gov/cber/gdlns/ctclin.pdf.
- 40.Carter B. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005;16:541–50. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- 41.Carter B, Burstein H, Peluso R. AAV vectors for gene delivery. In: Templeton N, editor. Gene and cell therapy: therapeutic mechanisms and strategies. 2. New York: Marcel Dekker; 2004. pp. 55–101. [Google Scholar]
- 42.Schnepp B, Jensen R, Chen C, Johnson P, Clark K. Characterization of adeno-associated virus genomes isolated from human tissues. J Virol. 2005;79:14793–803. doi: 10.1128/JVI.79.23.14793-14803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaiss A, Liu Q, Bowen G, Wong N, Bartlett J, Muruve D. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76:4580–90. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandalon Z, Bruckheimer E, Lustig K, Burstein H. Long-term suppression of experimental arthritis following intramuscular administration of a pseudotyped AAV2/1-TNFR:Fc vector. Mol Ther. 2007;15:264–9. doi: 10.1038/sj.mt.6300043. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Xie J, Lu H, Chen L, Hauck B, Samulski R, et al. Existence of transient functional double-stranded DNA intermediates during recombinant AAV transduction. Proc Natl Acad Sci U S A. 2007;104:13104–9. doi: 10.1073/pnas.0702778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang H, Pierce G, Ozelo M, de Paula E, Vargas J, Smith P, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14:452–5. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Sasano T, Kikuchi K, McDonald A, Lai S, Donahue J. Targeted high-efficiency, homogeneous myocardial gene transfer. J Mol Cell Cardiol. 2007;42:954–61. doi: 10.1016/j.yjmcc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maack C, O’Rourke B. Excitation-contraction coupling and mitochondrial energetics. Basic Res Cardiol. 2007;102:369–92. doi: 10.1007/s00395-007-0666-z. [DOI] [PMC free article] [PubMed] [Google Scholar]