Abstract
Introduction
A novel alpha-melanocyte stimulating hormone peptide analog CHX-A”-Re(Arg11)CCMSH, that targeted the melanocortin-1 receptor (MC1-R) over-expressed on melanoma cells, was investigated for its biodistribution and tumor imaging properties.
Methods
The metal bifunctional chelator CHX-A” was conjugated to the melanoma targeting peptide (Arg11)CCMSH and cyclized by Re incorporation to yield CHX-A”-Re(Arg11)CCMSH. CHX-A”-Re(Arg11)CCMSH was labeled with 111In, 86Y and 68Ga and the radiolabeled peptides were examined in B16/F1 melanoma bearing mice for their pharmacokinetic as well as their tumor targeting properties using small animal SPECT and PET.
Results
The radiolabeling efficiencies of the 111In, 86Y and 68Ga labeled CHX-A”-Re(Arg11)CCMSH peptides were > 95%, resulting in specific activities of 4.44 GBq/μg, 3.7 GBq/μg and 1.85 GBq/μg, respectively. Tumor uptake of the 111In, 86Y and 68Ga labeled peptides was rapid with 4.17±0.94 % ID/g, 4.68±1.02 % ID/g and 2.68±0.69 % ID/g present in the tumors 2 h post injection, respectively. Disappearance of radioactivity from the normal organs and tissues was rapid with the exception of the kidneys. Melanoma tumors were imaged with all three radiolabeled peptides 2 h post injection. MC1-R specific uptake was confirmed by competitive receptor blocking studies.
Conclusions
Melanoma tumor uptake and imaging was exhibited by the 111In, 86Y and 68Ga labeled -Re(Arg11)CCMSH peptides, although the tumor uptake was moderated by low specific activity. The facile radiolabeling properties of CHX-A”-Re(Arg11)CCMSH allow it to be employed as a melanoma imaging agent with little or no purification after 111In, 86Y and 68Ga labeling.
Keywords: Melanoma, Imaging, α-MSH, Peptide, CHX-A”
1. Introduction
Radiolabeled alpha-melanocyte stimulating hormone (α-MSH) peptide analogs have shown great promise as melanoma imaging [1-11] and radio-therapeutic agents [12-14] in preclinical studies and early clinical trials [15,16]. Native α-MSH is a tridecapeptide hormone, derived from the pro-opiomelanocortin gene, binds the G-protein linked melanocortin receptor family of proteins, namely the melanocortin-1 receptor (MC1-R) [17]. Melanocortin receptors are involved in the regulation of numerous physiological functions including, skin pigmentation (MC1-R), stress response (MC2-R), energy balance, feeding behavior and neurological roles (MC3-R, MC4-R and MC5-R) [17-18]. The MC1-R is primarily expressed on skin melanocytes and exhibits specificity and nanomolar to subnanomolar affinities for α-MSH and many of its analogs [17-19]. Over-expression of MC1-R on the surfaces of melanoma tumor cells has stimulated the development of α-MSH analogs for diagnostic imaging and therapeutic applications. Analyses of melanoma cell lines demonstrated that receptor numbers vary from approximately 900 to 7000 receptors per cell [19-22]. Upon MC1-R binding, radiolabeled peptide-receptor complexes are internalized, sequestering the radionuclide in the cytoplasm of the targeted melanoma tumor cell [3-14]. Rapid melanoma uptake and retention of MC1-R-targeted radionuclides, coupled with rapid whole body excretion of the radiolabeled targeting peptide, lead to high tumor to normal tissue ratios of radioactivity.
One of the most widely used strategies for radiolabeling α-MSH peptides is to introduce a metallic radionuclide into a peptide-chelator conjugate. The most commonly used metal chelator has been 1,4,7,10-tetraazacyclodocane-1,4,7,10-tetraacetic acid (DOTA), based largely on its ability to coordinate numerous radionuclides [23]. DOTA conjugated α-MSH peptides were radiolabeled with the imaging radionuclides 111In [4,24,25], 68Ga [5,6,9], 86Y [7] and 64Cu [7,10] and with the therapeutic radionuclides 212Pb/212Bi [13], 177Lu [14] and 90Y [26]. The DOTA chelator is particularly effective at stable coordination of the 3+ lanthanide series radiometals, yttrium, indium and bismuth. The introduction of metallic radionuclides into the DOTA chelator generally requires elevated temperatures (75-95°C) and extended incubation times (30-60 min). While elevated metal chelation temperatures are not usually detrimental to peptides, they can result in denaturation and inactivation of proteins, such as antibodies and antibody fragments. Elevated radionuclide chelation temperatures and extended incubation times do present potential obstacles to simple routine on-site formulation in nuclear pharmacies, especially with short-half life radionuclides.
The use of the metal chelator diethylenetriaminepentaacetic acid (DTPA) is an attractive alternative to DOTA, since metal ions can be chelated under mild conditions and shorter incubation times [23]. DTPA is a five-pendent polyaminocarboxylate chelator, as opposed to the constrained cyclic structure of DOTA, which contributes to its rapid radionuclide chelation kinetics. The development of a preorganized cyclohexyl derivative of DTPA, N-(2-aminoethyl)-trans-1,2-diaminocyclohexane-N,N',N”-pentaacetic acid (CHX-A”), resulted in a bifunctional chelator with improved metal affinity and metal complex stability [27]. The CHX-A” bifunctional chelator has been used successfully to radiolabel antibodies and peptides with a number of radionuclides including, 213Bi [28] 212Bi [29], 90Y [30,31], 177Lu [32] and 111In [31]. Recently, a novel protected version of the CHX-A” chelator was described that was suitable for solid-phase peptide synthesis [33]. A mono-N-hydroxysuccinimidyl penta-tert-butyl ester derivative of CHX-A” with a glutaric acid spacer enabled efficient coupling to peptides immobilized on solid supports or in solution. This advance greatly facilitated the utility of CHX-A” in the development of radiolabeled peptide imaging and therapeutic agents.
In this report we describe the synthesis and preclinical characterization of the MC1-R targeting peptide CHX-A”-Re(Arg11)CCMSH. The glutaric acid succinimidyl ester derivative of the penta-tert-butyl CHX-A” bifunctional chelator was coupled to the free-N-terminal protected linear peptide (Arg11)CCMSH in solution. CHX-A”-(Arg11)CCMSH was cyclized by site-specific rhenium incorporation to yield the final product CHX-A”-Re(Arg11)CCMSH. Purified CHX-A”-Re(Arg11)CCMSH was radiolabeled with 111In, 86Y, and 68Ga and the radiolabeled peptides were examined for their biodistribution and tumor imaging properties in B16/F1 melanoma bearing mice. The results of the biodistribution and imaging studies with the 111In, 86Y and 68Ga radiolabeled CHX-A”-Re(Arg11)CCMSH peptides were compared to published results of radiolabeled DOTA-Re(Arg11)CCMSH peptides. These studies demonstrated that radiolabeled CHX-A”-Re(Arg11)CCMSH peptides were able to selectively target and image tumors in the B16/F1 mouse melanoma model and that tumor uptake and biodistribution were greatly influenced by specific activity of each preparation as well as the chelate attached to the targeting peptide.
2. Materials and methods
2.1 Materials
All chemicals unless otherwise stated were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Water was purified and deionized (18 MΩ/cm2) via a Milli-Q water filtration system (Millipore Corp., Milford, MA). 111In was purchased from Mallinckrodt (St. Louis, MO). 86Y was produced on a CS-15 biomedical cyclotron at Washington University School of Medicine [34]. 68Ga was obtained from a 68Ge/68Ga radionuclide generator (Cyclotron Co., Ltd., Obninsk, Russia). The parent, 68Ge, was accelerator produced and decays with a half life of 270.8 days by electron capture. The carrier of the generator was TiO2 and it was eluted with high purity 0.1 M HCl (TraceMetal concentrated HCl was obtained from Fisher Scientific and diluted with Mill-Q water). At the time of the experiments the generator was over 9 months old and the average elution was 462.5 MBq (12.5 mCi) in 5 mL of elution (of which 80-90% of the activity was collected in a 1.5 mL fraction). Radioactivity was counted with a Beckman Gamma 8000 counter containing a NaI crystal (Beckman Instruments, Inc., Irvine, CA). Radio-TLC detection was accomplished using a BIOSCAN AR2000 Imaging Scanner (Washington DC, USA). Amino acid monomers were obtained from Novabiochem, EMD Biosciences, Inc. (San Diego, CA).
2.2 CHX-A”-Re(Arg11)CCMSH synthesis
The (Arg11)CCMSH sequence (CCEH-dPhe-RWCRPV-NH2) was synthesized by standard solid phase peptide synthesis using an Fmoc strategy on acid labile 2ClTrt resin. The free N-terminal amino fully protected peptide was cleaved from the resin with 2 × 5 min treatments of 25% 1,1,1,3,3,3-hexafluoroisopropanol (HFIP) and 5% triisopropylsilane (TIS) in dichloromethane (DCM). Conjugation with CHX-A” was performed by reacting the (Arg11)CCMSH peptide with the NHS ester of the CHX-A”-glutarate ligand (2 molar excess) in dimethylformamide (DMF) and in the presence of diisopropylethylamine (DIEA, 4 molar excess) for 2 h. After diethylether precipitation, the peptide was treated with 80% TFA and scavengers for 2 h to yield the final deprotected crude conjugate. CHX-A”-(Arg11)CCMSH was cyclized by site-specific rhenium (Re) coordination [8], purified by preparative HPLC and lyophilized. The final product CHX-A”-Re(Arg11)CCMSH (MW 2280 daltons) was subjected to LC-MS analysis, yielding a homogeneous peptide conjugate with the expected mass.
2.3 Competitive Binding Assay
The IC50, or concentration of peptide required to inhibit 50% of radioligand binding, was determined for the CHX-A”-Re-(Arg11)CCMSH peptide in a competitive binding assays with 125I-(Tyr2)-NDP, a radioiodinated α-MSH analog with picomolar affinity for the MC1-R [35]. B16/F1cells were plated in 24-well tissue culture plates and incubated overnight to yield a final cell count of 0.5 million cells per well. Individual wells were incubated at 25°C for 3 h with approximately 50,000 cpm 125I-(Tyr2)-NDP in 0.5 mL binding medium (modified Eagle's medium with 25 mmol/L N-(2-hydroxyethyl)-piperazine-N'-(2-ethanesulfonic acid), 0.2% BSA, and 0.3 mmol/L 1,10-phenanthroline) with concentrations of CHX-A”-(Arg11)CCMSH ranging from 10-13 to 10-5 mol/L. The radioactivity in the cells and in the medium was separately collected and measured. The data were processed, and the IC50 value of the CHX-A”-Re(Arg11)CCMSH peptide were calculated with the Kell software package (Biosoft, Ferguson, MO).
2.4 Radiolabeling of CHX-A”-Re(Arg11)CCMSH with 111In, 86Y and 68Ga
44.4 MBq (1.2 mCi) of 111InCl3 in 60 μL of 0.05 M HCl was added to 20 μg of CHX-A”-Re(Arg11)CCMSH in 120 μL 0.2M ammonium acetate (pH 5.5). The reaction mixture was heated at 40 °C for 40 min. Radiochemical purity was confirmed by reverse-phase HPLC using a Vydac 218TP54, 250 mm column (P J Cobert Associates, Inc, MO, USA) eluted with a gradient of 22% B to 32% B in 20 min at a flow rate of 1.0 mL /min (Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: 0.1% trifluoroacetic acid in acetonitrile). Under these HPLC conditions, the retention times of 111InCl3 and the product 111In- CHX-A”-Re(Arg11)CCMSH were 3 min and 10.4 min, respectively. The specific activity of 111In-CHX-A”-Re(Arg11)CCMSH was 4.44 MBq/μg (120 μCi/μg) or 10.62 GBq/μmol (287 mCi/μmol).
37 MBq (1.0 mCi) of 86Y in 10 μL of 0.1 M HCl was added to 10 μg of CHX-A”-Re(Arg11)CCMSH in 100 μL 0.5 M ammonium acetate (pH 5.5). The reaction mixture was heated at 75 °C for 30 min. Product formation was monitored by radio-TLC using Whatman MKC18F TLC plates developed with 30:70 10 % NH4OAc to methanol (86Y: Rf = 0; 86Y-CHX-A”-DTPA-ReCCMSH(Arg11): Rf = 0.3). Radiochemical purity was also confirmed with reverse-phase HPLC using a Microsorb C18, 4.6 × 250 mm column (Varian Inc. Lake Forest, CA) eluted with a gradient of 10% B to 20% B in 10 min, 20% B in 10 min, 20% B to 40% B in 60 min at a flow rate of 1.0 mL/min (Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: 0.1% trifluoroacetic acid in acetonitrile). Under these HPLC conditions, the retention times of uncomplexed 86Y and the product 86Y-CHX-A”-Re(Arg11)CCMSH were 2 min and 33 min respectively. The specific activity of 86Y-CHX-A”-Re(Arg11)CCMSH was approximately 3.7 MBq/μg (100 μCi/μg) or 8.77 GBq/μmol
74 MBq (2.0 mCi) 68GaCl3 in 300 μL 0.1 M HCl was added to 40 μg of CHX-A”-Re(Arg11)CCMSH in 300 μL 0.1 M ammonium acetate (pH 8). The reaction mixture was incubated at 85 °C for 20 min. Radiochemical purity was >95% as indicated by thin-layer chromatography using instant TLC (ITLC-SG) glass microfiber sheets developed with 50:50 10% NH4OAc to methanol (68GaCl3: Rf = 0; 68Ga-CHX-A”-Re(Arg11)CCMSH Rf = 0.9). Radiochemical purity was also confirmed with reverse-phase HPLC using a Microsorb C18, 4.6 × 250 mm column (Varian Inc. Lake Forest, CA) eluted with a gradient of 1% B to 30% B in 15 min then 30% B to 70% B in 5 min at a flow rate of 1.0 mL/min (Solvent A: 0.1% trifluoroacetic acid in water, Solvent B: 0.1% trifluoroacetic acid in acetonitrile). Under these HPLC conditions, the retention times of uncomplexed 68Ga and the product 68Ga-CHX-A”-Re(Arg11)CCMSH were 1.5 min and 19.6 min respectively. The specific activity of 68Ga-CHX-A”-Re(Arg11)CCMSH was approximately 1.85 MBq/μg (50 μCi/μg) or 4.33 GBq/μmol (117 mCi/μmol).
2.5 Biodistribution Studies
All animal experiments were conducted in compliance with the Guidelines for the Care and Use of Research Animals established by the Institutional Animal Care and Use Committees at Washington University, The University of Missouri, and Harry S. Truman Veterans Hospital. Biodistribution studies were carried out on 18-23 g female C57BL/6 mice (Charles River Laboratories, Wilmington, MA) that were implanted with 1 × 106 cultured B16/F1 murine melanoma cells in 100 μL subcutaneously into the nape of the neck. Tumors were allowed to grow for 10 days.
111In-CHX-A”-Re(Arg11)CCMSH
Biodistribution studies of 111In- CHX-A”-Re(Arg11)CCMSH were performed in B16/F1 murine melanoma-bearing C57 female mice. Approximately 0.111 MBq (3 μCi) of 111In-CHX-A”-Re(Arg11)CCMSH (16.7 ng, 7.3 pmol) was injected into each mouse through the tail vein for biodistribution studies. Groups of 5 mice per each time point were used for the biodistribution studies. The mice were sacrificed at 30 min, 1, 2, 4 and 24 h after 111In- CHX-A”-Re(Arg11)CCMSH injection. Tumor specific uptake of 111In-CHX-A”-Re(Arg11)CCMSH was determined by blocking tumor uptake with the injection of 20 μg of [Nle4,D-Phe7]α-MSH (NDP), a linear α-MSH peptide analog with picomolar affinity for the MC1 receptor present on melanoma cells [35], 15 min prior to injection of 111In-CHX-A”-Re(Arg11)CCMSH.
86Y-CHX-A”-Re(Arg11)CCMSH
Mice bearing 10-day B16/F1 tumors received ~0.296 MBq (~8 μCi) of 86Y-CHX-A”-Re(Arg11)CCMSH (~80 ng, 35 pmol) in 100 μL of saline via lateral tail vein injection. Four groups of mice (n = 5 per group) were examined at 30 min, 2 , 4 and 24 h p.i. To examine in vivo uptake specificity, a fifth group of mice (2 h time point) was pre-injected with 20 μg of NDP to act as a receptor block immediately prior to injecting of 86Y-CHX-A”-Re(Arg11)CCMSH. The NDP peptide represented a ~250-fold increase over the mass of peptide injected with 86Y-CHX-A”-Re(Arg11)CCMSH.
68Ga-CHX-A”-DTPA-Re(Arg11)CCMSH
The mice bearing 10-day B16/F1 tumors received ~0.37 MBq (~10 μCi) of 68Ga-CHX-A”-Re(Arg 11)CCMSH (~200 ng, 91 pmol) in 100 μL of saline via the lateral tail vein injection. Three groups of mice (n = 5 per group) were examined at 30 min and 1 and 2 h p.i. To examine in vivo uptake specificity, a forth group of mice (2 h time point) was pre-injected with 60 μg of NDP to act as a receptor block immediately prior to injecting of 68Ga-CHX-A”- Re(Arg11)CCMSH. The NDP peptide block represented a ~300-fold increase over the mass of the injected 68Ga-CHX-A”- Re(Arg11)CCMSH.
In all studies following euthanasia, tissues and organs of interest were removed and weighed and the radioactivity was measured in a γ-counter. Blood values were taken as 6.5% of the whole-body weight. The results were expressed as percentage injected dose/gram (%ID/g) and as percentage injected dose (%ID).
2.6 Small-Animal SPECT and PET Studies
The SPECT images were obtained using the micro-CAT II SPECT/CT (Siemens Medical Solutions) unit equipped with high-resolution 2-mm pinhole collimators. B16/F1 melanoma-bearing C57 mice were injected with 12.95 MBq of 111In-CHX-A”-Re(Arg11)CCMSH (350 μCi, 1.94 μg, 0.85 nmol) via the tail vein 10 days after B16/F1 cell implantation. The mice were anesthetized with 1% to 2% isoflurane 2 h p.i. for the SPECT/CT imaging studies. The SPECT data were collected immediately after CT data collection. The blocked mouse received 20 μg of NDP 15 min prior to the administration of 111In-CHX-A”-Re(Arg11)CCMSH.
Whole body small animal PET imaging was performed on a microPET-Focus scanner (Concorde Microsystems, Knoxville, TN). Imaging studies were carried out on C57BL/6 mice bearing 10-day B16/F1 murine melanoma tumors. The mice were injected via the tail vein with 4.44 MBq of 86Y-CHX-A”-Re(Arg11)CCMSH (120 μCi, 1.2 μg, 0.52 nmol) or 3.7 MBq of 68Ga-CHX-A”-Re(Arg11)CCMSH (100 μCi, 2 μg, 0.9 nmol). These mice were imaged side by side with mice that had been treated with 20 μg of NDP prior to injection of 86Y-CHX-A”-Re(Arg11)CCMSH or 60 μg of NDP prior to injection of 68Ga-CHX-A”-Re(Arg11)CCMSH. At different time points, the mice were anesthetized with 1% to 2% isoflurane, positioned supine, immobilized and imaged. Ten minute static data sets were collected at each time point.
2.7 Statistical Methods
All of the data are presented as mean ± SD. For statistical classification, a Student's t-test was performed using GraphPad PRISM (San Diego, CA). Differences at the 95% confidence level (p < 0.05) were considered significant.
3. Results
3.1 Preparation and radiolabeling of CHX-A”-Re(Arg11)CCMSH
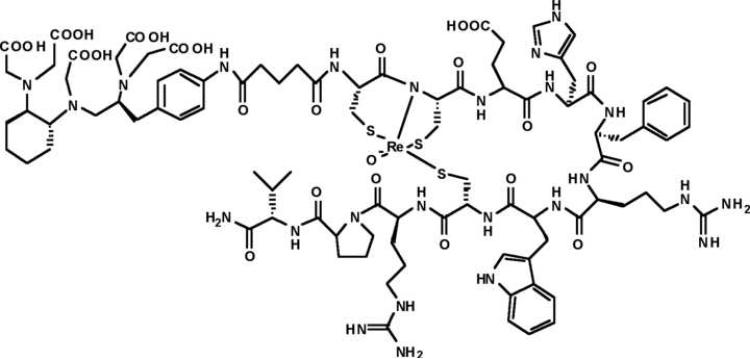
The peptide (Arg11)CCMSH was synthesized using standard Fmoc solid phase peptide chemistry, cleaved from its support resin and stored with sidechain protecting groups remaining in place. The mono-N-hydroxysuccinimidyl glutaric acid ester derivative of penta-tert-butyl protected CHX-A” was reacted with protected (Arg11)CCMSH in DMF to yield protected CHX-A”-(Arg11)CCMSH, which was deprotected and HPLC purified. CHX-A”-(Arg11)CCMSH was cyclized by site-specific rhenium coordination using previously published conditions [8] to yield the final compound CHX-A”-Re(Arg11)CCMSH (Fig.1). The molecular weights of the final product and synthetic intermediates were determined by LC-MS. Competitive binding studies with 125I-NDP and various concentrations of CHX-A”-Re(Arg11)CCMSH ranging from 10-5 to 10-13 molar. The IC50 of CHX-A”-Re(Arg11)CCMSH was determined to be 3.8 nanomolar, which is similar to the 2.1 nanomolar IC50 reported for DOTA-Re(Arg11)CCMSH [24].
Figure 1.
The structure of CHX-A”-Re(Arg11)CCMSH
3.2 Biodistribution studies of 111In, 86Y and 68Ga labeled CHX-A”-Re(Arg11)CCMSH
The biodistribution data for 111In-CHX-A”-Re(Arg11)CCMSH at 0.5 , 1, 2, 4, and 24 h post injection (p.i.) are presented in Table 1. Melanoma bearing mice were injected with 0.111 MBq (3 μCi; 7.3 pmol) of 111In-CHX-A”-Re(Arg11)CCMSH. Tumor uptake was rapid, reaching 4.38±0.72 % ID/g at 0.5 h p.i. and 4.59±0.69 % ID/g at 1 h p.i., then remained nearly constant to 4 h p.i. Disappearance of radioactivity from the blood, muscle and major organs was rapid, with the exception of the kidneys. The tumor-to-blood and tumor-to-muscle ratios were 8.9 and 38 at 2 h p.i. Kidney uptake was high at 23.34±4.46 % ID/g at 0.5 h p.i. and remained high at later time points (22.16±2.49 % ID/g at 4 h p.i. and 10.08±2.21 % ID/g at 24 h p.i.). The blocking study at 2 h p.i yielded similar biodistribution data to the 2 h time point except that tumor uptake was significantly reduced to 1.19±0.23 % ID/g (p < 0.001), confirming that the radioactivity in the tumor was receptor mediated. These data also showed that radioactivity in the kidneys was not receptor specific.
Table 1.
Biodistribution of 111In-CHX-A”-Re(Arg11)CCMSH, 68Ga-CHX-A”-Re(Arg11)CCMSH, 86Y-CHX-A”-Re(Arg11)CCMSH in B16/F1 murine melanoma bearing C57 mice. The data are presented as percent injected dose/gram (Mean±SD, n = 5).
| Tissues | Blood | Lung | Liver | Spleen | Kidneys | Muscle | Heart | Tumor | Skin | |
|---|---|---|---|---|---|---|---|---|---|---|
| 111In-CHX-A”-Re(Arg11) CCMSH | 30 min | 3.78 ± 2.51 | 4.84 ± 1.73 | 1.74 ± 0.65 | 1.67 ± 0.76 | 23.34 ± 4.46 | 0.84 ± 0.28 | 1.59 ± 0.96 | 4.38 ± 0.72 | 5.29 ± 1.24 |
| 1 hour | 2.02 ± 0.94 | 3.21 ± 1.14 | 0.94 ± 0.16 | 1.14 ± 0.42 | 19.60 ± 1.78 | 0.41 ± 0.16 | 0.73 ± 0.34 | 4.59 ± 0.69 | 3.47 ± 0.89 | |
| 2 hour | 0.47 ± 0.22 | 1.59 ± 0.62 | 0.73 ± 0.06 | 0.87 ± 0.33 | 21.14 ± 6.45 | 0.11 ± 0.04 | 0.21 ± 0.04 | 4.17 ± 0.94 | 1.53 ± 1.18 | |
| 2 hr. block | 0.29 ± 0.07 | 0.50 ± 0.11 | 0.84 ± 0.24 | 1.40 ± 0.51 | 18.07 ± 3.98 | 0.08 ± 0.02 | 0.16 ± 0.05 | 1.19 ± 0.23 | 0.77 ± 0.22 | |
| 4 hour | 0.18 ± 0.05 | 1.09 ± 0.42 | 0.77 ± 0.09 | 0.92 ± 0.61 | 22.16 ± 2.49 | 0.04 ± 0.01 | 0.10 ± 0.02 | 3.87 ± 1.03 | 0.34 ± 0.24 | |
| 24 hour | 0.03 ± 0.01 | 0.08 ± 0.01 | 0.39 ± 0.10 | 0.82 ± 0.68 | 10.08 ± 2.21 | 0.02 ± 0.00 | 0.03 ± 0.01 | 1.35 ± 0.43 | 0.10 ± 0.04 | |
| 86Y-CHX-A”-Re(Arg11) CCMSH | 30 min | 4.65 ± 0.98 | 3.44 ± 0.43 | 4.23 ± 0.36 | 1.16 ± 0.25 | 10.95 ± 3.01 | 1.24 ± 0.20 | 2.23 ± 0.46 | 3.99 ± 0.54 | 2.71 ± 0.56 |
| 2 hour | 1.09 ± 0.24 | 1.24 ± 0.18 | 3.30 ± 0.54 | 0.53 ± 0.13 | 8.19 ± 1.92 | 0.23 ± 0.03 | 0.88 ± 0.21 | 4.68 ± 1.02 | 0.87 ± 0.36 | |
| 2 hr. block | 1.07 ± 0.44 | 1.16 ± 0.42 | 2.99 ± 1.12 | 0.59 ± 0.27 | 7.91 ± 2.03 | 0.39 ± 0.26 | 0.79 ± 0.21 | 2.71 ± 0.46 | 1.23 ± 0.73 | |
| 4 hour | 0.46 ± 0.08 | 0.82 ± 0.14 | 3.00 ± 0.42 | 0.46 ± 0.08 | 8.78 ± 1.69 | 0.19 ± 0.06 | 0.61 ± 0.05 | 4.18 ± 0.45 | 0.60 ± 0.29 | |
| 24 hour | 0.06 ± 0.02 | 0.51 ± 0.17 | 3.06 ± 0.34 | 0.30 ± 0.03 | 7.13 ± 3.19 | 0.05 ± 0.03 | 0.33 ± 0.09 | 2.79 ± 0.44 | 0.21 ± 0.03 | |
| 68Ga-CHX-A”-Re(Arg11) CCMSH | 30 min. | 1.72 ± 0.24 | 2.64 ± 0.65 | 1.38 ± 0.30 | 0.92 ± 0.20 | 10.24 ± 0.94 | 1.47 ± 0.51 | 0.96 ± 0.44 | 2.46 ± 0.41 | 3.49 ± 1.13 |
| 1 hour | 1.16 ± 0.52 | 1.73 ± 0.39 | 0.99 ± 0.39 | 0.58 ± 0.16 | 6.98 ± 1.29 | 0.46 ± 0.19 | 0.41 ± 0.09 | 2.16 ± 0.36 | 1.33 ± 0.43 | |
| 2 hour | 0.58 ± 0.14 | 0.67 ± 0.10 | 0.79 ± 0.24 | 0.31 ± 0.12 | 7.39 ± 2.13 | 0.28 ± 0.24 | 0.31 ± 0.11 | 2.68 ± 0.69 | 2.10 ± 0.47 | |
| 2 hr. block | 0.65 ± 0.14 | 0.83 ± 0.15 | 0.69 ± 0.17 | 0.22 ± 0.09 | 7.95 ± 0.87 | 0.50 ± 0.32 | 0.38 ± 0.20 | 0.61 ± 0.19 | 2.14 ± 1.09 |
Biodistribution data for 86Y-CHX-A”-Re(Arg11)CCMSH in melanoma bearing mice are shown in Table 1. Tumor bearing mice were injected with 0.296 MBq (8 μCi; 35 pmol) of 86Y-CHX-A”-Re(Arg11)CCMSH and biodistribution data were collected at 0.5, 2, 4 and 24 h p.i. Accumulation of radioactivity in the tumor was rapid reaching a maximum of 4.68±1.02 % ID/g at 2 h p.i. and remained constant (4.18±0.45 % ID/g) to 4 h p.i. The tumor-to-blood and tumor-to-muscle ratios were 4.3 and 20 at 2 h p.i. and 9.1 and 22 at 4 h p.i. The blocking study at 2 h p.i yielded similar biodistribution data to the 2 h time point except that tumor uptake was significantly reduced to 2.71 ± 0.46 % ID/g (p < 0.02). Radioactivity in the major organs and tissues was not significantly different between the blocked and non-blocked mice. Clearance of radioactivity from the blood and major organs was rapid with the exception of the liver and kidneys. Kidney uptake of 86Y-CHX-A”-Re(Arg11)CCMSH was 10.95±3.0 % ID/g at 0.5 h p.i. and 8.78±1.69 % ID/g at 4 h p.i., which was approximately 2 and 3 times less than 111In-CHX-A”-Re(Arg11)CCMSH at the same time points. Liver uptake was significantly higher with 86Y-CHX-A”-Re(Arg11)CCMSH, with 3.30±0.54 % ID/g at 2 h p.i. compared to 111In-CHX-A”-Re(Arg11)CCMSH (0.73±0.06; p < 0.002) or 68Ga-CHX-A”-Re(Arg11)CCMSH (0.79±0.04 % ID/g; p < 0.004). The amount of radioactivity in the liver 2 h p.i. was not significantly different between the block and non-blocked animals indicating that it was not MC1-R mediated.
The 68Ga-CHX-A”-Re(Arg11)CCMSH peptide biodistribution data in B16/F1 tumor bearing mice are presented in Table 1. The melanoma bearing mice were injected with 0.37 MBq (10 μCi; 91 pmol) of 68Ga-CHX-A”-Re(Arg11)CCMSH and biodistribution data were collected at 0.5, 1, and 2 h p.i. Tumor uptake was rapid reaching 2.46±0.41 % ID/g at 0.5 h p.i. and a maximum of 2.68±0.69 % ID/g relatively at 2 h p.i. The tumor to blood and muscle ratios were 4.6 and 9.6 at 2 h p.i. Co-injection of excess non-radioactive NDP peptide significantly reduced tumor uptake to 0.61±0.19 % ID/g (p < 0.05) confirming that tumor uptake was specific. The addition of non-radioactive peptide did not significantly affect the levels of radioactivity in any other organs. The disappearance of radioactivity from the major organs was rapid with the exception of the kidneys, which exhibited uptake values of 10.24±0.94 % ID/g at 0.5 h p.i. and 7.39±2.13 % ID/g at 2 h p.i. The level of radioactivity in the kidneys was not significantly different between the 2 h study and the 2 h blocking study, indicating that radioactivity retention in the kidneys was non-specific. At 2 h p.i there was also radioactivity in the skin (2.10±0.47 % ID/g), however, this was not receptor specific, since the value remained the same during the 2 h blocking study.
3.2 Melanoma imaging with 111In, 68Ga and 86Y labeled CHX-A”-Re(Arg11)CCMSH
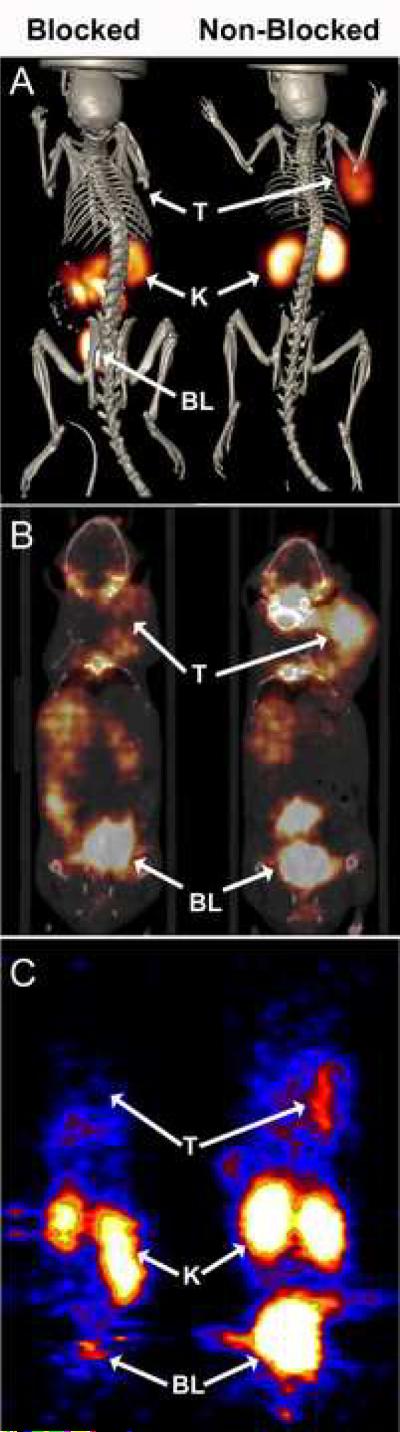
SPECT and PET images of B16/F1 melanoma bearing mice were obtained with 111In, 86Y and 68Ga labeled CHX-A”-Re(Arg11)CCMSH. Figure 2A shows SPECT / CT images of 111In-CHX-A”-Re(Arg11)CCMSH (12.95 MBq; 350 μCi) in melanoma bearing C57 mice 2 h p.i. The melanoma tumor located over the right shoulder was easily visualized. Injection of 20 μg of non radiolabeled CHX-A”-Re(Arg11)CCMSH blocked tumor uptake of the radiolabeled compound confirming receptor specificity. Uptake of 111In-CHX-A”-Re(Arg11)CCMSH in the kidneys was clearly visible in both blocked and non-blocked animals, confirming that it was not receptor specific. Radioactivity in the GI of the blocked mouse was potentially due to the addition of excess non-radioactive peptide, which altered the excretion pathway to include heptobiliary - GI route.
Figure 2.
Whole body SPECT/CT, PET/CT, and PET images of B16 melanoma tumor bearing C57 mice 2 h post tail vein injection of radiolabeled CHX-A”-Re(Arg11)CCMSH. (A) SPECT/CT images of tumor bearing mice inject i.v with 12.95 MBq (350 μCi) of 111In-CHX-A”-Re(Arg11)CCMSH with (Blocked) or without (Non-Blocked) a 20 μg non-radiolabeled peptide block. PET/CT and PET imaging of melanoma bearing mice 2 h post tail vein injection of (B) 4.44 MBq (120 μCi) of 86Y-CHX-A”-Re(Arg11)CCMSH with a 20 μg NDP block (Blocked) and without block (Non-Blocked) or (C) 3.7 MBq (100 μCi) of 68Ga-CHX-A”-Re(Arg11)CCMSH with (Blocked) and without (Non-Blocked) a 60 μg NDP block, respectively. Tumor (T), kidney (K) and (BL) bladder locations are highlighted on for each mouse.
PET/CT imaging of 86Y labeled CHX-A”-Re(Arg11)CCMSH in B16/F1 melanoma bearing mice 2 h p.i. is shown in Figure 2B. Tumor bearing mice were injected with 4.44 MBq (120 μCi) of 86Y-CHX-A”-Re(Arg11)CCMSH with or without 20 μg of non-radiolabeled NDP for the blocking study. Coronal micro-PET / CT images of the melanoma bearing mice show tumor specific uptake of 86Y-CHX-A”-Re(Arg11)CCMSH at 2 h p.i. A reduction in tumor radioactivity was observed in the animal receiving the non-radioactive NDP block demonstrating receptor specificity. Kidney uptake of radioactivity was evident in both blocked and non-blocked animals. The radioactivity present in the bladders of the mice was due to the rapid whole body clearance of the radiolabeled peptide via the kidneys. PET imaging of 68Ga-CHX-A”-Re(Arg11)CCMSH in melanoma bearing mice is shown in Figure 2C. Mice bearing melanoma tumors over their right shoulders were injected i.v. with 3.7 MBq (100 μCi) of 68Ga labeled CHX-A”-Re(Arg11)CCMSH and imaged at 2 h p.i. Coronal micro-PET images show tumor uptake of 68Ga-CHX-A”-Re(Arg11)CCMSH, which was blocked by co-injection of non-radiolabeled NDP peptide. The peptide block did not affect uptake of radioactivity in the kidneys demonstrating that kidney uptake was non-receptor specific. Radioactivity present in the bladder of the mouse imaged with 68Ga-CHX-A”-Re(Arg11)CCMSH was due to the mouse not voiding its bladder prior to the imaging study.
4. Discussion
The CHX-A” bifunctional chelator has been used to radiolabel antibodies and other proteins [28-31], but it has not been readily applied to peptides due to difficulties with conjugation chemistry and stability. The advent of the protected-CHX-A”-bifunctional chelator has fostered the use of CHX-A” for solid phase and solution phase conjugation with peptides [32]. In this study, conjugation of protected-CHX-A” to the amino terminus of protected-(Arg11)CCMSH peptide was found to be much more efficient in solution than when the protected peptide was still immobilized on the synthetic resin. It is possible that steric constraints imposed on the peptide by being bound to the synthetic resin or by substitution density on the resin limited the solid phase conjugation reaction. Conjugation of protected-CHX-A” to protected-(Arg11)CCMSH in DMF via the NHS activated glutaric acid linker was performed in solution yielding CHX-A”-(Arg11)CCMSH, which was then deprotected and cyclized by site-specific Re incorporation to yield the final product CHX-A”-Re(Arg11)CCMSH.
The acyclic CHX-A” ligand was examined due to its rapid and efficient metal chelation properties. DTPA and CHX-A” have been reported to chelate yttrium and other trivalent metal cations nearly instantaneously at a chelator:metal ratio of 1:1 at 22°C [36]. Rapid chelation kinetics are desirable for instant kit formulation of the imaging agent, while a minimal chelator:radiometal ratio insures that the imaging agent formulation would be at a high enough specific activity as to not saturate the target receptors. These CHX-A” chelation properties facilitated the goal of developing a MC1-R targeted melanoma radiolabeled imaging agent that could be prepared in a single step without subsequent purification. The cyclic chelator DOTA was reported to have superior kinetic stability to CHX-A” over time, however, DOTA:radiometal ratios of 3:1 were necessary to drive the chelation reaction to completion [36]. In our experience, a minimum molar ratio of DOTA-Re(Arg11)CCMSH: radiometal of 20:1 and incubation at ≥75°C are necessary for efficient peptide labeling. Without post labeling purification of radiolabeled DOTA-Re(Arg11)CCMSH, the large excess amount of unlabeled peptide remaining in the final mixture would effectively compete for or saturate receptor binding.
The radiolabeling efficiencies of 111In, 86Y and 68Ga labeled CHX-A”-Re(Arg11)CCMSH peptide preparations were greater than 95%. 111In-CHX-A”-Re(Arg11)CCMSH was partially purified using reverse phase HPLC to yield a final product with a similar specific activity to the 86Y and 68Ga compounds. Based on HPLC peptide UV absorbance, approximately 50% of the unlabeled peptide was not separated from the labeled peptide fraction yielding a specific activity of 4.44 MBq/mg (120 μCi/mg) or 10.62 MBq/μmol (287 μCi/μmol) for 111In-CHX-A”-Re(Arg11)CCMSH. The 86Y and 68Ga labeled CHX-A”-Re(Arg11)CCMSH peptides were used without additional HPLC purification. The lack of HPLC purification post 86Y and 68Ga labeling resulted in lower specific activities, but was particularly important for 68Ga-CHX-A”-Re(Arg11)CCMSH preparation due to the short half life of 68Ga (t1/2 = 68 min). The specific activities of the 86Y and 68Ga labeled CHX-A”-(Arg11)CCMSH peptide preparations were 8.77 GBq/μmol (237 mCi/μmol) and 4.33 GBq/μmol (117 mCi/μmol), respectively.
Despite the lower specific activities of the 111In, 86Y and 68Ga labeled CHX-A”-Re(Arg11)CCMSH peptides, melanoma selective uptake of the radiolabeled peptide was evident from competitive blocking studies. The tumor uptake values of the 111In, 86Y and 68Ga labeled CHX-A”-Re(Arg11)CCMSH peptides at 2 h p.i. were significantly reduced to 29%, 58% and 23%, respectively, of the corresponding non-blocked tumor uptake values by co-injection of 20 μg of non-radioactive NDP peptide. The NDP peptide is a superpotent MC1-R agonist with picomolar affinity for the receptor [35]. These results demonstrated that the radioactivity in the tumors was MC1-R mediated and that the MC1-receptors were not completely saturated by the lower specific activity preparations of the radiolabeled peptides. There were no significant differences in radioactivity uptake in normal organ or tissue in mice receiving the radiolabeled peptide or radiolabeled peptide plus NDP, with the exception of lung uptake for 111In-CHX-A”-Re(Arg11)CCMSH. It is not clear why apparent MC1-R uptake was observed for 111In-CHX-A”-Re(Arg11)CCMSH in the lung. In the current study there were no statistical differences in blocked and non-blocked lung uptake with the 86Y and 68Ga labeled CHX-A”-Re(Arg11)CCMSH peptides, nor was there a difference in blocked and unblocked lung values for 111In-DOTA-Re(Arg11)CCMSH [24]. MC1-recptors are not known to be expressed in lung tissue [22], but have been reported to be upregulated on macrophages and involved in regulation of inflammatory response [37]. Gross examination of the lungs at the time of necropsy did not show signs of abnormalities, but we cannot rule out an inflammatory response without a histological examination.
The biodistribution studies with the 111In, 86Y and 68Ga labeled CHX-A”-Re(Arg11)CCMSH peptides showed a positive correlation between specific activity and tumor uptake. The lower the specific activity of the radionuclide the greater the mass of peptide injected. The 111In (10.62 GBq/μmol; 287 mCi/μmol) and 86Y (8.77 GBq/μmol; 237 mCi/μmol) labeled peptides had higher specific activities and showed approximately 2 times the tumor uptake %ID/g as the lower specific activity 68Ga (4.33 GBq/μmol; 117 mCi/μmol) labeled CHX-A”-Re(Arg11)CCMSH. These results highlight the importance of the use of high-specific activity radiolabeled peptides for imaging, especially when the numbers of target receptors is low. We have previously published that the MC1-R density on the surface of B16/F1 murine melanoma cells is ~7,000 receptors/cell, while our laboratory and others have published that human melanoma tumor cells have ~900-5000 receptors/cell [19-22]. Agonistic ligand binding to MC1-receptors results in the internalization of the receptor-ligand complex. We have previously demonstrated that upon binding to the MC1-R, radiolabeled Re(Arg11)CCMSH analogs were internalized [3,4,26,38]. Receptor-ligand internalization has the benefit of sequestering the peptide-targeted radionuclide in the tumor cell's cytoplasm, increasing its resonance time. However, once the MC1-R ligand complex is internalized, it takes nearly 96 hr in vitro [3] and up to 48 h in vivo [12] for the receptor population to return to its normal density. Therefore, it is important that the radiolabeled Re(Arg11)CCMSH peptide analogs be prepared at high specific activities, since there is essentially a one pass opportunity for MC1-R mediated binding and uptake.
The closely related DOTA-Re(Arg11)CCMSH peptide has been radiolabeled with 111In, 86Y and 68Ga. 111In and 86Y labeled DOTA-Re(Arg11)CCMSH peptides were HPLC purified after the labeling reaction to yield specific activities of 811 MBq/μg (21,892 μCi/μg) [25] and 111 MBq/μg (3000 μCi/μg) [7], respectively. The 68Ga-labeled peptide was not subjected to additional purification, so the specific activity of 68Ga-DOTA-Re(Arg11)CCMSH (1.74 MBq/μg; 47μCi/μg) was approximately the same as 68Ga-CHX-A”-Re(Arg11)CCMSH (1.85 MBq/μg; 50 μCi/μg). There was also a significant positive correlation between the specific activity of the radiolabeled peptide and tumor uptake for the radiolabeled DOTA-Re(Arg11)CCMSH peptides. For example, tumor uptake of 111In and 86Y labeled DOTA-Re(Arg11)CCMSH 2 h p.i was 17.29±2.49 % ID/g and 9.83±2.27 % ID/g compared to 111In and 86Y labeled CHX-A”-Re(Arg11)CCMSH that were 4.17±0.94 % ID/g and 4.68±1.02 % ID/g. As expected the tumor uptake value of 68Ga-DOTA-Re(Arg11)CCMSH was approximately the same as 68Ga-CHX-A”-Re(Arg11)CCMSH, since both compounds were not HPLC purified before the biodistribution studies.
Non-specific uptake of radioactivity by the kidneys appeared to be related to the radionuclide and to the specific activity of the radiolabeled peptide preparation. Even though retention of radioactivity in the kidney was not MC1-R mediated there appeared to be a peptide mass effect. The lower specific activity 68Ga labeled CHX-A”-Re(Arg11)CCMSH peptide had less kidney uptake (7.39±2.13 % ID/g) than the higher specific activity 111In labeled peptide (21.14±6.45 % ID/g) 2 h p.i. Radioactivity in the kidneys was not significantly different between the 68Ga and 86Y labeled CHX-A”-Re(Arg11)CCMSH peptides, although there was a large difference in kidney uptake between 86Y-CHX-A”-Re(Arg11)CCMSH (8.19±1.92 % ID/g, 3.7 MBq/μg; 100 μCi/μg) and 86Y-DOTA-Re(Arg11)CCMSH (18.36±3.62 % ID/g, 111 MBq/μg; 3000 μCi/μg) 2 h p.i. A positive correlation between specific activity and kidney uptake was also present amongst the 111In [24], 86Y [7], 68Ga [9] and 64Cu [7] labeled DOTA-Re(Arg11)CCMSH peptides, where the higher specific activity preparations exhibited the greatest kidney uptake values. At lower specific activities, the increased amount of peptide circulating appears to help block some non-specific retention of radioactivity in the kidney. Blocking studies performed with the 111In, 86Y, 68Ga and 64Cu labeled DOTA-Re(Arg11)CCMSH compounds showed no MC1-R selective uptake in the kidneys. The nonspecific uptake in kidney can often hinder the in vivo application of radiolabeled peptides. It has been reported repeatedly that the renal accumulation of peptides or proteins can be reduced by administration of certain amino acids such as lysine and arginine [4,9,38,39]. For example, the kidney uptake of 68Ga-DOTA-ReCCMSH(Arg11) was reduced by D-lysine administration where the kidney uptake at 2 h post-injection was reduced 53% (from 6.80 ± 1.72 to 3.17 ± 1.41 %ID/g, p < 0.01) when 15 mg D-lysine was pre-injected with the radiolabeled peptide, while radioactivity in tumor and other organs was not significantly affected [9].
Even with the efficient radiolabeling associated with the CHX-A” conjugated peptides, the specific activities of the radiolabeled products were not sufficient for optimal tumor uptake without separation of unlabeled peptide. Higher specific activity preparations of radiolabeled CHX-A”- Re(Arg11)CCMSH could be produced by HPLC purification, which should result in higher tumor uptake values and superior tumor images. Reverse phase HPLC separation of 111In- CHX-A”-Re(Arg11)CCMSH from free 111In was successful, however, separation of radiolabeled peptide from non labeled peptide was not complete. These results indicate that differences between the metal bound and metal free CHX-A”-Re(Arg11)CCMSH peptide are subtle and that additional separation techniques will be necessary to achieve optimal specific activity. While HPLC purification could be accommodated for the 111In and 86Y labeled CHX-A”- Re(Arg11)CCMSH conjugates, it may not be practical for 68Ga-CHX-A”-Re(Arg11)CCMSH. The short half-life of 68Ga necessitates rapid radiolabeling and purification for practical dose preparation. It is possible that HPLC purification of 68Ga-CHX-A”-Re(Arg11)CCMSH could be accommodated if microwave radiolabeling technology dramatically shortened the labeling time.
In this study the concept of high specific activity, as related to the radiolabeled peptide, was important due to the relatively low number of MC1-R receptors. Low numbers of target receptors/cell can easily be saturated even at low total injected mass of peptide ligand. Both the specific activity of the radionuclide (classical definition, Bq•kg-1) and the apparent specific activity (radioactivity • isolated mass-1) of the final product are relevant to radiolabeled CHX-A”-Re(Arg11)CCMSH, [40]. The importance of high specific activity radionuclide production is underscored by the fact that it sets the upper limit of the final product's apparent specific activity. Moreover, contaminating metals that reduce radionuclide specific activity can dramatically reduce radiolabeling efficiencies for CHX-A” and DOTA. For bioconjugate imaging agents, high apparent specific activity is necessary to insure that the administered mass does not alter the biochemistry of the system or saturate low density molecular targets such as G-protein linked receptors. HPLC purification of an imaging agent is often necessary for optimal tumor localization, if the apparent specific activity of the radiolabeled product and or the number of molecular targets is low.
5. Conclusion
The synthesis of CHX-A”-Re(Arg11)CCMSH and its radiolabeling with 111In, 86Y and 68Ga were straightforward and efficient. The 111In, 86Y and 68Ga labeled CHX-A”-Re(Arg11)CCMSH peptides were able to selectively target melanoma tumors with little or no purification. Melanoma tumors were clearly visualized by all 3 radiolabeled CHX-A”-Re(Arg11)CCMSH peptides underscoring their potential as a melanoma imaging agents.
Acknowledgements
The authors would like to acknowledge support from the National Cancer Institute P50-103130, R24 CA86307, the NIH Clinical Biodetective Graduate Training Grant R90DK071510 and the Harry S. Truman Veterans Administration Hospital Biomolecular Imaging Center, Columbia MO. Small animal PET imaging was supported by an NIH/NCI SAIRP grant (R24 CA86060) with additional support from the Small Animal Imaging Core of the Alvin J. Siteman Cancer Center at Washington University and Barnes-Jewish Hospital. The SAIC is supported by an NCI Cancer Center Support Grant P30 CA91842. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations
- α-MSH
alpha-melanocyte stimulating hormone
- CHX-A”
N-(2-aminoethyl)-trans-1,2-diaminocyclohexane-N,N',N”-pentaacetic acid
- Re(Arg11)CCMSH
[Re(Cys3,4,10), D-Phe7, Arg11]α-MSH3-13
- NDP
[Nle4, D-Phe7]α-MSH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Giblin MF, Wang N, Hoffman TJ, Jurisson SS, Quinn TP. Design and characterization of alpha-melanotropin peptide analogs cyclized through rhenium and technetium metal coordination. Proc of the Natl Acad Sci USA. 1998;95:12814–12818. doi: 10.1073/pnas.95.22.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vaidyanathan G, Zalutsky MR. Fluorine-18-labeled [Nle4,D-Phe7]-alpha-MSH, an alpha-melanocyte stimulating hormone analogue. Nucl Med Biol. 1997;24:171–8. doi: 10.1016/s0969-8051(96)00211-9. [DOI] [PubMed] [Google Scholar]
- [3].Chen JQ, Cheng Z, Hoffman TJ, Jurisson SS, Quinn TP. Melanoma-Targeting Properties of 99mTechnetium-Labeled Cyclic α-Melanocyte Stimulating Hormone Peptide Analogues. Cancer Res. 2000;60:5649–5658. [PubMed] [Google Scholar]
- [4].Chen J-Q, Cheng Z, Owen NK, Hoffman TJ, Miao Y, Jurisson SS, et al. Evaluation of an 111In-DOTA-Rhenium Cyclized α-MSH Analog: A Novel Cyclic-Peptide Analog with Improved Tumor Targeting Properties. J Nucl Med. 2001;42:1847–1855. [PubMed] [Google Scholar]
- [5].Froidevaux S, Calame-Christe M, Tanner H, Sumanovski L, Eberle AN. A novel DOTA-α-melanocyte-stimulating hormone analog for metastatic melanoma diagnosis. J Nucl Med. 2002;43:1699–1706. [PubMed] [Google Scholar]
- [6].Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, et al. A gallium-labeled DOTA-alpha-melanocyte-stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med. 2004;45:116–123. [PubMed] [Google Scholar]
- [7].McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, Lewis JS. Imaging of melanoma using 64Cu- and 86Y- DOA-ReCCMSH(Arg11), a cyclic peptide analogue of α-MSH. J Med Chem. 2005;48:2985–92. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]
- [8].Wei L, Butcher C, Miao Y, Gallazzi F, Quinn TP, Welch MJ, et al. Synthesis and biological evaluation of 64Cu-labled rhenium-cyclized α-MSH peptide analogs using a cross-bridged cyclam chelator. J Nucl Med. 2007;48:64–72. [PubMed] [Google Scholar]
- [9].Wei, Miao Y, Gallazzi F, Quinn TP, Welch MJ, Vavere AL, et al. Ga-68 labeled DOTA-rhenium cyclized α-MSH analog for imaging of malignant melanoma. Nucl Med Biol. 2007;34:945–953. doi: 10.1016/j.nucmedbio.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. 64Cu-labeled alpha-melanocyte-stimulating hormone analog for microPET imaging of melanocortin 1 receptor expression. Bioconjugate Chemistry. 2007;18:765–72. doi: 10.1021/bc060306g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cheng Z, Zhang L, Graves E, Xiong Z, Dandekar M, Chen X, et al. Small-animal PET of melanocortin-1 receptor expression using a 18F-labeled α-melanocyte stimulating hormone analog. J Nucl Med. 2007;48:987–94. doi: 10.2967/jnumed.107.039602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miao Y, Owen NK, Darrell R, Fisher DR, Hoffman TJ, Quinn TP. Therapeutic Efficacy of a 188Re Labeled α-Melanocyte Stimulating Hormone Peptide Analogue in Murine and Human Melanoma-bearing Mouse Models. J Nucl Med. 2005;46:121–9. [PubMed] [Google Scholar]
- [13].Miao Y, Hylarides M, Fisher DR, Shelton T, Moore H, et al. Melanoma Therapy via Peptide-Targeted Alpha-Radiation. Clin Cancer Res. 2005;11:5616–5621. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- [14].Miao Y, Shelton T, Quinn TP. Therapeutic Efficacy of a 177Lu Labeled DOTA Conjugated α-Melanocyte Stimulating Hormone Peptide in a Murine Melanoma-bearing Mouse Model. Cancer Biotherapy and Radiopharm. 2007;22:333–341. doi: 10.1089/cbr.2007.376.A. [DOI] [PubMed] [Google Scholar]
- [15].Bard DR, Knight CG, Page-Thomas DP. A chelating derivative of α-melanocyte stimulating hormone as a potential imaging agent for malignant melanoma. Br J Cancer. 1990;62:919–922. doi: 10.1038/bjc.1990.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wraight EP, Bard DR, Maughan TS, Knight CG, Page-Thomas DP. The use of a chelating derivative of alpha melanocytes stimulating hormone for the clinical diagnosis of malignant melanoma. British J Radiol. 1992;65:112–8. doi: 10.1259/0007-1285-65-770-112. [DOI] [PubMed] [Google Scholar]
- [17].Hruby VJ, Cai M, Grieco P, Han G, Kavarana M, Trivedi D. Exploring the stereostructural requirements of peptide ligands for the melanocortin receptors. Ann NY Acad Sci. 2003;994:12–20. doi: 10.1111/j.1749-6632.2003.tb03157.x. [DOI] [PubMed] [Google Scholar]
- [18].Schioth HB, Haitina T, Ling MK, Ringholm A, Fredriksson R, Cerda-Reverter JM, et al. Evolutionary conservation of the structural, Pharmacological and genomic characteristics of melanocortin receptor subtypes. Peptides. 2005;26:1886–1900. doi: 10.1016/j.peptides.2004.11.034. [DOI] [PubMed] [Google Scholar]
- [19].Roberts DW, Newton RA, Beaumont KA, Leonard JH, Strum RA. Quantitative analysis of MC1R gene expression in human skin cell cultures. Pigment Cell Res. 2005;19:76–89. doi: 10.1111/j.1600-0749.2005.00286.x. [DOI] [PubMed] [Google Scholar]
- [20].Siegrist W, Solca F, Stutz S, Giuffre L, Carrel S, Girard J, et al. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989;49:6352–8. [PubMed] [Google Scholar]
- [21].Tatro JB, Atkins M, Mier JW, Hardarson S, Wolfe H, Smith T, et al. Melanotropin receptors demonstrated in situ in human melanoma. Journal of Clinical Investigation. 1990;85:1825–32. doi: 10.1172/JCI114642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Miao Y, Whitener D, Feng W, Owen NK, Chen J-Q, Quinn TP. Evaluation of the human melanoma targeting properties of radiolabeled α-melanocyte stimulating hormone peptide analogues. Bioconjugate Chem. 2003;14:1177–1184. doi: 10.1021/bc034069i. [DOI] [PubMed] [Google Scholar]
- [23].Schubiger PA, Alberto R, Smith A. Vehicles, chelators and radionuclides: Choosing the “building blocks” of an effective therapeutic radioimmunoconjugate. Bioconjugate Chem. 1996;7:165–179. doi: 10.1021/bc950097s. [DOI] [PubMed] [Google Scholar]
- [24].Cheng Z, Chen J, Owen N, Miao Y, Quinn TP, Jurisson SS. Modification of the structure of a metallopeptide: Synthesis and biological evaluation of 111In labeled DOTA conjugated rhenium cyclized α-MSH analogs. J Med Chem. 2002;45:3048–3056. doi: 10.1021/jm010408m. [DOI] [PubMed] [Google Scholar]
- [25].Miao Y, Benwell K, Quinn TP. 99mTc and 111In-labeled α-melanocyte stimulation hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J Nucl Med. 2007;48:73–80. [PubMed] [Google Scholar]
- [26].Miao Y, Hoffman TJ, Quinn TP. Tumor targeting properties of 90Y and 177Lu labeled alpha-melanocyte stimulating hormone peptide analogues in a murine melanoma model. Nuc Med Biol. 2005;32:485–493. doi: 10.1016/j.nucmedbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- [27].Brechbiel MW, Gansow OA, Pippen CG, Rogers RD, Planalp RP. Preparation of the novel chelating agent N-(2-aminoethyl)-trans-1,2-diaminocyclohexane N,N',N”-pentaacetic acid (H5CyDTPA), a preorganized analogue of diethylenetriaminepentaacetic acid (H5DTPA), and the structures of BiIII(CyDTPA)2- and BiIII(H2DTPA) complexes. Inorg Chem. 1996;35:6343–6348. [Google Scholar]
- [28].Milenic DE, Garmestani K, Brady ED, Albert PS, Ma DS, Abdulla A, et al. Targeting of HER2 antigen for the treatment of disseminated peritoneal disease. Clin Cancer Res. 2004;10:7834–7841. doi: 10.1158/1078-0432.CCR-04-1226. [DOI] [PubMed] [Google Scholar]
- [29].Huneke RB, Pippin CG, Squire RA, Brechbiel MW, Gansow OA, Strand M. Effective α-particle-mediated radioimmunotherapy of murine leukemia. Cancer Res. 1992;52:5818–20. [PubMed] [Google Scholar]
- [30].Lee FT, Mountain AJ, Kelly MP, Hall C, Rigopoulos A, Johns TG, et al. Enhanced efficacy of radioimmunotherapy with 90Y-CHX-A”-DTPA-hu3S193 by inhibition of epidermal growth factor receptor (EGFR) signaling with EGFR tyrosine kinase inhibitor AG1478. Clin Cancer Res. 2005;11:7080–7086. doi: 10.1158/1078-0432.CCR-1004-0019. [DOI] [PubMed] [Google Scholar]
- [31].Blend MJ, Stastny JJ, Swanson SM, Brechbiel MW. Labeling anti-HER2/neu monoclonal antibodies with 111In and 90Y using a bifunctional DTPA chelating agent. Cancer Biother Radiopharm. 2003;18:355–363. doi: 10.1089/108497803322285107. [DOI] [PubMed] [Google Scholar]
- [32].Milenic DE, Garmestani K, Chappell LL, Dadachova E, Yordanov A, Ma D, et al. In Vivo Comparison of Macrocyclic and Acyclic Ligands for Radiolabeling of Monoclonal Antibodies with 177Lu for Radioimmunotherapeutic Applications. Nucl Med Biol. 2002;29:431–442. doi: 10.1016/s0969-8051(02)00294-9. [DOI] [PubMed] [Google Scholar]
- [33].Clifford T, Boswell CA, Biddlecombe GB, Lewis JS, Brechbiel MW. Validation of a novel CHX-A” derivative suitable for peptide conjugation: small animal PET/CT imaging using yttrium-86-CHX-A”-Octreotide. J Med Chem. 2007;49:4297–4304. doi: 10.1021/jm060317v. [DOI] [PubMed] [Google Scholar]
- [34].Yoo J, Tang L, Perkins TA, Rowland DJ, Laforest R, Lewis JS, et al. Preparation of High Specific Activity 86Y Using a Small Biomedical Cyclotron. Nuc Med Biol. 2005;32:891–897. doi: 10.1016/j.nucmedbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- [35].Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MN, Heward CE, Burnett JB, et al. [Nle4,D-Phe7] α-melanocyte stimulating hormone: A highly potent α-melanotropin with ultralong biological activity. Proc Natl Acad Sci USA. 1980;77:5754–5758. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stimmel JB, Stockstill ME, Kull FC. Yttrium-90 chelation properties of tetraazatetraacetic acid macrocycles, diethylenetriaminepenataacetic acid analogues, and a novle terpyridine acyclic chelator. Bioconjug Chem. 1995:219–225. doi: 10.1021/bc00032a010. [DOI] [PubMed] [Google Scholar]
- [37].Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- [38].Miao Y, Figueroa SD, Fisher DR, Moore HA, Testa RF, Hoffman TJ, et al. 203Pb-labeled α-melanocyte stimulating hormone peptide as an imaging probe for melanoma detection. J Nucl Med. 2008;49:823–9. doi: 10.2967/jnumed.107.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bernard BF, Krenning EP, Breeman WAP, Rolleman EJ, Bakker WH, Visser TJ, et al. D-lysine reduction of indium-111 octreotide and yttrium-90 octreotide renal uptake. J Nucl Med. 1997;38:1929–33. [PubMed] [Google Scholar]
- [40].Eckelman WC, Volkert WA, Bonardi M. True radiotracers: are we approaching the theoretical specific activity with Tc-99m and I-123? Nucl Med Biol. 2008;35:523–27. doi: 10.1016/j.nucmedbio.2008.03.005. [DOI] [PubMed] [Google Scholar]