Abstract
Ischemic heart disease, which remains the leading cause of morbidity and mortality in the Western world, is invariably characterized by impaired cardiac function and disturbed Ca2+ homeostasis. Because enhanced inhibitor-1 (I-1) activity has been suggested to preserve Ca2+ cycling, we sought to define whether increases in I-1 activity in the adult heart may ameliorate contractile dysfunction and cellular injury in the face of an ischemic insult. To this end, we generated an inducible transgenic mouse model that enabled temporally controlled expression of active I-1 (T35D). Active I-1 expression in the adult heart elicited significant enhancement of contractile function, associated with preferential phospholamban phosphorylation and enhanced sarcoplasmic reticulum Ca2+ -transport. Further phosphoproteomic analysis revealed alterations in proteins associated with energy production and protein synthesis, possibly to support the increased metabolic demands of the hyperdynamic hearts. Importantly, on ischemia/reperfusion-induced injury, active I-1 expression augmented contractile function and recovery. Further examination revealed that the infarct region and apoptotic as well as necrotic injuries were significantly attenuated by enhanced I-1 activity. These cardioprotective effects were associated with suppression of the endoplasmic reticulum stress response. The present findings indicate that increased I-1 activity in the adult heart enhances Ca2+ cycling and improves mechanical recovery, as well as cell survival after an ischemic insult, suggesting that active I-1 may represent a potential therapeutic strategy in myocardial infarction.
Keywords: ischemia, reperfusion, protein phosphatase-1 inhibitor-1, phospholamban, ER stress
Ischemic heart disease remains the leading cause of cardiovascular disease and mortality in the Western world. In the United States alone, the incidence of myocardial infarction has reached an alarming 8.1 million.1 Even though restoration of coronary flow alleviates the detriment of the ischemic insult, it is invariably accompanied by reperfusion-induced contractile dysfunction and cellular damage. The causes for these deleterious effects are multifactorial, but disturbed Ca2+ homeostasis has been proposed as a central contributor to postischemic injury.2,3
The sarcoplasmic reticulum (SR) is a crucial regulator of Ca2+ handling in the cardiomyocyte, and elucidation of its role in ischemia/reperfusion (I/R)-induced injury has been the focus of several investigations. Experimental evidence indicates that SR Ca2+ cycling is depressed in the postischemic myocardium4,5; yet the functional manifestations of this alteration remain controversial. On the one hand, it has been reported that intracellular Ca2+ overload, during reperfusion, and the subsequent futile cycles of Ca2+ release and reuptake by the SR are critical events that lead to contractile dysfunction, necrosis, and mitochondrial-dependent apoptosis.6–8 As such, depressed SR function may be an adaptive mechanism aimed at alleviating these adverse effects. Conversely, the SR may act as an intracellular sink for excess cytosolic Ca2+, and its compromised function may reduce the capacity of the cardiomyocyte to cope with intracellular Ca2+ overload.3,9 Further efforts put forth to unravel the role of SR Ca2+ -handling proteins in I/R become very important not only to advance our fundamental understanding of SR function in the postischemic heart but also to identify novel targets, with potential clinical application.
An attractive protein in this respect is protein phosphatase (PP)1 inhibitor-1, which was the first recognized endogenous regulator of this phosphatase. Early studies revealed that, similarly to other PP1-interacting proteins, inhibitor-1 contains an RVXF motif sequence, which facilitates its interaction with PP1. Further studies indicated that inhibitor-1 is widely expressed in mammalian tissues. In fact, its significance has been described extensively in the brain and skeletal muscle, where it has been implicated in synaptic plasticity and hormonal regulation of glycogen metabolism, respectively.10 In the heart, inhibitor-1 has been postulated as an integrator of multiple neurohormonal pathways associated with Ca2+ homeostasis and proper contractile function. In particular, on stimulation of the β-adrenergic axis, protein kinase (PK)A phosphorylates Thr35 in inhibitor-1, resulting in PP1 inhibition and amplification of the contractile response.11,12 Inactivation of inhibitor-1 occurs by dephosphorylation of Thr35 by PP2A and PP2B, leading to relief of PP1 inhibition and restoration of basal function.13 Importantly, inhibitor-1 can also be phosphorylated at Ser67 and Thr75 by PKC, but these phosphorylations enhance PP1 activity and diminish contractility.14,15 Altogether, these data emphasize a key regulatory role for inhibitor-1 in cardiac physiology.
Notably, previous studies have shown that chronic increases in an active (T35D) and truncated (amino acids 1 to 65) form of inhibitor-1 that lacks the PKC sites, which mitigate the beneficial effects of inhibitor-1,14,15 enhance Ca2+cycling, and preserve cardiac performance in the failing heart.12 In light of the contentious role of SR function in postischemic injury, we sought to address the effects of enhanced Ca2+ cycling, mediated by increased inhibitor-1 activity in the adult heart, on an ischemic insult. Our findings herein further support a beneficial role for enhanced Ca2+ cycling in I/R and implicate active inhibitor-1 as a potential therapeutic strategy for ischemic heart disease.
Materials and Methods
Generation of an Inducible Mouse Model With Expression of Active Inhibitor-1 (I-1c)
I-1c (amino acids 1 to 65; T35D) single transgenic mice (TG2) were crossed with mice carrying the tetracycline-controlled transactivator (tTA) gene (TG1) to generate double transgenic (DTG) mice, with inducible I-1c expression. Animals were handled as approved by the Institutional Animal Care and Use Committee at the University of Cincinnati.
An expanded Materials and Methods section can be found in the online data supplement at http://circres.ahajournals.org.
Results
Generation of an Inducible Mouse Model With Expression of Active Inhibitor-1
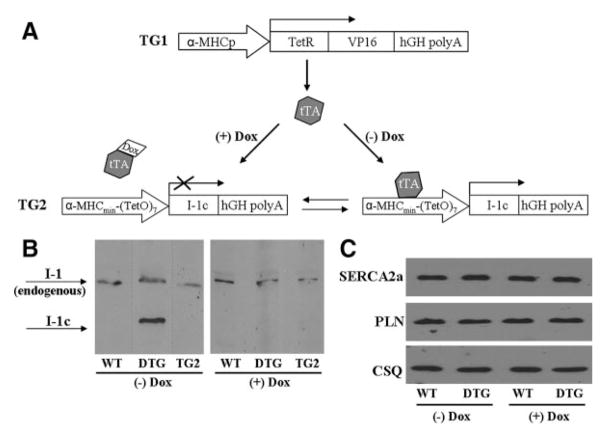
To elucidate the effects of enhanced I-1 activity in the adult heart, we generated a mouse model with temporally regulated and cardiac-specific expression of a constitutively active and truncated (T35D; amino acids 1 to 65) form of I-1 (I-1c), using the Tet-off system. Double transgenic (DTG) mice are expected to express I-1c only on withdrawal of doxycycline (Dox) (Figure 1A). Control mice were also kept on the same diet regimen to eliminate any potential differential effects resulting from Dox administration. Immunoblotting confirmed the fidelity of this system. Specifically, in the absence of Dox, I-1c expression was effectively induced, at 1.65-fold of endogenous I-1 (Figure 1B, left blot), whereas in the presence of Dox, expression was successfully suppressed, in the DTG mice (Figure 1B, right blot). Importantly, the single transgenic I-1c mice (TG2) did not express I-1c either in the presence or absence of Dox (Figure 1B), indicating that the I-1c attenuated promoter did not exhibit leakage neither in the DTG mice, when the transactivator was sequestered by Dox, nor in the single I-1c transgenic mice, where the transactivator was absent. In addition, another line, line 2, was generated with similar (1.5-fold) increases in I-1c, compared to endogenous I-1 (data not shown). Importantly, Dox administration was not associated with any alterations in the levels of the Ca2+-ATPase pump SERCA2a and phospholamban (PLN) (Figure 1C), further supporting the fidelity of the Tet-off system.
Figure 1.
Generation of a mouse model with inducible expression of I-1c. A, DTG mice were generated using the Tet-off system. TG1 drives expression of the tetracycline-regulated transactivator (tTA) by using the traditional α-myosin heavy chain promoter (α-MHCp). TG2 drives I-1c expression, using the attenuated α-MHCp, which is only active in the absence of Dox. B, Immunoblot analysis, after trichloroacetic acid precipitation of ≈400 mg of tissue, confirmed the validity of the system. DTG mice expressed I-1c at 1.65-fold of endogenous I-1 (WT, n=3; DTG, n=3) on Dox withdrawal for 8 weeks (left blot), whereas I-1c expression was suppressed in the presence of Dox (right blot). Single transgenic I-1c (TG2) mice did not express I-1c either in the presence (right blot) or absence of Dox (left blot). Dotted lines represent different blots. C, Immunoblot analysis for SERCA2a and PLN revealed that Dox administration did not have any off-target effects. Calsequestrin (CSQ) was used as a loading control (WT, n=4; DTG, n=4).
Active I-1 Expression in the Adult Heart Enhances Basal Contractility
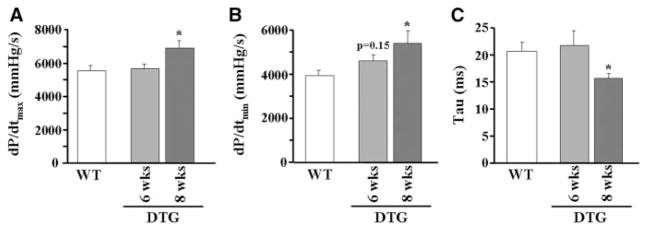
Because I-1 has been proposed as a modulator of cardiac contractility, left ventricular contractile indices were assessed by use of invasive catheterization in vivo, to delineate any functional effects of I-1c expression in the adult heart. The results indicated that contractility was enhanced in a time- dependent manner, on Dox withdrawal. Specifically, a trend for enhanced relaxation was observed at 6 weeks (dP/dtmin was 3927±256 and 4583±291 mm Hg/sec, for wild-type [WT] and DTG respectively) and significant increases in basal contraction (25% increase in dP/dtmax; Figure 2A) and relaxation (37% increase in dP/dtmin [Figure 2B]; 24% decrease in Tau [Figure 2C]) parameters were obtained at 8 weeks in DTGs, as compared to WTs. This timeline of expression is similar to a previous report, using the Tet-off system.16 Based on these results, subsequent experiments were conducted after 8 weeks of Dox withdrawal. In addition, contractility was enhanced to a similar extent in line 2 (Figure I in the online data supplement). Overall, these findings indicate that I-1c expression in the adult heart enhances cardiac performance.
Figure 2.
I-1c expression in the adult heart enhances basal contractility. Dox was withdrawn from the diet of the mice for either 6 or 8 weeks, and cardiac function was examined using in vivo catheterization. Hearts expressing I-1c for 8 weeks exhibited enhanced rates of contraction (A) and relaxation (B), as well as a decreased time constant of relaxation τ (C) (WT, n=11; DTG [6 weeks], n=4; DTG [8 weeks], n=8). *P<0.05 vs WT.
Effects of Active I-1 Expression on Key Regulatory Phosphoproteins
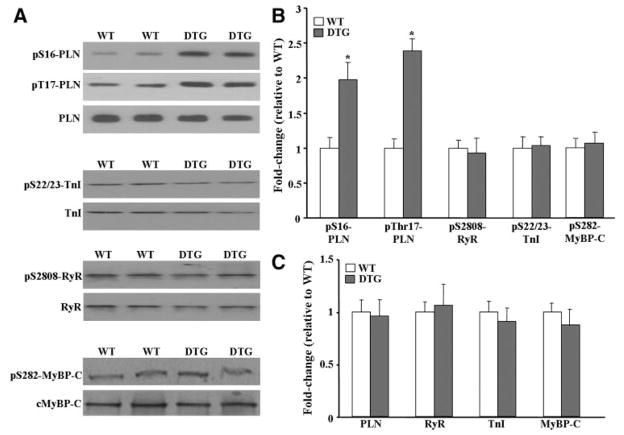
Because phosphorylation of PLN, the ryanodine receptor, troponin I, and myosin-binding protein-C constitutes an important regulatory mechanism that governs cardiac contractility, we investigated these phosphoproteins as potential I-1c substrates. Quantitative immunoblotting indicated that phosphorylation of PLN at Ser16 and Thr17 was increased by 2-fold and 2.3-fold, respectively, in DTG, as compared to WT hearts. Interestingly, no changes were observed in the phosphorylation status of the other phosphoproteins examined (Figure 3A and 3B). Furthermore, no compensation was observed at the total protein level of PLN, the ryanodine receptor, troponin I, or myosin-binding protein-C (Figure 3A and 3C). These results were confirmed independently in line 2 (data not shown), suggesting that I-1c shows remarkable specificity for PLN in vivo.
Figure 3.
Ca2+ regulatory phosphoproteins in DTG hearts. A, Immunoblot analysis revealed that phosphorylation of PLN was increased in DTG hearts. No changes were observed in the levels of PLN, ryanodine receptor (RyR), troponin I (TnI), and myosin-binding protein-C (MyBP-C). B, Quantitative analysis of the phosphorylation levels of proteins shown in A, normalized to their respective total protein levels. C, Quantitative analysis of the total protein levels shown in A, normalized to calsequestrin (CSQ). Bars represent means±SEM. *P<0.05 vs WT.
Active I-1 Enhances Ca2+ Uptake Into the Sarcoplasmic Reticulum
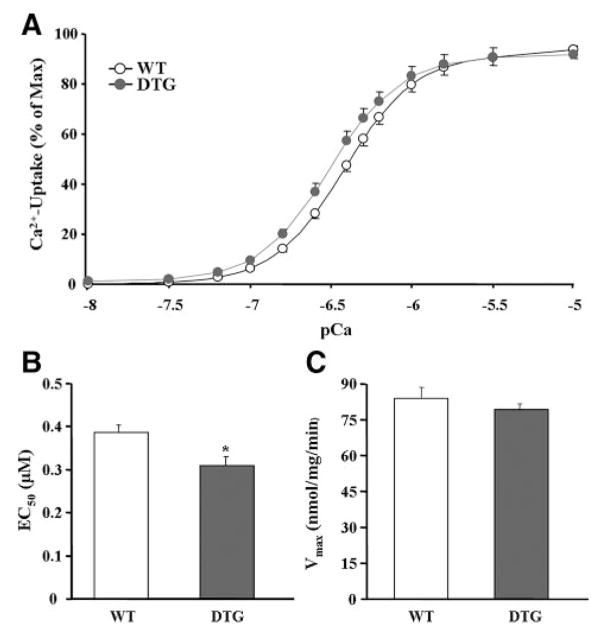
Because the degree of PLN phosphorylation profoundly affects the activation state of SERCA2a and contractility, oxalate-supported SR Ca2+-transport was assessed in cardiac homogenates over a wide range of Ca2+ concentrations, similar to those present in vivo during relaxation and contraction. The results showed that I-1c expression significantly enhanced the affinity of SERCA2a for Ca2+ (the EC50 value decreased by 20.5%; Figure 4A and 4B). This alteration was similar to that obtained in PLN-heterozygous hearts but much smaller than the shift in the EC50 value in PLN-deficient hearts (58% decrease, compared to WT).17 Furthermore, no differences in the maximal velocity of Ca2+ uptake were noted between the 2 groups (Figure 4A and 4C). These findings indicate that I-1c expression mediates disinhibition of SERCA2a by enhancing phosphorylation of PLN.
Figure 4.
I-1c expression enhances SERCA2a activity. A, The initial SERCA2a Ca2+ uptake rates were assessed in cardiac homogenates over a wide range of Ca2+ concentrations. The data were analyzed with Origin software, and the affinity of SERCA for Ca2+ (B) and the maximal velocity (C) were calculated.
Phosphoproteomic Analysis of I-1c–Expressing Hearts
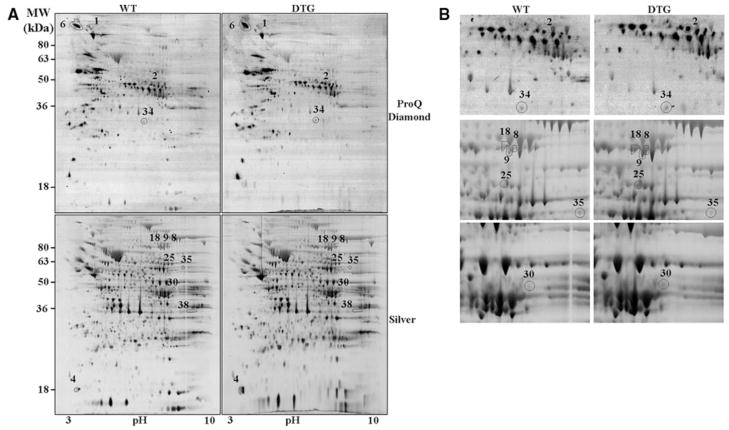
To gain further insights into the apparent specificity of I-1c for PLN, a phosphoproteomic approach was used to identify other potential I-1c–regulated targets. In particular, 2D gels were stained with a phosphospecific dye, namely ProQ Diamond, for detection of the phosphoproteome profile. Subsequently, the same gels were silver-stained to visualize any alterations at the whole proteome level. Analysis of 512 phosphoprotein and 1423 protein spots revealed that only 4 and 8 were altered in I-1c hearts, respectively (Figure 5A and 5B). Interestingly, none of the 4 phosphoproteins appeared to correspond to any of the altered proteins. Mass spectroscopic analysis positively identified 10 of these 12 spots. The altered proteins were grouped into 2 major categories: energy production and protein synthesis. In the first category, the phosphorylation levels of enoyl coenzyme A hydratase 1 (spot 34) and the protein levels of electron-transferring flavoprotein (spot 25), both of which are involved in the high-yield metabolic pathway of β-oxidation of fatty acids, were increased. Furthermore, creatine kinase (spot 30), which catalyzes the conversion of the high-energy compound phosphocreatine, was also increased. Interestingly, the level of glucose phosphate isomerase (spot 35), an important enzyme in glycolysis, the less efficient metabolic pathway, was downregulated. In the second category, the phosphorylation of the Tu translation elongation factor (spot 2), which is involved in protein translation, and the levels of inner mitochondrial membrane protein, which is involved in protein import, were increased. Notably, 3 different spots (spots 8, 9, and 18) were identified as inner mitochondrial membrane protein, which may represent previously reported spliced variants or posttranslational modifications.18 The protein synthesis machinery was possibly increased to support assembly of the aforementioned energy production proteins. Finally, the phosphorylation of contrapsin (spot 6) was increased in I-1c hearts. Contrapsin is a serine-proteinase inhibitor, whose function is not well understood.19 Although the observed phosphoprotein changes may be attributable to a direct action of I-1, this seems unlikely. Changes in these pathways were also observed in hyperdynamic PLN-deficient hearts,20 and they may represent compensatory alterations to accommodate the increased energetic demands of these hearts.
Figure 5.
Phosphoproteomic analysis of I-1c hearts. A, Representative images of ProQ-Diamond- and silver-stained 2D gels. B, Enlargement of some of the spots shown in A. The spot number, identity and fold change are indicated in Online Table I. WT, n=3; DTG, n=3.
Active I-1 Attenuates Contractile Dysfunction and Myocardial Infarction After I/R In Vivo
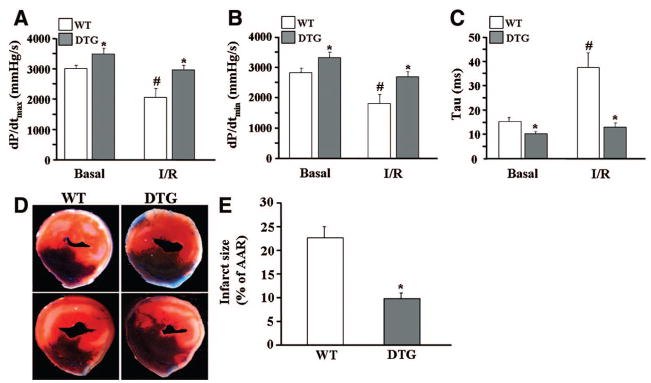
To examine the hypothesis that I-1c may be protective against postischemic injury, WT and DTG mice were subjected to I/R in vivo. WT hearts presented with contractile dysfunction post-I/R, as evidenced by depressed contraction (31% decrease in dP/dtmax; Figure 6A) and relaxation (37% decrease in dP/dtmin [Figure 6B]; 244% increase in Tau [Figure 6C]) parameters. However, DTG hearts exhibited enhanced cardiac function post-I/R and their contractile parameters were similar to those in WT hearts at preischemic conditions.
Figure 6.
I-1c attenuates contractile dysfunction and infarct size after I/R in vivo. Cardiac function was assessed after 30 minutes of ischemia, followed by 60 minutes of reperfusion, using in vivo catheterization. I-1c expression enhanced the rates of contraction (A) and relaxation (B) and decreased τ (C). *P<0.05 vs WT under similar conditions, #P<0.05 vs WT basal (WT, n=4; DTG, n=4). D, Myocardial infarct size was assessed after 30 minutes of ischemia, followed by 24 hours of reperfusion. Hearts were infused with 2,3,5-triphenyltetrazolium chloride, followed by phthalo blue, and cut transversely into 2- to 3-mm-thick slices. Two representative myocardial sections from 1 heart in each group are shown. E, Quantification of infarct size. Bars represent means±SEM (WT, n=8; DTG, n=5). *P<0.05 vs WT.
In addition to contractile dysfunction, the postischemic heart presents with irreversible cellular injury. To delineate any effects by I-1c in postischemic damage, I/R-induced myocardial infarction was assessed. The infarct-to-risk region ratio was 22.7±2.3 in WT hearts post-I/R, similar to previous reports,21,22 whereas it was significantly attenuated (9.8±1.5, P<0.005) in DTG hearts (Figure 6D and 6E). These results indicate that enhanced I-1 activity attenuates postischemic contractile dysfunction and myocardial infarct size in vivo.
Active I-1 Improves Functional Recovery After I/R Ex Vivo
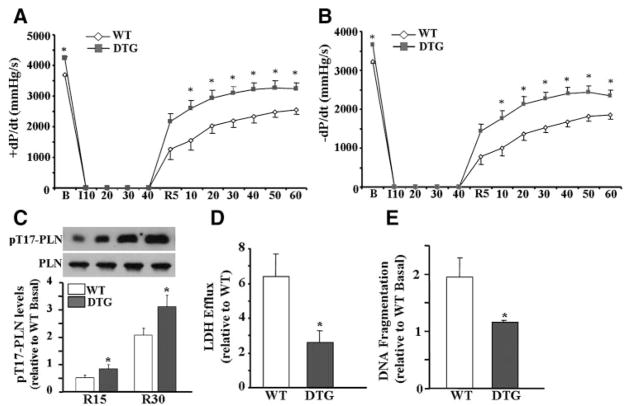
To further examine the potential benefits of I-1c, in the absence of neurohormonal influences, hearts were Langendorff-perfused and subjected to 40 minutes of global ischemia followed by 60 minutes of reperfusion. During the stabilization period, DTG hearts exhibited higher contractile parameters (Figure 7A and 7B), consistent with our results from in vivo recordings (Figure 2). On coronary flow interruption, contractile parameters dropped essentially to 0 in both groups. However, on reperfusion, DTG hearts exhibited better functional recovery, as compared to WT. Remarkably, after the first 5 minutes of reperfusion, contractile and relaxation parameters were increased by 1.7-fold and 1.8-fold, respectively, as compared to WT hearts, and function remained elevated over the 60 minutes of the reperfusion period (Figure 7A and 7B). Notably, the rates of contraction and relaxation, normalized to their preischemic values, were 51±0.07% and 39±0.05% in DTG hearts and only 34±0.09% and 24±0.06% in WT hearts, after 5 minutes of reperfusion, whereas no differences were noted after 60 minutes of reperfusion (Online Figure II, A and B). To elucidate the mechanisms, which may contribute to improved functional recovery, the phosphorylation levels of PLN, the target substrate of I-1c (Figure 3), were assessed. Phosphorylation at Ser16-PLN was not detectable post-I/R in either group, in agreement with the lack of β-adrenergic stimulation in ex vivo perfused hearts. However, phosphorylation at Thr17-PLN was significantly elevated in DTG hearts at 15 and 30 minutes, compared to WTs (Figure 7C), whereas there were no differences at 60 minutes postreperfusion. Collectively, these results show that I-1c ameliorates I/R-induced contractile dysfunction, at least partly, by increased PLN phosphorylation at its Thr17 site during early reperfusion.
Figure 7.
I-1c improves postis-chemic cardiac performance in isolated perfused hearts. DTG hearts displayed enhanced maximal rates of contraction (A) and relaxation (B) post-I/R. Values represent means±SEM (WT, n=9; DTG, n=10). *P<0.05 vs WT. C, Immunoblot analysis of pThr17-PLN on 15 and 30 minutes of reperfusion. Bars represent means±SEM (WT, n=3; DTG, n=3). *P<0.05 vs WT. D, Lactate dehydrogenase (LDH) release, determined from the perfusate of hearts after 15 minutes of reperfusion. Bars represent means±SEM (WT, n=8; DTG, n=6). *P<0.05 vs WT. E, DNA fragmentation, assessed at 120 minutes post-reperfusion. Bars represent means±SEM (WT, n=4; DTG, n=4). *P<0.05 vs WT.
Active I-1 Alleviates Cell Death After I/R by Attenuating the Endoplasmic Reticulum Stress Response
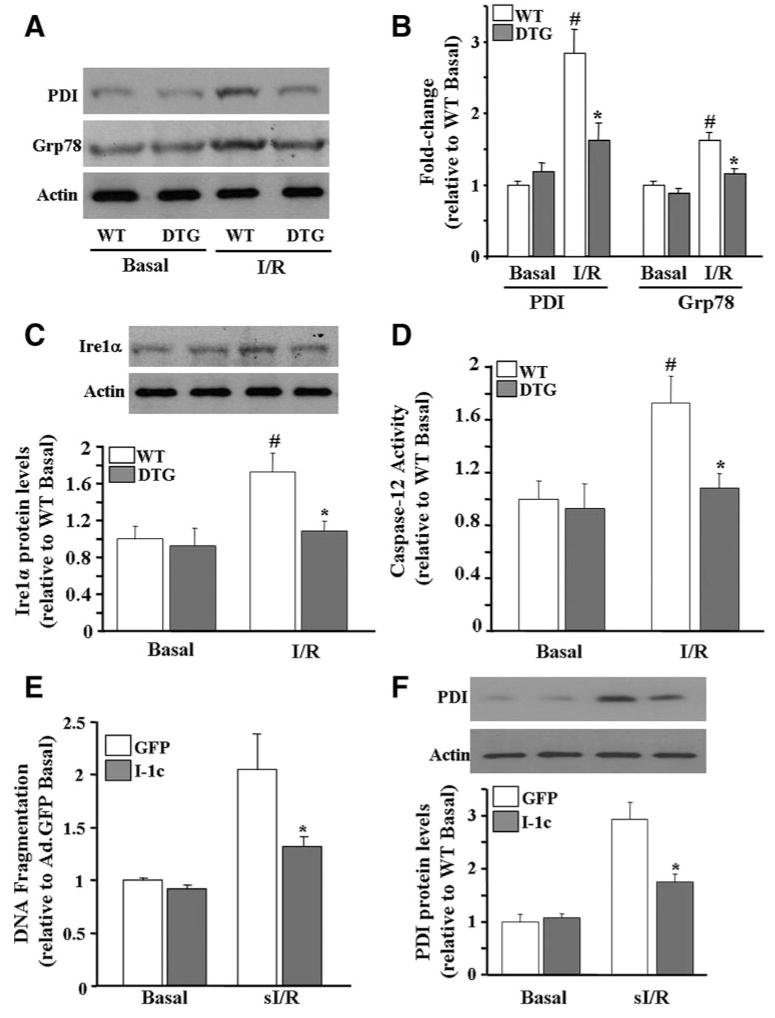
To delineate any cardioprotective effects conferred by I-1c in postischemic cellular damage, which may also contribute to the improved functional recovery, the extent of necrotic and apoptotic cell death was examined after I/R ex vivo. Lactate dehydrogenase efflux, an index of necrotic injury, was decreased by 60% (Figure 7D), and the extent of apoptosis, as assessed using an ELISA-based DNA fragmentation assay, was reduced to preischemic levels in DTG hearts post-I/R (Figure 7E). Because it has been previously reported that the endoplasmic reticulum (ER) stress response, is induced in the ischemic heart, at least partly by altered Ca2+ homeostasis, and contributes to cardiomyocyte apoptosis,23 we hypothesized that improved Ca2+ cycling, mediated by I-1c expression, may attenuate the ER stress response. Thus, the ER stress response was evaluated at 15, 30, and 60 minutes postreperfusion. Although there was no induction observed at 15 and 30 minutes, there were significant increases in the levels of protein disulphide isomerase and Grp78 (glucose-regulated protein 78) by 2.8-fold and 1.6-fold, respectively, after 60 minutes (Figure 8A and 8B). Importantly, expression of both proteins was reduced to preischemic values in DTG hearts after 60 minutes of reperfusion, suggesting that induction of the ER stress response was prevented in DTG hearts. Consistent with activation of the ER stress response, expression of a downstream target, inositol-requiring enzyme 1α, was increased by 1.7-fold in WT hearts at this time point, whereas it was unaltered in DTG hearts (Figure 8C). Inositol-requiring enzyme 1α has been shown to be involved in the proteolytic processing of pro–caspase 12 into its active form, caspase-12. Consistently, caspase-12 activity was increased by 1.7-fold in WT hearts at 60 minutes postreperfusion, whereas it was attenuated to preischemic values in DTG hearts (Figure 8D).
Figure 8.
I-1c attenuates the I/R-induced ER stress response. A, Immunoblot analysis of protein disulphide isomerase (PDI) and Grp78 revealed that the ER stress response was induced in WT hearts, whereas it was unaltered in DTG hearts on I/R ex vivo. B, Quantitative analysis of the blots in A, normalized to actin. C, Inositol-requiring enzyme 1α (Ire1α) increased in WT hearts post-I/R but remained unaltered in DTG hearts. D, Caspase-12 activity was increased post-I/R in WT hearts, but it was reduced to preischemic values in DTG hearts. Bars represent means±SEM (WT, n=4; DTG, n=4). *P<0.05 vs WT I/R, #P<0.05 vs preischemic values in each group. E, DNA fragmentation was attenuated in Ad.I-1c–infected myocytes after sI/R (1 hour of ischemia/3 hours of reperfusion). F, ER stress induction was attenuated in Ad.I-1c–infected myocytes. Bars represent means±SEM (WT, n=4; DTG, n=4). *P<0.05 vs Ad.GFP basal.
Furthermore, because mitochondrial-mediated apoptosis is a major determinant of cell injury in I/R, the potential effects of enhanced I-1 activity on this pathway were examined. Because it has been previously reported that Bad, an important regulator of mitochondrial-mediated cell death, is a PP1 substrate,24 this protein was investigated as a potential I-1c–regulated phosphoprotein. The phosphorylation levels of Bad at Ser116 and Ser136 were similar between I-1c and WT hearts basally and post-I/R (Online Figure III, A and B). To further address a possible effect of I-1c on mitochondrial-mediated apoptosis, the activity of caspase-9, the downstream target of this pathway, was assessed. No differences were observed between the 2 groups at basal levels and after I/R (Online Figure III, C), suggesting that the antiapoptotic effects of I-1c may be primarily mediated through prevention of ER stress- and not mitochondrial-mediated cell death.
To further delineate the effects of I-1c on ER stress at the cellular level, adult rat cardiomyocytes were infected with Ad.I-1c and Ad.GFP (green fluorescent protein), as a control, and subjected to simulated I/R (sI/R). The extent of apoptosis, as assessed by DNA fragmentation, was increased by 2-fold in Ad.GFP-infected cells after sI/R, whereas it was reduced by 64.4% in I-1c–expressing myocytes (Figure 8E). Importantly, these effects were associated with an attenuated ER stress response, as evidenced by decreased (59.8%) levels of protein disulphide isomerase (Figure 8F). Overall, these results suggest that increased I-1 activity effectively attenuates induction of the ER stress response, which may mediate the cardioprotective effects of I-1c.
Discussion
Ischemic injury inevitably manifests in contractile dysfunction, impaired Ca2+ homeostasis, and cell death, on restoration of coronary flow. We provide evidence herein to show, for the first time, that augmenting Ca2+ cycling in the SR/ER, by enhancing inhibitor-1 activity in the adult heart, ameliorates the postischemic detriment at least at 2 different levels by facilitating mechanical recovery and ameliorating cell injury, through suppression of the ER stress response. These beneficial effects may be associated with enhanced PLN phosphorylation in the SR. These findings are consistent with the notion that, even though protein phosphatase-1 is present in multiple cellular compartments, it is differentially regulated by auxiliary proteins, such as inhibitor-1, which define its localization, substrate specificity, and catalytic activity.25
The observation that augmented SR Ca2+ transport and cycling, mediated by active inhibitor-1, improved postischemic injury has important implications on the role of Ca2+ cycling in myocardial I/R. Indeed, even though it is generally accepted that Ca2+ homeostasis is impaired and Ca2+ uptake into the SR is depressed in myocardial I/R,4,5 the effects of enhanced Ca2+ uptake into the SR and concomitantly increased SR Ca2+ load, in the face of an ischemic insult remain controversial. On the one hand, it has been reported that such a maneuver is beneficial as it ameliorates postischemic injury. Consistent with this notion, transgenic expression of SERCA1a,26 which exhibits higher kinetics than SERCA2a, as well as gene transfer of SERCA2a in rat and porcine animal models,27,28 alleviated postischemic cardiac dysfunction and injury, whereas SERCA2a deficiency impaired relaxation and increased infarction on I/R.29 In addition, it has been reported that phosphorylation of PLN at Thr17 is essential in facilitating recovery of contractility during early reperfusion,30 further supporting the beneficial effects of dis-inhibition of the SR Ca2+ pump. On the other hand, there exists evidence that increasing SR Ca2+ cycling in the ischemic heart may be detrimental. Pharmacological inhibition of SERCA2a improved postischemic recovery and overexpression of histidine-rich Ca2+ -binding protein, an endogenous SERCA2a inhibitor, improved functional recovery and cellular damage in the midst of I/R-induced injury.21,31 In addition, PLN ablation exacerbated I/R-induced dysfunction,32,33 further supporting the notion that enhancing Ca2+ cycling may have deleterious effects. It is intriguing to postulate that the extent of Ca2+ uptake and level of Ca2+ load in the SR are important determinants of the final outcome and that a tight balance needs to be attained. Indeed, in the present study, active inhibitor-1 moderately enhanced SR Ca2+ cycling, with beneficial effects. Future studies, using a gene therapy approach in higher mammalian species, may delineate the benefits of active I-1 therapy in ischemic heart disease.
The antiapoptotic effects elicited by active inhibitor-1 were associated with attenuation of ER stress-activated caspase-12 activity, whereas mitochondrial-dependent caspase-9 activity was similar in both groups. These results suggested that active inhibitor-1-mediated cardioprotection may be primarily dependent on attenuation of the ER stress or unfolded protein response (UPR). The initial intent of the UPR is to adapt to changing cellular conditions and reestablish proper ER function. Thus, adaptive, cytoprotective mechanisms are induced, including activation of transcriptional programs to increase the folding capacity of the ER, inhibition of protein synthesis, and degradation of misfolded proteins. However, prolonged or persistent induction of the ER stress pathways becomes maladaptive and initiates host defense mechanisms, including activation of the apoptotic cascade, which may lead to pathological disease states.34 In fact, recent studies have reported that the UPR is induced in the failing heart and may be causally related to heart failure induction.35,36
Various stimuli, including disturbed Ca2+ homeostasis, have been shown to induce the UPR.34,37 In fact, depletion of ER Ca2+ stores using thapsigargin, an inhibitor of SERCA2a, is a classic pharmacological way of inducing ER stress. The apparent importance of constant Ca2+ levels lies in the Ca2+ -dependent nature of ER chaperones, such as Grp78, for activation. As such, the altered Ca2+ homeostasis in the ischemic heart,2,3 may adversely affect their function and induce the UPR.38 In fact, it has been previously reported that ischemic insults induce the UPR in the cardiomyocyte, which activates apoptotic pathways.23 Consistently, we found that the ER stress response was induced both in isolated cardiomyocytes and in whole hearts following I/R. However, active inhibitor-1 effectively attenuated ER stress activation. This may be attributable to its ability to, at least partially, restore Ca2+ homeostasis in the SR/ER by augmenting Ca2+ cycling in this organelle.
Collectively, the present findings indicate that enhancing Ca2+ cycling in the SR/ER by increasing inhibitor-1 activity alleviates postischemic injury by improving contractile recovery and attenuating cellular injury, suggesting that active inhibitor-1 may represent a novel therapeutic strategy in myocardial infarction.
Supplementary Material
Acknowledgments
We thank K.D. Greis and M.A. Wyder from the University of Cincinnati Proteomics Laboratory for performing the phosphoproteomic studies.
Sources of Funding
This work was supported by NIH grants HL-26507, HL-64018, and HL-77101 (to E.G.K.); the Leducq Foundation (to E.G.K.); and AHA Predoctoral Fellowship 0715500B (to P.N.).
Footnotes
Disclosures
E.G.K. is a scientific founder of Nanocor. R.J.H. is a founder of Celladon and Nanocor.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2008 update. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Chen L, Lu XY, Li J, Fu JD, Zhou ZN, Yang HT. Intermittent hypoxia protects cardiomyocytes against ischemia-reperfusion injury-induced alterations in Ca2+ homeostasis and contraction via the sarcoplasmic reticulum and Na+/Ca2+ exchange mechanisms. Am J Physiol Cell Physiol. 2006;290:C1221–C1229. doi: 10.1152/ajpcell.00526.2005. [DOI] [PubMed] [Google Scholar]
- 3.Valverde CA, Mundiña-Weilenmann C, Reyes M, Kranias EG, Escobar AL, Mattiazzi A. Phospholamban phosphorylation sites enhance the recovery of intracellular Ca2+ after perfusion arrest in isolated, perfused mouse heart. Cardiovasc Res. 2006;70:335–345. doi: 10.1016/j.cardiores.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Krause SM, Jacobus WE, Becker LC. Alterations in cardiac sarcoplasmic reticulum calcium transport in the postischemic “stunned” myocardium. Circ Res. 1989;65:526–530. doi: 10.1161/01.res.65.2.526. [DOI] [PubMed] [Google Scholar]
- 5.Zucchi R, Ronca-Testoni S, Di Napoli P, Yu G, Gallina S, Bosco G, Ronca G, Calafiore AM, Mariani M, Barsotti A. Sarcoplasmic reticulum calcium uptake in human myocardium subjected to ischemia and reperfusion during cardiac surgery. J Mol Cell Cardiol. 1996;28:1693–1701. doi: 10.1006/jmcc.1996.0159. [DOI] [PubMed] [Google Scholar]
- 6.Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundiña-Weilenmann C, Mattiazzi A. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res. 2007;73:689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, Berretta R, Potts ST, Marsh JD, Houser SR. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ -overload causes mitochondrial dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama H, Chen X, Baines CP, Klevitsky R, Zhang X, Zhang H, Jaleel N, Chua BH, Hewett TE, Robbins J, Houser SR, Molkentin JD. Ca2+ - and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure. J Clin Invest. 2007;117:2431–2444. doi: 10.1172/JCI31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdallah Y, Gkatzoflia A, Gligorievski D, Kasseckert S, Euler G, Schlüter KD, Schäfer M, Piper HM, Schäfer C. Insulin protects cardiomyocytes against reoxygenation-induced hypercontracture by a survival pathway targeting SR Ca2+ storage. Cardiovasc Res. 2006;70:346–353. doi: 10.1016/j.cardiores.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Oliver CJ, Shenolikar S. Physiologic importance of protein phosphatase inhibitors. Front Biosci. 1998;3:D961–D972. doi: 10.2741/a336. [DOI] [PubMed] [Google Scholar]
- 11.El-Armouche A, Rau T, Zolk O, Ditz D, Pamminger T, Zimmermann WH, Jäckel E, Harding SE, Boknik P, Neumann J, Eschenhagen T. Evidence for protein phosphatase inhibitor-1 playing an amplifier role in beta-adrenergic signaling in cardiac myocytes. FASEB J. 2003;1:437–439. doi: 10.1096/fj.02-0057fje. [DOI] [PubMed] [Google Scholar]
- 12.Pathak A, del Monte F, Zhao W, Schultz JE, Lorenz JN, Bodi I, Weiser D, Hahn H, Carr AN, Syed F, Mavila N, Jha L, Qian J, Marreez Y, Chen G, McGraw DW, Heist EK, Guerrero JL, DePaoli-Roach AA, Hajjar RJ, Kranias EG. Enhancement of cardiac function and suppression of heart failure progression by inhibition of protein phosphatase-1. Circ Res. 2005;96:756–766. doi: 10.1161/01.RES.0000161256.85833.fa. [DOI] [PubMed] [Google Scholar]
- 13.El-Armouche A, Bednorz A, Pamminger T, Ditz D, Didié M, Dobrev D, Eschenhagen T. Role of calcineurin and protein phosphatase-2A in the regulation of phosphatase inhibitor-1 in cardiac myocytes. Biochem Biophys Res Commun. 2006;346:700–706. doi: 10.1016/j.bbrc.2006.05.182. [DOI] [PubMed] [Google Scholar]
- 14.Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, Kimball TF, Lorenz JN, Nairn AC, Liggett SB, Bodi I, Wang S, Schwartz A, Lakatta EG, DePaoli-Roach AA, Robbins J, Hewett TE, Bibb JA, Westfall MV, Kranias EG, Molkentin JD. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10:248–254. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez P, Mitton B, Nicolaou P, Chen G, Kranias EG. Phosphorylation of human inhibitor-1 at Ser67 and/or Thr75 attenuates stimulatory effects of protein kinase A signaling in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H762–H769. doi: 10.1152/ajpheart.00104.2007. [DOI] [PubMed] [Google Scholar]
- 16.Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, Molkentin JD. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo W, Wolska BM, Grupp IL, Harrer JM, Haghighi K, Ferguson DG, Slack JP, Grupp G, Doetschman T, Solaro RJ, Kranias EG. Phospholamban gene dosage effects in the mammalian heart. Circ Res. 1996;78:839–847. doi: 10.1161/01.res.78.5.839. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Romero C, Calamia V, Mateos J, Carreira V, Martínez-Gomariz M, Fernández M, Blanco FJ. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: a decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol Cell Proteomics. 2009;8:172–189. doi: 10.1074/mcp.M800292-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida K, Suzuki Y, Sinohara H. Molecular cloning and sequence analysis of C57BL/6 mouse contrapsin cDNA. DNA Seq. 2001;12:289–291. doi: 10.3109/10425170109025005. [DOI] [PubMed] [Google Scholar]
- 20.Chu G, Kerr JP, Mitton B, Egnaczyk GF, Vazquez JA, Shen M, Kilby GW, Stevenson TI, Maggio JE, Vockley J, Rapundalo ST, Kranias EG. Proteomic analysis of hyperdynamic mouse hearts with enhanced sarcoplasmic reticulum calcium-cycling. FASEB J. 2004;18:1725–1727. doi: 10.1096/fj.04-2025fje. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Fan GC, Ren X, Waggoner JR, Gregory KN, Chen G, Jones WK, Kranias EG. Overexpression of histidine-rich Ca-binding protein protects against ischemia/reperfusion-induced cardiac injury. Cardiovasc Res. 2007;75:487–497. doi: 10.1016/j.cardiores.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, Li H, Kirshenbaum LA, Hahn HS, Robbins J, Jones WK, Dorn GW. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007;117:2825–2833. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szegezdi E, Duffy A, O’Mahoney ME, Logue SE, Mylotte LA, O’brien T, Samali A. ER stress contributes to ischemia-induced cardiomyocyte apoptosis. Biochem Biophys Res Commun. 2006;349:1406–1411. doi: 10.1016/j.bbrc.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Garcia A, Cayla X, Guergnon J, Dessauge F, Hospital V, Rebollo MP, Fleischer A, Rebollo A. Serine/threonine protein phosphatases PP1 and PP2A are key players in apoptosis. Biochimie. 2003;85:721–726. doi: 10.1016/j.biochi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Cohen PT. Protein phosphatase 1-targeted in many directions. J Cell Sci. 2002;115:241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 26.Talukder MA, Kalyanasundaram A, Zhao X, Zuo L, Bhupathy P, Babu GJ, Cardounel AJ, Periasamy M, Zweier JL. Expression of SERCA isoform with faster Ca2+ -transport properties improves post-ischemic cardiac function and Ca2+ -handling and decreases myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H2418–H2428. doi: 10.1152/ajpheart.00663.2007. [DOI] [PubMed] [Google Scholar]
- 27.del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, Hajjar RJ. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium-cycling. Proc Natl Acad Sci U S A. 2004;101:5622–7. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prunier F, Kawase Y, Gianni D, Scapin C, Danik SB, Ellinor PT, Hajjar RJ, Del Monte F. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ -ATPase pump overexpression in a porcine model of ischemia/reperfusion. Circulation. 2008;118:614–24. doi: 10.1161/CIRCULATIONAHA.108.770883. [DOI] [PubMed] [Google Scholar]
- 29.Talukder MA, Kalyanasundaram A, Zuo L, Velayutham M, Nishijima Y, Periasamy M, Zweier JL. Is reduced SERCA2a expression detrimental or beneficial to postischemic cardiac function and injury? Evidence from heterozygous SERCA2a knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H1426–H1434. doi: 10.1152/ajpheart.01016.2007. [DOI] [PubMed] [Google Scholar]
- 30.Mattiazzi A, Mundiña-Weilenmann C, Vittone L, Said M. Phosphorylation of phospholamban in ischemia-reperfusion injury: functional role of Thr17 residue. Mol Cell Biochem. 2004;263:131–136. [PubMed] [Google Scholar]
- 31.du Toit EF, Opie LH. Inhibitors of Ca2+ -ATPase pump of sarcoplasmic reticulum attenuate reperfusion stunning in isolated rat heart. J Cardiovasc Pharmacol. 1994;24:678–684. doi: 10.1097/00005344-199410000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Cross HR, Kranias EG, Murphy E, Steenbergen C. Ablation of PLB exacerbats ischemic injury to a lesser extent in female than male mice: protective role of NO. Am J Physiol Heart Circ Physiol. 2003;284:H683–H690. doi: 10.1152/ajpheart.00567.2002. [DOI] [PubMed] [Google Scholar]
- 33.DeSantiago J, Maier LS, Bers DM. Phospholamban is required for CaMKII-dependent recovery of Ca transients and SR Ca-reuptake during acidosis in cardiac myocytes. J Mol Cell Cardiol. 2004;36:67–74. doi: 10.1016/j.yjmcc.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 34.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 36.Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, Takada Y, Suzuki M, Nishino T, Nakaya H, Koseki H, Aoe T. Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol. 2004;24:8007–17. doi: 10.1128/MCB.24.18.8007-8017.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Görlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 38.Corbett EF, Oikawa K, Francois P, Tessier DC, Kay C, Bergeron JJ, Thomas DY, Krause KH, Michalak M. Ca2+ regulation of interactions between endoplasmic reticulum chaperones. J Biol Chem. 1999;274:6203–6211. doi: 10.1074/jbc.274.10.6203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.