Abstract
To investigate the neural basis of age-related source memory (SM) deficits, young and older adults were scanned with fMRI while encoding faces, scenes, and face-scene pairs. Successful encoding activity was identified by comparing encoding activity for subsequently remembered versus forgotten items or pairs. Age deficits in successful encoding activity in hippocampal and prefrontal regions were more pronounced for SM (pairs) compared to item memory (faces and scenes). Age-related reductions were also found in regions specialized in processing faces (fusiform face area) and scenes (parahippocampal place area), but these reductions were similar for item and SM. Functional connectivity between the hippocampus and the rest of the brain was also affected by aging; whereas connections with posterior cortices were weaker in older adults, connections with anterior cortices including prefrontal regions were stronger in older adults. Taken together, the results provide a link between SM deficits in older adults and reduced recruitment of hippocampal and prefrontal regions during encoding. The functional connectivity findings are consistent with a posterior-anterior shift with aging (PASA), previously reported in several cognitive domains and linked to functional compensation.
Keywords: fMRI, episodic memory, hippocampus, prefrontal cortex, fusiform face area, parahippocampal place area
One of the most consistent findings in the cognitive aging literature is that memory deficits in healthy older adults are larger for source memory than for item memory (for a review, see Spencer & Raz, 1995). Item memory (IM) refers to remembering what happened in the past whereas source memory (SM) refers to remembering where, when, and how it happened. More broadly, SM is involved whenever a task requires memory for individual features or details associated with the encoding event – such as distinguishing between internally or externally generated events (i.e., reality monitoring, Johnson & Raye, 1981) or in which voice a sentence was heard (for a review see Johnson, Hashtroudi, & Lindsay, 1993). One of the key aspects of SM involves the concept of binding individual features of an experience, such as content and context. One common, real-world example of SM involves the everyday need to remember when and where you meet people. In any given day you may experience many different events such as being at work or the grocery store or a cocktail party, during which time you may meet several new people in each context. Without the ability to form a link between the people and the location in which you meet them (i.e., bind item and source information) it would be impossible to later recall where you know someone from.
Greater age-related impairments for SM than for IM have been demonstrated for a variety of stimuli and experimental paradigms (e.g., Chalfonte & Johnson, 1996; Kausler & Puckett, 1981a, , 1981b; e.g., Naveh-Benjamin & Craik, 1995; Park & Puglisi, 1985; Park, Puglisi, & Lutz, 1982; Spaniol, Madden, & Voss, 2006). These findings are consistent with evidence that older adults are more impaired in recollection than in familiarity (Bastin & Van der Linden, 2003; Davidson & Glisky, 2002; Parkin & Walter, 1992), which is a closely related distinction. However, very little is known regarding the neural basis of SM decline in older adults. To investigate this issue, we used functional magnetic resonance imaging (fMRI) to measure brain activity in young and older adults while they encoded faces, scenes, and face-scene pairs. To isolate activity related to successful encoding operations, we compared encoding activity for items that were subsequently remembered versus those that were forgotten (Paller & Wagner, 2002). We focused on three brain regions assumed to play an important role in SM, the medial temporal lobes (MTL), the prefrontal cortex (PFC), and the inferior temporal (IT) cortex, as well as on their interactions.
Within MTL, the region assumed to be most critical for source memory is the hippocampus. The hippocampus is assumed to bind different aspects of a complex event into an integrated memory trace, thereby allowing source memory and recollection (Eichenbaum, Yonelinas, & Ranganath, 2007; Johnson, Hashtroudi, & Lindsay, 1993). This idea is supported by evidence that amnesiacs with hippocampal damage are more impaired in SM than in IM (Giovanello, Verfaellie, & Keane, 2003; Kan, Giovanello, Schnyer, Makris, & Verfaellie, 2007; Kroll, Knight, Metcalfe, Wolf, & Tulving, 1996; Turriziani, Fadda, Caltagirone, & Carlesimo, 2004) (but see Stark & Squire, 2003), and by functional neuroimaging evidence that the hippocampus shows greater activity during the encoding of associations among items than during the encoding of single items (Davachi & Wagner, 2002; Henke, Weber, Kneifel, Wieser, & Buck, 1999; Prince, Daselaar, & Cabeza, 2005; Sperling, Chua et al., 2003; Sperling et al., 2001). Also, functional neuroimaging studies have linked the hippocampus to recollection rather than to familiarity (e.g., Daselaar, Fleck, & Cabeza, 2006; Ranganath et al., 2004). Although there is substantial evidence that the structural integrity of the hippocampus declines during aging (for a review see Raz, 2000; Raz et al., 2005), evidence linking SM deficits in older adults to hippocampal dysfunction is scarce. Mitchell and colleagues (2000) found an age-related reduction in hippocampal activity during feature binding, but this study focused on working memory (encoding) and did not assess long-term episodic memory. A previous study from our laboratory (Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006) linked age-related reductions in recollection to attenuated hippocampal activity, but this study focused on retrieval rather than encoding. Thus, the first goal of the present study was to investigate the effects of aging on hippocampal activity during SM encoding.
PFC regions are assumed to be critical for SM because of their role in executive and organizational functions. During encoding, PFC is believed to organize incoming information thereby enhancing binding in the hippocampus (Johnson, Hashtroudi, & Lindsay, 1993; Moscovitch, 1992). The role of PFC in SM is supported by evidence that frontal patients generally display greater deficits for SM than for IM (Butters, Kaszniak, Glisky, Eslinger, & Schacter, 1994; Janowsky, Shimamura, & Squire, 1989; Milner, Corsi, & Leonard, 1991; Shimamura, Janowsky, & Squire, 1990). Similarly, functional neuroimaging studies have shown that PFC is more activated during retrieval for SM than for IM, including the retrieval of temporal order, spatial, location, and modality of presentation (Cabeza, Locantore, & Anderson, 2003; Cabeza, Mangels et al., 1997; Hayes, Ryan, Schnyer, & Nadel, 2004; Henson, Shallice, & Dolan, 1999; Kostopoulos & Petrides, 2003; Nolde, Johnson, & D'Esposito, 1998; Ranganath, Johnson, & D'Esposito, 2000; Rugg, Fletcher, Chua, & Dolan, 1999). Finally, recollection has been shown to elicit greater activity than familiarity in several PFC regions (Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006; Dobbins, Rice, Wagner, & Schacter, 2003; Henson, Rugg, Shallice, Josephs, & Dolan, 1999). As in the case of the hippocampus, evidence linking SM deficits in older adults to PFC dysfunction is meager. In older adults, PFC regions show pronounced atrophy (Pfefferbaum, Sullivan, Rosenbloom, Mathalon, & Lim, 1998; Raz et al., 2005; Resnick, Pham, Kraut, Zonderman, & Davatzikos, 2003) and reduced functional activity (for a review see Dennis & Cabeza, 2008). However, very few functional neuroimaging studies have directly linked reduced PFC activations in older adults to SM encoding deficits. Two early studies found age-related reductions in PFC activity during the encoding of word pairs (Anderson et al., 2000; Cabeza, Grady et al., 1997), and a more recent study found them during the encoding of face-name associations (Sperling, Bates et al., 2003). However, these studies used blocked designs, and hence, could not directly link changes in encoding activity to differences in successful encoding operations. Accordingly, the second goal of the present study was to investigate the effects of aging on PFC activity during SM encoding.
In addition to binding processes mediated by the hippocampus and organizational processes mediated by PFC, SM depends on successful perceptual processing in IT cortex. Given the use of faces and scenes stimuli in the present study, two relevant regions are the fusiform face area (FFA) and the parahippocampal place area (PPA). FFA is involved not only in face perception (Kanwisher, McDermott, & Chun, 1997; McCarthy, Puce, Gore, & Allison, 1997) but also in face encoding (Bernstein, Beig, Siegenthaler, & Grady, 2002; Kuskowski & Pardo, 1999; Sperling, Chua et al., 2003; Sperling et al., 2001). Likewise, PPA contributes to both scene processing (Epstein & Kanwisher, 1998) and scene encoding (e.g., Kohler, Crane, & Milner, 2002; Kohler, Moscovitch, Winocur, & McIntosh, 2000). Of particular relevance to the issue of SM, there is evidence that visual regions that mediate stimulus-specific processing also support accurate SM for these stimuli (e.g., Cansino, Maquet, Dolan, & Rugg, 2002; Kensinger & Schacter, 2006; Sommer, Rose, Weiller, & Buchel, 2005). Although the anatomy of ventral pathway regions is well preserved in older adults (Raz, 2000), there are functional changes in these regions during aging. First, activity in posterior brain regions including IT is reduced in older adults across a wide range of cognitive tasks (for a review see Dennis & Cabeza, 2008). These reductions are often accompanied by age-related increases in PFC regions (Posterior-Anterior Shift in Aging—PASA), which have been attributed to functional compensation (Davis, Dennis, Daselaar, Fleck, & Cabeza, in press; Grady et al., 1994). Second, the specialization of IT regions for different kind of stimuli (e.g., faces vs. places) is also reduced by aging (Park et al., 2004; Payer et al., 2006), a process that has been attributed to age-related neural dedifferentiation. Thus, the third goal of the study was to investigate if age-related changes in IT cortex contribute to SM decline in older adults.
Finally, given that the foregoing regions cannot function in isolation, SM is assumed to depend on functional connectivity between MTL, PFC, and IT regions. This idea is supported by recent neuroimaging studies in which increased functional connectivity of these regions during successful encoding has been observed (Dickerson et al., 2007; Ranganath, Heller, Cohen, Brozinsky, & Rissman, 2005). Regarding the effects of aging, there is evidence that changes in functional connectivity between PFC and the rest of the brain contribute to age-related encoding deficits (Cabeza, Grady et al., 1997). There is also evidence that changes in hippocampal connectivity contributes to memory impairments in older adults (Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006; Grady, McIntosh, & Craik, 2003; Gutchess et al., 2005). However, the specific role of hippocampal connectivity to age-related SM decline is unknown. Therefore, the fourth goal of the study was to investigate this issue.
Traditionally, studies of source memory have been restricted to a large number of items tested as originating from a limited number of sources. While this has many practical laboratory benefits, it is not completely analogous to what is experienced in the real world. Furthermore, although many source memory studies present information from one of two sources (e.g., a male or female voice, the left or right side of the computer screen), there is nothing inherent in the definition of source memory, as described by Johnson and colleagues, that would preclude the use of a unique source (a scene) with a unique item (a face). In fact, there have been studies (e.g., Schacter, Osowiecki, Kaszniak, Kihlstrom, & Valdiserri, 1994) showing that age-related deficits in source memory do not change as a function of the number of items associated with each source.
The current study aims to expand upon previous work, by making the current task more ecologically valid with the use of many unique stimuli pairings, based upon the everyday example we discussed early, remembering where you met someone. The paradigm used in the current study is depicted in Figure 1. Participants encoded faces, scenes, and face-scene pairs in different scans. Thus, unlike previous source memory tasks we did not link a limited number of sources with many unique items but used many unique face-scene pairings in the SM task. Furthermore, to effectively contrast SM with IM we chose to use faces and scenes as our stimuli for item encoding. This allowed us to investigate not only the encoding of face-scene pairings, but to parse out the separate contributions or demands associated with face and scene encoding in isolation.
Figure 1.
Example of stimuli in the item memory (e.g., face, scene) and source memory (face/scene pairs) conditions – for both encoding and retrieval. During encoding participants engaged in a 2-back working memory task and during retrieval they performed a recognition task with three responses: Definitely old (Def. Old), Probably Old (Prob. Old), or New.
To ensure attention to all stimuli, participants performed an N-back task. Only a few stimuli repeated, and they were not included in fMRI analyses. During retrieval, participants recognized faces, scenes, and pairs in different scans. In the case of faces and scenes, participants distinguished between studied and non-studied stimuli, whereas in the case of face scene pairs, they had to identify if the source was the same or different (i.e., was the face-scene pairing identical to that which was presented during encoding). Delay between encoding and retrieval scans was varied across conditions and groups to attenuate differences in memory performance and control for potential differences in task difficulty. As noted above, successful encoding activity was isolated by comparing encoding activity for items that were subsequently remembered versus those that were forgotten, a difference know as Dm (Difference in memory). Thus, our paradigm provided three different types of Dm, Face Dm, Scene Dm, and Source Dm. When we refer to both Face and Scene Dm, we use the term Item Dm.
As noted previously, the study had four main goals. The first goal was to investigate the role of MTL regions in SM decline in older adults. Based on previous research, we predicted a greater age-related reduction of Source Dm than Item Dm in the hippocampus. The second goal was to examine the role of PFC in source memory age-related SM deficits. Again, previous research led us to predict that some PFC regions would show greater age-related reductions in Source Dm than Item Dm. The third goal was to investigate the contribution of IT cortex to SM difficulties in older adults. In particular, we examined whether age-related changes in FFA and PPA would differentially influence Source Dm versus Item Dm. Finally, the fourth goal was to explore age-related changes in hippocampal connectivity with PFC and IT regions during SM encoding.
METHOD
Participants
Fourteen healthy young adults (6 females, 8 males; mean age = 19.4 yrs; mean education = 13.2 yrs) and 14 healthy older adults (9 females, 5 males; mean age = 68.4 yrs; mean education = 17.1 yrs), screened for contraindications to MRI, participated in the study (see Table 1 for neuropsychological test results). All participants gave written informed consent and received financial compensation. All experimental procedures were approved by the Duke University institutional review board.
Table 1.
Mean demographic and neuropsychological data (standard deviation) from young and older adults.
| Young Adults | Older Adults | |||
|---|---|---|---|---|
| raw score | z-score1 | raw score | z-score | |
| Age (years) | 19.4 (1.3) | 68.4 (7.1) | ||
| Education (years) | 13.2 (1.1) | 17.1 (1.2) | ||
| Pattern Recognition Memory: percent correct | 96.7 (3.7) | 1.1 (0.34) | 92.3 (4.9) | 0.70 (0.47) |
| Paired Associates Learning: total errors adjusted | 4.1 (8.9) | 0.12 (1.2) | 12.5 (7.0) | 0.66 (0.48) |
| Spatial Span: span length | 7.8 (1.1) | 0.60 (0.80) | 5.7 (1.3) | 0.57 (1.4) |
| Spatial Working Memory: between errors | 6.1 (8.8) | 0.52 (0.78) | 34.8 (25.5) | 0.36 (1.2) |
| Spatial Working Memory: strategy | 24.6 (5.2) | 0.88 (1.0) | 31.6 (7.4) | 0.57 (1.3) |
| Reaction Time: reaction time | 292.8 (33.6) | 0.85 (0.65) | 376.1 (52.4) | 0.04 (1.00) |
| Reaction Time: movement time | 320.1 (30.5) | 1.61 (0.29) | 401.5 (71.5) | 0.90 (0.79) |
| Rapid Information Processing: A' | 0.96 (0.03) | 1.1 (0.62) | 0.90 (0.07) | −0.40 (1.4) |
| Set Shifting: stages completed | 9 (0) | 0.28 (0.001) | 8.8 (0.58) | 0.33 (0.43) |
| Set Shifting: total errors adjusted | 10.6 (5.0) | 0.38 (0.27) | 21.3 (15.6) | 0.39 (0.48) |
Z-score computation was based on normative data from age matched controls.
Materials
See Figure 1 for examples of stimulus materials. Face stimuli consisted of 693 faces gathered from the following databases: the Color FERET database (Phillips, Moon, Rizvi, & Rauss, October2000), adult face database from Dr. Denise Park’s lab (Minear & Park, 2004), the AR face database (Martinez & Benavente, 1998), and the FRI CVL Face Database (Solina, Peer, Batageli, Juvan, & Kovac, 2003). Scene stimuli consisted of 693 indoor and outdoor scenes gathered from the internet. Using Adobe Photoshop CS2 version 9.0.2 and Irfanview 4.0 (http://www.irfanview.com/), face stimuli were edited to a uniform size (320 × 240 pixels) and background (black) and scene stimuli were standardized to 576 × 432 pixels. Face-scene pairs were created using a custom MatLab (version 6.1) script that overlaid faces on scenes, and images were standardized to 576 × 432 pixels. Neuropsychological test materials were comprised of the Cambridge Neuropsychological Test Automated Battery (CANTABeclipse, version 2.0; Cambridge Cognition Ltd), a computerized battery of neuropsychological tests designed to assess verbal and visual episodic and working memory, executive functions, attention, and language using a PC equipped with a touch screen display.
Procedure
Completion of the experiment required three visits to the lab. During the first visit, neuropsychological testing was completed using the CANTAB. A brief pilot version of the experimental task was also administered to ensure sufficient memory performance during the scanning sessions. Participants whose neuropsychological test performance was two or more standard deviations below age-matched normative data on two or more CANTAB sub-tests or whose pilot test performance fell below chance or resulted in too few miss trials were excluded from the scanning sessions.
Scanning consisted of two sessions, scheduled approximately 24 hours apart. For both scanning sessions, participants were placed supine on the MRI table, fitted with earplugs and earphones, and had their heads stabilized with cushions. The participants were moved into the bore of the scanner, and 3-plane localizer scans were collected, followed by functional scanning. Stimuli were presented via a mirror in the head coil and a rear projection system using a PC computer programmed with Cogent, a stimulus presentation toolbox within Matlab. Button responses and response time were recorded using a magnetically shielded 4-button box held in the participant’s right hand. After completion of the second scanning session, participants were debriefed.
Session 1 consisted of collection of diffusion weighted and high-resolution structural images and one 8 run set of event-related functional imaging (see Image Acquisition for details). Session 2 consisted of two more 8 run sets of event-related functional scanning, separated by a 5 minute break. Each 8 run set consisted of four encoding runs [1 scene, 1 face, and 2 source (face-scene pair)] and four recognition runs [1 scene, scene, 1 face, and 2 source].
During encoding participants performed an N-back (N=2) working memory task. That is, for every trial presented at encoding, participants had to determine whether the current stimulus matched the stimulus presented 2 trials earlier. The goal of using the N-back task during encoding was to assure that participants were attending to the stimuli (and in the case of the source trials, attending to both items). Furthermore, in the case of the source trials, distractors during encoding were presented such that sometimes only the face or only the scene was repeated, but the other item was new. This was done to further ensure that participants encoded both the item and source within each encoding trial. To eliminate the effects of multiple presentations N-back trials (or those trials that repeated during encoding) repeated items were not brought to retrieval and thus not used in the subsequent memory analysis. Each item (face and scene) encoding run consisted of 48 trials, 32 of which were critical encoding trials and thus used as targets during retrieval (this resulted in a total of 96 targets in each of the item conditions); source encoding runs consisted of 39 trials, 21 of which were critical encoding trials (consisting of unique face-scene pairings) and thus used as targets during retrieval (this resulted in a total of 126 targets in the source memory condition). Again source retrieval consisted of either (a) targets in which the identical face-scene pairing from encoding were presented, (b) distractors where only one of the elements was old and the other new or (c) distractors where both elements were old (previously presented during encoding), but recombined to form a new face-scene pairing.
During recognition, participants made memory judgments using a 3-point scale: definitely old, probably old, or new. Each item recognition run consisted of 32 memory targets and 25 lures (i.e., new faces or scenes), and each source recognition run was composed of 21 memory targets and 8 lures (i.e., recombination of previous face-scene pairings presented during encoding, but not as part of any target trial). Inter-trial jitter ranged from 750 to 1500ms to facilitate deconvolution of the hemodynamic response (Dale, 1999). Presentation rate and delay between encoding and recognition were varied between conditions to equate for memory performance within and between participants/groups, and were based on extensive piloting. Delay between Scene encoding and recognition was approximately 31 min for both groups. The delay between Face encoding and recognition was approximately 23 min for young adults, and 14 min for older adults, and for Source trials, 9 min and 4 min, respectively. By manipulating delay we sought to reduce the possibility that age differences were due to increased task difficulty for SM relative to IM.
Image Acquisition
Images were collected on a General Electric 3.0 Tesla Signa Excite HD short bore scanner (Milwaukee, WI), equipped with an 8 channel head coil. Session 1 total scan time was approximately 90 minutes. Following acquisition of the high-resolution anatomical images (450-ms repetition time (TR), a 3-ms echo time (TE), a 24-cm field of view (FOV), a 2562 matrix, and a slice thickness of 1.9-mm), whole-brain functional images were acquired parallel to the anterior–posterior commissure plane using an inverse spiral sequence (direction = interleaved, matrix = 642, FOV = 24 cm, TR = 2000 ms, TE = 30 ms, sections = 34, thickness = 3.8 mm, inter-scan spacing = 0). Functional scanning lasted approximately 45 minutes, and occurred in 8 runs (item encoding runs = 3min 7sec; source encoding runs = 3 min 13sec; item recognition runs =4min 36 sec; source recognition runs= 3min 13sec). After completion of functional scanning, diffusion-weighted images, which are not reported here, were collected. Image acquisition during Session 2 was identical to Session 1, although in Session 2 functional scanning consisted of 16 runs and diffusion weighted images were not collected. Total scan time in Session 2 was approximately 120 min.
Image Processing
Functional data were preprocessed and analyzed with SPM2 (Statistical Parametric Mapping; Wellcome Department of Cognitive Neurology, http://www.fil.ion.ucl.ac.uk/spm). Time-series were corrected for differences in slice acquisition times and realigned. Functional images were spatially normalized to a standard stereotaxic space, using the Montreal Neurological Institute (MNI) templates implemented in SPM2 and resliced to a resolution of 3.75 mm3. The coordinates were later converted to Talairach space (Talairach & Tournoux, 1988). Finally, the volumes were spatially smoothed using an 8 mm isotropic Gaussian kernel and proportionally scaled to the whole-brain signal. The hemodynamic response for each trial was modeled using the canonical hemodynamic response function. Serial correlations were estimated using an autoregressive AR (1) model. Data were high pass filtered using a cutoff of 128 Hz, and global effects were removed (session specific grand mean scaling, global scaling). Head motion was assessed prior to pre-processing. No individual moved more than 3 mm in any direction, in any run. Thus, no data was eliminated in either age group due to motion artifacts.
Statistical Analyses
In each condition (scene, face, and source) subsequently remembered trials (i.e., encoding trials that lead to a high-confidence “old” recognition response) and subsequently forgotten trials (i.e., encoding trials that lead to a “new” or a low-confidence “old” recognition response) were modeled separately. The inclusion of low-confidence recognition responses with forgotten trials was based upon the observed chance performance associated with this trial type (see below).1 Repeated encoding trials and recognition trials as well as confounding factors such as head motion and scanner drift were also included in the model and treated as regressors of no interest. Retrieval data will be reported in subsequent publication. Within each group, Dm activations were identified by directly comparing subsequently remembered and forgotten trials at p < 0.005 with minimum cluster size of 10 contiguous voxels. These results were subsequently used as an inclusive mask for identifying aging effects at p <.05 and 10 contiguous voxels. Thus, we only considered activations that passed two thresholds: (1) they had to show a significant Dm effect (remember > forgotten) within one of the groups (p < .005); (2) an age group difference in the Dm effect (p < .05). The conjoint probability following inclusive masking approaches p = .00025 (Fisher, 1950; Lazar, Luna, Sweeney, & Eddy, 2002), but this estimate should be taken with caution given that the contrasts were not completely independent. This analysis approach was used in order to ensure that age differences were associated specifically with those regions that were critical to performance in a given age group. Furthermore, given our goals, we concentrated on the following regions: MTL, PFC, FFA and PPA. The results of the whole brain analyses were masked with these four regions, further reducing the probability of false positives. The masks for the MTL and PFC were defined using an anatomical library (Tzourio-Mazoyer et al., 2002). The masks for FFA and PPA were functionally defined by contrasting raw face with raw scene activity, and raw scene with raw face activity, respectively, across all 28 participants (young plus old) at a threshold of p < .001. Given that this contrast collapsed over encoding differences (remembered and forgotten trials) and age differences (young and older groups), the resulting masks were not biased by these factors.
Connectivity Analyses
To assess connectivity within the SM encoding network, we used the hippocampus as a seed region and examined correlations in whole brain activity with hippocampal activity. The seed hippocampal voxel used was that which showed the greatest age difference in the event-related analysis for Source Dm. This was done to identify whether age differences in connectivity between hippocampal activity and the rest of the brain also exist. A new model was created such that each trial in the model was uniquely coded as a separate event. This allowed us to correlate the time series activity of the seed region with the rest of the voxels in the brain – on a trial by trial basis. The validity of this design has been confirmed in previous studies (Daselaar, Fleck, & Cabeza, 2006; Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006; Rissman, Gazzaley, & D'Esposito, 2004). The goal of this exploratory analysis was to assess connectivity for SM; therefore, the analysis was constrained to subsequently remembered trials in the source memory condition. As a second step, group averages (of hippocampal connectivity on SM trials) were calculated by employing a one-sample t-test on the resulting correlation maps (random effects). Group differences were again calculated using a multiple contrast approach. The between group two-sample t-test was conducted at p < .05 with a minimum cluster size of 20 voxels, inclusively masking for effects of interest within each group (p < .05 and a minimal cluster size of 20 voxels). Thus, like the previous analyses, the resulting activity showing age-related differences in the functional connectivity with the hippocampus also had to be confirmed by differences observed in individual groups.
RESULTS
Behavioral Results
Memory Performance
As described in the Methods section, to attenuate performance differences, study-test delays were manipulated across conditions and across groups. Thus, the accuracy data in Table 2 do not reflect naturally occurring differences but the combined effect of these differences and our experimental manipulations. Despite our attempt to equate memory performance in young and older adults, a 2 (group: young vs. old) × 2 (confidence: high vs. low) × 3 (stimulus type: face vs. scene vs. source) analysis of variance (ANOVA) on corrected recognition scores (hits – false alarms) revealed a significant group effect, F(1, 26) = 4.36, p < .05, reflecting better performance in young than in older adults. This analyses yielded also a stimulus type × confidence interaction, F(2, 52) = 21.68, p < .001, due to an effect of stimulus type for high confidence responses (scenes > faces > source, all p’s < .01) but not for low confidence responses (all p’s N.S.).
Table 2.
Behavioral performance by confidence level of young and older adults.
| High Confidence | Low Confidence | ||||||
|---|---|---|---|---|---|---|---|
| Group | Condition | Hits | False Alarms | CR | Hits | False Alarms | CR |
| Young | Face | 0.49 (.04) | 0.07 (.02) | 0.42 (.04) | 0.25 (.03) | 0.24 (.04) | 0.01 (.03) |
| Scene | 0.61(.03) | 0.04 (.01) | 0.57 (.03) | 0.17 (.02) | 0.16 (.04) | 0.01 (.02) | |
| Source | 0.45 (.04) | 0.10 (.02) | 0.35 (.04) | 0.29 (.04) | 0.25 (.03) | 0.04 (.02) | |
| Older | Face | 0.50 (.04) | 0.16 (.02) | 0.34 (.04) | 0.25 (.02) | 0.24 (.02) | 0.01 (.02) |
| Scene | 0.59 (.05) | 0.07 (.02) | 0.52 (.05) | 0.16 (.02) | 0.18 (.05) | −0.02 (.06) | |
| Source | 0.51 (.05) | 0.26 (.04) | 0.25 (.05) | 0.23 (.03) | 0.23 (.03) | 0.00 (.02) | |
CR = Corrected recognition score (Hits – False Alarms)
Given that participants’ corrected recognition scores for low confidence responses were essentially zero (see rightmost column in Table 2), we formally investigated memory discrimination by comparing hits and false alarms. A 2 (group: young vs. older) × 2 (confidence: high vs. low) × 2 (memory trial: hits vs. false alarms) ANOVA yielded a confidence × memory trial interaction, F(1, 26) = 113.84, p <.001. Follow-up t-tests indicated that for high confidence responses, hits were greater than false alarms, t(27) = 14.70, p < .001, whereas for low confidence, they did not differ, t(27) < 1, p = ns. Thus, only high confidence responses showed a good level of memory accuracy, whereas low confidence responses were essentially guesses. Therefore, to ensure that our fMRI analyses represented successful memory encoding, only high confidence hits were classified as remembered, and low confidence hits were classified as forgotten together with the misses. This approach has been used in other subsequent memory fMRI studies (Prince, Daselaar, & Cabeza, 2005; Schon, Hasselmo, Lopresti, Tricarico, & Stern, 2004; Sperling, Chua et al., 2003). Furthermore, there were no group differences in either high or low confidence hit rate for each trial type (i.e., face, scene, source). We can thus be assured that levels of estimation efficiency were comparable across groups.
It should be noted, that despite our efforts to equate memory performance across groups and conditions, older adults still performed more poorly than younger adults and both groups performed more poorly on source than item memory tasks. While the current study attempted to control for difficulty as it relates to successful performance by focusing on high confidence trials in the neuroimaging analyses, it is certainly the case that differences in effort or difficulty may have contributed to the subsequent neuroimaging results. More work is necessary to equate performance without reducing the episodic quality of the memory task.
Encoding Response Times
To confirm that subsequent memory effects were not influenced by response times (RTs) during encoding, we analyzed encoding RTs based on subsequent memory performance (see Table 3). A 2 (group: young vs. old) × 2 (encoding success: remembered vs. forgotten) × 3 (stimulus type: face vs. scene vs. source) ANOVA revealed a significant group effect, F(1, 26) = 10.04, p < .01, due to slower responses in older adults. This analysis revealed a significant Group × Stimulus Type interaction, F(2, 52) = 6.12, p < .01. Follow-up pairwise comparisons indicated that this interaction was driven by significant differences across conditions in older adults (source > scene > face, all p’s < .01), but not in younger adults (all p’s N.S.). Importantly, the main effect of encoding success and the interactions involving this factor were all nonsignificant (all F’s < 1), indicating that Dm effects do not reflect RT differences.
Table 3.
Response times (standard error) during encoding
| Subsequently Remembered |
Subsequently Forgotten |
||
|---|---|---|---|
| Young | Face | 955 (57) | 927 (50) |
| Scene | 915 (47) | 949 (59) | |
| Source | 971 (41) | 1000 (47) | |
| Older | Face | 1154 (69) | 1155 (81) |
| Scene | 1235 (73) | 1235 (68) | |
| Source | 1355 (100) | 1379 (114) |
Subsequently remembered included high confidence hits, and subsequently forgotten, low confidence hits and all misses
fMRI Results
Whole Brain Results
Although the focus of the present study is in four a priori regions (MTL, PFC, FFA and PPA), the results of whole brain analyses are reported for sake of completion. Table 4 displays significant individual group effects for each encoding condition. Table 5 displays regions showing significant age-related differences in Face, Scene, and Source Dm. To isolate regions selectively associated with Source Dm, Face Dm and Scene Dm were masked out at lenient threshold (p < .05). As indicated by Table 5, selective age-related reductions in Source Dm were found in hippocampal/parahippocampal regions and PFC regions. All following results represent age differences in activations (as opposed to deactivations); specifically results represent age differences in Dm activity or the difference in activations associated with subsequent memory success compared to subsequent memory failures.
Table 4.
Individual Group Effects in Item and Source Dm
| Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| H | BA | x | Y | z | t | voxels | |
| Face Dm | |||||||
| Young | |||||||
| Occipitotemporal ctx | R | 37/19/18/17 | 48 | −51 | −7 | 6.12 | 430 |
| L | 19/37/18 | −41 | −76 | 4 | 6.84 | 244 | |
| Dorsolateral PFC | L | 9 | −41 | 9 | 38 | 3.97 | 14 |
| Ventrolateral PFC | L | 45/46 | −45 | 33 | 9 | 3.34 | 13 |
| Amygdala | L | −22 | 3 | −16 | 5.11 | 18 | |
| R | 26 | −8 | −9 | 4.08 | 18 | ||
| Thalamus | M | −7 | −26 | −8 | 4.6 | 36 | |
| Older | |||||||
| Occipitotemporal ctx | L | 19/37 | −33 | −70 | −6 | 5.02 | 70 |
| R | 19/37 | 33 | −55 | −13 | 3.74 | 15 | |
| Scene Dm | |||||||
| Young | |||||||
| Occipitotemporal ctx | R | 37/19 | 33 | −48 | −7 | 7.81 | 645 |
| L | 37/19 | −33 | −44 | −7 | 7.35 | 568 | |
| Superior Frontal Gyrus | L | 6 | −7 | 18 | 62 | 3.44 | 15 |
| Inferior Frontal Gyrus | L | 9 | −30 | 2 | 35 | 3.75 | 22 |
| Hippocampus/PHG | L | −22 | −15 | −9 | 4.3 | 12 | |
| Anterior PHG | R | 20 | 33 | −12 | −28 | 5.23 | 17 |
| Posterior PHG | L | 27 | −30 | −40 | 9 | 3.23 | 13 |
| Precuneus | L | 7 | −19 | −63 | 56 | 6.66 | 10 |
| Posterior Cingulate | L | 29 | −15 | −47 | 9 | 3.92 | 11 |
| Insula | L | −33 | −2 | 25 | 4.24 | 66 | |
| Older | |||||||
| Occipitotemporal ctx | R | 18/19/37/39 | 37 | −80 | −2 | 6.89 | 251 |
| L | 18/19 | −22 | −87 | 15 | 5.91 | 87 | |
| L | 20/37 | −48 | −41 | −17 | 4.14 | 10 | |
| Occipital ctx | L | 18/17 | −30 | −84 | 1 | 4.32 | 15 |
| Dorsolateral PFC | L | 8 | −22 | 31 | 44 | 6.17 | 81 |
| Mid-dorsolateral PFC | R | 44 | 45 | 16 | 20 | 4.06 | 31 |
| L | 44 | −45 | 15 | 17 | 4.01 | 14 | |
| Sensorimotor ctx | R | 4 | 26 | −23 | 50 | 4.84 | 11 |
| L | 4 | −26 | −26 | 54 | 4.76 | 34 | |
| L | 3/4 | −41 | −62 | −6 | 4.71 | 33 | |
| Postcentral Gyrus | L | 3/4 | −41 | −9 | 42 | 3.9 | 26 |
| Superior Frontal Gyrus | R | 6 | 15 | 10 | 56 | 4.08 | 10 |
| Caudate | L | −11 | 8 | 17 | 6.8 | 25 | |
| Posterior Cingulate | L | 30/29 | −15 | −57 | 17 | 5.2 | 38 |
| R | 30/29 | 7 | −47 | 6 | 4.04 | 11 | |
| R | 30/29 | 22 | −54 | 17 | 3.92 | 13 | |
| Lateral Parietal ctx | R | 40/7 | 33 | −52 | 52 | 3.94 | 32 |
| Source Dm | |||||||
| Young | |||||||
| Dorsolatral PFC | L | 44/9 | −41 | 12 | 24 | 6.32 | 74 |
| R | 44/9 | 48 | 9 | 31 | 4.45 | 23 | |
| Occipto/tempora/parietal | L | 19/37/18/20 | −33 | −40 | −8 | 7.69 | 513 |
| Hippocampus (subpeak) | L | −33 | −40 | −4 | 6.45 | 22 | |
| Occipto/tempora/parietal | R | 19/37/18/20 | 37 | −73 | −6 | 7.36 | 564 |
| Hippocampus/PHG (subpeak) | R | 33 | −23 | −21 | 3.89 | 12 | |
| Hippocampus/Amygdala | L | −37 | −16 | −25 | 5.68 | 27 | |
| Older | |||||||
| Occipto-temporal | R | 19/37 | 52 | −59 | −7 | 6.75 | 118 |
| L | 37 | −30 | −51 | −7 | 5.54 | 28 | |
| Occipital Cortex | R | 19 | 33 | −76 | 18 | 4.43 | 35 |
H=hemisphere; BA=Brodmann Area; L=left; R=right; M=medial
PFC= prefrontal cortex; ctx=cortex
Table 5.
Age-related differences on successful encoding activity (Dm)
| H | BA | x | y | Z | t | voxels | |
|---|---|---|---|---|---|---|---|
| Face Dm | |||||||
| Young > Older | |||||||
| Occipitotemporal ctx | R | 37/19/18/17 | 45 | −51 | −7 | 4.77 | 290 |
| L | 18/19/37 | −26 | −84 | 4 | 4.49 | 151 | |
| Dorsolateral PFC | L | 9 | −41 | 9 | 38 | 3.3 | 13 |
| Ventrolateral PFC | L | 45/46 | −48 | 30 | 9 | 2.95 | 10 |
| Thalamus | M | −7 | −15 | 1 | 3.87 | 18 | |
| Amygdala | L | −26 | −1 | −16 | 3.22 | 12 | |
| Scene Dm | |||||||
| Young > Older | |||||||
| Occipitotemporal ctx | L | 30/19/37/36 | −37 | −69 | −3 | 5.21 | 270 |
| R | 19/37/35 | 41 | −44 | −7 | 4.29 | 260 | |
| Older > Young | |||||||
| Dorsolateral PFC | L | 8 | −22 | 35 | 37 | 4.29 | 70 |
| Mid-dorsolateral PFC | R | 44 | 52 | 15 | 17 | 3 | 28 |
| Sensorimotor ctx | L | 4 | −26 | −26 | 54 | 4.44 | 34 |
| L | 3/4 | −41 | −19 | 50 | 4.44 | 26 | |
| R | 4 | 26 | −23 | 50 | 3.41 | 10 | |
| Lateral Parietal ctx | R | 39 | 52 | −61 | 21 | 4.33 | 15 |
| R | 40/7 | 37 | −49 | 48 | 2.81 | 14 | |
| Source Dm (Item Dm masked out) | |||||||
| Young > Older | |||||||
| Dorsolateral PFC | L | 44/9 | −41 | 16 | 31 | 4.55 | 25 |
| R | 44/9 | 48 | 9 | 31 | 3.97 | 23 | |
| Hippocampus | L | −30 | −37 | −5 | 2.76 | 14 | |
| Parahippocampal gyrus | L | 36 | −26 | −47 | −1 | 3.42 | 14 |
H=hemisphere; BA=Brodmann Area; L=left; R=right; M=medial
PFC= prefrontal cortex; ctx=cortex
Medial Temporal Lobes
Item Dm
No age-related differences were found in the hippocampus for Face or Scene Dm. Age-related decreases were found in the left amygdala (peak: −26, −1, −16) for Face Dm. When the extent threshold was lowered to 5 voxels, an age-related decrease was found in bilateral posterior parahippocampal cortex (right peak: 19, −37, −8; left peak: −30, −37, −8) for Scene Dm. No MTL region showed age-related increases in either Face or Scene Dm.
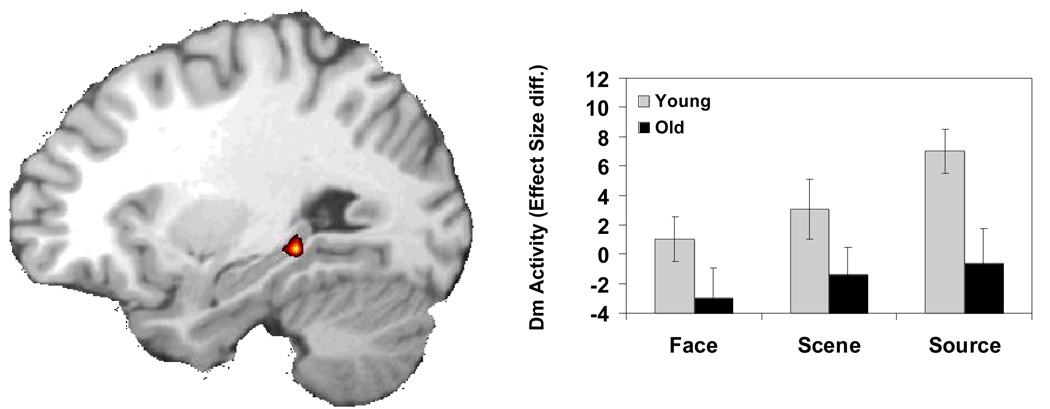
Source Dm
Consistent with our first prediction, an age-related reduction in Source Dm was found in the left hippocampus (peak: −30, −37, −5). This activation is displayed in Figure 2 and 4a. To determine if this age-related reduction in hippocampal activity was unique to the Source Dm condition, we masked out voxels that showed either Face Dm or Scene Dm at a lenient p <.05 threshold. Note that because we are using a lenient threshold for the exclusive mask, that this in fact makes the criteria for Source DM more stringent. After excluding Face and Scene DM activation, the age differences in hippocampal Source Dm remained significant. Peak activity within this cluster was analyzed with a 3 (condition: Face vs. Scene vs. Source) × 2 (Dm: remembered vs. forgotten) × 2 (age group: young vs. older) ANOVA. Although the 3-way interaction was not significant, separate ANOVAs on each condition showed only a significant Age × Dm interaction in the Source condition, F(1, 26) = 17.15, p = .0003, but not in the Face, F(1,26) = 2.48, p = .13 or the Scene, F(1,26) = 2.79, p = .11 condition. Thus, Source Dm in the hippocampus was significantly attenuated by aging, whereas the effect of aging on Item Dm in the hippocampus was not significant.
Figure 2.
Age-related hippocampal deficit in source memory encoding. The bar graphs display differences in the effect size (difference in parameter estimates) of activations for subsequent high confidence hits versus subsequently forgotten trials (low confidence hits and misses) during encoding for each memory condition. Activations were extracted from the peak voxel of the SM encoding condition. Diff, Difference.
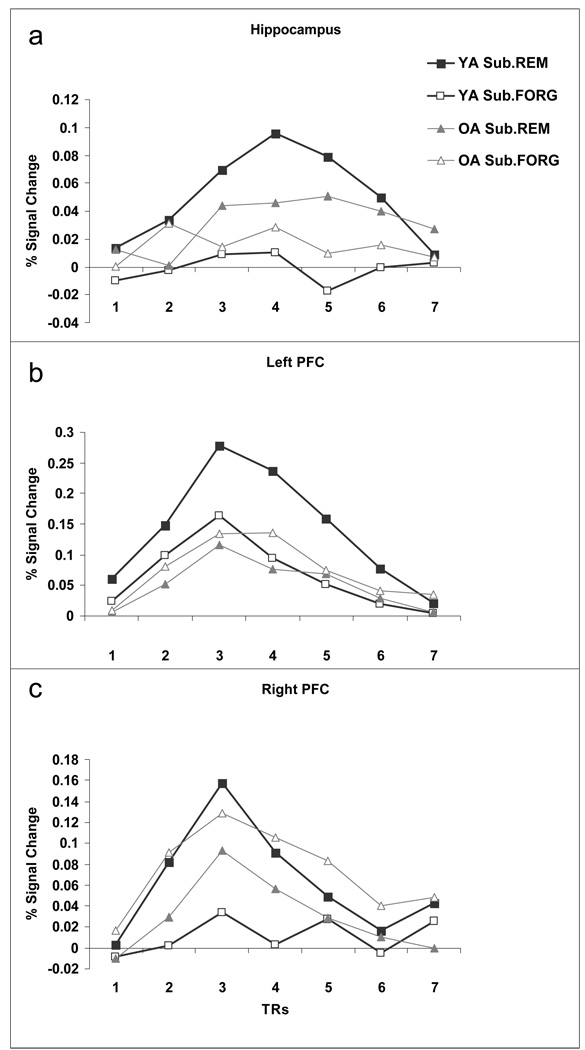
Figure 4.
Percent signal change for subsequently remembered (Sub.REM) and subsequently forgotten (Sub.FORG) trials for both young and older adults in the Source Memory condition. Timecourses were extracted from the three critical ROIs discussed in the SM condition: (a) hippocampus and (b) left and (c) right PFC.
Prefrontal Cortex
Item Dm
Age-related decreases for Face Dm were found in left dorsolateral (peak: −41, 9, 38) and ventrolateral (peak: −48, 30, 9) PFC regions. No frontal region showed an age-related increase in Face Dm activity. Conversely, age-related reductions in Scene Dm were not observed, but age-related increases were observed in several PFC regions including dorsolateral regions in both hemispheres (left: −23, 35, 37; right: 52, 15, 17).
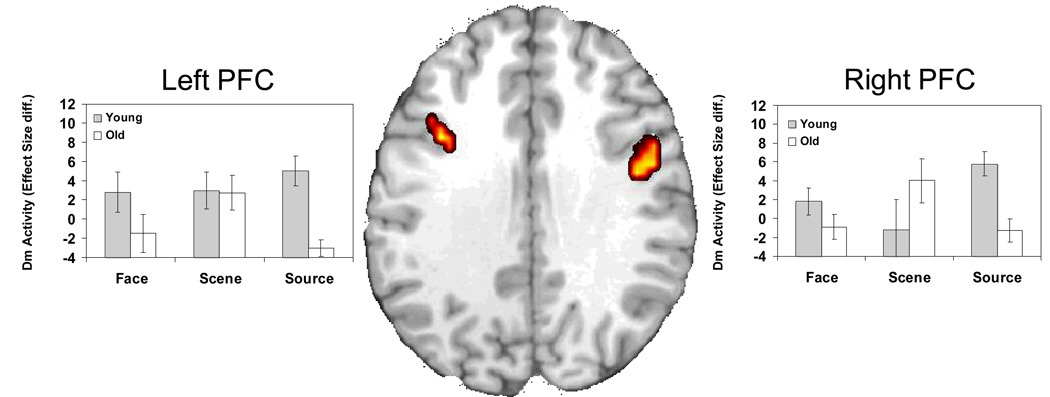
Source Dm
Consistent with our second prediction, an age-related reduction in Source Dm was found in bilateral dorsolateral PFC (left peak: −45, 20, 27; right peak: 48, 9, 31) and medial superior frontal gyrus (peak: −4, 10, 62). Again, to assess if this difference was specific to Source Dm, we excluded voxels that also showed Face or Scene Dm activity at a lenient p < .05 threshold. After excluding these shared age-effects, the majority of PFC activations showing age-related Source Dm reductions remained significant (Figure 3; 4b and c) (with the left peak shifting to −41, 16, 31). Separate Condition × Dm × Age ANOVAs examined peak activity for the left and right PFC. In left PFC, the 3-way interaction was marginally significant, F(1, 26) = 2.51, p = .09, but separate ANOVAs on each condition showed a significant Age × Dm interaction only for the Source task, F(1, 26) = 20.73, p = .0001, but not the Face, F(1,26) = 2.23, p = .15 or the Scene, F(1,26) = .01, p = .94 condition. Right PFC showed both a significant 3-way interaction, F(1, 26) = 4.59, p = .01, and a significant Age × Dm interaction for the Source condition, F(1, 26) = 15.79, p = .0005, but not the Face, F(1,26) = 1.83, p = .19 or the Scene, F(1,26) = 1.72, p = .20 condition. Thus, older adults showed weaker Source Dm in dorsolateral PFC regions and this effect was significant in the source condition but not in the Item Dm conditions.
Figure 3.
Age-related frontal deficits in source memory encoding. The bar graphs display differences in the effect size (difference in parameter estimates) of activations for subsequent high confidence hits versus subsequently forgotten trials (low confidence hits and misses) during encoding for each memory condition. Activations were extracted from the peak voxel of the SM encoding condition. Diff, Difference.
Inferior Temporal Cortex
Item Dm
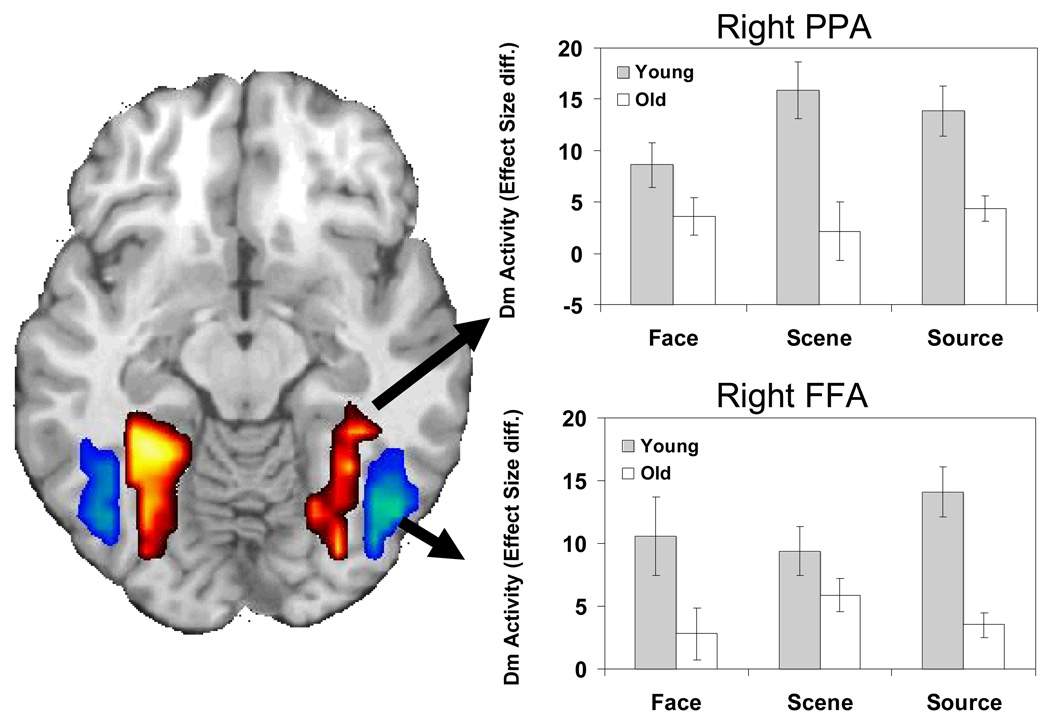
Both Face and Scene Dm were characterized by age-related activity reductions in visual processing regions. The FFA and PPA ROIs were used to isolate activity in each region (see Figure 5). Specifically, age-related decreases for Face Dm were observed within bilateral FFA (right peak: 45, −51, −7; left peak: −26, −84, 4). For Scene Dm, older adults again demonstrated a reduction in activity in ventral visual cortex, spanning bilateral parahippocampal gyrus, fusiform gyrus, and specifically PPA (left peak: −30, −48, −7; right peak: 30, −52, −13). Older adults showed no age-related increases in activity in IT cortex relative to young adults.
Figure 5.
Age-related deficits in both FFA (blue) and PPA (red). For display purposes activations were extracted from the Face and Scene Dm conditions for FFA and PPA respectively and masked for Face>Scene activity and Scene>Face activity, respectively. The bar graphs display differences (Diff.) in the effect size (difference in parameter estimates) of activations for subsequent high confidence hits versus subsequently forgotten trials during encoding for each memory condition. Activations were extracted from the peak voxel of the SM encoding condition.
Source Dm
Older adults exhibited decreased activity throughout IT cortex during the Source Dm condition. After masking out Face and Scene Dm, no region in posterior visual cortex showed age-related reductions specific to Source Dm. Thus, the effects of aging on FFA and PPA were not specific to Source Dm and were shared with Face or Scene Dm. This idea was further investigated with Age × Dm ANOVAs performed on activity extracted from bilateral FFA and PPA regions. In bilateral FFA regions, significant Age × Dm interactions were found not only in the Source condition [left: F(1, 26) = 13.03, p = .0013; right: F(1, 26) = 22.82, =.0001] but also in the Face condition [left: F(1, 26) = 10.33, p = .0035; right: F(1, 26) = 19.86, p = .0001]. Likewise, in bilateral PPA regions, significant a Age × Dm interaction were found not only in the Source condition [left: F(1, 26) = 20.09, p = .0001; right: F(1, 26) = 12.29, p = .0017] but also in the Scene condition [left: F(1, 26) = 16.38, p = .0004; right: F(1, 26) = 17.52, p = .0003]. Thus, age-related Dm reductions in visual processing regions were not specific for Source Dm and were shared with Face Dm (FFA) and Scene Dm (PPA).
Connectivity Analyses
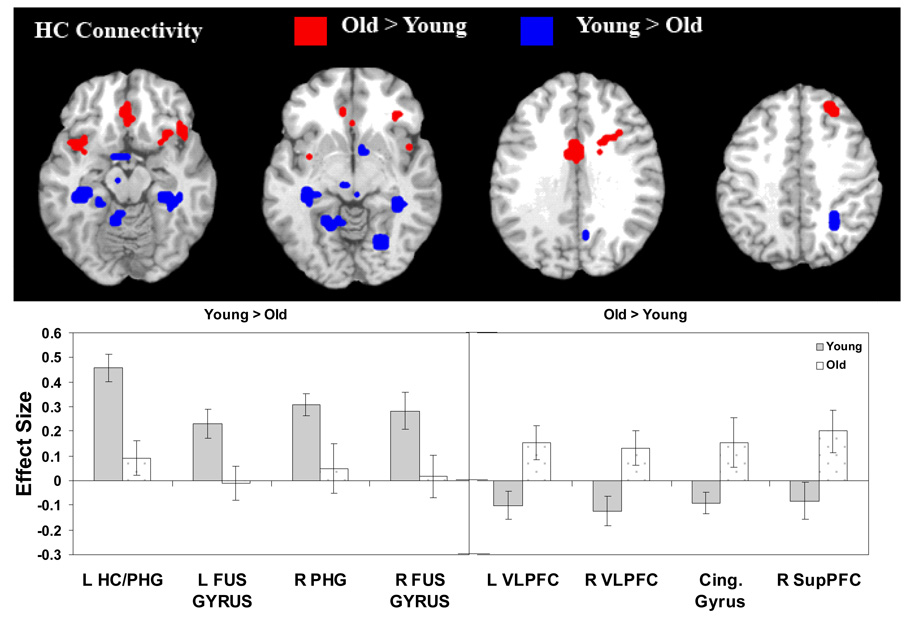
As listed in Table 6, young compared to older adults exhibited greater connectivity between the hippocampus and posterior brain regions including bilateral MTL, posterior cingulate, right parietal, and IT regions. In contrast, older adults exhibited greater hippocampal connectivity with anterior brain regions, including anterior cingulate, bilateral ventrolateral PFC, right dorsolateral PFC, and superior frontal cortex (see Figure 6). These results suggest a posterior-anterior shift in the pattern of age-related differences (PASA) in regions that show co-activation with the hippocampus.
Table 6.
Age-Related Differences in Hippocampal Connectivity
| Coordinates | |||||||
|---|---|---|---|---|---|---|---|
| H | BA | x | y | z | t | voxels | |
| Young > Older | |||||||
| Occipitotemporal ctx | L | 36/37/19 | −34 | −37 | −5 | 45.99 | 62 |
| R | 31/18 | 26 | −69 | 21 | 7.24 | 51 | |
| Parahippocampal gyrus | L | 30 | −23 | −40 | 6 | 11.55 | 40 |
| L | 37 | −23 | −45 | −7 | 8.16 | 31 | |
| Fusiform gyrus | R | 37/36 | 38 | −37 | −8 | 8.52 | 28 |
| Superior Parietal | R | 40/7 | 26 | −49 | 48 | 7.93 | 21 |
| Thalamus | R | 8 | −26 | 1 | 7.15 | 24 | |
| Hypothalamus | M | 0 | 11 | −1 | 6.15 | 25 | |
| Posterior Cingulate | M | 31 | 4 | −58 | 27 | 4.37 | 45 |
| Cerebellum | R | 34 | −53 | −32 | 3.9 | 37 | |
| Older > Young | |||||||
| Ventrolateral PFC | R | 47 | 34 | 18 | −17 | 3 | 33 |
| L | 47/38 | −41 | 10 | −13 | 2.93 | 43 | |
| Superior PFC | R | 8 | 30 | 35 | 44 | 2.82 | 21 |
| Mid-dorsolateral PFC | R | 44/46 | 34 | 20 | 24 | 2.81 | 29 |
| Orbitofrontal ctx | M | 11 | 0 | 32 | −14 | 4.01 | 35 |
| Cingulate Gyrus | M | 24 | 0 | 1 | 28 | 2.6 | 23 |
| Fusiform gyrus | R | 37 | 49 | −53 | −20 | 4.06 | 21 |
PFC= prefrontal cortex; ctx=cortex; H=hemisphere; BA=Brodmann Area; L=left; R=right; M=medial
Figure 6.
Age differences in hippocampal (HC) connectivity. L = Left; R = Right; VLPFC = ventrolateral prefrontal cortex; Cing. = Cingulate; SupPFC = Superior prefrontal cortex; HC = hippocampus; PHG = parahippocampal gyrus; FUS = fusiform.
DISCUSSION
The current study used event-related fMRI to examine the effects of aging on the neural correlates of SM and IM encoding. The study yielded four main findings. First, hippocampal activity associated with successful SM encoding was reduced in older adults compared to young adults. Second, PFC activity associated with successful SM encoding was also reduced in older adults compared to young adults. In both hippocampus and PFC, age-related reductions in successful encoding activity were significant for SM but not for IM. Third, compared to young adults, older adults also showed reduced successful encoding activity in bilateral FFA and PPA regions. Unlike reductions in hippocampal and PFC regions, reductions in FFA and PPA reductions were similar for SM and IM encoding. Taken together the first three findings indicate that age-related changes in hippocampal and PFC functions have a greater impact on SM compared to IM, whereas age-related changes in ventral visual regions have similar effects on SM and IM. Finally, functional connectivity analyses indicated that hippocampal connectivity shifts from stronger interactions with posterior cortices in young adults, to stronger interaction with anterior cortices, including PFC, in older adults. The latter change may reflect functional compensation for a decline in hippocampal function. The four main findings are discussed in separate sections below.
Medial Temporal Lobes
Consistent with our first prediction, the hippocampus showed an age-related reduction in successful encoding activations (Dm) in the SM condition (see Figure 2; 4a). The hippocampal reduction was significant in the SM condition but not in the IM conditions (face and scene). These findings are consistent with the assumption that age-related changes in hippocampal function have a greater impact on SM than on IM. It should be noted, however, that even if the age-related Dm reductions in the hippocampus were significant for SM but not for IM, differences between SM and IM conditions were more graded than absolute. A graded pattern is not inconsistent with the idea that the hippocampus plays a more critical role in SM than in IM, in part because memory conditions are never pure. For example, single faces may elicit mental associations (e.g., “this person seems friendly”), which may contribute to the ability to recognize the face in a subsequent test (i.e., recollection). Also, although we classified memory for scenes as IM, scenes are complex stimuli involving multiple associations, which is an important component of SM.
At a theoretical level, the finding that older adults showed reduced Source Dm in the hippocampus is consistent with accounts that emphasize the role of binding deficits in explaining older adults' difficulties with SM (Johnson, Hashtroudi, & Lindsay, 1993; Naveh-Benjamin, 2000). For example, Naveh-Benjamin's (2000) association deficit hypothesis posits that older adults have a difficulty in forming and retrieving associations between different pieces of information. While this behavioral theory posits no functional or anatomical conjectures as to the neural basis of this hypothesized deficit, subsequent research has firmly linked the hippocampus to both binding (e.g., Davachi & Wagner, 2002; Prince, Daselaar, & Cabeza, 2005; Sperling et al., 2001) and relational memory processes (Eichenbaum, 1999). Thus, given the presumed role of the hippocampus in relational memory, our first finding is consistent with previous behavioral studies suggesting that older adults have a specific deficit in binding individual items into a coherent representation (Chalfonte & Johnson, 1996; Johnson, Hashtroudi, & Lindsay, 1993). Combined with recent work indicating that age-related differences in SM are unaffected by culture (Chua, Chen, & Park, 2006), the current study suggests that, despite differences in stimuli processing across cultural boundaries (Gutchess, Welsh, Boduroglu, & Park, 2006), age deficits in SM associated with the hippocampus may be universal (see also Goh et al., 2007) and a direct result of neural and functional changes in aging.
At an empirical level, our first finding extends available functional neuroimaging evidence linking SM decline to age-related changes in hippocampal function. For example, the fMRI study by Mitchell et al (2000) found an age-related reduction in hippocampal activity during binding in working memory. Young participants showed greater hippocampal activity while simultaneously maintaining object and location information than while maintaining these features independently. Older adults did not show this difference. The present study extends this finding in two ways: (1) by means of the subsequent memory paradigm, it directly links age-related reduction in hippocampal activity specifically to a deficit in successful episodic encoding operations, and (2) by testing memory after a longer delay, it demonstrates the contribution of the hippocampal reduction to long-term SM decline in older adults. Our first finding also extends the results of a previous study from our laboratory (Daselaar, Fleck, & Cabeza, 2006), in which we found an age-related reduction in hippocampal activity related to the recollection of single words during retrieval. The present finding broadens this finding by demonstrating a hippocampal reduction during encoding rather than during retrieval, and by testing memory for associations between distinct items (faces and scenes).
One interesting difference between our word recollection fMRI study (Daselaar, Fleck, & Cabeza, 2006) and the present study is that in the former study age-related reductions in recollection-related activity in the hippocampus were accompanied by an age-related increase in familiarity-related activity in rhinal cortex. We interpreted the latter finding as reflecting functional compensation. The dissociation between the effects of aging on the two MTL regions is consistent with their presumed roles in recollection and familiarity (e.g., Brown & Aggleton, 2001) and their differential sensitivity to age-related atrophy (Raz et al., 2005). One possible explanation for the absence of an age-related increase in rhinal activity in the present study could be that this effect is specific to retrieval. Another possibility is that familiarity cannot compensate for reductions in recollection in source memory tests (e.g., identical vs. recombined face-scene pairs), because these tests measure mainly recollection (Hockley & Consoli, 1999; Yonelinas, 1997). The lack of MTL (or PFC) compensatory activations may also suggest that while older adults do not bind memories as effectively as younger adults during encoding, they may engage in compensatory processes during recognition when retrieving memory details (i.e., item and source). We are presently investigating this theory. At any rate, further research is required to determine the conditions under which older adults do or do not recruit familiarity-related rhinal activity to compensate for reductions in recollection-related hippocampal activity.
Prefrontal Cortex
Consistent with our second prediction, PFC regions showed an age-related reduction in successful encoding activations (Dm) in the SM condition (see Figure 3; 4b and c). Within the PFC regions that showed age-related reductions in Source Dm, age-related decreases in Item Dm (face and scene) were not significant. These findings are consistent with the assumption that PFC decline in older adults have a greater impact on SM than on IM. However, there were other PFC regions where significant age-related Dm reductions were also found for IM (see Table 5). Thus, we conclude that changes in PFC function contribute to SM decline in older adults, but they may also contribute to their IM decline.
The finding that older adults showed reduced Source Dm in PFC is consistent with accounts that emphasize the contribution of frontal lobe decline to cognitive deficits in older adults (Moscovitch & Winocur, 1995; West, 1996). Within the memory domain, this finding is consistent with the hypothesis that PFC regions mediate the organization of incoming information during encoding (e.g., Johnson, 1992; Moscovitch, 1992). Among other findings, this idea is supported by evidence that age-related decline in binding is augmented when participants are given intentional encoding instructions, which tend to benefit young adults more than older adults (Naveh-Benjamin, 2000). Also, there is evidence that older adults do not spontaneously use encoding strategies or use only shallow strategies (e.g., Naveh-Benjamin, 2000; Smith, Park, Earles, Shaw, & Whiting, 1998). In the present study, participants were not provided with an explicit strategy for the N-back task, and hence, it is likely that older adults were not as effective as young adults in integrating the faces and the scenes in the Source condition.
Additionally, a theory posited by Simons and colleagues suggests that limitations in executive and attentional resources, mediated by the PFC, contribute to SM deficits in aging (Simons, Dodson, Bell, & Schacter, 2004). This theory builds on that of Spencer and Raz (1995) who also showed that attention was a necessary component of SM. While both theories are based primarily on behavioral evidence, the current study lends neuroimaging support, finding age deficits in right PFC for SM greater than IM. With executive and attentional processes linked to frontal functioning (for a review see Giesbrecht, Kingstone, Handy, Hopfinger, & Mangun, 2006), the current findings support the idea that limited resources in older adults hinder processes necessary for forming complex source memories. While recent evidence also indicates that age-related attentional deficits in this region occur during IM (Dennis, Daselaar, & Cabeza, 2007), previous research concludes that SM places greater demands on these resources (Gagnon, Soulard, Brasgold, & Kreller, 2007). It is also possible that frontal regions mediate other tasks, such as binding in the hippocampus, possibly through top-down control mechanisms. While this theory is not incompatible with the aforementioned theory, more work is necessary to distinguish the exact role of frontal cortices in SM encoding.
Previous research has also indicated that older adults often recruit additional PFC regions not activated by young adults (for reviews see Cabeza, 2002; Dennis & Cabeza, 2008). In the present study, age-related Dm increases within PFC were found in the Scene condition but not in the Face and Source conditions. A possible explanation is that scenes are highly memorable stimuli that allowed older adults to deploy compensatory strategies (Smith & Park, 1991), whereas faces and face-scene pairs are more demanding stimuli that left older adults with fewer cognitive resources available for compensation. One reason why scenes are easier to remember is that they provide rich and distinctive perceptual information, and at the same time they are nameable (e.g., pine forest, beach) and easier to link to preexisting knowledge (e.g., pine forests are found in colder climates). Thus, one may speculate that in the case of scenes, older adults were able to recruit compensatory processes based on their preserved knowledge and semantic processing abilities (Burke & Shafto, in press). In contrast, unfamiliar faces are meaningless stimuli that older adults find difficult to remember (Bartlett & Leslie, 1986; Bartlett, Leslie, Tubbs, & Fulton, 1989; Boutet & Faubert, 2006; Grady, Bernstein, Beig, & Siegenthaler, 2002), and hence, less amenable to compensation. Finally, face-scene pairs are completely arbitrary and very demanding in terms of processing resources, leaving few resources available for compensation. The assumption that processing of scenes was easier is supported by our pilot studies, which lead to the decision of using longer study-test delays for scenes than for faces and pairs in order to attenuate performance differences across conditions and groups. This assumption is also supported by smaller age-related reductions in brain activity for scenes than for faces and pairs. The finding of age-related increases in PFC activity in the easiest memory condition is consistent with a functional neuroimaging study (Cabeza, Anderson, Houle, Mangels & Nyberg, 2000) in which older adults showed greater PFC activity than young adults in the IM condition (old/new recognition) but not in the SM condition (recency discrimination). Thus, older adults may show compensatory PFC activity only when enough resources are available.
Inferior Temporal Cortex
Although IT regions may not play a direct role in binding per se, they are critical for the identification and differentiation of stimuli, which in turn is critical for forming distinctive memory representations. The current study found age-related reductions in source Dm in bilateral PPA and FFA (see Figure 5). However, when age-related reductions in item Dm were excluded, the difference in source Dm disappeared, indicating that age-related Dm reductions in PPA and FFA were common for SM and IM encoding.
Age-related reduction in PPA and FFA activity are consistent with previous subsequent memory fMRI studies of IM and aging (Dennis, Daselaar, & Cabeza, 2007; Gutchess et al., 2005). This finding suggests that older adults may experience age-related reductions in encoding or differentiating fine details necessary for later memory. Such reductions in item-specific processing are not uncommon in aging. As noted, previous research suggests that older adults rely less on item-specific details in memory, and more on familiarity or gist processing (e.g., Dennis, Kim, & Cabeza, 2007). While this age-related shift from recollection and detail-oriented memory processing to a more familiarity-based strategy has been most often linked to changes in hippocampal function, it may also be driven by a deficit in the initial visual processing of item-specific details. With younger adults showed greater activity in perceptual processing regions such as FFA and PPA for all memory conditions, including SM, evidence suggests that the quality of the incoming information may simply be greater in young adults. These results in turn lend support to Marcia Johnson’s Source Monitoring Framework (Johnson, Hashtroudi, & Lindsay, 1993), which posits that SM depends on the quality of incoming information, and hence, any factor that impoverishes this information will also tend to reduce the ability to make accurate SM decisions. Moreover, research based on this framework finds that SM depends on stronger differentiation than IM (Johnson, Kounios, & Reeder, 1994), and a high degree of perceptual similarity between encoding events impairs older adults’ SM performance (Ferguson, Hashtroudi, & Johnson, 1992). At the same time, young adults showed greater connectivity between these perceptual processing regions and the region assumed to bind such incoming information, the hippocampus. To the extent that the current study used many faces and scenes as stimuli, older adults may have experienced difficulty encoding distinctive perceptual details of a given trial. As such, the impoverished perceptual trace may not have been enough to aid in SM judgments. Reduced perceptual encoding may arise from failures of item-specific processing regions. Dedifferentiation of visual cortices in aging (Chee et al., 2006; Park et al., 2004; Payer et al., 2006) may contribute to this encoding impairment.
Connectivity
Functional neuroimaging studies have shown that age-related cognitive changes reflect not only altered activity in individual brain regions but also changes in functional connectivity among these regions (Cabeza, McIntosh, Tulving, Nyberg, & Grady, 1997; Della-Maggiore et al., 2000; Grady et al., 1994; McIntosh et al., 1999). Thus, in order to understand the age-related decline in SM, it is not only important to examine age-related neural differences associated with each component of the SM network, but also to examine differences in connectivity between regions. Because the hippocampus lies at the center of this network, we assessed age differences in whole-brain connectivity with function in this binding region. Results showed that older adults exhibited enhanced functional coupling between the hippocampus and PFC regions, but reduced functional connectivity with IT regions involved in perceptual processing. Taken together with the aforementioned age-related Dm reductions in PPA and FFA, this finding suggests that whereas younger adults are able to bind visual information, perhaps with more efficient use of perceptually driven or automatic processes, older adults are less efficient in these processes. Increased frontal connectivity in older adults may therefore reflect a shift in the recruited SM encoding network. This shift may be related to strategic differences employed by older adults in order to perform the task successfully. However, while older adults exhibit increased hippocampal-frontal coupling, they do no exhibit increased frontal activations, compared to young adults. This evidence suggests that while they may be attempting to employ their ‘typical’ compensatory strategy, i.e., increased frontal engagement (for a review see Cabeza, 2002; Dennis and Cabeza, 2008; Davis et al., in press), already drained resources (resources that are at their limit under SM conditions) may limit the effectiveness of this strategy.
This age-related change in neural connectivity with the hippocampus is consistent with a Posterior to Anterior Shift in Aging (PASA) that has been reported in many functional neuroimaging studies across a wide range of cognitive processes, including visual perception, attention, encoding, and retrieval (for a review see Dennis & Cabeza, 2008). One possible interpretation of PASA is that older adults compensate for processing deficits in posterior brain regions by recruiting more anterior brain regions, particularly the frontal lobes. While our analyses of Source Dm activity did not yield age-related increase in PFC activity, connectivity results suggest that older adults do show increased engagement of hippocampal-frontal interactions associated with source encoding, consistent with PASA. The similarities and differences between recruiting a region by increasing its interactions with other components of the network versus recruiting a region by increasing its level of activity are unknown and deserve further study in both young and older adults.
CONCLUSIONS
This is the first study to investigate the neural mechanisms that underlie age-related impairments in episodic SM binding. Our findings indicate that older adults exhibit a more pronounced difficulty in recruiting hippocampal and PFC regions to support successful SM encoding, compared to that seen for successful IM encoding. These results are consistent with cognitive hypotheses that proposes that, compared to young adults, older adults are impaired in binding or associating units of information together to form a cohesive memory (Chalfonte & Johnson, 1996; Naveh-Benjamin, 2000; Naveh-Benjamin, Brav, & Levy, 2007). While automatic binding is assumed to be mediated by the hippocampus, strategic operations managing this binding process are assumed to be mediated by PFC regions (Johnson, Hashtroudi, & Lindsay, 1993). Older adults also exhibited age-related reductions in stimulus-specific processing regions for both IM and SM encoding. Consistent with previous research indicating age deficits in stimulus-specific processing (Park et al., 2004) results suggest an age deficit in processing incoming information necessary for binding. Finally, connectivity analyses using the hippocampus as a seed region revealed a posterior-anterior shift in age-related activity that is co-activated with the hippocampus. The age-related increase hippocampal-prefrontal connectivity may serve a compensatory function (Dennis & Cabeza, 2008). Taken together, the results suggest that disruptions in the neural processes mediating successful SM encoding may contribute to SM deficits in older adults.
Acknowledgments
Nancy A. Dennis, Center for Cognitive Neuroscience, Duke University, and Center for the Study of Aging and Human Development, Duke University Medical Center; Scott M. Hayes, Center for Cognitive Neuroscience, Duke University; Steven E. Prince, Center for Cognitive Neuroscience, Duke University, and Brain Imaging and Analysis Center, Duke University Medical Center; David J. Madden, Center for the Study of Aging and Human Development, and Brain Imaging and Analysis Center, Duke University Medical Center; Scott A. Huettel, Center for Cognitive Neuroscience, Duke University, and Brain Imaging and Analysis Center, Duke University Medical Center; Roberto Cabeza, Center for Cognitive Neuroscience, Duke University, Brain Imaging and Analysis Center, and Center for the Study of Aging and Human Development, Duke University Medical Center.
This work was supported by NIA grants R01 AG019731 and R01 AG23770 awarded to RC, NIA grant R01 AG011622 awarded to DJM, and R01 MH-70685 awarded to SAH. NAD was supported by NIA grant T32 AG000029. SMH was supported by NIA grant F32 AG029738. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, National Institute of Mental Health, or the National Institutes of Health. The authors wish to thank Susanne Harris and Christy Krupa for assistance with recruiting, Amber Tarter for assistance with piloting and testing, James Kragel for assistance with analysis, and Simon W. Davis, Peggy St. Jacques, Elsa Baena, and Micah Adams for assistance with preparation of the manuscript. Portions of the research in this paper use the Color FERET database of facial images collected under the FERET program.
Footnotes
The currents set of analyses were repeated contrasting subsequent high-confidence hits to subsequent miss trials (excluding low-confidence hits from the analysis); all main findings were replicated. The analysis was also repeated fitting temporal derivatives to the model. Again, all main findings were replicated.
REFERENCES
- Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FI. The effects of divided attention on encoding- and retrieval-related brain activity: A PET study of younger and older adults. Journal of Cognitive Neuroscience. 2000;12(5):775–792. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Leslie JE. Aging and memory for faces versus single views of faces. Memory & Cognition. 1986;14(5):371–381. doi: 10.3758/bf03197012. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Leslie JE, Tubbs A, Fulton A. Aging and memory for pictures of faces. Psychology & Aging. 1989;4(3):276–283. doi: 10.1037//0882-7974.4.3.276. [DOI] [PubMed] [Google Scholar]
- Bastin C, Van der Linden M. The contribution of recollection and familiarity to recognition memory: a study of the effects of test format and aging. Neuropsychology. 2003;17(1):14–24. [PubMed] [Google Scholar]
- Bernstein LJ, Beig S, Siegenthaler AL, Grady CL. The effect of encoding strategy on the neural correlates of memory for faces. Neuropsychologia. 2002;40(1):86–98. doi: 10.1016/s0028-3932(01)00070-7. [DOI] [PubMed] [Google Scholar]
- Boutet I, Faubert J. Recognition of faces and complex objects in younger and older adults. Memory & Cognition. 2006;34(4):854–864. doi: 10.3758/bf03193432. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience. 2001;2(1):51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Burke DM, Shafto M. Aging and Language Production. Current Directions in Psychological Science. doi: 10.1111/j.0963-7214.2004.01301006.x. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Kaszniak AW, Glisky EL, Eslinger PJ, Schacter DL. Recency discrimination deficits in frontal lobe patients. Neuropsychology. 1994;8:343–353. [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, et al. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. Journal of Neuroscience. 1997;17(1):391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Locantore JK, Anderson ND. Lateralization of prefrontal activity during episodic memory retrieval: evidence for the production-monitoring hypothesis. J Cogn Neurosci. 2003;15(2):249–259. doi: 10.1162/089892903321208187. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Mangels J, Nyberg L, Habib R, Houle S, McIntosh AR, et al. Brain regions differentially involved in remembering what and when: a PET study. Neuron. 1997;19(4):863–870. doi: 10.1016/s0896-6273(00)80967-8. [DOI] [PubMed] [Google Scholar]
- Cabeza R, McIntosh AR, Tulving E, Nyberg L, Grady CL. Age-related differences in effective neural connectivity during encoding and recall. Neuroreport. 1997;8(16):3479–3483. doi: 10.1097/00001756-199711100-00013. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cerebral Cortex. 2002;12(10):1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory & Cognition. 1996;24(4):403–416. doi: 10.3758/bf03200930. [DOI] [PubMed] [Google Scholar]
- Chee MW, Goh JO, Venkatraman V, Tan JC, Gutchess A, Sutton B, et al. Age-related changes in object processing and contextual binding revealed using fMR adaptation. Journal of Cognitive Neuroscience. 2006;18(4):495–507. doi: 10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Chua HF, Chen W, Park DC. Source memory, aging and culture. Gerontology. 2006;52(5):306–313. doi: 10.1159/000094612. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza RE. Triple Dissociation in the Medial Temporal Lobes: Recollection, Familiarity, and Novelty. Journal of Neurophysiology. 2006 doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cerebral Cortex. 2006;16(12):1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. Journal of Neurophysiology. 2002;88(2):982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Davidson PS, Glisky EL. Neuropsychological correlates of recollection and familiarity in normal aging. Cognitive, Affective & Behavioral Neuroscience. 2002;2(2):174–186. doi: 10.3758/cabn.2.2.174. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SA, Fleck MS, Cabeza R. Qué PASA? The Posterior-Anterior Shift in Aging. Cerebral Cortex. doi: 10.1093/cercor/bhm155. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, Sekuler AB, Grady CL, Bennett PJ, Sekuler R, McIntosh AR. Corticolimbic interactions associated with performance on a short-term memory task are modified by age. Journal of Neuroscience. 2000;20(22):8410–8416. doi: 10.1523/JNEUROSCI.20-22-08410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of Healthy Cognitive Aging. In: Salthouse TA, Craik FEM, editors. Handbook of Aging and Cognition. 3rd edition. New York: Psychological Press; 2008. pp. 1–56. [Google Scholar]
- Dennis NA, Daselaar S, Cabeza R. Effects of aging on transient and sustained successful memory encoding activity. Neurobiology of Aging. 2007;28:11. doi: 10.1016/j.neurobiolaging.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Kim HK, Cabeza R. Effects of aging on the neural correlates of true and false memory formation. Neuropsychologia. 2007;45:3157–3166. doi: 10.1016/j.neuropsychologia.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Miller SL, Greve DN, Dale AM, Albert MS, Schacter DL, et al. Prefrontal-hippocampal-fusiform activity during encoding predicts intraindividual differences in free recall ability: An event-related functional-anatomic MRI study. Hippocampus. 2007 doi: 10.1002/hipo.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Rice HJ, Wagner AD, Schacter DL. Memory orientation and success: separable neurocognitive components underlying episodic recognition. Neuropsychologia. 2003;41(3):318–333. doi: 10.1016/s0028-3932(02)00164-1. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behavioural Brain Research. 1999;103(2):123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The Medial Temporal Lobe and Recognition Memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392(6676):598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Hashtroudi S, Johnson MK. Age differences in using source-relevant cues. Psychology and Aging. 1992;7(3):443–452. doi: 10.1037//0882-7974.7.3.443. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical methods for Research Workers. London: Oliver and Boyd; 1950. [Google Scholar]
- Gagnon S, Soulard K, Brasgold M, Kreller J. Effects of normal aging on memory for multiple contextual features. Brain and Cognition. 2007;64(3):208–216. doi: 10.1016/j.bandc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Kingstone A, Handy TC, Hopfinger JB, Mangun GR. Functional neuroimaging of attention. In: Kingstone A, Cabeza R, editors. Handbook on Functional Neuroimaging of Cognition. Cambridge, MA: MIT Press; 2006. pp. 85–112. [Google Scholar]
- Giovanello KS, Verfaellie M, Keane MM. Disproportionate deficit in associative recognition relative to item recognition in global amnesia. Cognitive, Affective & Behavioral Neuroscience. 2003;3(3):186–194. doi: 10.3758/cabn.3.3.186. [DOI] [PubMed] [Google Scholar]
- Goh JO, Chee MW, Tan JC, Venkatraman V, Hebrank A, Leshikar ED, et al. Age and culture modulate object processing and object-scene binding in the ventral visual area. Cogn Affect Behav Neurosci. 2007;7(1):44–52. doi: 10.3758/cabn.7.1.44. [DOI] [PubMed] [Google Scholar]
- Grady CL, Bernstein LJ, Beig S, Siegenthaler AL. The effects of encoding task on age-related differences in the functional neuroanatomy of face memory. Psychology and Aging. 2002;17(1):7–23. doi: 10.1037//0882-7974.17.1.7. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, et al. Age-related changes in cortical blood flow activation during visual processing of faces and location. Journal of Neuroscience. 1994;14(3 Pt 2):1450–1462. doi: 10.1523/JNEUROSCI.14-03-01450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13(5):572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Boduroglu A, Park DC. Cultural differences in neural function associated with object processing. Cogn Affect Behav Neurosci. 2006;6(2):102–109. doi: 10.3758/cabn.6.2.102. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, et al. Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial-temporal activity. Journal of Cognitive Neuroscience. 2005;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Ryan L, Schnyer DM, Nadel L. An fMRI study of episodic memory: retrieval of object, spatial, and temporal information. Behavioral Neuroscience. 2004;118(5):885–896. doi: 10.1037/0735-7044.118.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke K, Weber B, Kneifel S, Wieser HG, Buck A. Human hippocampus associates information in memory. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(10):5884–5889. doi: 10.1073/pnas.96.10.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. Journal of Neuroscience. 1999;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain. 1999;122(Pt 7):1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Hockley WE, Consoli A. Familiarity and recollection in item and associative recognition. Memory and Cognition. 1999;27(4):657–664. doi: 10.3758/bf03211559. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27(8):1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Johnson MK. MEM: Mechanisms of recollection. Journal of Cognitive Neuroscience. 1992;4(3):268–280. doi: 10.1162/jocn.1992.4.3.268. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Hashtroudi S, Lindsay DS. Source monitoring. Psychological Bulletin. 1993;114(1):3–28. doi: 10.1037/0033-2909.114.1.3. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Kounios J, Reeder JA. Time-course studies of reality monitoring and recognition. Journal of Experimental Psychology: Learning, Memory and Cognition. 1994;20(6):1409–1419. doi: 10.1037//0278-7393.20.6.1409. [DOI] [PubMed] [Google Scholar]
- Kan IP, Giovanello KS, Schnyer DM, Makris N, Verfaellie M. Role of the medial temporal lobes in relational memory: Neuropsychological evidence from a cued recognition paradigm. Neuropsychologia. 2007;45(11):2589–2597. doi: 10.1016/j.neuropsychologia.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17(11):4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausler DH, Puckett JM. Adult age differences in memory for modality attributes. Experimental Aging Research. 1981a;7(2):117–125. doi: 10.1080/03610738108259794. [DOI] [PubMed] [Google Scholar]
- Kausler DH, Puckett JM. Adult age differences in memory for sex of voice. J Gerontol. 1981b;36(1):44–50. doi: 10.1093/geronj/36.1.44. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with the successful encoding of item, but not source, information for positive and negative stimuli. Journal of Neuroscience. 2006;26(9):2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Crane J, Milner B. Differential contributions of the parahippocampal place area and the anterior hippocampus to human memory for scenes. Hippocampus. 2002;12(6):718–723. doi: 10.1002/hipo.10077. [DOI] [PubMed] [Google Scholar]
- Kohler S, Moscovitch M, Winocur G, McIntosh AR. Episodic encoding and recognition of pictures and words: role of the human medial temporal lobes. Acta Psychol (Amst) 2000;105(2–3):159–179. doi: 10.1016/s0001-6918(00)00059-7. [DOI] [PubMed] [Google Scholar]
- Kostopoulos P, Petrides M. The mid-ventrolateral prefrontal cortex: insights into its role in memory retrieval. European Journal of Neuroscience. 2003;17(7):1489–1497. doi: 10.1046/j.1460-9568.2003.02574.x. [DOI] [PubMed] [Google Scholar]
- Kroll NEA, Knight RT, Metcalfe J, Wolf FS, Tulving E. Coehension failure as a source of memory illusions. Journal of Memory & Language. 1996;35:176–196. [Google Scholar]
- Kuskowski MA, Pardo JV. The role of the fusiform gyrus in successful encoding of face stimuli. Neuroimage. 1999;9(6 Pt 1):599–610. doi: 10.1006/nimg.1999.0442. [DOI] [PubMed] [Google Scholar]
- Lazar NA, Luna B, Sweeney JA, Eddy WF. Combining brains: a survey of methods for statistical pooling of information. Neuroimage. 2002;16(2):538–550. doi: 10.1006/nimg.2002.1107. [DOI] [PubMed] [Google Scholar]
- Martinez AM, Benavente R. The AR face database. CVC Technical Report #24. 1998 [Google Scholar]
- McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. Journal of Cognitive Neuroscience. 1997;9(5):605–610. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Sekuler AB, Penpeci C, Rajah MN, Grady CL, Sekuler R, et al. Recruitment of unique neural systems to support visual memory in normal aging. Current Biology. 1999;9(21):1275–1278. doi: 10.1016/s0960-9822(99)80512-0. [DOI] [PubMed] [Google Scholar]
- Milner B, Corsi P, Leonard G. Frontal-lobe contribution to recency judgements. Neuropsychologia. 1991;29(6):601–618. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- Minear M, Park DC. A lifespan database of adult facial stimuli. Behav Res Methods Instrum Comput. 2004;36(4):630–633. doi: 10.3758/bf03206543. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D'Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain Research: Cognitive Brain Research. 2000;10(1–2):197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: a component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Winocur G. Frontal lobes, memory, and aging. Annals of the New York Academy of Sciences. 1995;769:119–150. doi: 10.1111/j.1749-6632.1995.tb38135.x. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory and Cognition. 2000;26(5):1170–1187. doi: 10.1037//0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Brav TK, Levy O. The associative memory deficit of older adults: the role of strategy utilization. Psychology and Aging. 2007;22(1):202–208. doi: 10.1037/0882-7974.22.1.202. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M, Craik FI. Memory for context and its use in item memory: comparisons of younger and older persons. Psychology and Aging. 1995;10(2):284–293. doi: 10.1037//0882-7974.10.2.284. [DOI] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, D'Esposito M. Left prefrontal activation during episodic remembering: an event-related fMRI study. Neuroreport. 1998;9(15):3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6(2):93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(35):13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Puglisi JT. Older adults' memory for the color of pictures and words. Journal of Gerontology. 1985;40(2):198–204. doi: 10.1093/geronj/40.2.198. [DOI] [PubMed] [Google Scholar]
- Park DC, Puglisi JT, Lutz R. Spatial memory in older adults: effects of intentionality. Journal of Gerontology. 1982;37(3):330–335. doi: 10.1093/geronj/37.3.330. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychology and Aging. 1992;7:290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Payer D, Marshuetz C, Sutton B, Hebrank A, Welsh RC, Park DC. Decreased neural specialization in old adults on a working memory task. Neuroreport. 2006;17(5):487–491. doi: 10.1097/01.wnr.0000209005.40481.31. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Archives of General Psychiatry. 1998;55(10):905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Phillips PJ, Moon H, Rizvi SA, Rauss PJ. The FERET Evaluation Methodology for Face Recognition Algorithms. IEEE Trans Pattern Analysis and Machine Intelligence. October2000;22:1090–1104. [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. J Neurosci. 2005;25(5):1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Heller A, Cohen MX, Brozinsky CJ, Rissman J. Functional connectivity with the hippocampus during successful memory formation. Hippocampus. 2005;15(8):997–1005. doi: 10.1002/hipo.20141. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D'Esposito M. Left anterior prefrontal activation increases with demands to recall specific perceptual information. J Neurosci. 2000;20(22):RC108. doi: 10.1523/JNEUROSCI.20-22-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42(1):2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Raz N. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In: Craik FI, Salthouse TA, editors. The Handbook of Aging and Cognition. Mahwah, NJ: Erlbaum; 2000. [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. Journal of Neuroscience. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23(2):752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Chua PM, Dolan RJ. The role of the prefrontal cortex in recognition memory and memory for source: an fMRI study. Neuroimage. 1999;10(5):520–529. doi: 10.1006/nimg.1999.0488. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Osowiecki D, Kaszniak AW, Kihlstrom JF, Valdiserri M. Source memory: extending the boundaries of age-related deficits. Psychol Aging. 1994;9(1):81–89. doi: 10.1037//0882-7974.9.1.81. [DOI] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24(49):11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28(8):803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Simons JS, Dodson CS, Bell D, Schacter DL. Specific- and partial-source memory: effects of aging. Psychol Aging. 2004;19(4):689–694. doi: 10.1037/0882-7974.19.4.689. [DOI] [PubMed] [Google Scholar]
- Smith AD, Park DC. The failure to find adult age differences in scene memory. Exp Aging Res. 1991;17(2):92. [PubMed] [Google Scholar]
- Smith AD, Park DC, Earles JL, Shaw RJ, Whiting WLt. Age differences in context integration in memory. Psychol Aging. 1998;13(1):21–28. doi: 10.1037//0882-7974.13.1.21. [DOI] [PubMed] [Google Scholar]
- Solina F, Peer P, Batageli B, Juvan S, Kovac J. Color-based face detection in the “15 seconds of fame” art installation. Paper presented at the Conference on Computer Vision / Computer Graphics Collaboration for Model-based Imaging, Rendering, Image Analysis and Graphical Special Effects, INRIA Rocquencourt; 2003, March 10–11; France. [Google Scholar]
- Sommer T, Rose M, Weiller C, Buchel C. Contributions of occipital, parietal and parahippocampal cortex to encoding of object-location associations. Neuropsychologia. 2005;43(5):732–743. doi: 10.1016/j.neuropsychologia.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Madden DJ, Voss A. A diffusion model analysis of adult age differences in episodic and semantic long-term memory retrieval. J Exp Psychol Learn Mem Cogn. 2006;32(1):101–117. doi: 10.1037/0278-7393.32.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychology and Aging. 1995;10(4):527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, et al. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003;20(2):1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, et al. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. Journal of Neurol Neurosurg Psychiatry. 2003;74(1):44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Cocchiarella AJ, Schacter DL, Rosen BR, Albert MS. Encoding novel face-name associations: a functional MRI study. Human Brain Mapping. 2001;14(3):129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Hippocampal damage equally impairs memory for single items and memory for conjunctions. Hippocampus. 2003;13(2):281–292. doi: 10.1002/hipo.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart; 1988. [Google Scholar]
- Turriziani P, Fadda L, Caltagirone C, Carlesimo GA. Recognition memory for single items and for associations in amnesic patients. Neuropsychologia. 2004;42(4):426–433. doi: 10.1016/j.neuropsychologia.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin. 1996;120(2):272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Recognition memory ROCs for item and associative information: the contribution of recollection and familiarity. Memory and Cognition. 1997;25(6):747–763. doi: 10.3758/bf03211318. [DOI] [PubMed] [Google Scholar]