Abstract
OBJECTIVE
The purpose of this work was to evaluate therapy for patent ductus arteri-osus as a risk factor for death or neurodevelopmental impairment at 18 to 22 months, bronchopulmonary dysplasia, or necrotizing enterocolitis in extremely low birth weight infants.
METHODS
We studied infants in the National Institute of Child Health and Human Development Neonatal Research Network Generic Data Base born between 2000 and 2004 at 23 to 28 weeks’ gestation and at <1000-g birth weight with patent ductus arteriosus. Patent ductus arteriosus therapy was evaluated as a risk factor for outcomes in bivariable and multivariable analyses.
RESULTS
Treatment for subjects with patent ductus arteriosus (n = 2838) included 403 receiving supportive treatment only, 1525 treated with indomethacin only, 775 with indomethacin followed by secondary surgical closure, and 135 treated with primary surgery. Patients who received supportive therapy for patent ductus arteriosus did not differ from subjects treated with indomethacin only for any of the outcomes of interest. Compared with indomethacin treatment only, patients undergoing primary or secondary surgery were smaller and more premature. When compared with indomethacin alone, primary surgery was associated with increased adjusted odds for neurodevelopmental impairment and bronchopulmonary dysplasia in multivariable logistic regression. Secondary surgical closure was associated with increased odds for neurodevelopmental impairment and increased adjusted odds for bronchopulmonary dysplasia but decreased adjusted odds for death. Risk of necrotizing enterocolitis did not differ among treatments. Indomethacin prophylaxis did not significantly modify these results.
CONCLUSIONS
Our results suggest that infants treated with primary or secondary surgery for patent ductus arteriosus may be at increased risk for poor short- and long-term outcomes compared with those treated with indomethacin. Prophylaxis with indomethacin in the first 24 hours of life did not modify the subsequent outcomes of patent ductus arteriosus therapy.
Keywords: patent ductus arteriosus, bronchopulmonary dysplasia, necrotizing enterocolitis, neurodevelopmental impairment, therapy ductus arteriosus
What’s Known on This Subject.
Optimal therapy for PDA remains controversial. Some ELBW infants with PDA may not require treatment, and the risk of complications of failed indomethacin therapy and subsequent surgical ligation or primary surgical ligation have not been studied extensively.
What This Study Adds.
Our analysis of 2838 infants with PDA in the NRN GDB showed that those treated with indomethacin, when compared with those with supportive care, had similar outcomes, and those with surgical ligation had more complications.
Clinically significant patent ductus arteriosus (PDA) occurs in ~49% of extremely low birth weight (ELBW) infants with weights of 501 to 750 g and 38% of infants with weights of 751 to 1000 g.1,2 Infants with PDA may be at increased risk for necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), or intraventricular hemorrhage (IVH).3–7 The most premature infants are more likely to have a significant PDA, least likely to respond to indomethacin, and more likely to reopen the ductus.8–15
Optimal therapy for PDA remains controversial. The only direct comparison of primary medical versus surgical treatment did not include infants <29 weeks’ gestation and was completed before the widespread use of antenatal glucocorticoids and postnatal surfactant.16,17 The risk of complications of failed indomethacin therapy has not been extensively studied.16–22 Some ELBW infants with PDA may not require treatment.12,23,24 Prophylactic indomethacin therapy has not proven beneficial in large studies, and its relationship to subsequent PDA therapy and outcome are controversial.21,22,25–27
The purpose of this study was to determine associations of treatment for PDA with short- and long-term outcomes in a cohort of ELBW infants. We examined the National Institute of Child Health and Human Development Neonatal Research Network Generic Data Base (NRN GDB) to evaluate the association of treatment type, including supportive medical therapy, indomethacin alone, indomethacin followed by surgery, and primary surgery, on the outcomes of death plus neurodevelopmental impairment (NDI) at 18 to 22 months’ corrected age, BPD, and NEC. We also examined the impact of indomethacin prophylaxis on the outcomes of these treatments. We hypothesized that outcomes would be poorer after surgical closure and that this increased risk would be amplified in infants with birth weights of <750 g.
METHODS
We performed a cohort study using multicenter data prospectively collected for the NRN GDB. Inclusion of infants in the NRN GDB was approved by the institutional review board for each of the sites. The NRN GDB includes infants born at 23 to 28 weeks’ gestation, with birth weights 401 to 1000 g, born at or transferred to 1 of the NRN GDB centers between January 1, 2000, and December 31, 2004. All of the subjects must have survived >72 hours, developed clinically significant PDA, and had 18- to 22-month neurodevelopmental follow-up before October 27, 2006. We excluded subjects with congenital heart disease or chromosomal abnormalities or if they had insufficient follow-up data to determine outcome at 18 to 22 months.
Demographic details and clinical information related to presence of, and treatment for, PDA, as well as outcomes, were collected. Clinically significant PDA was defined as clinical evidence of left to right shunt or echocardiographic evidence of PDA with documentation of left-to-right ductal shunting. Although not required by the NRN GDB, all of the NRN GDB centers confirmed clinical symptoms with echocardiography for the presence of PDA. Therapy for PDA included indomethacin administered for PDA and/or surgical closure of PDA or supportive therapy only. The number of indomethacin courses, dates of administration, and ibuprofen administration were not available data in the NRN GDB, and the study period predated Food and Drug Administration–approved use of ibuprofen in the United States. The mode of PDA treatment was selected by the caretakers on the basis of clinical circumstances, caretaker preferences, or unit guidelines.
Infants were divided into 4 groups reflecting PDA therapy: supportive treatment, indomethacin therapy, indomethacin followed by surgical closure, or primary surgical closure. The supportive treatment group included patients who met the clinical criteria for significant PDA and who received no indomethacin treatment or surgical ligation for PDA. Some patients who received supportive treatment received prophylactic indomethacin before the diagnosis of PDA.
Information was collected regarding indomethacin administration within the first 24 hours of life, whether for PDA or IVH prophylaxis. Details regarding clinicians’ choice of therapy, several complications of prematurity, and therapy for PDA other than indomethacin or surgery were not available. Illness severity scores were unavailable; therefore, the presence of RDS and the number of doses of surfactant were used as markers of illness severity.
Detailed information regarding outcomes was collected, including mortality, BPD defined as use of oxygen >0.21 at 36 weeks’ corrected gestational age, and NEC stage 2 or greater.28 NDI was defined as having ≥1 of the following: moderate-to-severe cerebral palsy, no useful vision in either eye, hearing aids required in each ear, or a score of <70 on either the mental development index or psychomotor development index of the Bayley Scales of Neurodevelopment II. IVH and PVL were not included as outcomes, because they were considered to be strongly correlated with NDI and on the causal pathway of NDI, and these complications occurred in some patients before PDA therapy.
Data Analysis
We compared the 4 PDA treatment groups in bivariable analyses using analysis of variance for continuous demographic variables and χ2 for categorical variables. Subjects who received indomethacin prophylactically were evaluated in bivariable analyses in comparison with those who did not. Next, for each of the outcomes of interest, bivariable analyses were completed using continuity-adjusted χ2 tests for dichotomous predictor variables and binary logistic regression for continuous variables. Subsequently, multivariable logistic regression was performed to evaluate relationships between treatment groups and outcomes. Three pairwise comparisons were planned for each outcome; namely, each nonreference treatment level (supportive treatment or secondary or primary surgery) was compared with the reference treatment level (indomethacin only). To maintain a study-wise type I error rate of ≤5%, a Bonferroni approach was taken, where a P value for a single comparison was considered significant only if <.05/3, or .017. Similarly, confidence intervals for odds ratios (ORs) were given for a 98.3% level of confidence.
All of the models were adjusted for center, gestational age, birth weight, gender, the use of prophylactic indomethacin within the first 24 hours of life, presence or absence of labor, Apgar score at 5 minutes, respiratory distress syndrome (RDS) further categorized as presence of RDS, RDS treated with 1 dose of surfactant or >1 dose of surfactant, growth restriction, antenatal steroids, antenatal (toxoplasmosis, other infections, rubella, cytomegalovirus, herpes simplex virus [TORCH]) and postnatal infection or culture proven sepsis, and maternal marital status and age. Analyses were completed first on the entire cohort described, and then a planned subgroup analysis was performed for infants who weighed <750 g at birth because of the higher risk of adverse outcomes in this group.
RESULTS
Demographics and Descriptive Characteristics
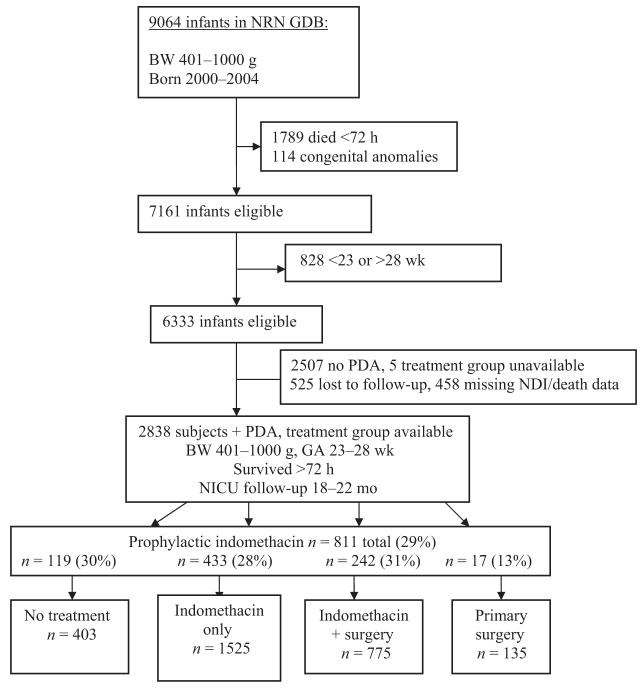
Clinically significant PDA developed in 2838 ELBW infants who met study criteria (Fig 1). A total of 403 infants received supportive treatment only for their PDA, 1525 were treated with indomethacin only, 775 received indomethacin followed by secondary surgical closure, and 135 underwent primary surgical closure. Of the subjects, 811 (29% of the cohort) received indomethacin prophylaxis within the first 24 hours of life, and a statistically significant difference in rates of prophylaxis between treatment groups was found (P <.001; Table 1). A total of 807 infants (28%) survived >72 hours but died before discharge, 396 (14%) developed NEC stage 2 or greater, and 1385 (62%) met criteria for BPD at 36 weeks’ corrected gestational age. Neurodevelopmental impairment was demonstrated at 18 to 22 months’ corrected age in 898 infants (44%). The combined outcome of neurodevelopmental impairment or death occurred in 1705 subjects, or 60% of the cohort. In the cohort of subjects who did not have clinically significant PDA, only 47% had NDI or death.
FIGURE 1.
Study derivation. BW indicates birth weight; GA, gestational age.
TABLE 1.
Demographics Relative to Treatment Groups
| Variable | No Treatment (N = 403) | Indomethacin Only (N = 1525) | Indomethacin and Surgery (N = 775) | Primary Surgery (N = 135) | P |
|---|---|---|---|---|---|
| Birth weight, mean ± SD, g | 758 ± 148 | 745 ± 139g | 719 ± 134 | 726 ± 133 | <.001 |
| Gestational age, mean ± SD, wk | 25.6 ± 1.5 | 25.4 ± 1.3 | 24.8 ± 1.3 | 25.1 ± 1.4 | <.001 |
| Apgar score at 5 min, median (first quartile, third quartile) | 7 (5, 8) | 7 (5, 8) | 6 (5, 8) | 6 (5, 8) | .001 |
| Mother’s age, mean ± SD, y | 26.8 ± 6.3 | 26.8 ± 6.7 | 26.8 ± 6.6 | 26.6 ± 7.5 | .99 |
| Male, n (%) | 211 (52) | 758 (50) | 397 (51) | 77 (57) | .35 |
| Small for gestational age, n (%) | 41 (10) | 150 (10) | 47 (6) | 11 (8) | .02 |
| Antenatal steroids, n (%) | 304 (76) | 1194 (78) | 580 (76) | 101 (75) | .36 |
| Mother single, n (%) | 217 (54) | 795 (52) | 412 (54) | 68 (51) | .86 |
| Indomethacin within 24 h, n (%) | 119 (30) | 433 (28) | 242 (31) | 17 (13) | <.001 |
| RDS, n (%) | .31 | ||||
| None | 80 (20) | 278 (18) | 144 (19) | 29 (22) | |
| Yes, no surfactant | 11 (3) | 69 (5) | 28 (4) | 3 (2) | |
| 1 dose surfactant | 81 (21) | 343 (23) | 145 (19) | 26 (19) | |
| >1 dose surfactant | 222 (56) | 822 (54) | 449 (59) | 76 (57) | |
| Died, n (%) | 140 (35) | 476 (31) | 151 (19) | 40 (30) | <.001 |
P values are from an analysis of variance (means), Kruskal-Wallis test (medians), or χ2 tests.
Bivariable Analyses
Comparison of Baseline Demographic Characteristics on the Basis of Treatment Strategy
Subjects’ descriptive characteristics and demographic information were compared relative to their PDA treatment group (Table 1). When compared with subjects who were treated with indomethacin only (the reference group), infants who received supportive therapy only were almost identical in demographic characteristics, and infants who underwent primary or secondary surgical closure were less mature, smaller at birth, had lower Apgar scores, and were less likely to be small for gestational age.
Comparison of Subjects With and Without NDI/Death, BPD, and NEC
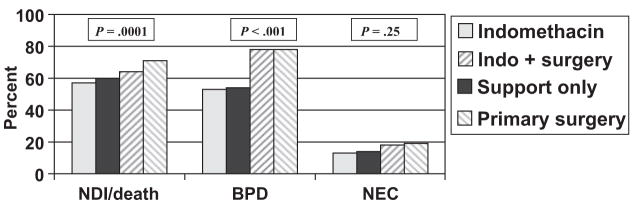
Bivariable analysis revealed statistically significant differences between the treatment groups for NDI or death and for BPD: NDI or death was more likely for infants undergoing primary or secondary surgical closure (ORs: 1.89 and 1.39, respectively) than for infants treated with indomethacin alone or supportive care (OR for supportive treatment: 1.17; Fig 2). Similarly, BPD was more frequent for infants undergoing primary surgery or secondary surgery (ORs: 3.3 and 3.2, respectively; OR for supportive therapy: 1.07; Fig 2). The PDA treatment groups were not significantly different with regard to the incidence of NEC (Fig 2).
FIGURE 2.
Bivariate analysis: treatment group and outcomes. P values are from Pearson χ2 tests of outcome by PDA.
In all of the bivariable analyses for outcomes by treatment, outcomes were similar for the indomethacin only and supportive therapy groups and for the primary and secondary surgery groups. In general, infants with NDI or death, BPD, or NEC were more immature, had lower birth weights, and were more likely to be boys than infants without these outcomes. They were more likely to have had postnatal sepsis or infection and were more likely to have received indomethacin prophylaxis. To control for these factors, a multivariable analysis was done.
Multivariable Logistic Regression Analyses
The risk of NDI or death, BPD, or NEC for subjects receiving supportive treatment alone was not different from those receiving indomethacin therapy (Table 2).
TABLE 2.
Multivariable Analyses Based on Pairwise Comparison Error Rate of 0.017
| Variable | OR Estimate | 98.3% CI | P |
|---|---|---|---|
| NDI/death, overall P = .16 | |||
| Primary surgery vs indomethacin only | 1.54 | 0.90–2.63 | .055 |
| Indomethacin + surgery vs indomethacin only | 1.03 | 0.80–1.33 | .80 |
| No treatment vs indomethacin only | 1.20 | 0.87–1.64 | .18 |
| NDI, overall P = .001 | |||
| Primary surgery vs indomethacin only | 1.79 | 0.998–3.21 | .017 |
| Indomethacin + surgery vs indomethacin only | 1.53 | 1.16–2.03 | <.001 |
| No treatment vs indomethacin only | 1.11 | 0.76–1.63 | .51 |
| Death, overall P <.0001 | |||
| Primary surgery vs indomethacin only | 0.75 | 0.44–1.30 | .22 |
| Indomethacin + surgery vs indomethacin only | 0.46 | 0.35–0.62 | <.0001 |
| No treatment vs indomethacin only | 1.24 | 0.89–1.72 | .13 |
| BPD, overall P <.0001 | |||
| Primary surgery vs indomethacin only | 2.19 | 1.16–4.15 | .003 |
| Indomethacin + surgery vs indomethacin only | 3.10 | 2.26–4.26 | <.001 |
| No treatment vs indomethacin only | 0.92 | 0.63–1.35 | .61 |
| NEC, overall P = .62 | |||
| Primary surgery vs indomethacin only | 1.22 | 0.67–2.24 | .43 |
| Indomethacin + surgery vs indomethacin only | 0.94 | 0.67–1.31 | .65 |
| No treatment vs indomethacin only | 1.16 | 0.75–1.79 | .42 |
The overall P value is from the type 3 effects of the logistic regression. It indicates the effect of treatment on the outcome. Note that the confidence limits for the ORs were calculated by using a level of significance of .017. This was found by setting the study-wise error rate at 0.05 and dividing by this rate by 3 (for the 3 outcomes of interest). Hence, a P value of >.017 should not be considered significant, although a P value of greater than but close to .017 may still be noteworthy. All of the multivariable analyses are adjusted for potential confounders including: center, gestational age, birth weight, gender, prophylactic indomethacin, Apgar score, severe RDS, growth restriction, antenatal steroids, antenatal/postnatal infection, maternal marital status, and age.
NDI or Death
Infants undergoing primary or secondary surgery were not different from infants who received indomethacin alone relative to their risk of NDI or death (Table 2). When NDI alone was evaluated as an outcome, patients undergoing secondary ligation had a higher risk of NDI (OR: 1.53) and a borderline increase in NDI with primary surgery (OR: 1.79; Table 2). Prophylaxis was not associated with a difference in the risk of NDI or death with subsequent treatment in multivariable analysis.
Bronchopulmonary Dysplasia
BPD was more likely to occur in infants who underwent secondary surgical closure after indomethacin therapy compared with indomethacin alone (OR: 3.10; Table 2). Infants who had primary surgical closure had odds of BPD >2 times the odds for those who were treated with indomethacin alone (OR: 2.19; Table 2). Indomethacin prophylaxis did not affect the risk of BPD in any of the treatment groups when examined in multivariable analysis.
Necrotizing Enterocolitis
NEC stage 2 or greater did not differ between the treatment groups (Table 2). In a model that included a borderline significant interaction between indomethacin prophylaxis and therapy, a near-significant increased risk of NEC for infants who received indomethacin prophylaxis was found in those who were subsequently treated with indomethacin therapy alone (OR: 1.49; 98.3% confidence interval: 0.99–2.22; P = .053). All of the other infants receiving prophylaxis followed by other therapies had no difference in NEC risk.
Subgroup Analysis, Infants <750 g
NDI alone was more likely to occur with secondary surgery (Table 3). The odds of having BPD for infants <750 g treated with indomethacin followed by secondary surgery were ~3 times those for infants treated with indomethacin alone (Table 3). Those who had primary surgical closure were not different but trended toward significantly increased risk. Patients <750 g undergoing secondary surgery were less likely to die when compared with those receiving indomethacin treatment alone (Table 3). Infants with primary surgery showed a trend toward a similar benefit when compared with indomethacin alone, but this was not statistically significant.
TABLE 3.
Multivariable Regression, Subgroup (<750 g) Analysis
| Variable | OR Estimate | 98.3% CI | P |
|---|---|---|---|
| NDI/death, overall P = .58 | |||
| Primary surgery vs indomethacin only | 1.10 | 0.52–2.33 | .77 |
| Indomethacin + surgery vs indomethacin only | 1.02 | 0.71–1.46 | .92 |
| No treatment vs indomethacin only | 1.33 | 0.81–2.17 | .17 |
| NDI, overall P = .019 | |||
| Primary surgery vs indomethacin only | 1.25 | 0.55–2.87 | .52 |
| Indomethacin + surgery vs indomethacin only | 1.71 | 1.14–2.58 | .002 |
| No treatment vs indomethacin only | 1.20 | 0.66–2.18 | .48 |
| Death, overall P <.0001 | |||
| Primary surgery vs indomethacin only | 0.63 | 0.32–1.26 | .11 |
| Indomethacin + surgery vs indomethacin only | 0.43 | 0.30–0.62 | <.0001 |
| No treatment vs indomethacin only | 1.24 | 0.79–1.93 | .26 |
| BPD, overall P <.0001 | |||
| Primary surgery vs indomethacin only | 2.14 | 0.85–5.35 | .048 |
| Indomethacin + surgery vs indomethacin only | 2.92 | 1.84–4.63 | <.0001 |
| No treatment vs indomethacin only | 0.85 | 0.46–1.58 | .53 |
| NEC, overall P = .64 | |||
| Primary surgery vs indomethacin only | 0.78 | 0.33–1.84 | .48 |
| Indomethacin + surgery vs indomethacin only | 1.04 | 0.68–1.58 | .85 |
| No treatment vs indomethacin only | 1.27 | 0.71–2.29 | .33 |
The overall P value is from the type 3 effects of the logistic regression. It indicates the effect of treatment on the outcome. Note that the confidence limits for the ORs were calculated by using a level of significance of .017. This was found by setting the study-wise error rate at 0.05 and dividing by this rate by 3 (for the 3 outcomes of interest). Hence, a P value of >.017 should not be considered significant, although a P value of greater than but close to .017 may still be noteworthy.
DISCUSSION
In this large, multicenter cohort study, we found that infants who received supportive therapy alone for PDA had no difference in risk of NDI/death, BPD, or NEC when compared with indomethacin treatment. The risk of NDI was higher for both primary and secondary surgical ligation. BPD was also much more common among patients who underwent surgical closure when compared with indomethacin therapy. We found no difference in the incidence of NEC with respect to PDA therapy and no effect of indomethacin prophylaxis on subsequent outcomes relative to treatment for PDA. Whether the risks of the outcomes of interest were primarily attributable to the PDA therapy, the failure of PDA therapy in some cases, the inherent risks of surgery, or the underlying risks of PDA cannot be fully clarified.
The best approach to understanding the optimal treatment for PDA would be a randomized, controlled trial. Our study does not provide a randomized comparison of treatment modalities but adds important information regarding PDA therapy and its outcomes for clinical use and for future research. Although prospectively collected data were used in this study, the analysis was retrospective. The timing of particular therapies for PDA was not available in the NRN GDB, which would have added significantly to our analysis of relationships between the risk of BPD, NEC, and the length of ductal patency. The NRN GDB lacks formal illness severity scores, which required the use of proxies for severe illness in multivariable analysis; this may not have fully captured the clinical stability of particular patients. Reasons underlying decisions for particular treatment assignments were also not available or controlled for in the analysis.
Little information is available regarding neurodevelopmental outcomes and mortality relative to treatment for PDA. Patients who fail primary medical therapy and undergo secondary surgical closure, or are assigned to primary surgical closure, are likely to be more immature and are more likely to die or develop neurodevelopmental impairment. This confounding by indication has been addressed only with statistical analysis in observational studies, rather than through random assignment. Recent opinion has begun to readdress the question of whether PDA, its therapies, or the underlying immaturity associated with PDA is most responsible for PDA-associated morbidities.29 One study reported a higher risk of NDI in patients undergoing surgical closure when compared with indomethacin therapy.22 Another trial, in which all of the subjects received prophylactic indomethacin followed by nonrandomized choice of indomethacin versus surgery, demonstrated no differences in rates of NDI.21 Doyle et al30 theorized several reasons for the association between surgery and neurodevelopmental impairment, including underlying brain injury preceding surgery, higher illness severity leading to the assignment to surgery, and intraoperative and anesthetic complications, which may lead to neurologic injury.
In our study, which included significantly larger numbers of subjects than the studies above, the risk for NDI was significantly higher in patients undergoing secondary surgical closure but was of borderline significance for patients undergoing primary surgical closure when compared with indomethacin alone. We did not find a difference in the risk of NDI in combination with death relative to PDA therapy type in the entire cohort or the <750-g subgroup.
Clinically significant PDA has long been theorized to affect the risk for BPD.31–34 Whether this risk is related to prolonged ductal patency, the risk of the medical or surgical therapy, or both has yet to be clarified in randomized trials. Observational studies have reported conflicting results regarding the relationship between surgical closure and the risk for BPD, with higher risk in subjects undergoing secondary surgery.19,21 These studies differ from ours in that all of the patients were treated with prophylactic indomethacin before surgical therapy; however, they corroborate our finding of increased risk of BPD in patients after surgery. This may be attributable to the effects of prolonged ductal patency in combination with underlying risks of lung injury with surgical closure. We found a slightly decreased risk of BPD with primary surgery than with secondary surgical closure, again raising the possibility that surgery alone exposes infants to risks that are associated with chronic lung injury. The possible protective effect of indomethacin as an anti-inflammatory agent is a consideration with the risk of BPD relative to medical versus surgical therapy, yet we found no difference in the risk of BPD when comparing the indomethacin only group with supportive therapy.35
The risk for NEC after primary medical therapy as compared with primary or secondary surgical therapy for PDA has been examined in only a few studies, which reported conflicting results.18–20 We found no difference in the risk of NEC relative to treatment for PDA; however, the timing of treatments was not available in the NRN GDB, and this may have affected our ability to fully assess the risk of NEC in this group.
Of significant interest, we found in both bivariable and multivariable analysis, that outcomes for death, NDI, BPD, and NEC were nearly identical for the indomethacin only and the supportive therapy group. This raises the possibility that outcomes with watchful waiting, even in this extremely premature cohort, may be the same as with indomethacin therapy. Others have theorized that PDA is common and that benefits of treatment may not outweigh the risks, and they point to conservative therapy as a viable option for PDA in pre-term infants.24,36 Our findings raise the possibility that supportive therapy only may be comparable to indomethacin therapy with regard to the risk of NDI/death, BPD, and NEC and equal to indomethacin in decreased risk of these outcomes as compared with surgical therapy.
Twenty-nine percent of the cohort from our study received indomethacin prophylaxis within the first 24 hours of life, and we found no effect of prophylaxis on subsequent outcomes, with the exception of the borderline increase of NEC in subjects receiving prophylaxis followed by indomethacin therapy. Prophylaxis was strongly associated with the center and was more often used for patients who were less mature, smaller, and male. This confirms findings from several randomized, controlled trials that found few long-term risks or benefits of prophylaxis, with a trend toward benefit at 4-year follow-up in 1 trial.26,27,37–39
CONCLUSIONS
We found that infants in our cohort treated surgically for PDA had a poorer outcome than those treated medically and that outcomes among subjects who received no specific treatment were comparable with the indomethacin only group. Secondary surgical closure was significantly associated with an increased risk for neurodevelopmental impairment in the cohort as a whole. When compared with indomethacin alone, both primary and secondary surgeries were associated with an increased risk of BPD. Indomethacin prophylaxis had no effect on short- or long-term outcomes studied, but a trend toward higher rates of NEC was demonstrated in patients who received prophylaxis followed by indomethacin therapy. Outcome is likely to be better in ELBW infants with a PDA that successfully responds to medical therapy alone, with or without the use of indomethacin, compared with those who undergo surgical ligation of the ductus.
Acknowledgments
This work was supported by grants from the National Institute of Child Health and Human Development (U01 HD36790, U10 HD21364, U10 HD21373, U10 HD21385, U10 HD21397, U10 HD27851, U10 HD27853, U10 HD27856, U10 HD27871, U10 HD27880, U10 HD27904, U10 HD34216, U10 HD40461, U10 HD40492, U10 HD40498, U10 HD40521, and U10 HD40689) and the National Institutes of Health (GCRC M01 RR30, GCRC M01 RR32, GCRC M01 RR39, GCRC M01 RR44, GCRC M01 RR70, GCRC M01 RR80, GCRC M01 RR125, GCRC M01 RR633, GCRC M01 RR750, GCRC M01 RR16587, GCRC M01 RR 6022, GCRC M01 RR7122, and GCRC M01 RR8084).
The following investigators participated in the National Institute of Child Health and Human Development Neonatal Research Network Generic Database and Follow-up Studies (2001–2006): Cincinnati Children’s Hospital Medical Center: A. Jobe, MD, PhD, Chair; Brown University: Women and Infants Hospital of Rhode Island: A. R. Laptook, MD; W. Oh, MD; A. Hensman, BSN, RNC; B. Vohr, MD; L. Noel, RN; Case Western Reserve: University Rainbow Babies and Children’s Hospital: A. A. Fanaroff, MB, BCh; M. C. Walsh, MD, MS; N. S. Newman, BA, RN; D. Wilson-Costello, MD; BS Siner, RN; Duke University: University Hospital, Alamance Regional Medical Center, Duke Raleigh Hospital, and Durham Regional Hospital: R. N. Goldberg, MD; C. M. Cotten, MD; K. Auten, BS; R. Goldstein, MD; M. Lohmeyer, RN; Emory University: Grady Memorial Hospital, Emory Crawford Long Hospital, and Children’s Healthcare of Atlanta: B. J. Stoll, MD; L. Jain, MD; S. Buchter, MD; E. Hale, RN, BS: Indiana University: Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services: B. B. Poindexter, MD, MS; J. A. Lemons, MD; D. D. Appel, RN, BSN; L. Miller, RN, BSN, CCRC; A. M. Dusick, MD; L. Richard, RN; National Institute of Child Health and Human Development: R. D. Higgins, MD; L. L. Wright, MD; Research Triangle Institute: A. Das, PhD; W. K. Poole, PhD; B. Hastings; E. McClure, MEd; C. Petrie Huitema, MS; K. Zaterka-Baxter, RN; Stanford University: Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital: D. K. Stevenson, MD; K. P. Van Meurs, MD; MB Ball, BS, CCRC; S. R. Hintz, MD, MS; University of Alabama at Birmingham: Health System and Children’s Hospital of Alabama: W. A. Carlo, MD; M. V. Collins, RN, BSN; S. S. Cosby, RN, BSN; M. Peralta-Carcelen, MD; V. Phillips, RN, BSN; University of California San Diego: Medical Center and Sharp Mary Birch Hospital for Women: N. N. Finer, MD; M. R. Rasmussen, MD; D. Kaegi, MD; C. Henderson, RCP, CRTT; K. Arnell, RN; W. Rich, BS, RRT, CCRC; Y. E. Vaucher, MD, MPH; M. Fuller, RN, MSN; University of Cincinnati: University Hospital, Cincinnati Children’s Hospital Medical Center, and Good Samaritan: E. F. Donovan, MD; K. Schibler, MD; B. Alexander, RN; C. Grisby, BSN, CCRC; H. Mincey, RN; J. Shively, RN; J. Steichen, MD; T. Gratton, PA; University of Miami: Holtz Children’s Hospital: S. Duara, MD; C. R. Bauer, MD; R. Everett, RN, BSN; University of Rochester: Golisano Children’s Hospital at Strong: D. L. Phelps, MD; C. T. D’Angio, MD; R. Guillet, MD, PhD; L. Reubens, RN; G. Myers, MD; D. Hust, PNP; University of Texas at Dallas Southwestern Medical Center and Parkland Health and Hospital System: P. Sanchez, MD; C. Rosenfeld, MD PhD; G. Hensley, RN; N. Miller, RN; S. Madison, RN; R. Heyne, MD; S. Broyles, MD; J. Hickman, RN; J. Morgan, RN; University of Texas at Houston Health Science Center and Children’s Memorial Hermann Hospital: J. E. Tyson, MD, MPH; K. Kennedy, MD, MPH; A. E. Lis, RN, BSN; C. Y. Franco, RN, BNS, MSN, NNP; E. G. Akpa, RN, BSN; G. McDavid, RN; P. A. Cluff, RN; B. H. Morris, MD; P. J. Bradt, MD, MPH; Wake Forest University Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital: T. M. O’Shea, MD, MPH; L. Washburn, MD; N. Peters, RN; R. Dillard, MD; B. Jackson, RN; Wayne State University: Hutzel Women’s Hospital and Children’s Hospital of Michigan: S. Shankaran, MD; G. Muran, RN, BSN; R. Bara, RN, BSN; Y. Johnson, MD; D. Kennedy, RN; and Yale University: Bridgeport Hospital and Yale-New Haven Children’s Hospital: R. A. Ehrenkranz, MD; M. Konstantino, RN; P. Gettner, RN; E. Romano, RN.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Abbreviations
- PDA
patent ductus arteriosus
- ELBW
extremely low birth weight
- NEC
necrotizing enterocolitis
- BPD
bronchopulmonary dysplasia
- IVH
intraventricular hemorrhage
- NRN GDB
Neonatal Research Network Generic Data Base
- NDI
neurodevelopmental impairment
- OR
odds ratio
- RDS
respiratory distress syndrome
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
This work was presented in part at the annual meeting of the Pediatric Academic Societies; May 5– 8, 2007; Toronto, Ontario, Canada.
References
- 1.Fanaroff AA, Stoll BJ, Wright LL, et al. Trends in neonatal morbidity and mortality for very low birth weight infants. Am J Obstet Gynecol. 2007;196(2):147.e1–147.e8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Lee SK, McMillan DD, Ohlsson A, et al. Variations in practice and outcomes in the Canadian NICU network 1996–1997. Pediatrics. 2000;106(5):1070–1079. doi: 10.1542/peds.106.5.1070. [DOI] [PubMed] [Google Scholar]
- 3.Itabashi K, Ohno T, Nishida H. Indomethacin responsiveness of patent ductus arteriosus and renal abnormalities in preterm infants treated with indomethacin. J Pediatr. 2003;143(2):203–207. doi: 10.1067/S0022-3476(03)00303-2. [DOI] [PubMed] [Google Scholar]
- 4.Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with PDA: results of a national collaborative study. J Pediatr. 1983;102(6):895–906. doi: 10.1016/s0022-3476(83)80022-5. [DOI] [PubMed] [Google Scholar]
- 5.Grosfeld JL, Chaet M, Molinari F, Engle W, Engum SA, West KW. Increased risk of NEC in premature infants with PDA treated with indomethacin. Ann Surg. 1996;224(3):350–355. doi: 10.1097/00000658-199609000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotton RB, Stahlman MT, Kovar I, Catterton WZ. Medical management of small preterm infants with symptomatic patent ductus arteriosus. J Pediatr. 1979;92(3):467–473. doi: 10.1016/s0022-3476(78)80451-x. [DOI] [PubMed] [Google Scholar]
- 7.Satur CR, Walker DR, Dickinson DF. Day case surgical closure of PDA in preterm infants: a 10 year review. Arch Dis Child. 1991;66(4):477–480. doi: 10.1136/adc.66.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayanan M, Cooper B, Weiss H, Clyman RI. Prophylactic indomethacin: factors determining permanent ductus arteriosus closure. J Pediatr. 2000;136(3):330–337. doi: 10.1067/mpd.2000.103414. [DOI] [PubMed] [Google Scholar]
- 9.Little DC, Pratt TC, Blalock SE, Krauss DR, Cooney DR, Custer MD. PDA in micropreemies and full term infants: the relative merits of surgical closure versus indomethacin treatment. J Pediatr Surg. 2003;38(3):492–496. doi: 10.1053/jpsu.2003.50086. [DOI] [PubMed] [Google Scholar]
- 10.Quinn D, Cooper B, Clyman RI. Factors associated with permanent closure of the ductus arteriosus: a role for prolonged indomethacin therapy. Pediatrics. 2002;110(1) doi: 10.1542/peds.110.1.e10. Available at: www.pediatrics.org/cgi/content/full/110/1/e10. [DOI] [PubMed]
- 11.Sperandio M, Beedgen B, Feneberg R, et al. Effectiveness and side effects of an escalating, stepwise approach to indomethacin treatment for symptomatic PDA in premature infants below 33 weeks of gestation. Pediatrics. 2005;116(6):1361–1366. doi: 10.1542/peds.2005-0293. [DOI] [PubMed] [Google Scholar]
- 12.Koch J, Hensley G, Roy L, Brown S, Ramaciotti Cl, Rosenfeld C. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117(4):1113–1121. doi: 10.1542/peds.2005-1528. [DOI] [PubMed] [Google Scholar]
- 13.Jaillard S, Larrue B, Rakza T, Magnenant E, Warembourg H, Storme L. Consequences of delayed surgical closure of PDA in very premature infants. Ann Thorac Surg. 2006;81(1):231–234. doi: 10.1016/j.athoracsur.2005.03.141. [DOI] [PubMed] [Google Scholar]
- 14.Madan J, Fiascone J, Griffith J, Balasubramanian V, Hagadorn J. Predictors of successful ductal closure, necrotizing enterocolitis, and focal intestinal perforation in very low birth weight infants treated with indomethacin or surgical ligation for patent ductus arteriosus. Neonatology. 2008;94(1):45–51. doi: 10.1159/000113058. [DOI] [PubMed] [Google Scholar]
- 15.Keller RL, Clyman RI. Persistent Doppler flow predicts lack of response to multiple courses of indomethacin in premature infants with recurrent patent ductus arteriosus. Pediatrics. 2003;112(3 pt 1):583–587. doi: 10.1542/peds.112.3.583. [DOI] [PubMed] [Google Scholar]
- 16.Mavroudis C, Cook LN, Fleischaker JW, et al. Management of PDA in preterm infants; indomethacin versus surgical closure. Ann Thorac Surg. 1983;36(5):561–566. doi: 10.1016/s0003-4975(10)60686-8. [DOI] [PubMed] [Google Scholar]
- 17.Malviya M, Ohlsson A, Shah S. Surgical versus medical therapy with cyclooxygenase inhibitors for symptomatic PDA in preterm infants. Cochrane Database Syst Rev. 2003;(3):CD003951. doi: 10.1002/14651858.CD003951. [DOI] [PubMed] [Google Scholar]
- 18.O’Donovan DJ, Baetiong A, Adams K, et al. NEC and GI complications after indomethacin therapy and surgical closure in premature infants with PDA. J Perinatol. 2003;23(4):286–290. doi: 10.1038/sj.jp.7210911. [DOI] [PubMed] [Google Scholar]
- 19.Lee LC, Tillett A, Tulloh R, Yates R, Kelsall W. Outcome following PDA surgical closure in premature infants: a retrospective cohort analysis. BMC Pediatr. 2006;6:15. doi: 10.1186/1471-2431-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laughon M, Bose C, Clark R. Treatment strategies to prevent or close a PDA in preterm infants and outcomes. J Perinatol. 2007;27(3):164–170. doi: 10.1038/sj.jp.7211662. [DOI] [PubMed] [Google Scholar]
- 21.Chorne N, Leonard C, Piecuch R, Clyman RI. PDA and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics. 2007;119(6):1165–1174. doi: 10.1542/peds.2006-3124. [DOI] [PubMed] [Google Scholar]
- 22.Kabra NS, Schmidt B, Roberts RS, et al. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the Trial of Indomethacin Prophylaxis in Preterms. J Pediatr. 2007;150(3):229–234. 29. doi: 10.1016/j.jpeds.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 23.Vanhaesebrouck S, Zonnenberg I, Vandervoort P, Bruneel E, Van Hoestenberghe MR, Theyskens C. Conservative treatment for PDA in the preterm. Arch Dis Child Fetal Neonatal Ed. 2007;92(4):F244–F247. doi: 10.1136/adc.2006.104596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bose CL, Laughon MM. PDA: lack of evidence for common treatments. Arch Dis Child Fetal Neonatal Ed. 2007;92(6):F498–F502. doi: 10.1136/adc.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowlie PW, Davis PG. Prophylactic indomethacin for preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. 2003;88(6):F464–F466. doi: 10.1136/fn.88.6.F464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt B, Davis P, Moddemann D, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight-infants. N Engl J Med. 2001;344(26):1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 27.Ment LR, Vohr B, Oh W, et al. Neurodevelopmental outcome at 36 months’ corrected age of preterm infants in the Multi-center Indomethacin Intraventricular Hemorrhage Prevention Trial. Pediatrics. 1996;98(4 pt 1):714–718. [PubMed] [Google Scholar]
- 28.Bell MJ, Ternberg JL, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clyman RI, Chorne N. Patent ductus arteriosus: evidence for and against treatment. J Pediatr. 2007;150(3):216–219. doi: 10.1016/j.jpeds.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doyle LW Victorian Infant Collaborative Study Group. Outcome at 5 years of age of children 23 to 27 weeks’ gestation: refining the prognosis. Pediatrics. 2001;108(1):134–141. doi: 10.1542/peds.108.1.134. [DOI] [PubMed] [Google Scholar]
- 31.Gonzales A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E. Influence of infection on PDA and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr. 1996;12:470–478. doi: 10.1016/s0022-3476(96)70356-6. [DOI] [PubMed] [Google Scholar]
- 32.Merritt TA, Harris JP, Roghmann K, et al. Early closure of PDA in VLBW infants: a controlled trial. J Pediatr. 1981;99(2):281–286. doi: 10.1016/s0022-3476(81)80479-9. [DOI] [PubMed] [Google Scholar]
- 33.Rojas MA, Gonzales A, Bancalari E, Claure N, Poole C, Silva-Neto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr. 1995;126(4):605–610. doi: 10.1016/s0022-3476(95)70362-4. [DOI] [PubMed] [Google Scholar]
- 34.Zanardo V, Trevisanuto D, Dani C, et al. “Silent” PDA and BPD in VLBW infants. J Perinat Med. 1995;23(6):493–499. doi: 10.1515/jpme.1995.23.6.493. [DOI] [PubMed] [Google Scholar]
- 35.Watterberg K. Anti-inflammatory therapy in the NICU: present and future. Semin Fetal Neonatal Med. 2006;11(5):378–384. doi: 10.1016/j.siny.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Laughon MM, Simmons MA, Bose CL. Patency of the ductus arteriosus in the premature infant: is it pathologic? Should it be treated? Curr Opin Pediatr. 2004;16(2):146–151. doi: 10.1097/00008480-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Ment LR, Oh W, Ehrenkranz RA, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multi-center randomized trial. Pediatrics. 1994;93(4):542–550. [PubMed] [Google Scholar]
- 38.Cordero L, Nankervis CA, Delooze D, Giannone PJ. Indomethacin prophylaxis or expectant treatment of patent ductus arteriosus in extremely low birth weight infants? J Perinatol. 2007;27(3):158–163. doi: 10.1038/sj.jp.7211659. [DOI] [PubMed] [Google Scholar]
- 39.Ment LR, Vohr B, Allan W, et al. Outcome of children in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2000;105(3 pt 1):485–491. doi: 10.1542/peds.105.3.485. [DOI] [PubMed] [Google Scholar]