Abstract
Sphingosine-1-phosphate (S1P) is a potent sphingolipid mediator of diverse processes important for brain tumors, including cell growth, survival, migration, invasion, and angiogenesis. Sphingosine kinase 1 (SphK1), one of the two isoenzymes that produce S1P, is upregulated in glioblastoma and has been linked to poor prognosis in patients with glioblastoma multiforme (GBM). In the present study, we found that a potent isotype-specific SphK1 inhibitor, SK1-I, suppressed growth of LN229 and U373 glioblastoma cell lines and non-established human GBM6 cells. SK1-I also enhanced GBM cell death and inhibited their migration and invasion. SK1-I rapidly reduced phosphorylation of Akt but had no significant effect on activation of ERK1/2, another important survival pathway for GBM. Inhibition of the concomitant activation of the JNK pathway induced by SK1-I attenuated death of GBM cells. Importantly, SK1-I markedly reduced tumor growth rate of glioblastoma xenografts, inducing apoptosis and reducing tumor vascularization and enhanced the survival of mice harboring LN229 intracranial tumors. Our results support the notion that SphK1 may be an important factor in GBM and suggest that an isozyme-specific inhibitor of SphK1 deserves consideration as a new therapeutic agent for this disease.
Keywords: sphingosine-1-phosphate, sphingosine kinase type 1, glioblastoma, Akt
INTRODUCTION
Glioblastoma multiforme (GBM) is the most prevalent and lethal type of primary central nervous system tumors with a median survival of 10–12 months, even after aggressive surgery, radiation and advanced chemotherapy (1). Poor prognosis of patients with GBM has recently been correlated with elevated expression of sphingosine kinase type 1 (SphK1) (2, 3), one of the SphK isoenzymes that generates the pleiotropic lipid mediator, sphingosine-1-phosphate (S1P). S1P has been implicated in the etiology of GBM due to its involvement in various cell processes particularly important for tumorgenicity, including growth, survival, invasion, angiogenesis, and metastasis (4–7). The biological effects of this serum-borne lipid are mainly mediated by a family of five specific G protein-coupled receptors, designated S1P1–5 (8). Of those, S1P1–3, are expressed in the majority of human glioblastoma cell lines and are involved in S1P-mediated proliferation (4). Although S1P has no effect on matrix metalloproteinase secretion, it enhances glioblastoma cell adhesion and also stimulates their motility and invasiveness (9). Because S1P is present at high levels in brain tissue, it is possible that autocrine or paracrine signaling by S1P through its receptors enhances both glioma cell proliferation and invasiveness (10).
To explore the therapeutic implications of targeting SphK1 for treatment of GBM, we examined the effects of a newly developed isozyme-specific inhibitor of SphK1, SK1-1 (11), and found that it inhibits growth of GBM in vitro and in vivo. Our results suggest that specific SphK1 inhibitors might be useful for treatment, either alone or in combination with advanced chemotherapeutic agents.
MATERIALS AND METHODS
Cell culture
U373-MG and LN229 human glioblastoma cells (ATTC, Manassas, VA) were cultured in DMEM supplemented with 5% FCS. Primary human non-established glioblastoma GBM6 cells were kindly provided by Dr. C. David James and were passaged as subcutaneous tumors in nude mice and subcultured for 1 week following isolation from tumors in media containing 2% FCS to prevent growth of contaminating rodent fibroblasts and then cultured in 5% FCS as described (12). LN229 cells were transfected with H2B-EGFP plasmid and stable colonies were isolated following selection with 1 mg/ml of G418. LN229-H2B-EGFP cells were passaged as tumors as described above.
Xenograft tumors
Adult male NCI nu/nu mice were purchased from NCI (Frederick, MD). All animal studies were conducted in the Animal Research Core Facility at VCU School of Medicine in accordance with the institutional guidelines. LN229 cells (1 × 106) were injected in the flanks (4 sites per mouse). Palpable tumors appeared in about one week. Five days later, when tumors reached 3–4 mm in diameter, mice were randomly separated into 2 groups and injected i.p. with saline or SK1-I (10 mg/kg) every other day. Tumor measurements were made with calipers, and tumor volume was calculated using the formula: (π × [length in millimeters] × [width in millimeters]2)/6. At the end of the experiment, the animals were euthanized and the tumors removed, fixed in formalin and embedded in paraffin, or frozen in liquid nitrogen.
Intracranial LN229 xenograft tumors
Adult female NCI nu/nu mice were anesthetized and LN229-H2B-EGFP cells (2.5 × 104 in 1 μl PBS) were stereotactically implanted in the putamen region (1 mm anterior and 2.5 mm lateral to the Bregma at the depth of 3.5 mm at a rate of 0.1 μl/min). Mice were monitored for recovery until complete wakening. 20 days after implantation, mice were injected i.p. with SK1-I (20 mg/kg in PBS) every other day. Mice were observed daily following tumor implantation and were euthanized on reaching a moribund state.
In vitro assays
Immunoblotting, cell proliferation and cell death assays (13), SphK1 activity (11), and colony formation assays (14) were carried out following standard protocols as detailed previously.
Details about reagents, infection of cells with recombinant adenoviruses, and immunohistochemistry are presented as supplementary information.
RESULTS
SK1-I potently inhibits growth and survival of human glioblastoma cells
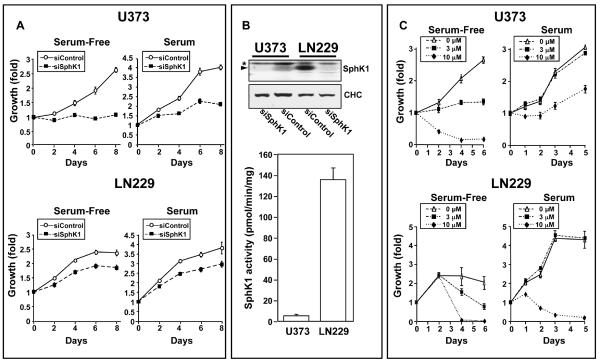
Previous studies demonstrated that S1P and SphK1, the kinase that produces it, play critical roles in growth and survival of glioblastoma cells (2, 4, 6). In agreement, downregulation of SphK1 expression decreased growth of both U373 cells, which express mutated PTEN, and LN229 cells expressing wild type PTEN, in serum-free medium as well as in the presence of serum, which greatly enhanced their growth (Fig. 1A). Expression of SphK1 in these cells was drastically reduced by siRNA targeted to a specific sequence of SphK1 mRNA, as detected by western blotting with a polyclonal anti-SphK1 antibody (Fig. 1A). The greater sensitivity of U373 cells to downregulation of SphK1 might be due to much lower SphK1 expression and enzymatic activity compared to LN229 cells (Fig. 1B).
Figure 1. Downregulation of SphK1 expression or its inhibition by SK1-I reduces growth of glioma cells.
(A,B) U373 and LN229 cells transfected with control siRNA or siRNA targeted to SphK1. (A) Cells were cultured for the indicated time in the absence or presence of 5% serum and cell growth was determined with WST-1. (B) Equal amounts of lysates were separated by SDS-PAGE and immunoblotted with anti-SphK1 antibody. Blots were stripped and re-probed with anti-clathrin heavy chain (CHC) antibody to confirm equal loading and transfer. * indicates non-specific band. SphK1 activity was measured in naïve U373 and LN229 cells. (C) U373 and LN229 cells were cultured for the indicated time in the absence or presence of SK1-I (3 and 10 μM) without or with 5% serum. Cell growth was determined by WST1 assay.
We recently described the first SphK1-specific inhibitor, SK1-I (11). SK1-I inhibited growth of both U373 and LN229 cells in a dose-dependent manner (Fig. 1C). A significant inhibitory effect was observed at 3 μM. SK1-I at 10 μM strongly inhibited growth of U373 and LN229 cells cultured in the absence of serum (Fig. 1C). SK1-I was less effective when cells were cultured in the presence of serum, which contains multiple growth factors and S1P. However, even in the presence of serum, within 2–4 days, there were severe reductions in cell numbers after treatment with 10 μM SK1-I (Fig. 1C).
SK1-I inhibits migration and invasion of glioblastoma cells
As S1P and SphK1 have been shown to regulate migration and invasion of glioblastoma cells (5, 7, 9, 15), and SphK1 regulates actin cytoskeletal dynamics (16) and lamellipodia formation (17), it was of interest to examine whether inhibition of SphK1 by SK1-I correlated with changes in reorganization of the actin cytoskeleton. F-actin was distributed across unstimulated U373 cells, as revealed by staining with Alexa488-conjugated phalloidin (Supplementary Fig. S1A). In response to PMA the actin cytoskeleton underwent dramatic reorganization, and more F-actin was condensed at the leading edge within lamellipodia (Supplementary Fig. S1A). In agreement with a previous study with human macrophages (16), downregulation of SphK1 markedly reduced the number of actin-rich lamellipodia produced by treatment with PMA (Supplementary Fig. S1A,C). Similarly, inhibiting SphK1 with SK1-I dramatically reduced PMA-stimulated F-actin reorganization at the leading edge as well as formation of lamellipodia and induced disassembly of filopodia (Supplementary Fig. S1B,D).
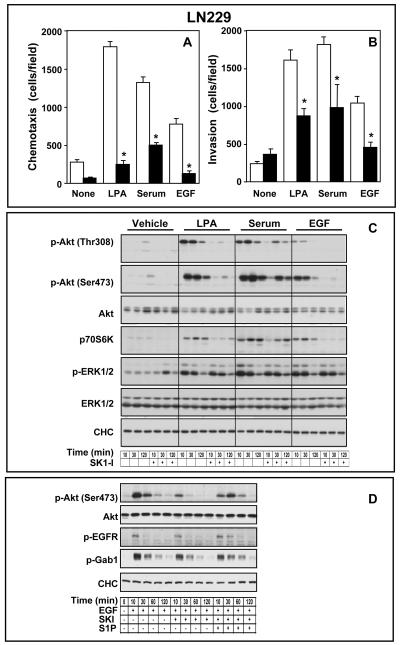
These results support the notion that SphK1 activity is required for actin filament dynamics (16). Therefore, we next examined the effect of SK1-I on migration and invasion of glioma cells. Directed motility (chemotaxis) of U373 cells toward serum or EGF in Boyden chamber assays was reduced by SK1-I (Supplementary Fig. S2A). Similarly, chemotaxis of LN229 cells, which show much greater rates of basal and stimulated migration toward serum and EGF than U373 cells, is also significantly inhibited by SK1-I (Fig. 2A). SK1-I also drastically inhibited chemotaxis of LN229 cells toward lysophosphatidic acid (LPA), another serum-borne lysophospholipid that has been shown to be a potent chemoattractant for certain glioblastoma cell lines, including LN229 cells (15) (Fig. 2A). LPA, serum, and EGF also stimulated in vitro invasion of LN229 cells (Fig. 2B), determined by their ability to invade the basement membrane matrix Matrigel, which was also greatly attenuated by SK1-I (Fig. 2B).
Figure 2. SK1-I attenuates migration and invasion of glioblastoma cells.
(A,B) LN229 cells pretreated with vehicle (open bars) or 10 μM SK1-I (filed bars) were allowed to migrate for 6 h through fibronectin-coated filters (A) or invade through Matrigel-coated filters (B) toward vehicle (None), LPA (1 μM), serum (5%), or EGF (25 nM), as indicated. Results are expressed as mean number of migrating cells per field ± S.D. * p ≤ 0.01. (C) Inhibition of SphK1 by SK1-I inhibits Akt phosphorylation. Serum starved LN229 cells were pre-treated with vehicle or SK1-I (10 μM) for 10 min prior to stimulation with vehicle, LPA, 5% serum, or EGF for 10, 30, and 120 min, as indicated. Cells were lysed and equal amounts of proteins analyzed by western blotting with the indicated antibodies. (D) S1P reverses SK1-I inhibitory effects on Akt phosphorylation. Serum starved LN229 cells were pre-treated with vehicle or SK1-I (10 μM) for 10 min prior to stimulation with vehicle, or EGF in the absence or presence of S1P as indicated. Cells were lysed and equal amounts of proteins analyzed by western blotting with the indicated antibodies.
SK1-I reduces basal and stimulated Akt phosphorylation
S1P-induced glioblastoma cell proliferation is greatly suppressed by inhibition of ERK1/2 and PI3K/Akt pathways (4). Thus, it was of interest to examine the effects of SK1-I on these signaling pathways. We utilized phospho-specific antibodies to examine phosphorylation of Akt at Thr308 in the activation loop and at Ser473 at the C-terminus, which are required for full activation (18). Consistent with the expression of wild-type PTEN, LN229 cells have low basal Akt phosphorylation, which was rapidly increased by serum, LPA, and EGF, to a lesser extent (Fig. 2C). SK1-I reduced Akt activation induced by all three stimuli. Treatment with SK1-I for only 20 min markedly suppressed phosphorylation of Akt at both Thr308 and Ser473 (Fig. 2C). SK1-I also reduced activation of p70S6K (Thr389), a downstream target of Akt. In sharp contrast, although serum, LPA, and EGF stimulated ERK1/2, in these short-term assays, SK1-I did not significantly affect stimulated ERK1/2 phosphorylation at Thr202/Tyr204 (Fig. 2C). Moreover, although Akt is active in U373 cells because, like many human gliomas they express a nonfunctional mutant form of PTEN that does not inhibit the PI3K/Akt pathway (18), SK1-I reduced their basal Akt phosphorylation at Thr308 and Ser473 (Supplementary Fig. S2B). A significant inhibitory effect was observed within 20 min (Supplementary Fig. S2B), which lasted for at least 24 hours (data not shown). As expected, serum and EGF enhanced phosphorylation of Akt, whereas SK1-I reduced it (Supplementary Fig. S2B). The inhibitory effect of SK1-I on Akt phosphorylation was not due to its degradation as there were no significant reductions in total Akt levels after treatment with SK1-I. However, SK1-I did not reduce EGF- and serum-induced ERK1/2 activation in both U373 (Supplementary Fig. S2B) and LN229 cells (Fig. 2C).
To substantiate that the effects of SK1-I were due to its ability to inhibit SphK1, S1P add-back experiments were carried out. Consistent with the reduction in levels of S1P by SK1-I (Fig 3A), inhibition of EGF-induced Akt phosphorylation by SK1-I was reversed by addition of S1P (Fig. 2D). EGF has been shown to activate PI3K/Akt by phosphorylating growth factor receptor–bound protein 2 (Grb2)–associated binder 1 (Gab1) (19). However, SK1-I did not affect EGF-induced tyrosine phosphorylation of EGFR or Gab1 (Fig. 2D), indicating that SK1-I did not directly interfere with EGFR activation. Thus, the SphK1 inhibitor SK1-I specifically inhibits phosphorylation and activation of Akt in GBM cells in a S1P-dependent manner.
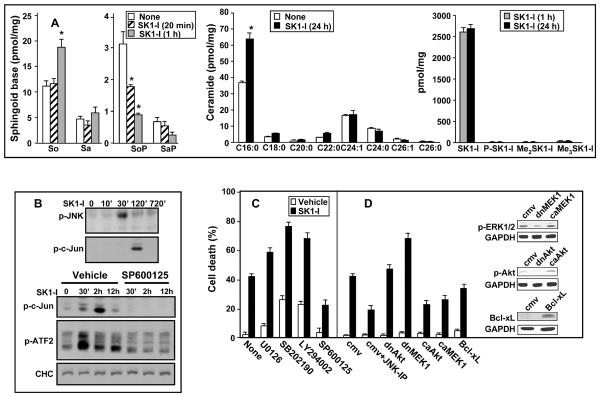
Figure 3. Effect of SK1-I on sphingolipid metabolites and JNK activation.
(A) LN229 cells were treated with vehicle or SK1-I (10 μM) for 20 min (stipple bars), 1 h (gray bars) or 24 h (black bars). Lipids were extracted and sphingosine (So), sphinganine (Sa), sphingosine-1-phosphate (SoP), sphinganine-1-phosphate (SaP), ceramide species as well as SK1-I and possible metabolites, including phosphorylated (P-SK1-I) and di- and tri-methyl SK1-I (Me2SK1-I and Me3SK1-I, respectively), were determined by ESI-MS/MS. Data are means of triplicate determinations and are expressed as picomol lipid per mg protein. Numbers indicate fatty acid chain length followed by the number of double bonds. * p ≤ 0.01. (B) LN229 cells were treated with vehicle or SK1-I (10 μM) for the indicated time. Where indicated, cells were pre-treated without or with SP600125 (10 μM) for 10 min. Cells were lysed and equal amounts of proteins analyzed by western blotting with anti-phospho-JNK (Thr183/Tyr185), anti-phospho-c-Jun (Ser63/73), anti-ATF-2 (Thr71), or anti-CHC antibodies. (C) LN229 cells were cultured for 24 h with vehicle or 10 μM SK1-I in the absence or presence of 1 μM SB202190, 1 μM SP600125, 1 μM U0126, or 10 μM LY294002 and cell death was assessed by trypan blue exclusion. (D) LN229 cells were infected with a control empty vector virus (CMV) or adenoviruses to express dominant-negative Akt (dnAkt), constitutively active Akt (caAkt), dnMEK1, caMEK1, or Bcl-xL. Cells were then treated with vehicle or 10 μM SK1-I and cell death was assessed by trypan blue exclusion 48h after drug exposure. Where indicated, cells were also treated with the JNK inhibitory peptide (JNK-PI, 10 μM) 30 min before addition of SK1-I.
Because downregulation of SphK1 not only decreases S1P, it also increases ceramide levels (20–23), it was of interest to examine the effects of inhibition of SphK1 with SK1-I on these sphingolipid metabolites that have been reported to have opposing effects on cell growth and apoptosis (24, 25). There was a significant reduction in S1P levels within 20 min after addition of SK1-I (Fig. 3A), which correlated with the rapid inhibition of Akt phosphorylation. Furthermore, within 1 h after addition of SK1-I, S1P levels were dramatically decreased by 70% that was accompanied by an increase in sphingosine levels without major changes in ceramide levels (Fig. 3A). However, after 24 h of treatment with SK1-I, ceramide levels increased markedly, particularly pro-apoptotic C16-ceramide. Unlike safingol (L-threo-dihydrosphingosine) (26), a pan SphK inhibitor, only less than 1% of SK1-I was converted to the tri-N-methyl metabolite after 24 h (Fig. 3A) and no other metabolites were detected. Moreover, in contrast to its structural analogue, the immunosuppressant drug FTY720, SK1-I is not readily phosphorylated, ruling out potential actions through S1P receptors.
Inhibition of c-Jun N-terminal kinase attenuates SK1-I-induced cell death
In agreement with many previous studies showing that downregulation of SphK1 and ceramide elevation are associated with increased apoptosis (reviewed in ((25, 27, 28)), treatment with SK1-I induced apoptosis of LN229 cells as demonstrated by increased cleavage of PARP (Supplementary Fig. S3A), a substrate for caspase-mediated proteolysis during apoptosis, increased fragmented and condensed nuclei (Supplementary Fig. S3B), and increased DNA strand breaks detected by TUNEL staining (Supplementary Fig. S3C). Moreover, SK1-I markedly suppressed long-term survival of LN229 cells in clonogenic assays (Supplementary Fig. S3D).
Sphingolipid metabolites, S1P versus sphingosine and ceramide, usually have opposing effects on Akt and the stress-related c-Jun NH2-terminal kinase (JNK) pathways (24, 25). Concomitant with the inactivation of the cytoprotective Akt pathway, exposure of LN229 cells to SK1-I was accompanied by delayed activation of JNK (Fig. 3B), without affecting p38 MAPK (data not shown). Increased phosphorylation of JNK after addition of SK1-I was accompanied by enhanced phosphorylation of its substrates, the transcription factors c-Jun (Ser63/73) and ATF-2 (Thr71) (Fig. 3B).
The output of ERK1/2 and Akt signaling versus JNK signaling represents a key homeostatic mechanism that in many cells regulates the balance between cell survival and cell death processes (29). Thus, we next examined the effects of a variety of agents that perturb these signaling pathways on SK1-I mediated lethality. Inhibition of MEK1/2, PI3K, and p38 by U0126, LY294002, SB202190, respectively, enhanced SK1-I lethality, whereas inhibition of JNK by SP600125 markedly attenuated the effects of SK1-I in both U373 and LN229 cells (Fig. 3C and data not shown). As expected, SP600125 efficiently blocked JNK activation, as demonstrated by inhibition of c-Jun and ATF-2 phosphorylation (Fig. 3B). Even at 1 μM, a concentration believed to specifically inhibit JNK without having non-specific effects on other kinases, SP600125 markedly reversed SK1-I-induced lethality (Fig. 3C). We further examined the importance of the JNK pathway using a specific JNK peptide inhibitor, which blocks the activation domain of JNK and prevents phosphorylation of c-Jun. This peptide also significantly reversed the cytotoxic effects of SK1-I (Fig. 3D), whereas the control peptide was ineffective. Similarly, expression of dominant-negative MEK1 also enhanced SK1-I-induced LN229 cell death, while dominant-negative Akt did not (Fig. 3D). Moreover, expression of constitutively-activated Akt or MEK1, or expression of Bcl-xL, suppressed cell death induced by SK1-I (Fig. 3D).
Effect of SK1-I on primary non-established glioblastoma
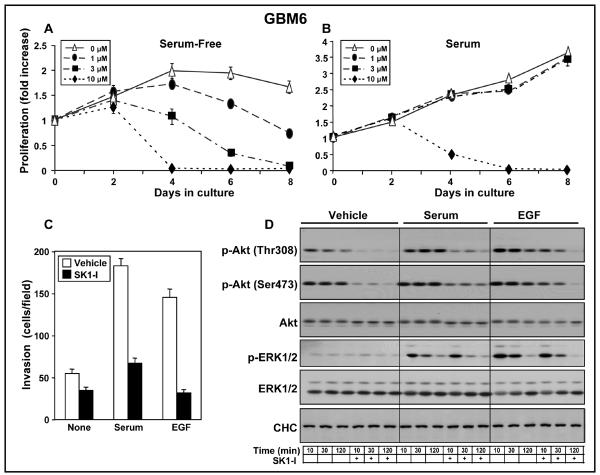
We expanded our observations with SK1-I to primary non-established human GBM6 glioblastoma cells, which have been shown to produce invasive, diffuse tumors in the brains of mice (14, 30). GBM6 express mutant p53, wild-type PTEN, and EGFRvIII, a constitutively activated mutant form of EGFR (14, 31). Similar to LN229 and U373 cells, growth of GBM6 cells was greatly reduced by SK1-I (Fig. 4A). In the absence of serum there was a dose-dependent effect of SK1-I and significant growth inhibition was observed at a concentration as low as 1 μM (Fig. 4A). Moreover, as with the glioblastoma cell lines, 10 μM SK1-I markedly reduced growth of GBM6 in the presence of serum (Fig. 4B). SK1-I also suppressed serum- and EGF-induced invasion of GBM6 (Fig. 4C). Similar to the effects on the established glioblastoma cell lines, SK1-I reduced basal as well as serum- and EGF-stimulated phosphorylation of Akt, without affecting pERK1/2 (Fig. 4D).
Figure 4. Effect of SK1-I on non-established glioblastoma cells.
GBM6 cells were cultured for the indicated time in the absence or presence of SK1-I (1, 3 and 10 μM) in the absence (A) or presence of 5% serum (B). Cell growth was determined with WST1. (C) GBM6 cells pretreated with vehicle (open bars) or 10 μM SK1-I (filed bars) were allowed to invade for 24 h through Matrigel-coated filters toward vehicle (None), serum (5%), or EGF (25 nM), as indicated. Results are expressed as mean number of migrating cells per field ± S.D. * p ≤ 0.01. (D) Serum starved GBM6 were pre-treated with SK1-I (10 μM) for 10 min and then stimulated with vehicle, serum (5%), or EGF (25 nM) for 10, 30, and 120 min, as indicated. Cells were lysed and equal amounts of proteins analyzed by western blotting with the indicated antibodies.
SK1-I reduces tumor growth in mice
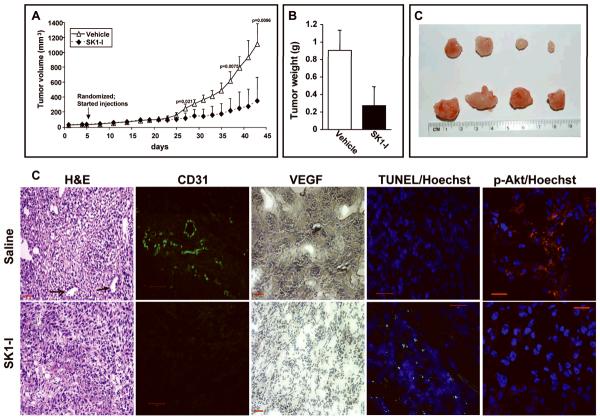
Encouraged by our findings, we investigated the effect of SK1-I on subcutaneous tumor growth of LN229 cells, which are fairly invasive and grow phenotypically similar to invasive gliomas in situ (32). Tumors appeared as palpable masses about one week after subcutaneous injection of one million cells in the flank of a mouse (Fig. 5A). Five days later, when the tumor size could be reliably measured (3–4 mm in diameter), animals were randomized and SK1-I was injected intraperitoneally every other day at a dose of 10 mg/kg. Tumors in control animals showed significant increases in volume as early as day 27, and growth accelerated thereafter. Statistical analysis (single factor ANOVA) revealed significantly smaller tumors in the SK1-I treatment group (Fig. 5A). After 43 days, animals had to be sacrificed due to the tumor burden in control mice. Tumors were excised, weighed, and histologically examined. In addition to the tumor volume and size (Fig. 5A,C), SK1-I treatment reduced tumor weight by almost 4-fold (Fig. 5B) and decreased vascularization in tumors, as shown by hematoxilin and eosin staining (Fig. 5D). Similar reductions in blood vessel density were observed by staining with antibodies to the mouse-specific endothelial cell marker CD31 (Fig. 5D). In agreement, immunohistochemistry for VEGF also revealed elevated expression of this angiogenic factor in vehicle treated tumors that was greatly reduced in SK1-I treated mice (Fig. 5D). The disruption of tumor cyto-architecture by SK1-I was accompanied by increased TUNEL positive apoptotic tumor cells (Fig. 5D). In agreement with attenuation of Akt phosphorylation in GBM cells by SK1-I, immunostaining for phosphorylated Akt in tumors was markedly decreased by treatment with SK1-I (Fig. 5D).
Figure 5. SK1-I decreases glioblastoma xenograft tumor growth in vivo.
LN229 cells (1 × 106) were injected in the flank of nu/nu mice. Palpable tumors appeared in two weeks. Five days later, tumor bearing mice were randomized into two groups and injected I.P. with saline or SK1-I (10 mg/kg) every other day. (A) Tumor volumes were measured on the indicated days and expressed as means ± S.D. (B,C) After 45 days, animals were killed and tumors excised and weighed. Mean weights (B) and representative photographs of typical tumors excised from mice treated without or with SK1-I are shown in C. (D) Tumor histology. Paraffin-embedded tumor sections were stained with H&E, or immunostained with CD31. Black arrows indicate tumor vasculature. VEGF expression was visualized by immunohistochemistry with anti-VEGF antibody (brown) and counterstained with hematoxylin (blue). Apoptotic cells were visualized by TUNEL staining (green) and counterstained with Hoechst 33342 (blue). Scale bars, 50 microns. Tumor sections were also immunostained with phospho-Akt (Ser473) antibody (red color) and counterstained with Hoechst. Scale bars, 20 microns.
SK1-I enhances survival of mice with LN229 orthotopic tumors
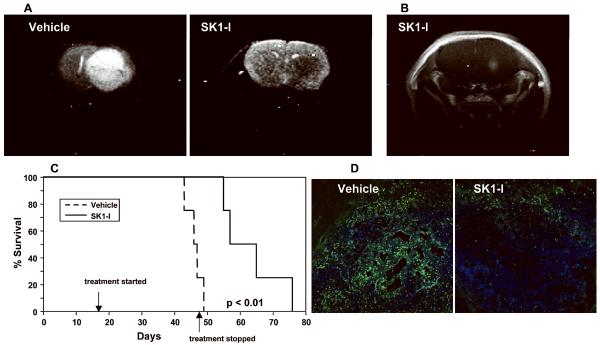
It was of interest to examine whether SK1-I was effective in a more clinically relevant orthotopic model of intracranially implanted LN229 cells. On the basis of trial growth rate analyses, intraperitoneal treatment with SK1-I was initiated at day 20 after intracranial implantation of GFP-labeled LN229 cells when the tumors would be established and the mice would be expected to be asymptomatic. Animals in the vehicle treated group began to show symptoms of tumor burden at day 40 and were euthanized on reaching a moribund state between day 43 and 49 (Fig. 6C). None of the SK1-I treated mice showed any symptoms at this point and SK1-I administration was then halted (Fig. 6C). At day 48, T2W MRI revealed the presence of a large tumor in the right hemisphere of vehicle treated mice (Fig. 6A), while no tumors were evident in SK1-I treated mice (Fig. 6A). Gadolinium enhancement revealed a small tumor in the brain of this SK1-I treated mouse at the site of the injection (Fig. 6B). Moreover, visualization of GFP-labeled LN229 cells in intracranial tumor sections showed significantly fewer invading cells and noticeable areas of necrosis in the middle of the tumors from SK1-I treated animals compared to vehicle treated animals (Fig. 6D). Kaplan-Meier survival analysis of intracranial glioblastoma xenografts showed significant survival benefit from SK1-I administration compared to vehicle control animals (Fig. 6C), indicating that SK1-I was remarkably efficacious in the brain even when administered intraperitoneally.
Figure 6. SK1-I enhances survival of mice with LN229 cells implanted intracranially.
(A–D) LN229-H2B-GFP cells were implanted into the brain of nu/nu mice 1 mm lateral to the Bregma and at a depth of 3.5 mm. Twenty days later, tumor bearing mice were randomized into two groups and injected i.p. with saline or SK1-I (10 mg/kg) every other day until day 49. (A) Representative MRI images at day 48 are shown: T2-weighted images of vehicle and SK1-I treated animals. Note the dramatic effect of SK1-I on tumor growth compared to the massive tumor in untreated animal. (B) Contrast-enhanced T1-weighted image of SK1-I treated animal. (C) Kaplan Meier survival curves for animals implanted with LN229 cells treated with vehicle or SK1-I, as described above. (D) Intracranial tumors of vehicle and SK1-I treated animals were visualized by confocal microscopy for GFP expression (green) after counterstaining with Hoechst 33342 (blue). Representative sections at the site of injection are shown. Scale bars, 100 microns.
DISCUSSION
Currently available therapies only minimally improve the prognosis of GBM patients and new therapeutic targets are desperately needed. Accumulating evidence suggests that SphK1 is an attractive new target. SphK1 message and protein levels are upregulated in GBM (2) and in astrocytoma tissues compared to adjacent normal brain (3). Patients whose tumors were among the highest one-third with regard to SphK1 expression survived a median of 102 days, whereas those within the lower two-thirds survived a median of 357 days (2). High expression of SphK1 was shown to be a predictor of poor prognosis for astrocytoma patients (3).
Here we show that targeting SphK1 with SK1-I suppressed proliferation of several human glioblastoma cell lines, including U373, LN229, U87, and U118 cells as well as non-established GBM6 cells. SK1-I also potently induced apoptosis and inhibited invasion of these cells. Similar to the effects of SK1-I, downregulation of SphK1 expression has been shown to reduce glioblastoma cell growth, survival, migration, and invasion (2). SK1-I was effective in GBM that are mutant for PTEN or p53 or have a constitutively activated form of EGFR. This is particularly important since more than 80% of GBMs show strong Akt activation, many due to lost or mutated PTEN. Activation of EGFR is also a critical pathogenetic event, with amplifications, mutations, or rearrangements commonly observed (33). SK1-I also showed significant antitumor activity in vivo, inducing GBM tumor cell apoptosis and reducing tumor vascularization.
We have begun to unravel the mechanisms by which inhibition of SphK1 by SK1-I so profoundly reduces proliferation and survival of GBM in vitro and inhibits tumor growth in vivo. SK1-I rapidly suppresses phosphorylation of Akt and its targets p70S6K and GSK3β, and thus interferes with signaling through the Akt pathway, which is frequently activated in gliomagenesis (33). This inhibition by SK1-I is not due to a direct effect on Akt, as it did not inhibit Akt activity in an in vitro kinase assay (11). It is also well accepted that S1P produced by activation of SphK1 is released from cells and stimulates its receptors that are linked to activation of Akt. Indeed, the reduction of S1P levels by SK1-I is rapid and could contribute to decreased phosphorylation of Akt. The effects of SK1-I may not be mediated solely by reduction of “inside-out signaling” by S1P but also by reduction of intracellular S1P. Our results are consistent with previous reports showing that SphK1 and intracellular S1P are critical for Akt activation and cell proliferation independently of S1P receptors (6, 34). Moreover, in 1321N1 glioblastoma cells, DNA synthesis and cyclin D expression was increased in a SphK1- and Akt-dependent manner independently of S1P receptors (6). In agreement, overexpression of SphK1 promotes cell survival and growth even in cells devoid of functional S1PRs (34). Similarly, overexpression of SphK1 is a S1P receptor-independent oncogenic event in progression of erythroleukemia that involves activation of Akt (35). In agreement with previous results in leukemia cells (11), SK1-I not only inhibited S1P production in glioma cells, it also increased levels of its pro-apoptotic precursor ceramide that has been shown to cause growth inhibition and apoptosis by inhibiting Akt (25). Thus, biphasic inhibition of Akt is likely due to a rapid decrease in intracellular S1P and later sustained increases in ceramide. Furthermore, a recent study in glioma cells showed that inhibition of the Akt pathway strongly upregulated ceramide levels by inhibiting conversion of ceramide to complex sphingolipids due to reduction of ER to Golgi trafficking of ceramide (36). Because ceramide in turn further inhibits Akt, this positive feedback loop amplifies the apoptotic effect of SK1-I. Activation of JNK may also be due to inhibition of Akt following SK1-I treatment as several studies raised the intriguing possibility that the ability of Akt to inhibit JNK signaling is due to phosphorylation of specific upstream targets in this pathway (37, 38).
Downregulation of SphK1, similar to SK1-I, causes a marked elevation in levels of ceramide (20–23). Consistent with the higher expression of SphK1 in GBM, ceramide levels are lower in human gliomas compared to surrounding brain tissue, and are inversely related to tumor progression and short patient survival (39). Thus, actions of SphK1 might be related to its role in regulation of ceramide levels.
The existence of redundant survival pathways suggests that targeting a single dysregulated pathway may not be sufficient to eliminate tumors. Indeed, it has been suggested that effective GBM therapy may require combinations of inhibitors targeting multiple signaling pathways (40). Our finding that inhibiting SphK1 with SK1-I further enhanced glioblastoma cell lethality induced by inhibitors of other important signaling pathways that are frequently desregulated in GBM may have implications for the design of protocols combining SphK1 inhibitors together with conventional anticancer agents or experimental therapeutics.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health R37GM043880 and R01CA61774 to SS and in part by R21NS063283 (TK) and by P01CA104177 (PD). Confocal microscopy was supported in part by NIH Grant P30 CA16059 to the Massey Cancer Center. We thank Drs. Panos Fatouros and Frank Corwin, Department of Radiology, for help with the MRI analyses.
REFERENCES
- 1.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–33. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 2.Van Brocklyn, JR, Jackson CA, Pearl DK, et al. Sphingosine kinase-1 expression correlates with poor survival of patients with glioblastoma multiforme: roles of sphingosine kinase isoforms in growth of glioblastoma cell lines. J Neuropathol Exp Neurol. 2005;64:695–705. doi: 10.1097/01.jnen.0000175329.59092.2c. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Guan HY, Gong LY, et al. Clinical significance of sphingosine kinase-1 expression in human astrocytomas progression and overall patient survival. Clin Cancer Res. 2008;14:6996–7003. doi: 10.1158/1078-0432.CCR-08-0754. [DOI] [PubMed] [Google Scholar]
- 4.Van Brocklyn J, Letterle C, Snyder P, et al. Sphingosine-1-phosphate stimulates human glioma cell proliferation through Gi-coupled receptors: role of ERK MAP kinase and phosphatidylinositol 3-kinase beta. Cancer Lett. 2002;181:195–204. doi: 10.1016/s0304-3835(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 5.Lepley D, Paik JH, Hla T, et al. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer Res. 2005;65:3788–95. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]
- 6.Radeff-Huang J, Seasholtz TM, Chang JW, et al. Tumor necrosis factor-alpha-stimulated cell proliferation is mediated through sphingosine kinase-dependent Akt activation and cyclin D expression. J Biol Chem. 2007;282:863–70. doi: 10.1074/jbc.M601698200. [DOI] [PubMed] [Google Scholar]
- 7.Young N, Van Brocklyn JR. Roles of sphingosine-1-phosphate (S1P) receptors in malignant behavior of glioma cells. Differential effects of S1P(2) on cell migration and invasiveness. Exp Cell Res. 2007;313:1615–27. doi: 10.1016/j.yexcr.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murph M, Mills GB. Targeting the lipids LPA and S1P and their signalling pathways to inhibit tumour progression. Expert Rev Mol Med. 2007;9:1–18. doi: 10.1017/S1462399407000476. [DOI] [PubMed] [Google Scholar]
- 9.Van Brocklyn JR, Young N, Roof R. Sphingosine-1-phosphate stimulates motility and invasiveness of human glioblastoma multiforme cells. Cancer Lett. 2003;199:53–60. doi: 10.1016/s0304-3835(03)00334-3. [DOI] [PubMed] [Google Scholar]
- 10.Anelli VV, Gault CR, Cheng AB, et al. Sphingosine kinase 1 is up-regulated during hypoxia in U87MG glioma cells: Role of hypoxia-inducible factors 1 and 2. J Biol Chem. 2008;283:3365–75. doi: 10.1074/jbc.M708241200. [DOI] [PubMed] [Google Scholar]
- 11.Paugh SW, Paugh BS, Rahmani M, et al. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112:1382–91. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yacoub A, Gupta P, Park MA, et al. Regulation of GST-MDA-7 toxicity in human glioblastoma cells by ERBB1, ERK1/2, PI3K, and JNK1-3 pathway signaling. Mol Cancer Ther. 2008;7:314–29. doi: 10.1158/1535-7163.MCT-07-2150. [DOI] [PubMed] [Google Scholar]
- 13.Sankala HM, Hait NC, Paugh SW, et al. Involvement of sphingosine kinase 2 in p53-independent induction of p21 by the chemotherapeutic drug doxorubicin. Cancer Res. 2007;67:10466–74. doi: 10.1158/0008-5472.CAN-07-2090. [DOI] [PubMed] [Google Scholar]
- 14.Yacoub A, Hamed H, Emdad L, et al. MDA-7/IL-24 plus radiation enhance survival in animals with intracranial primary human GBM tumors. Cancer Biol Ther. 2008;7:917–33. doi: 10.4161/cbt.7.6.5928. [DOI] [PubMed] [Google Scholar]
- 15.Malchinkhuu E, Sato K, Horiuchi Y, et al. Role of p38 mitogen-activated kinase and c-Jun terminal kinase in migration response to lysophosphatidic acid and sphingosine-1-phosphate in glioma cells. Oncogene. 2005;24:6676–88. doi: 10.1038/sj.onc.1208805. [DOI] [PubMed] [Google Scholar]
- 16.Kusner DJ, Thompson CR, Melrose NA, et al. The localization and activity of sphingosine kinase 1 are coordinately-regulated with actin cytoskeletal dynamics in macrophages. J Biol Chem. 2007;282:23147–62. doi: 10.1074/jbc.M700193200. [DOI] [PubMed] [Google Scholar]
- 17.Maceyka M, Alvarez SE, Milstien S, et al. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Mol Cell Biol. 2008;28:5687–97. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haas-Kogan D, Shalev N, Wong M, et al. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–8. doi: 10.1016/s0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 19.Mattoon DR, Lamothe B, Lax I, et al. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC Biol. 2004;2:24–35. doi: 10.1186/1741-7007-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maceyka M, Sankala H, Hait NC, et al. Sphk1 and Sphk2: Sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–29. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- 21.Pchejetski D, Golzio M, Bonhoure E, et al. Sphingosine kinase-1 as a chemotherapy sensor in prostate adenocarcinoma cell and mouse models. Cancer Res. 2005;65:11667–75. doi: 10.1158/0008-5472.CAN-05-2702. [DOI] [PubMed] [Google Scholar]
- 22.Taha TA, Kitatani K, El-Alwani M, et al. Loss of sphingosine kinase-1 activates the intrinsic pathway of programmed cell death: modulation of sphingolipid levels and the induction of apoptosis. FASEB J. 2006;20:482–84. doi: 10.1096/fj.05-4412fje. [DOI] [PubMed] [Google Scholar]
- 23.Berdyshev EV, Gorshkova IA, Usatyuk P, et al. De novo biosynthesis of dihydrosphingosine-1-phosphate by sphingosine kinase 1 in mammalian cells. Cell Signal. 2006;18:1779–92. doi: 10.1016/j.cellsig.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Cuvillier O, Pirianov G, Kleuser B, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–03. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 25.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 26.Coward J, Ambrosini G, Musi E, et al. Safingol (L-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-kinase pathway. Autophagy. 2009;5:184–93. doi: 10.4161/auto.5.2.7361. [DOI] [PubMed] [Google Scholar]
- 27.Cuvillier O. Downregulating sphingosine kinase-1 for cancer therapy. Expert Opin Ther Targets. 2008;12:1009–20. doi: 10.1517/14728222.12.8.1009. [DOI] [PubMed] [Google Scholar]
- 28.Shida D, Takabe K, Kapitonov D, et al. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662–73. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia Z, Dickens M, Raingeaud J, et al. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–31. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 30.Giannini C, Sarkaria JN, Saito A, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-oncol. 2005;7:164–76. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yacoub A, Mitchell C, Hong Y, et al. MDA-7 regulates cell growth and radiosensitivity in vitro of primary (non-established) human glioma cells. Cancer Biol Ther. 2004;3:739–51. doi: 10.4161/cbt.3.8.968. [DOI] [PubMed] [Google Scholar]
- 32.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 33.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 34.Olivera A, Rosenfeldt HM, Bektas M, et al. Sphingosine kinase type 1 Induces G12/13-mediated stress fiber formation yet promotes growth and survival independent of G protein coupled receptors. J Biol Chem. 2003;278:46452–60. doi: 10.1074/jbc.M308749200. [DOI] [PubMed] [Google Scholar]
- 35.Le Scolan E, Pchejetski D, Banno Y, et al. Overexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood. 2005;106:1808–16. doi: 10.1182/blood-2004-12-4832. [DOI] [PubMed] [Google Scholar]
- 36.Giussani P, Brioschi L, Bassi R, et al. Phosphatidylinositol 3-kinase/AKT pathway regulates the endoplasmic reticulum to golgi traffic of ceramide in glioma cells: a link between lipid signaling pathways involved in the control of cell survival. J Biol Chem. 2009;284:5088–96. doi: 10.1074/jbc.M808934200. [DOI] [PubMed] [Google Scholar]
- 37.Kim AH, Khursigara G, Sun X, et al. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barthwal MK, Sathyanarayana P, Kundu CN, et al. Negative regulation of mixed lineage kinase 3 by protein kinase B/AKT leads to cell survival. J Biol Chem. 2003;278:3897–902. doi: 10.1074/jbc.M211598200. [DOI] [PubMed] [Google Scholar]
- 39.Riboni L, Campanella R, Bassi R, et al. Ceramide levels are inversely associated with malignant progression of human glial tumors. Glia. 2002;39:105–13. doi: 10.1002/glia.10087. [DOI] [PubMed] [Google Scholar]
- 40.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–90. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.