Abstract
This article reviews current patterns of ascertainment, clinical characteristics and quality of care for girls with Turner syndrome, based on a cohort of 100 girls (aged 7–17 years) prospectively evaluated at the National Institute of Child Health since 2001. Approximately 25% were diagnosed prenatally or at birth owing to webbed neck and other features typical of fetal lymphedema, few were diagnosed during early childhood, with the majority undiagnosed until age 9 years or older. Major clinical features included thyroid autoimmunity (51%), congenital cardiovascular anomalies (44%), liver abnormalities (36%), hypertension (34%), hearing loss (30%) and renal anomalies (18%). Of the group, 75% were being or had been treated with growth hormone. These girls were an average of 5 cm taller and significantly less obese than the untreated group. We discuss new guidelines for the initiation of puberty and urgent research needed to promote the health and longevity of girls suffering from Turner syndrome as they become adults.
Keywords: aorta, bicuspid aortic valve, lymphedema, phenotype, short stature, Turner syndrome, X chromosome
Turner syndrome (TS) is a relatively common disorder of female development (affecting one out of 2000 births) caused by complete or partial monosomy for the X chromosome during embryonic development. The most consistent features are short stature and premature ovarian failure. Full-scale IQ is usually normal, with verbal scores typically greater than performance scores [1]. With timely diagnosis and intervention, girls with TS may gain improved height, benefit from attention to nonverbal learning difficulties early in school and have puberty at an age similar to their peers. Importantly, all these girls need comprehensive cardiovascular evaluation, since over a third have an underlying congenital cardiovascular defect that places them at an increased risk for life-threatening complications, such as pulmonary hypertension (e.g., partial anomalous pulmonary venous connection) or aortic dissection (i.e., bicuspid aortic valve or dilated ascending aorta). This article focuses on patterns of diagnosis and growth hormone (GH) use for TS girls participating in the NIH TS study from 2001 to 2007.
Ascertainment
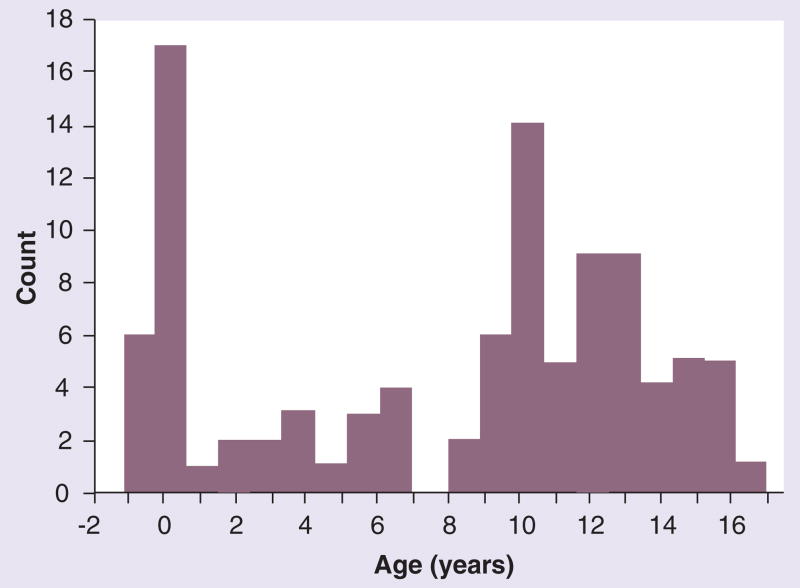
We have studied 100 girls aged 7–17 years with karyotype-proven TS. NIH study participant eligibility criteria included age 7 years or greater and a 50-peripheral white blood cell karyotype, in which over 70% of cells have a missing or fragmented sex chromosome. The actual karyotype distribution has been reported recently [2] and includes a few mosaics with approximately 10% normal cells. Approximately 25% of TS subjects have mosaicism for a 45,X cell line. With a 46,X abnormal X cell line, approximately 10% have 46,X,iXq, 5% have 46,X,delXp and the majority (59%) are 45,X. The average age of our pediatric study subjects is 12.5 years, with a median of 13 years. The age of diagnosis demonstrates a distinct biphasic pattern (Figure 1). Four girls were diagnosed prenatally, either incidentally during routine screening or because of an abnormal fetal ultrasound [2]. In total, 18 were diagnosed at birth because of neck webbing and associated signs of lymphedema and, in many cases, severe congenital heart disease. Surprisingly, very few girls were diagnosed during early childhood, despite the fact that growth failure is usually evident from shortly after birth [3], with 60% of girls not diagnosed until age 9 years or later.
Figure 1.
Age of diagnosis for 100 girls with Turner syndrome aged 7–17 years.
Phenotype
Common phenotypic features of the NIH pediatric TS study group are summarized in Table 1. Only 22% of the group had neck webbing, or pterygium colli. This feature is defined as the presence of (usually symmetric) redundant skin folds extending from mastoids to acromion processes but is sometimes confused with a simply short neck. This distinction is very important, since neck webbing results from tenting of the fetal skin over massively dilated jugular lymphatics (cystic hygroma) and is strongly associated with the coexistence of significant congenital cardiovascular defects, such as aortic coarctation and aortic valve defects [4,5]. The presence of neck webbing is usually obvious in children and leads to the diagnosis of TS in most cases; it may be more difficult to discern in obese adults. We did not attempt to assess short stature in this group because 75% received or had received GH treatment, nor did we assess the prevalence of premature ovarian failure because of the young age of our study group.
Table 1.
Phenotype of 100 girls with Turner syndrome.
| Feature | Total affected (%) |
|---|---|
| Webbed neck | 22 |
|
| |
| Congenital heart defects | 44 |
| – Bicuspid aortic valve | 29 |
| – Aortic coarctation | 11 |
| – Dilated ascending aorta | 22 |
| – Other* | 31 |
|
| |
| Renal anomalies | 18 |
| – Horseshoe kidney | 11 |
| – Renal agenesis | 3 |
| – Duplicated collecting ducts | 3 |
|
| |
| Thyroid disorder | 51 |
| – Hypothyroid | 33 |
| – Thyroid antibodies only | 18 |
|
| |
| Liver disorder | 36 |
| – Abnormal liver function tests‡ | 27 |
| – Fatty liver | 19 |
|
| |
| Diabetes (Type 1 or 2) | 0 |
|
| |
| Hypertension | 34 |
| – Prehypertension | 14 |
| – Hypertension | 20 |
|
| |
| Hearing loss | See text |
Data collected from the prospective evaluation of 100 consecutive pediatric participants in the NIH Turner syndrome study from 2001 to 2007. Diagnosis of congenital heart defects was by echocardiogram and cardiac MRI. Renal and hepatic imaging was assessed by ultrasound. Hypertension was determined by ambulatory blood pressure monitoring using height-based standards.
Includes partial anomalous pulmonary venous return, elongated transverse arch of the aorta (pseudocoarctation) and right aortic arch.
Refers to >10% elevation of alanine aminotransferase and/or aspartate aminotransferase.
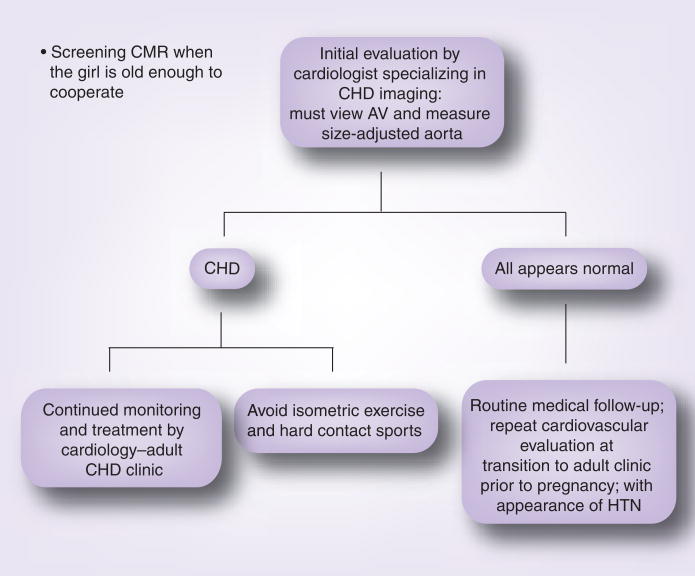
A major goal of the NIH protocol is detection of congenital cardiovascular defects or anomalies that might identify those at risk for life-threatening complications, such as aortic aneurysm, which foreshortens the lives of too many patients with TS [6,7]. All participants undergo in-depth cardiovascular evaluations, including echocardiography and MRI. The latter has been a revolutionary asset in visualizing cardiovascular anatomy in TS. With the aid of gadolinium contrast especially, major venous anomalies, including partial anomalous pulmonary venous connection, are easily detected. Even without contrast, the ability to see the entire thoracic aorta (i.e., the ascending directly compared with the descending) reveals dilation of the ascending aorta that may not be detected on ultrasound, and many anomalies of the great vessels, including the right-sided aortic arch and anomalous origins of the subclavian and carotid arteries, are very common in TS compared with controls [8]. Elongation of the transverse aortic arch and kinking of the lesser curvature are associated with dissection and are common in TS but not visible on transthoracic echocardiography [8]. Studies in diverse aortic aneurysm syndromes have found that the mere presence of a cardiovascular anomaly is coextensive with the risk for aortic dissection/rupture (i.e., a bicuspid aortic valve predicts aortic dissection, not because of secondary hemodynamic effects of abnormal flow through the valve, but because a shared developmental defect impairs correct development of valve and aorta) [9]. Hence, it is extremely important to fully characterize the cardiovascular anatomy in patients with TS. An algorithm for the screening and monitoring of cardiovascular disease in TS is summarized in Figure 2.
Figure 2. Screening for and monitoring of congenital cardiovascular disease in Tuner syndrome (TS).
The algorithm is based on an expert’s consensus conference held in 2006 and published in 2007, and general guidelines for care of patients at risk for aortic dissection, such as those with Marfan syndrome [21]. At the time of initial diagnosis, no matter what age, a comprehensive cardiovascular evaluation must be performed by a specialist in congenital heart disease. Routine transthoracic echocardiography rarely obtains adequate visualization of aortic valve, thoracic aorta and pulmonary veins, except perhaps in newborns with TS. CMR is more effective but requires sedation in the youngest patients and, thus, if clinical and echocardiography evaluations appear normal, we generally wait until the girl is old enough to cooperate with CMR (usually age 9–10 years). Certainly, by the age of 12 years, all girls with TS should have a screening CMR, thorough characterization of aortic valve structure, aortic diameters and investigation of other common anomalies [14]. If no anomalies are detected and systemic blood pressure is normal, we currently recommend routine medical care with re-evaluation of the cardiovascular system at least once during adulthood – especially prior to attempting pregnancy. If evidence of congenital defects are found during the initial cardiology evaluation, then follow-up is determined by the individual situation. However, at this time, it is clear that girls with known CHD (e.g., repaired aortic coarctation) should not be discharged from the pediatric cardiology clinic without further care but, instead, need to be transitioned to an adult CHD clinic, since they are at continued risk for aortic complications during adult years [7].
AV: Aortic valve; CHD: Congenital heart disease; CMR: Cardiac magnetic resonance; HTN: Hypertension.
Renal anomalies were found in 18% of girls with TS, including, most commonly, horseshoe kidney, a single unilateral kidney and a duplicated collecting system, usually unilateral. The anomalies do not cause problems in renal function, but collecting system obstruction should be considered in a TS patient with recurrent urinary tract infections. Autoimmune thyroid disease is extremely common in TS and over 50% of pediatric patients have either overt hypothyroidism or positive antithyroid antibodies, indicative of thyroiditis. Grave’s disease is an uncommon presentation of thyroid autoimmunity in TS. Liver function abnormalities, manifesting as isolated elevations of alanine aminotransferase and/or aspartate aminotransferase, were found in 27% of the participants. Most of these girls had normal hepatic ultrasound, while fatty infiltration of the liver was detected in 20%, most with normal liver function tests (Table 1). Hypertension was previously reported to affect approximately 30% of girls with TS, independent of congenital renal or cardiovascular defects [10], and we have confirmed this finding. It is important to use height-based normal values to evaluate blood pressure, since many girls are quite small. Hearing loss was evaluated in all these girls [11], but the pattern was too complex to summarize in Table 1. Basically, approximately 30% of girls aged 10 years or younger had conductive hearing loss. Girls between 11 and 20 years of age had improvement in conductive hearing, but onset of sensorineural loss occurred in approximately 10%. Thus, hearing impairment affected 20–30% of girls aged 7–20 years but was generally mild [11]. Attention-deficit disorder (ADD) or attention-deficit/hyperactivity disorder (ADHD) had been diagnosed elsewhere in 11% of our group, and all these girls were taking prescription medication for the disorder. Thyroid autoimmunity was slightly more prevalent in girls with an isoXq, as previously reported [12]; no effect of karyotype was detected for the prevalence of renal, cardiac or hearing defects.
GH therapy
A total of 75% of the girls in this study had been treated or were currently being treated with GH. The ages and heights of the subjects are summarized in Table 2. Most of the girls were treated in a community setting, usually by pediatric endocrinologists. The major reasons for not receiving GH treatment were:
Table 2.
Growth hormone use, body composition and glucose tolerance in Turner syndrome.
| Parameter | Growth hormone (n = 76) | No growth hormone (n = 26) | p-value |
|---|---|---|---|
| Age (years) | 13.9 ± 3.6 | 13.6 ± 3.7 | NS |
| Height (SD) | −1.93 ± 0.8 | −2.51 ± 1.3 | 0.009* |
| BF (%) | 28.2 ± 8.3 | 35 ± 7.7 | <0.0001‡ |
| SAT (cm3) | 99.5 ± 81.8 | 183.2 ± 37.5 | 0.001‡ |
| VAT (cm3) | 33 ± 13.7 | 49.8 ± 7.8 | 0.0009‡ |
| IGT (%) | 7 | 28 | 0.006 |
Group means were compared by ANCOVA using:
Age.
Age and BMI as covariates.
Prevalence of IGT was compared by χ2. Growth hormone was not administered the day before or day of metabolic testing. p-values remain significant at p < 0.01 after Bonferroni correction. Data are means ± standard deviation or proportion.
BF: Total body fat measured by dual energy X-ray absorptiometry; IGT: Impaired glucose tolerance; NS: Not significant; SAT: Subcutaneous abdominal fat;
SD: Standard deviation; VAT: Visceral abdominal fat.
Adapted from [2].
Delayed diagnosis (i.e., most of the girls that were diagnosed at age 14 years and older declined GH treatment in favor of pubertal induction);
Very recent diagnosis, with families still engaged in the initial evaluation and consideration of treatment.
The average age of starting GH was 8.7 ± 3.3 years and average treatment duration was 4.0 ± 3.31 years. A most remarkable finding was that glucose tolerance was actually better in the GH-treated group, an effect that was attributable to the more salutary body composition of the treated girls [2]. The average BMI, total body fat (dual energy X-ray absorptiometry) and abdominal fat (MRI) was significantly greater in never-treated girls than in girls currently receiving GH treatment and those that had finished GH treatment (Table 2). These unexpected findings were quite striking, and the statistical significance was very robust, even withstanding a rigorous correction for multiple comparisons.
Duration of GH treatment & pubertal induction
In the past, the onset of puberty was often delayed in girls with TS to allow for additional height gain before fusion of the epiphyses. However, in recent years, studies have shown that initiation of very low-dose estrogen does not seem to limit growth. Moreover, more attention is now focused on the psychosocial adjustment of these girls, which seems to be improved when they mature through puberty in concert with their peers [13]. The literature on this evolving issue, in addition to the current consensus recommendations have been summarized recently [14].
GH therapy & the heart
Owing to the prevalence of congenital cardiovascular defects in TS and the fact that GH has prominent effects upon the heart in acromegaly and some experimental models, there has been concern that pharmacological GH treatment could adversely affect the heart and/or aorta in girls with TS. In one recent study, we compared cardiac anatomy and function using transthoracic echocardiography in our groups of GH-treated and -untreated TS girls [15]. After adjusting for the larger somatic size of the GH-treated girls using body surface area, we found no difference in chamber size, wall thickness or ventricular function. We also investigated ascending aortic diameters determined by MRI and found that ascending aortic diameter was proportional to body size and not disproportionately enlarged in GH-treated girls [16]. However, a recent study using portable echocardiography at TS Society meetings reports a possible effect of GH treatment in enlarging the diameter of the aortic annulus but not other sites along the ascending aorta [17]. Dutch investigators used cardiac MRI to evaluate ventricular size and function in 30 young women that had received GH treatment and found that biventricular ejection fraction, mass, cardiac output and diastolic filling pattern were comparable to age-matched controls [18].
Safety of GH
A recent paper summarized adverse events from the National Cooperative Growth Study (NCGS, Genentech) database in regards to GH-treated girls with TS [19]. There were five deaths from aortic dissections recorded during the study that do not appear to be related to GH treatment. Intracranial hypertension occurred in 0.23% of girls with TS treated with GH, which is twofold higher than all GH-treated participants. Similarly, slipped capital femoral epiphyses occurred in 0.24% of TS girls, compared with only 0.15% of non-TS GH-treated girls. Scoliosis occurred in 0.7% of TS and 0.4% of non-TS girls during GH treatment. Pancreatitis was also increased among girls with TS, although the number of cases was extremely low (0.06%). New diagnoses of diabetes mellitus were increased among girls with TS, compared with non-TS girls (0.19 vs 0.10%). Finally, new-onset diagnosis of malignancy was higher among TS girls compared with non-TS GH-treated groups (0.11 vs 0.06%) [19]. The numbers of incident cases were so low in all groups that it is not possible to derive statistical significance from the data; yet, it should be suggested that TS girls are monitered very closely for these adverse effects while prescribing GH treatment.
Expert commentary
The use of GH to treat short stature in girls with TS has brought long-overdue attention to this common and still underdiagnosed developmental disorder of females. The jury is still out on the pros and cons and cost–benefit ratio for the use of GH to increase adult height by several inches. There are no major adverse consequences during follow-up of 5–10 years, and the reduction in adolescent obesity may have long-lasting positive effects [2]. Moreover, the attention of pediatric endocrinologists to this population has been an undiluted blessing, and one could only hope that adult endocrinologists will take up an interest in adult patients with this challenging endocrine disorder. Unfortunately, as it stands now, girls graduating from pediatric clinics seem to fall through the cracks of the medical system and are, in general, not receiving adequate care [20]. Adults with TS need expert management of ovarian hormone replacement and need expert diagnosis and treatment of autoimmune thyroid disease, hyperlipidemia, hypertension, diabetes and osteoporosis. Those with congenital cardiovascular defects need to be followed in adult congenital heart disease clinics.
Five-year view
Over the next 5 years, we expect to see major developments in diagnosis and care possibilities for children and adults with TS. First, modern high-throughput DNA technology will enable screening for sex chromosome anomalies at birth, thus greatly expanding the numbers and the spectrum of patients with TS and Klinefelter syndrome. Important progress in identifying girls at special risk for aortic complications is expected as a result of ongoing longitudinal and pathogenetic studies at the NIH. Clinical trials of medical and surgical treatments will follow. Pharmaceutical preparations designed originally for postmenopausal women may be used for the physiological normalization of estradiol levels in girls beginning approximately at 10–12 years of age.
Key issues
Obvious physical stigmata such as neck webbing affect only approximately 20% of girls with Turner syndrome (TS).
The presence of neck webbing and shield crest are indicative of fetal lymphedema and highly correlated with congenital cardiovascular defects.
TS is underdiagnosed in young girls with growth impairment.
The optimum age for initiation and total duration of growth hormone treatment in girls with TS are unknown.
Girls who experience puberty synchronously with their peers seem to make a better psychosexual adjustment.
50% of girls with TS have autoimmune thyroid disease, 30% have hypertension or prehypertension and 44% have congenital cardiovascular defects that require monitoring.
Cardiovascular screening using cardiac magnetic resonance angiography is essential for all girls with TS by age 12 years or sooner, if clinically indicated.
Acknowledgments
Financial & competing interests disclosure
This work was supported by the intramural research program of the NICHD, NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Contributor Information
Kateri McCarthy, Developmental Endocrinology Branch, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892, USA, Tel.: +1 301 496 4686, Fax: +1 301 402 0574.
Carolyn A Bondy, Developmental Endocrinology Branch, National Institute of Child Health and Human Development, CRC 1-3330, 10 Center Drive, National Institutes of Health, Bethesda, MD 20892, USA, Tel.: +1 301 496 4686, Fax: +1 301 402 0574, bondyc@mail.nih.gov.
References
- 1.Ross J, Roeltgen D, Zinn A. Cognition and the sex chromosomes: studies in Turner syndrome. Horm Res. 2006;65:47–56. doi: 10.1159/000090698. [DOI] [PubMed] [Google Scholar]
- 2.Wooten N, Bakalov VK, Hill S, Bondy CA. Reduced abdominal adiposity and improved glucose tolerance in GH-treated girls with Turner syndrome. J Clin Endocrinol Metab. 2008;93(6):2109–2114. doi: 10.1210/jc.2007-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport ML, Crowe BJ, Travers SH, et al. Growth hormone treatment of early growth failure in toddlers with Turner syndrome: a randomized, controlled, multicenter trial. J Clin Endocrinol Metab. 2007;92:3406–3416. doi: 10.1210/jc.2006-2874. [DOI] [PubMed] [Google Scholar]
- 4.Loscalzo ML, Van PL, Ho VB, et al. Association between fetal lymphedema and congenital cardiovascular defects in Turner syndrome. Pediatrics. 2005;115:732–735. doi: 10.1542/peds.2004-1369. [DOI] [PubMed] [Google Scholar]
- 5.Sachdev V, Matura LA, Sidenko S, et al. Aortic valve disease in Turner syndrome. J Am Coll Cardiol. 2008;51:1904–1909. doi: 10.1016/j.jacc.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Gravholt CH, Juul S, Naeraa RW, Hansen J. Morbidity in Turner syndrome. J Clin Epidemiol. 1998;51:147–158. doi: 10.1016/s0895-4356(97)00237-0. [DOI] [PubMed] [Google Scholar]
- 7.Matura LA, Ho VB, Rosing DR, Bondy CA. Aortic dilatation and dissection in Turner syndrome. Circulation. 2007;116:1663–1670. doi: 10.1161/CIRCULATIONAHA.106.685487. [DOI] [PubMed] [Google Scholar]
- 8.Ho VB, Bakalov VK, Cooley M, et al. Major vascular anomalies in Turner syndrome: prevalence and magnetic resonance angiographic features. Circulation. 2004;110:1694–1700. doi: 10.1161/01.CIR.0000142290.35842.B0. [DOI] [PubMed] [Google Scholar]
- 9.Braverman AC, Guven H, Beardslee MA, Makan M, Kates AM, Moon MR. The bicuspid aortic valve. Curr Prob Cardiol. 2005;30:470–522. doi: 10.1016/j.cpcardiol.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Nathwani NC, Unwin R, Brook CG, Hindmarsh PC. The influence of renal and cardiovascular abnormalities on blood pressure in Turner syndrome. Clin Endocrinol (Oxf) 2000;52:371–377. doi: 10.1046/j.1365-2265.2000.00961.x. [DOI] [PubMed] [Google Scholar]
- 11.King KA, Makishima T, Zalewski CK, et al. Analysis of auditory phenotype and karyotype in 200 females with Turner syndrome. Ear Hear. 2007;28:831–841. doi: 10.1097/AUD.0b013e318157677f. [DOI] [PubMed] [Google Scholar]
- 12.Elsheikh M, Wass JA, Conway GS. Autoimmune thyroid syndrome in women with Turner’s syndrome – the association with karyotype. Clin Endocrinol (Oxf) 2001;55:223–226. doi: 10.1046/j.1365-2265.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- 13.Carel J-C, Elie C, Ecosse E, et al. Self-esteem and social adjustment in young women with Turner syndrome – influence of pubertal management and sexuality: population-based cohort study. J Clin Endocrinol Metab. 2006;91:2972–2979. doi: 10.1210/jc.2005-2652. [DOI] [PubMed] [Google Scholar]
- 14.Bondy CA. Care of girls and women with Turner syndrome: a guideline of the Turner syndrome study group. J Clin Endocrinol Metab. 2007;92:10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 15.Matura LA, Sachdev V, Bakalov VK, Rosing DR, Bondy CA. Growth hormone treatment and left ventricular dimensions in Turner syndrome. J Pediatr. 2007;150:587–591. doi: 10.1016/j.jpeds.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bondy CA, Van PL, Bakalov VK, Ho VB. Growth hormone treatment and aortic dimensions in Turner syndrome. J Clin Endocrinol Metab. 2006;91:1785–1788. doi: 10.1210/jc.2005-2625. [DOI] [PubMed] [Google Scholar]
- 17.Lopez L, Arheart KL, Colan SD, et al. Turner syndrome is an independent risk factor for aortic dilation in the young. Pediatrics. 2008;121:e1622–e1627. doi: 10.1542/peds.2007-2807. [DOI] [PubMed] [Google Scholar]
- 18.van den Berg J, Bannink EMN, Wielopolski PA, et al. Cardiac status after childhood growth hormone treatment of Turner syndrome. J Clin Endocrinol Metab. 2008;93:2553–2558. doi: 10.1210/jc.2007-2313. [DOI] [PubMed] [Google Scholar]
- 19.Bolar K, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in Turner syndrome. J Clin Endocrinol Metab. 2008;93:344–351. doi: 10.1210/jc.2007-1723. [DOI] [PubMed] [Google Scholar]
- 20.Bondy C, Bakalov VK, Lange ED, Ceniceros I. Deficient medical care for adults with the Turner syndrome. Ann Intern Med. 2006;145:866–867. doi: 10.7326/0003-4819-145-11-200612050-00020. [DOI] [PubMed] [Google Scholar]
- 21.Milewicz DM, Dietz HC, Miller DC. Treatment of aortic disease in patients with Marfan syndrome. Circulation. 2005;111:e150–e157. doi: 10.1161/01.CIR.0000155243.70456.F4. [DOI] [PubMed] [Google Scholar]