Abstract
OBJECTIVE
Because of blood lipid concerns, diabetes associations discourage fructose at high intakes. To quantify the effect of fructose on blood lipids in diabetes, we conducted a systematic review and meta-analysis of experimental clinical trials investigating the effect of isocaloric fructose exchange for carbohydrate on triglycerides, total cholesterol, LDL cholesterol, and HDL cholesterol in type 1 and 2 diabetes.
RESEARCH DESIGN AND METHODS
We searched MEDLINE, EMBASE, CINAHL, and the Cochrane Library for relevant trials of ≥7 days. Data were pooled by the generic inverse variance method and expressed as standardized mean differences with 95% CI. Heterogeneity was assessed by χ2 tests and quantified by I2. Meta-regression models identified dose threshold and independent predictors of effects.
RESULTS
Sixteen trials (236 subjects) met the eligibility criteria. Isocaloric fructose exchange for carbohydrate raised triglycerides and lowered total cholesterol under specific conditions without affecting LDL cholesterol or HDL cholesterol. A triglyceride-raising effect without heterogeneity was seen only in type 2 diabetes when the reference carbohydrate was starch (mean difference 0.24 [95% CI 0.05–0.44]), dose was >60 g/day (0.18 [0.00–0.37]), or follow-up was ≤4 weeks (0.18 [0.00–0.35]). Piecewise meta-regression confirmed a dose threshold of 60 g/day (R2 = 0.13)/10% energy (R2 = 0.36). A total cholesterol–lowering effect without heterogeneity was seen only in type 2 diabetes under the following conditions: no randomization and poor study quality (−0.19 [−0.34 to −0.05]), dietary fat >30% energy (−0.33 [−0.52 to −0.15]), or crystalline fructose (−0.28 [−0.47 to −0.09]). Multivariate meta-regression analyses were largely in agreement.
CONCLUSIONS
Pooled analyses demonstrated conditional triglyceride-raising and total cholesterol–lowering effects of isocaloric fructose exchange for carbohydrate in type 2 diabetes. Recommendations and large-scale future trials need to address the heterogeneity in the data.
Although the National Cholesterol Education Program in the Adult Treatment Panel III guidelines (1) identified LDL cholesterol as the most atherogenic lipid fraction and primary target of cholesterol-lowering therapy for coronary heart disease, raised triglycerides (TGs) have been consistently associated with increased coronary heart disease risk even after adjustment for established coronary risk factors (2). The association is especially heightened in the context of a non–LDL cholesterol atherogenic dyslipidemia consisting of raised TGs and low HDL cholesterol, a pattern commonly manifest in type 2 diabetes (3).
Among sugars, fructose has been singled out in guidelines for its effect on blood lipids. Special concern has been expressed about using fructose as a nutritive sweetener in conditions that predispose to higher TG levels, such as diabetes. The American Diabetes Association (>15–20% energy) (4), Canadian Diabetes Association (CDA) (>60 g/day, ∼12% energy) (5), and European Association for the Study of Diabetes (17% energy) (6) discourage fructose at high intakes, citing its ability to affect lipids adversely. The data on which these recommendations are based, however, are inconsistent with new evidence that the dose threshold for TG effects may lie at >100 g/day (7), which exceeds even the 95th percentile of U.S. fructose intake (8). There is also paradoxical evidence that fructose may improve long-term glycemic control (7). To clarify the effect of fructose on lipid control in individuals with diabetes, we conducted a systematic review and meta-analysis of controlled, experimental trials assessing the effect of isocaloric, oral fructose exchange for carbohydrate on TGs, total cholesterol, LDL cholesterol, and HDL cholesterol in individuals with diabetes.
RESEARCH DESIGN AND METHODS
We followed the Cochrane Handbook for Systematic Reviews of Interventions for the planning and conduct of this meta-analysis (9). The reporting followed Quality of Reporting of Meta-Analyses guidelines (10).
Study selection
We conducted a search of MEDLINE (1950–20 February 2009), EMBASE (1980–5 February 2008), CINAHL (1982–5 February 2008), and the Cochrane Library, including the Cochrane Central Register of Controlled Trials (Clinical Trials; CENTRAL) database (1800-20 February 2009), using the following search terms and Boolean operators: fructose AND (triglyceride OR triacylglycerol OR VLDL OR VLDL OR lipemia OR lipaemia OR lipids OR cholesterol). The search was restricted to human research studies. No limit was placed on language. Manual searches supplemented the database search strategy. We included clinical intervention trials that investigated the chronic effect of exchanging oral fructose for carbohydrate on lipids in individuals with type 2 diabetes. Studies that had <7 days follow-up, administered fructose intravenously, lacked an adequate carbohydrate control, reported either hypercaloric, nonisoglucidic, or unbalanced comparisons, and/or reported only nonfasting results were excluded. If multiple publications existed for the same study, the article with the most information was included.
Data extraction
Two investigators (J.L.S., A.J.C.) independently extracted relevant data on study characteristics and outcomes using a standardized proforma. These data included information about study design (parallel, crossover, factorial, and others), randomization, blinding, sample size and subject characteristics (age, sex, BMI, and diabetes status), fructose format, dose, reference carbohydrates used as controls (starch, sucrose, or mixed carbohydrates [undefined combination]), follow-up, and macronutrient profile of the background diet. Means ± SEM posttreatment values for TGs, total cholesterol, LDL cholesterol, and HDL cholesterol were extracted as the main end points. Studies that did not report mean and/or SEM values had these values imputed from SD, 95% CI, P values, or t, F, or Tukey honestly significant difference statistics, using standard formulas (11). If these data were unavailable, then SEM was extrapolated by imputing the pooled SEM from the other studies included in the meta-analysis (12,13). For studies that differed in units, units were converted using standard conversion factors. The investigators also assessed the quality of each study using the Heyland score (14), which assigns a score from 0–1 or 0–2 over nine categories of quality related to study design, sampling procedures, and interventions for a total of 13 points. Studies that reported 100% follow-up data were scored as intent-to-treat analyses. Metabolically controlled designs were also recorded as a measure of study quality. Disagreements were reconciled by consensus after discussion with other investigators (R.J.D., D.J.A.J.). Authors were not contacted to request additional information.
Statistical analyses
Data were analyzed using Review Manager (RevMan 5.0.16; Cochrane Library software, Oxford, U.K.). Separate pooled analyses were conducted for any diabetes, type 1 diabetes, and type 2 diabetes using the generic inverse variance fixed method. Outcomes included end differences for TG, total cholesterol, LDL cholesterol, and HDL cholesterol. Random-effect models were applied when heterogeneity was significant with fixed-effects models being used otherwise. Paired analyses were applied to all crossover trials (13), necessitating that data be expressed as standardized mean differences (SMDs) with 95% CI, where <0.4 represents a small effect size, 0.4–0.7 represents a moderate effect size, and >0.7 represents a large effect size. To address a unit-of-analysis error from including trials with multiple intervention arms, we combined arms to create single pairwise comparisons. Interstudy heterogeneity was tested by Cochrane's Q (χ2) (P < 0.10) and quantified by I2, where I2 ≥ 50% is evidence of substantial heterogeneity and I2 ≥ 75% is evidence of considerable heterogeneity (11). Sources of heterogeneity were investigated by sensitivity analyses and a priori subgroup analyses, investigating the effect of reference carbohydrates (starch, sucrose, and mixed carbohydrates), fructose format (crystalline, fluid, and mixed format), dose (CDA thresholds ≤60 g/day or >60 g/day [5]), length of follow-up (≤4 weeks or >4 weeks), study quality (Heyland Methodological Quality Score [MQS] <8 and ≥8 [14]), and randomization. Additional post hoc subgroup analyses were undertaken to investigate the effect of feeding control (metabolic or nonmetabolic), design (paralleled or crossover), washout in crossover studies (yes or no), and background diet. Inverse variance weighted piecewise polynomial regression models were used to estimate dose thresholds. To assess independent predictors of the isocaloric exchange of fructose for carbohydrate, we used multiple regression models with inverse variance weighting selected by all possible regression using the R2 criterion (NCSS [Number Cruncher Statistical System] software, Kaysville, Utah). Publication bias was investigated by inspection of funnel plots.
RESULTS
Search results
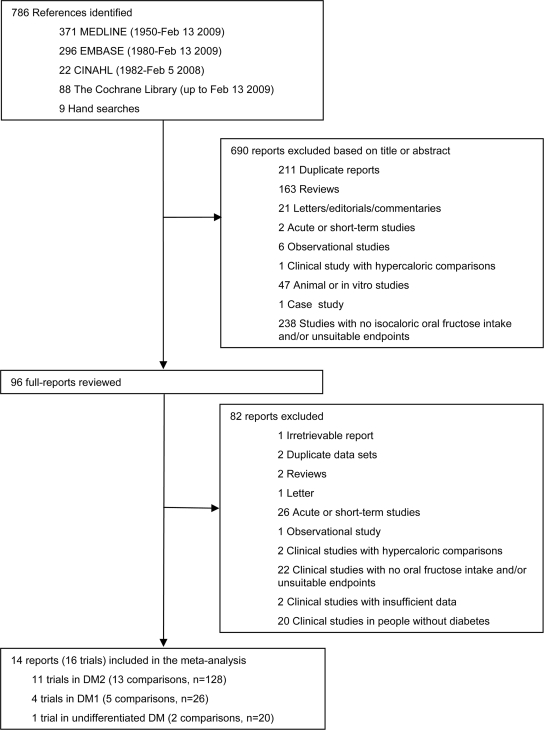
Figure 1 shows the flow of the literature applying the systematic search and selection strategies to identify eligible reports; 786 reports were identified by the search. Of these, 690 were determined to be irrelevant on review of the titles and abstracts. The remaining 96 reports were retrieved and reviewed in full, of which 82 were excluded. A total of 14 reports (16 trials) were selected for pooled analyses.
Figure 1.
Flow of the literature.
Trial characteristics
Table 1 shows the characteristics of the 16 included trials, which contained 20 comparisons in 236 subjects with type 1 diabetes (4 trials, n = 54), type 2 diabetes (11 trials, n = 156), and undifferentiated type 1 and type 2 diabetes (1 trial, n = 26) (15–28). Nine trials were randomized. Eleven trials used crossover designs. Starch, sucrose, or mixed carbohydrates were used as the reference carbohydrate (comparator). Fructose was administered in crystalline, liquid, or mixed formats at doses from 30 to 160 g/day, with six trials exceeding the CDA threshold of 60 g/day. Eight trials were metabolically controlled, providing all foods consumed. Background diets were 40–55% carbohydrate, 25–38% fat, and 15–20% protein. Follow-up was from 8 days to 52 weeks. The Heyland MQS ranged from 4 to 8 with nine trials considered to be of high quality (MQS ≥8).
Table 1.
Characteristics of experimental trials of the effect of fructose exchange for carbohydrate on blood lipids
| Study | Subjects | Design* | Randomization | Fructose dose (g/day) | Fructose form | Reference carbohydrate | Diet† | Follow-up | MQS‡ |
|---|---|---|---|---|---|---|---|---|---|
| Type 1 diabetes | |||||||||
| Akerblom et al., 1972 (15) | 16 DM1 children | C | No | ∼40 (20% E) | Mixed | Starch | 45:35:20 | 1 week | 4 |
| Pelkonen et al., 1972 (16) | 8 DM1 | C | No | 75 (15% E) | Crystalline | Starch | 40:40:20 | 10 days | 7 MF |
| Bantle et al., 1986 (18) | 12 DM1 (6 M/6 F) | C | Yes | 84–109 (21% E) | Mixed | Starch | 55:30:15 | 8 days | 8 MF |
| 12 DM1 (6 M/6 F) | C | Yes | 84–109 (21% E) | Mixed | Sucrose | 55:30:15 | 8 days | 8 MF | |
| Bantle et al., 1992 (25) | 6 DM1 (3 M/3 F) | C | Yes | 80–160 (20% E) | Crystalline | Starch | 55:30:15 | 4 weeks | 8 MF |
| Type 2 diabetes | |||||||||
| Crapo et al., 1986 (17) | 7 DM2 (3 M/4 F) | C | No | 80–115 (13.2% E) | Mixed | Sucrose | 55:30:15 | 2 weeks | 7 MF |
| Bantle et al., 1986 (18) | 12 DM2 (5 M/7 F) | C | Yes | 84–109 (21% E) | Mixed | Starch | 55:30:15 | 8 days | 8 MF |
| 12 DM2 (5 M/7 F) | C | Yes | 84–109 (21% E) | Mixed | Sucrose | 55:30:15 | 8 days | 8 MF | |
| McAteer et al., 1987 (19) | 10 DM2 | C | No | 50 (11.6% E) | Liquid | Mixed | 42:38:20 | 4 weeks | 7 |
| Osei et al., 1987 (20) | 18 DM2 (15 M/3 F) | P | Yes | 60 (10% E) | Crystalline | Mixed | 50:35:15 | 12 weeks | 8 |
| Grigoresco et al., 1988 (21) | 8 DM2 (5 M/3 F) | C | Yes | 30 (8% E) | Crystalline | Starch | 50:30:20 | 8 weeks | 8 |
| Thorburn et al., 1989 (22) | 8 DM2 (4 M/4 F) | P | No | 76–124 (13% E) | Mixed | Sucrose | 55:30:15 | 12 weeks | 6 MF |
| Anderson et al., 1989 (23) | 14 DM2 (14 M/0 F) | C | No | 50–60 (12% E) | Mixed | Mixed | 55:25:20 | 23 weeks | 8 |
| Osei and Bossetti, 1989 (24) | 13 DM2 (5 M/8 F) | C | Yes | 60 (7.5% E) | Crystalline | Mixed | 50:35:15 | 26 weeks | 8 |
| Bantle et al., 1992 (24) | 12 DM2 (4 M/8 F) | C | Yes | 80–160 (20% E) | Crystalline | Starch | 55:30:15 | 4 weeks | 8 MF |
| Koivisto and Yki-Jarvinen, 1993 (26) | 10 DM2 (4 M/6 F) | C | Yes | 45–65 (20% E) | Liquid | Starch | 50:30:20 | 4 weeks | 9 MF |
| Malerbi et al., 1996 (26) | 16 DM2 (7 M/9 F) | C | No | 63.2 (20% E) | Liquid | Starch | 55:30:15 | 4 weeks | 7 |
| 16 DM2 (7 M/9 F) | C | No | 63.2 (20% E) | Liquid | Sucrose | 55:30:15 | 4 weeks | 7 | |
| Undifferentiated diabetes | |||||||||
| Blayo et al., 1990 (28) | 14 DM (11 DM1, 3 DM2) | P | Yes | 20–30 (∼5% E) | Crystalline | Mixed | 55:30:15 | 52 weeks | 7 |
| Blayo et al., 1990 (28) | 12 DM (8 DM1, 4 DM2) | P | Yes | 20–30 (∼5% E) | Crystalline | Sucrose | 55:30:15 | 52 weeks | 7 |
*C denotes crossover, and P denotes parallel.
†Values are for the ratio of carbohydrates-to-fat-to-protein.
‡Study quality was assessed by the Heyland MQS (13). MF denotes studies with metabolic feeding control. DM, diabetes; DM1, type 1 diabetes; DM2, type 2 diabetes; E, energy; F, female; M, male.
Primary analyses
Table 2 shows the effect of isocaloric fructose exchange for carbohydrate on TG, total cholesterol, LCL cholesterol, and HDL cholesterol as assessed in the 14 included trials in individuals with any, type 1, or type 2 diabetes. No effect of isocaloric exchange of fructose for carbohydrate was seen for any outcome. There was, however, evidence of considerable interstudy heterogeneity for TG, total cholesterol, and HDL cholesterol (I2 ≥ 75%, P < 0.10). Systematic removal of each trial during sensitivity analyses, however, explained some of the heterogeneity. Removal of Pelkonen et al. (16) for TG in the type 1 diabetes analysis, Osei et al. (20) for TG, and Osei and Bossetti (24) for HDL cholesterol in the type 2 diabetes analyses eliminated the evidence for heterogeneity without altering the conclusions.
Table 2.
Primary pooled analyses of the effect of fructose exchange for carbohydrate on blood lipids
| Outcome | No. (studies) | No. (subjects) | Effect estimate |
Heterogeneity |
|||
|---|---|---|---|---|---|---|---|
| SMD (95% CI) | P | χ2 | I2 (%) | P | |||
| TG | |||||||
| Any diabetes | 16 | 236 | 0.01 (−0.19–0.21) | 0.9 | 36.75 | 59 | 0.001 |
| Type 1 diabetes | 4 | 54 | −0.03 (−0.51–0.46) | 0.91 | 10.96 | 73 | 0.01* |
| Type 2 diabetes | 11 | 156 | 0.06 (−0.17–0.30) | 0.61 | 23.22 | 57 | 0.01† |
| Total cholesterol | |||||||
| Any diabetes | 14 | 172 | −0.02 (−0.18–0.14) | 0.79 | 40.96 | 71 | <0.0001 |
| Type 1 diabetes | 2 | 14 | 0.17 (−0.32–0.67) | 0.49 | 5.45 | 82 | 0.02 |
| Type 2 diabetes | 11 | 132 | −0.08 (−0.26–0.10) | 0.37 | 32.29 | 72 | 0.0002 |
| LDL cholesterol | |||||||
| Any diabetes | 7 | 99 | 0.02 (−0.07–0.11) | 0.69 | 7.00 | 14 | 0.32 |
| Type 1 diabetes | 1 | 6 | 0.25 (−0.03–0.53) | 0.08 | — | — | — |
| Type 2 diabetes | 6 | 93 | −0.01 (−0.10–0.09) | 0.86 | 3.97 | 0 | 0.55 |
| HDL cholesterol | |||||||
| Any diabetes | 12 | 164 | 0.02 (−0.05–0.10) | 0.51 | 48.01 | 77 | <0.00001‡ |
| Type 1 diabetes | 1 | 6 | −0.01 (−0.46–0.44) | 0.96 | — | — | — |
| Type 2 diabetes | 10 | 132 | 0.02 (−0.05–0.10) | 0.53 | 47.95 | 81 | <0.00001‡ |
Analyses were performed by the generic inverse variance method using fixed-effects or random-effects (if heterogeneity was significant at P < 0.10) models with paired analyses applied for crossover trials (12). —, no available data for subgroup analyses or, if there are no data for heterogeneity in the presence of a corresponding effect estimate, data only available from one study, precluding calculation of I2 and the corresponding P value.
*Removal of Osei et al. (20) during sensitivity analyses explained heterogeneity (I2 = 0%, P = 0.59).
†Removal of Osei and Bossetti (24) during sensitivity analyses explained heterogeneity (I2 = 0%, P = 0.88 for all diabetes; I2 = 0%, P = 0.76 for type 2 diabetes).
‡Removal of Pelkonen et al. (16) during sensitivity analyses explained heterogeneity (I2 = 10%, P = 0.33).
Type 1 diabetes subgroup analyses
A priori and post hoc subgroup analyses were used to explore the effect of sources of heterogeneity in type 1 diabetes (supplementary Table A1, available at http://care.diabetesjournals.org/cgi/content/full/dc09-0619/DC1). None of the subgroup analyses were significant for any outcome, except for total cholesterol, for which they were driven by the single study (25) included in the analysis. Data from only one study were included in the LDL cholesterol and HDL cholesterol pooled analyses. Evidence for heterogeneity was significant only in the one category of each subgroup analysis containing Pelkonen et al. (16), the removal of which reduced heterogeneity to nonsignificant levels (I2 < 50%, P ≥ 0.10) without altering conclusions.
Type 2 diabetes subgroup analyses
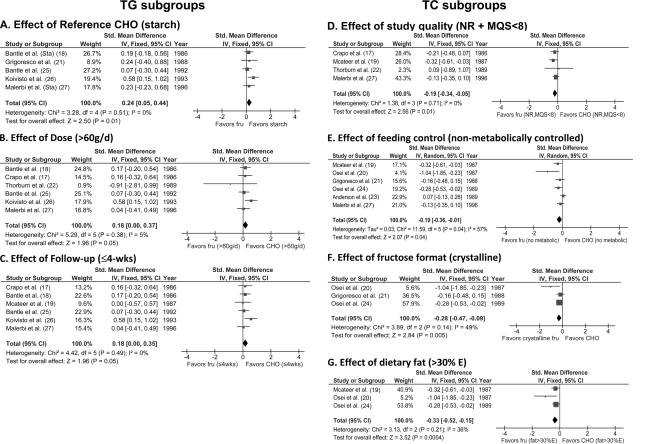
A priori and post hoc subgroup analyses were used to explore the effect of sources of heterogeneity in type 2 diabetes (supplementary Table A2, available in an online appendix). These analyses showed a small TG-raising effect of fructose exchange for carbohydrate under specific conditions: starch as the reference carbohydrate, dose >60 g/day (lower dose limit), or follow-up ≤4 weeks (Fig. 2). Dose thresholds were further explored by piecewise polynomial meta-regression (quadratic-quadratic) models, which confirmed the same dose breakpoint of 60 g/day (R2 = 0.13) or 10% energy (R2 = 0.36). Conversely, a TG-lowering effect was seen only in trials with parallel designs. The interstudy heterogeneity seen for TGs was largely explained by these subgroups. It became nonsignificant in every subgroup category with the exception of those categories containing Osei et al. (20), the removal of which explained heterogeneity (I2 = 0–5%, P ≥ 0.10) without altering conclusions.
Figure 2.
Forest plots of significant subgroup analyses of the effect of isocaloric exchange of fructose for carbohydrate on TGs (A–C) and total cholesterol (TC) (D–G) in subjects with type 2 diabetes reported in 11 trials. Paired analyses were applied to all crossover trials, according to Elbourne et al. (13). Data are SMDs with 95% CI, where an SMD is interpreted as follows: <0.4 represents a small effect size; 0.4–0.7 represents a moderate effect size; and >0.7 represents a large effect size. P values are for generic inverse variance fixed- and random-effects models. Interstudy heterogeneity was tested by Cochrane's Q (χ2) at a significance level of P < 0.10 and quantified by I2, where I2 ≥ 50% is considered to be evidence of substantial heterogeneity and I2 ≥ 75% is considered to be considerable heterogeneity (11). Study quality was assessed by the Heyland MQS, where MQS ≥ 8 is considered high quality (range 0–13) (14). Because the trials that were nonrandomized (NR) were identical to those that were scored as low quality (MQS < 8), the two subgroups were presented as a single forest plot. Fru, fructose; FU, follow-up; E, energy.
A total cholesterol–lowering effect was seen only in type 2 diabetes under specific conditions. These included no randomization and poor study quality, no metabolic feeding control, crystalline fructose, or dietary fat >30% energy (Fig. 2). Among these significant subgroup analyses, there was evidence of interstudy heterogeneity in only the analysis for no metabolic feeding control. Interstudy heterogeneity remained unexplained for this subgroup analysis and the other nonsignificant subgroup analyses.
No effect of subgroup analyses was seen on LDL cholesterol or HDL cholesterol. Interstudy heterogeneity was only significant for HDL cholesterol in each category containing the study of Osei and Bossetti (24), the removal of which during sensitivity analyses explained heterogeneity (I2 = 0%, P ≥ 0.10) without altering conclusions.
Multivariate analyses
To explore further the heterogeneity identified in univariate analyses for TG and total cholesterol, multiple regression models assessed the independent predictors of these outcomes in the complete dataset combining subjects with type 1 and type 2 diabetes (supplementary Table A3, available in an online appendix). The models were significant (P ≤ 0.05) for both outcomes, explaining 72–86% of the variability in outcomes. A crossover design was found to be the strongest independent predictor of TG followed by metabolic feeding control, follow-up, fluid format, dose, and starch reference carbohydrates. Follow-up was found to be the strongest independent predictor of total cholesterol followed by dose.
Publication bias
Funnel plots for each of the analyses were inspected for the presence of publication bias (supplementary Fig. A1, available in an online appendix). There was limited evidence of funnel plot asymmetry for TG, with two small trials reporting large effects and error estimates that favored fructose but no small trials favoring any carbohydrate. No asymmetry was observed for total cholesterol, LDL cholesterol, and HDL cholesterol.
CONCLUSIONS
The present pooled analyses of 16 controlled experimental trials in 236 subjects with type 1 and type 2 diabetes demonstrate heterogeneous lipid effects of isocaloric exchange of fructose for other carbohydrates. Conditional TG-increasing and total cholesterol–lowering effects were seen only in subjects with type 2 diabetes. No other lipid effects were seen. Expressed as mean differences (mean difference = SMD × pooled SD), the modest SMD increases in TG of ∼0.17–0.23 mmol/l and decreases in total cholesterol of ∼−0.17 to −0.29 mmol/l were dependent on specific trial conditions.
Fructose was found to raise TG in isocaloric exchange for starch but not for sucrose or mixed carbohydrate sources in type 2 diabetes. These observations fit with proposed mechanisms. Fructose, unlike glucose, bypasses the major rate-limiting step of glycolysis (phophofructokinase), allowing fructose to serve as an unregulated substrate for de novo lipogenesis (DNL). This model of metabolic handling, however, is only supported by hypercaloric fructose feeding (>20% excess energy) trials (29,30). Other evidence suggests a mechanism whereby fructose decreases TG clearance by lipoprotein lipase, due to decreased glucose-stimulated insulin secretion and increased chylomicron remnants (31). On the other hand, the observed lack of effect compared with sucrose and mixed carbohydrates is probably explained by both sources necessarily containing fructose as part of the sucrose molecule. The implication is that fructose may be no worse than other fructose-containing nutritive sweeteners in the diabetic diet.
A dose threshold for the effect of fructose on TG was observed in type 2 diabetes. Only at fructose doses greater than the CDA threshold of >60 g/day (>12% energy for a 2,000-kcal diet) (5) was a TG-raising effect observed in subgroup analyses. This threshold is consistent with the 100 g/day identified across different clinical states (7) and findings from hypercaloric feeding trials with fructose intakes at 25% excess energy in healthy humans, the only trials in which a TG-raising effect has been observed reliably (29,30,32–35). Although our threshold is lower than these estimates, it is higher than the estimated U.S. intake of total fructose of 9.1% energy (45.5 g/day for a 2,000-kcal diet) (8). The inability of low doses to stimulate a quantitatively meaningful DNL response may explain this threshold. Whereas DNL contributes 60–70% TG in rodents (36), it only contributes <5% TG in humans, under longer term, isocaloric, high-carbohydrate feeding conditions (37). Alternatively, the threshold may relate to the benefit of catalytic fructose doses (<10 g/meal) in decreasing acute postprandial glycemic and insulinemic responses in type 2 diabetes (38), mediated by increased hepatic glucose clearance via increased glycogen synthase-flux (39). The suggestion is that the benefit of fructose on carbohydrate metabolism seen at lower doses may mitigate adverse lipid effects, which require high doses to become manifest.
An effect of follow-up was also observed in type 2 diabetes. Follow-up was identified as the strongest independent predictor of TG and total cholesterol, explaining 22–36% of the variation in these outcomes. Only at follow-up ≤4 weeks was a TG-raising effect seen. The lack of effect on TG beyond 4 weeks may relate to a metabolic adaptation to prolonged fructose feeding or decreased compliance due to fructose malabsorption. In stark contrast to animal models fed fructose at >60% energy (40) to elicit metabolic derangements without malabsorption, 80% of humans fed 50 g/day (∼10% energy) show evidence of malabsorption, which is mitigated by glucose (41). Alternatively, trials that were ≤4 weeks may have had protocols that better favored compliance. In this regard, 8 of 11 trials that were ≤4 weeks were also metabolically controlled, an independent predictor explaining 10% of the variation in TG.
Other factors affecting lipids in type 2 diabetes include aspects of study quality, design, and protocol. Although not significant in univariate subgroup analyses, crossover design was the strongest independent predictor of TG. Fluid fructose format was also positively associated with TG. The main drivers of the total cholesterol–lowering effect were crystalline fructose, no metabolic control, dietary fat intake >30% energy, no randomization, and low study quality (MQS >8) in univariate analyses and dose and follow-up, as independent predictors in multivariate analyses.
Interpretation of these pooled analyses is complicated by several caveats. First, only five trials lasted ≥12-weeks. Whether the lack of effect on TG seen in studies of >4 weeks persists over a longer term, therefore, is uncertain. Second, we did not identify any eligible trials investigating the effect of high-fructose corn syrup, a main source of fructose as a sweetener. This absence was surprising as high-fructose corn syrup has become one of the dominant sweeteners in the U.S., owing to its increased functionality and lower cost (42). Third, the subgroup analyses were underpowered to assess differences in one factor across the different levels of the others. We did, however, attempt to explore the relative contribution of the subgroup factors with multiple regression models, although we are aware of the limitation posed by performing these analyses with so few studies. Finally, because only published studies were included, publication bias remains a possibility, although funnel plots showed this to be improbable.
In summary, fructose used as a nutritive sweetener in isocaloric exchange for carbohydrate seems to have only a modest TG-raising effect in type 2 diabetes at doses >60 g/day with follow-up of ≤4 weeks or when the reference carbohydrate is starch. This effect is in addition to a modest total cholesterol–lowering effect driven by markers of poor study quality, crystalline fructose, and dietary fat intake >30% energy. No other lipid effects were detected. These data suggest that in the context of low fructose intake as a nutritive sweetener in exchange for other sugars, concerns relating to adverse lipid effects may not be justified. Nevertheless, there is a clear imperative for well-powered, long-term (>6 months) randomized controlled trials that investigate fructose exchange for starch and sucrose over a wide dose range in individuals with type 2 diabetes to address the sources of heterogeneity identified in the data. Further meta-analyses of additional metabolic parameters, including body weight, glycemic control, and blood pressure, are planned. Separate analyses of hypercaloric feeding trials are also warranted. In the meantime, the heterogeneity in the data should be considered in the formulation of guidelines.
Supplementary Material
Acknowledgments
J.L.S. was supported in this work by a Province of Ontario Postdoctoral Fellowship and the Edie Steinberg Scholarship Fund and the Edward Christie Stevens Fellowship in Medicine. D.J.A.J. was funded by the Government of Canada through the Canada Research Chair Endowment.
Travel funding for presentation of this work at the first two meetings mentioned below was provided by an unrestricted grant from The Coca-Cola Company (Atlanta, GA). J.L.S. has received travel fees and honorarium from Archer Daniels Midland and consultant fees from Pulse Canada via BDSK Consulting, Toronto, Ontario, Canada. C.W.C.K. serves on the scientific advisory board for Pulse Canada, has received consultant fees from Pulse Canada via BDSK Consulting, Toronto, Ontario, Canada, and has served on the scientific advisory board, received research support, travel support, consultant fees, or honoraria from Barilla, Solae, Unilever, Hain Celestial, Loblaws, Oldways Preservation Trust, the Almond Board of California, the International Nut Council, Paramount Farms, the California Strawberry Commission, and the Canola and Flax Councils of Canada. D.J.A.J. has served on the scientific advisory board for/or received research support, consultant fees, or honoraria from Barilla, Solae, Unilever, Hain Celestial, Loblaws, Sanitarium Company, Herbalife International, Pacific Health Laboratories, Metagenics/MetaProteomics, Bayer Consumer Care, Oldways Preservation Trust, the Almond Board of California, the California Strawberry Commission, Orafti, and the Canola and Flax Councils of Canada. No other potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 26th International Symposium on Diabetes and Nutrition, Varna, Bulgaria, 26–29 June 2008; the Canadian Diabetes Association and Canadian Society for Endocrinology and Metabolism Professional Conference and Annual Meetings, Montreal, Quebec, Canada, 15–18 October 2008; and the 7th International Symposium on Multiple Risk Factors in Cardiovascular Disease, Venice, Italy, 22–25 October 2008.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497 [DOI] [PubMed] [Google Scholar]
- 2. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V: Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation 2007;115:450–458 [DOI] [PubMed] [Google Scholar]
- 3. Lorenzo C, Williams K, Hunt KJ, Haffner SM: The National Cholesterol Education Program-Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care 2007;30:8–13 [DOI] [PubMed] [Google Scholar]
- 4. Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML: Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl. 1):S61–S78 [DOI] [PubMed] [Google Scholar]
- 5. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2008;32:S1–S201 [DOI] [PubMed] [Google Scholar]
- 6. Mann JI, De Leeuw I, Hermansen K, Karamanos B, Karlstrom B, Katsilambros N, Riccardi G, Rivellese AA, Rizkalla S, Slama G, Toeller M, Uusitupa M, Vessby B: Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis 2004;14:373–394 [DOI] [PubMed] [Google Scholar]
- 7. Livesey G, Tagami H: Interventions to lower the glycemic response to carbohydrate foods with a low-viscosity fiber (resistant maltodextrin): meta-analysis of randomized controlled trials. Am J Clin Nutr 2009;89:114–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marriott BP, Cole N, Lee E: National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009;139:1228S–1235S [DOI] [PubMed] [Google Scholar]
- 9. Higgins JPT, Green S. (Eds.). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 [updated September 2008]. Oxford, U.K., Cochrane Collaboration, 2008. [Google Scholar]
- 10. Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF: Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-Analyses. Lancet 1999;354:1896–1900 [DOI] [PubMed] [Google Scholar]
- 11. Higgins JPT, Green S. (Eds.). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0. Oxford, U.K., Cochrane Collaboration, 2008. [Google Scholar]
- 12. Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N: Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006;59:7–10 [DOI] [PubMed] [Google Scholar]
- 13. Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A: Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002;31:140–149 [DOI] [PubMed] [Google Scholar]
- 14. Heyland DK, Novak F, Drover JW, Jain M, Su X, Suchner U: Should immunonutrition become routine in critically ill patients? A systematic review of the evidence. JAMA 2001;286:944–953 [DOI] [PubMed] [Google Scholar]
- 15. Akerblom HK, Siltanen I, Kallio AK: Does dietary fructose affect the control of diabetes in children? Acta Med Scand Suppl 1972;542:195–202 [DOI] [PubMed] [Google Scholar]
- 16. Pelkonen R, Aro A, Nikkila EA: Metabolic effects of dietary fructose in insulin dependent diabetes of adults. Acta Med Scand Suppl 1972;542:187–193 [DOI] [PubMed] [Google Scholar]
- 17. Crapo PA, Kolterman OG, Henry RR: Metabolic consequence of two-week fructose feeding in diabetic subjects. Diabetes Care 1986;9:111–119 [DOI] [PubMed] [Google Scholar]
- 18. Bantle JP, Laine DC, Thomas JW: Metabolic effects of dietary fructose and sucrose in types I and II diabetic subjects. JAMA 1986;256:3241–3246 [PubMed] [Google Scholar]
- 19. McAteer EJ, O'Reilly G, Hadden DR: The effects of one month high fructose intake on plasma glucose and lipid levels in non-insulin-dependent diabetes. Diabet Med 1987;4:62–64 [DOI] [PubMed] [Google Scholar]
- 20. Osei K, Falko J, Bossetti BM, Holland GC: Metabolic effects of fructose as a natural sweetener in the physiologic meals of ambulatory obese patients with type II diabetes. Am J Med 1987;83:249–255 [DOI] [PubMed] [Google Scholar]
- 21. Grigoresco C, Rizkalla SW, Halfon P, Bornet F, Fontvieille AM, Bros M, Dauchy F, Tchobroutsky G, Slama G: Lack of detectable deleterious effects on metabolic control of daily fructose ingestion for 2 mo in NIDDM patients. Diabetes Care 1988;11:546–550 [DOI] [PubMed] [Google Scholar]
- 22. Thorburn AW, Crapo PA, Beltz WF, Wallace P, Witztum JL, Henry RR: Lipid metabolism in non-insulin-dependent diabetes: effects of long-term treatment with fructose-supplemented mixed meals. Am J Clin Nutr 1989;50:1015–1022 [DOI] [PubMed] [Google Scholar]
- 23. Anderson JW, Story LJ, Zettwoch NC, Gustafson NJ, Jefferson BS: Metabolic effects of fructose supplementation in diabetic individuals. Diabetes Care 1989;12:337–344 [DOI] [PubMed] [Google Scholar]
- 24. Osei K, Bossetti B: Dietary fructose as a natural sweetener in poorly controlled type 2 diabetes: a 12-month crossover study of effects on glucose, lipoprotein and apolipoprotein metabolism. Diabet Med 1989;6:506–511 [DOI] [PubMed] [Google Scholar]
- 25. Bantle JP, Swanson JE, Thomas W, Laine DC: Metabolic effects of dietary fructose in diabetic subjects. Diabetes Care 1992;15:1468–1476 [DOI] [PubMed] [Google Scholar]
- 26. Koivisto VA, Yki-Jarvinen H: Fructose and insulin sensitivity in patients with type 2 diabetes. J Intern Med 1993;233:145–153 [DOI] [PubMed] [Google Scholar]
- 27. Malerbi DA, Paiva ES, Duarte AL, Wajchenberg BL: Metabolic effects of dietary sucrose and fructose in type II diabetic subjects. Diabetes Care 1996;19:1249–1256 [DOI] [PubMed] [Google Scholar]
- 28. Blayo A, Fontveille A-M, Rizkalla S, Bruzzo F, Slama G: Effets Metaboliques de la Consommation Quotidienne Pednant un an de Saccharose ou de Fructose par des Diabetiques. Med Nut 1990;26:909–913 [Google Scholar]
- 29. Diraison F, Yankah V, Letexier D, Dusserre E, Jones P, Beylot M: Differences in the regulation of adipose tissue and liver lipogenesis by carbohydrates in humans. J Lipid Res 2003;44:846–853 [DOI] [PubMed] [Google Scholar]
- 30. Faeh D, Minehira K, Schwarz JM, Periasamy R, Park S, Tappy L: Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 2005;54:1907–1913 [DOI] [PubMed] [Google Scholar]
- 31. Chong MF, Fielding BA, Frayn KN: Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 2007;85:1511–1520 [DOI] [PubMed] [Google Scholar]
- 32. Abdel-Sayed A, Binnert C, Le KA, Bortolotti M, Schneiter P, Tappy L: A high-fructose diet impairs basal and stress-mediated lipid metabolism in healthy male subjects. Br J Nutr 2008;100:393–399 [DOI] [PubMed] [Google Scholar]
- 33. Couchepin C, Le KA, Bortolotti M, da Encarnacao JA, Oboni JB, Tran C, Schneiter P, Tappy L: Markedly blunted metabolic effects of fructose in healthy young female subjects compared with male subjects. Diabetes Care 2008;31:1254–1256 [DOI] [PubMed] [Google Scholar]
- 34. Le KA, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L: A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr 2006;84:1374–1379 [DOI] [PubMed] [Google Scholar]
- 35. Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ: Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murphy EJ: Stable isotope methods for the in vivo measurement of lipogenesis and triglyceride metabolism. J Anim Sci 2006;84(Suppl.):E94–E104 [DOI] [PubMed] [Google Scholar]
- 37. Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK: Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest 1999;104:1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moore MC, Davis SN, Mann SL, Cherrington AD: Acute fructose administration improves oral glucose tolerance in adults with type 2 diabetes. Diabetes Care 2001;24:1882–1887 [DOI] [PubMed] [Google Scholar]
- 39. Petersen KF, Laurent D, Yu C, Cline GW, Shulman GI: Stimulating effects of low-dose fructose on insulin-stimulated hepatic glycogen synthesis in humans. Diabetes 2001;50:1263–1268 [DOI] [PubMed] [Google Scholar]
- 40. Lewis GF, Uffelman K, Naples M, Szeto L, Haidari M, Adeli K: Intestinal lipoprotein overproduction, a newly recognized component of insulin resistance, is ameliorated by the insulin sensitizer rosiglitazone: studies in the fructose-fed Syrian golden hamster. Endocrinology 2005; 146:247–255 [DOI] [PubMed] [Google Scholar]
- 41. Skoog SM, Bharucha AE: Dietary fructose and gastrointestinal symptoms: a review. Am J Gastroenterol 2004;99:2046–2050 [DOI] [PubMed] [Google Scholar]
- 42. White JS: Misconceptions about high-fructose corn syrup: is it uniquely responsible for obesity, reactive dicarbonyl compounds, and advanced glycation endproducts? J Nutr 2009;139:1219S–1227S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.