Abstract
OBJECTIVE
The Indian Diabetes Prevention Programme-1 (IDPP-1) showed that lifestyle modification (LSM) and metformin were effective for primary prevention of diabetes in subjects with impaired glucose tolerance (IGT). Among subjects followed up for 3 years (n = 502), risk reductions versus those for the control group were 28.5, 26.4, and 28.2% in LSM, metformin (MET), and LSM plus MET groups, respectively. In this analysis, the roles of changes in secretion and action of insulin in improving the outcome were studied.
RESEARCH DESIGN AND METHODS
For this analysis, 437 subjects (93 subjects with normoglycemia [NGT], 150 subjects with IGT, and 194 subjects with diabetes) were included. Measurements of anthropometry, plasma glucose, and plasma insulin at baseline and at follow-up were available for all of them. Indexes of insulin resistance (homeostasis model assessment of insulin resistance) and β-cell function (insulinogenic index [ΔI/G]: 30-min fasting insulin divided by 30-min glucose) were also analyzed in relation to the outcome.
RESULTS
Subjects with IGT showed a deterioration in β-cell function with time. Individuals with higher insulin resistance and/or low β-cell function at baseline had poor outcome on follow-up. In relation to no abnormalities, the highest incidence of diabetes occurred when both abnormalities coexisted (54.9 vs. 33.7%, χ2 = 7.53, P = 0.006). Individuals having abnormal insulin resistance (41.1%) or abnormal ΔI/G (51.2%, χ2 = 4.87, P = 0.027 vs. no abnormalities) had lower incidence. Normal β-cell function with improved insulin sensitivity facilitated reversal to NGT, whereas deterioration in both resulted in diabetes. The beneficial changes were better with intervention than in the control group. Intervention groups had higher rates of NGT and lower rates of diabetes.
CONCLUSIONS
In the IDPP-1 subjects, beneficial outcomes occurred because of improved insulin action and sensitivity caused by the intervention strategies.
Primary prevention studies in diabetes have been done in subjects with a high risk for diabetes, such as those with impaired glucose tolerance (IGT) (1–6) or with a history of gestational diabetes mellitus (7). Lifestyle modification (LSM) (1–5) and/or pharmacological agents such as metformin (MET) (1,5) and glitazones (6) have been shown to be effective in reducing the rate of conversion of IGT to diabetes in different ethnic groups. The benefits are seen in association with weight reduction in the obese population (1,2) or without significant weight changes in relatively nonobese population (3,5). The mechanisms that result in the beneficial changes are associated with two important pathophysiological components, namely impaired secretion and impaired action of insulin.
The Indian Diabetes Prevention Programme-1 (IDPP-1) had shown that moderate, but consistent, LSM or use of MET reduced the risk of deterioration of IGT to diabetes by 28% in relation to that in a control group who had no intervention in a 3-year follow-up period (5). Combining LSM with MET showed no added benefit.
IGT, an intermediate state in the natural history of type 2 diabetes, is characterized by a worsening in insulin resistance and insulin secretion (8). Asian Indians have higher rates of insulin resistance than Europeans and other white populations despite being relatively nonobese (9,10).
The chief pathophysiological components of type 2 diabetes, namely impaired secretion and action of insulin are detectable many years before the diagnosis of clinical diabetes (11). A combined occurrence of both defects due to gradual deterioration, eventually results in diabetes. This analysis was done to identify the changes in insulin secretion and insulin action that produced the improved outcome with the primary prevention strategies in the IDPP-1 cohort.
RESEARCH DESIGN AND METHODS
In the IDPP-1, 531 subjects (421 male and 110 female) aged 35–55 years were recruited (5). Screening was performed using 2-h postglucose capillary glucose measurement, and confirmatory diagnosis was made by a standard oral glucose tolerance test (OGTT) with a 75-g glucose load. Subjects found to have IGT on two occasions (2-h postglucose levels of ≥7.8–11.1 mmol/l) according to the criteria of the World Health Organization (12) were included in the program. All eligible subjects were randomly assigned consecutively, as follows: group 1 (control), standard health care advice; group 2, advice on LSM; group 3, treatment with 500 g/day MET; and group 4, LSM plus MET. The primary outcome measure was new-onset type 2 diabetes. The measurements were done during semiannual reviews. If a diagnosis of diabetes was made, it was confirmed by an OGTT as per the World Health Organization criteria (12). Over a median follow-up period of 30 months, 502 subjects were available for follow-up, and the cumulative incidences of diabetes were 55, 39.3, 40.5, and 39.5%, respectively, in the four groups; the risk reduction relative to the control group was 28.5% with LSM, 26.4% with MET, and 28.2% with LSM plus MET. Numbers (percentage) of subjects with normoglycemia (NGT) in the four groups were 32 (24.1%), 35 (35.8%), 39 (30.5%), and 38 (31.4%), respectively. The study protocol was approved by the ethics committee of the institution. Informed consent was obtained for all subjects.
Plasma glucose was measured using the glucose-oxidase peroxidase method on a Hitachi autoanalyzer 912 with the reagents supplied by Roche Diagnostics (Mannheim, Germany). A1C was estimated by the immunoturbidimetric method (Roche Reagents). The cutoff value for normal was 6.0%.
Plasma insulin was measured using a radioimmunoassay kit (DiaSorin, Saluggia, Italy). The assay had a sensitivity of <24 pmol/l, and the intra- and interassay coefficient of variations were <10%. Insulin resistance was calculated using homeostasis model assessment (HOMA-IR) (13). The insulinogenic index (ΔI/G) was calculated using the difference in the values of 30-min and fasting plasma insulin (picomoles per liter) divided by the 30-min glucose value (millimoles per liter) (14). Cutoff values for normal HOMA-IR were <4.1 (15) and ≥28 for ΔI/G (16).
The relative risk reduction in the incidence of diabetes compared with that in the control group was similar in the three intervention groups. Hence, the present analysis was done in the control group versus all intervention groups combined.
All of the relevant data for this analysis were available for 437 subjects of the 502 subjects followed up for a median of 30 months. Comparative analyses of baseline and 3rd-year data were done for subjects with NGT and IGT. For the diabetic group, the baseline data were compared with the corresponding values recorded at the time of diagnosis of diabetes. The numbers of subjects with NGT, IGT, and diabetes were 93, 150, and 194, respectively.
Variables included in the analysis were BMI, waist circumference, plasma glucose, plasma insulin values (0, 30, and 120 min during an OGTT), HOMA-IR values, and ΔI/G. These baseline variables in the subgroup of 437 analyzed were similar to those of the original cohort of 531 subjects. The differences at the 3rd year from the baseline value were calculated. Data were computed for control and intervention groups.
Changes in plasma glucose, insulin, HOMA-IR, and ΔI/G were analyzed annually in the total group (control and intervention) in relation to their glucose tolerance status. In addition, annual HOMA-IR and ΔI/G were compared with the respective baseline values for each intervention group.
Statistical analysis
Means ± SD of the variables were calculated for normally distributed variables. Inter- and intragroup variations were tested using paired or unpaired t tests as relevant. Data for plasma insulin, HOMA-IR, and ΔI/G had skewed distribution and hence median values are shown. Inter- and intragroup comparisons were done using a Mann-Whitney U test and Wilcoxon's signed-rank test, respectively. A χ2 test was done to compare intergroup results. Cox regression analyses were done to identify the variables predictive of conversion to diabetes or to NGT. For diabetes, baseline and the corresponding values recorded at the time of diagnosis were used for analysis. For NGT and IGT, baseline and 3rd-year values were analyzed. Cox regression analyses showed that the outcome measures were influenced in the control and intervention groups by similar baseline and follow-up variables. However, in the control group a few variables showed weaker association, which failed to reach statistical significance (P = 0.078 for HOMA-IR). This was probably due to the smaller sample size in this group. Hence, considering the uniformity of results, the final regression analysis was done in the total sample, combining control and intervention groups. The control group was included as an independent variable. The statistical package SPSS for Windows (version 10.0; SPSS, Chicago, IL) was used. P < 0.05 was considered to be significant.
RESULTS
For this analysis, 437 subjects were included. At the end of the study at the 3rd year, subjects with NGT, IGT, and diabetes were 93, 150, and 194, respectively. Table 1 shows the comparative data at baseline and at the 3rd-year review in the control (n = 116) versus the intervention groups (n = 321). The baseline values of anthropometry and biochemical variables were similar in both groups except for a higher plasma insulin value at 120 min in the control group (P = 0.021). At the review, BMI was higher in the control versus the intervention group (P = 0.036). Plasma glucose increased significantly in both groups in the 3rd year. As expected, the increase was more significant in the control group. At the 3rd year, the total A1C value increased in the control group (not significant), whereas it showed a significant (P = 0.016) reduction in the intervention group. Significant decreases in plasma insulin values were seen in both groups. Although the median HOMA-IR values showed no significant changes, the ΔI/G decreased significantly in both groups.
Table 1.
Comparison of data at baseline and at 3rd-year review in control versus intervention groups
| Control | Intervention |
P
|
|||
|---|---|---|---|---|---|
| Intergroup | Intragroup control | Intragroup intervention | |||
| n | 116 | 321 | |||
| BMI (kg/m2) | |||||
| Basal | 26.0 ± 3.0 | 25.5 ± 3.4 | 0.185 | ||
| Follow-up | 26.4 ± 3.1 | 25.7 ± 3.3 | 0.036 | <0.0001 | |
| Waist circumference (cm) | |||||
| Basal | 89.3 ± 7.4 | 89.1 ± 8.8 | 0.815 | ||
| Follow-up | 90.8 ± 7.6 | 90.0 ± 8.7 | 0.426 | <0.007 | 0.002 |
| Glucose (mmol/l) | |||||
| Fasting | |||||
| Basal | 5.5 ± 0.8 | 5.4 ± 0.7 | 0.305 | ||
| Follow-up | 6.5 ± 1.8 | 6.1 ± 1.5 | 0.025 | <0.0001 | <0.0001 |
| 30-min | |||||
| Basal | 9.3 ± 1.7 | 9.5 ± 1.9 | 0.174 | ||
| Follow-up | 11.0 ± 2.9 | 10.6 ± 2.5 | 0.114 | <0.0001 | <0.0001 |
| 120-min | |||||
| Basal | 8.6 ± 0.7 | 8.5 ± 0.7 | 0.198 | ||
| Follow-up | 10.9 ± 4.0 | 9.9 ± 3.2 | 0.009 | <0.0001 | <0.0001 |
| A1C (%) | |||||
| Basal | 6.2 ± 0.5 | 6.2 ± 0.5 | 0.481 | ||
| Follow-up | 6.4 ± 1.2 | 6.1 ± 0.9 | 0.016 | 0.140 | 0.215 |
| Insulin (pmol/l) | |||||
| Fasting | |||||
| Basal | 120 | 114 | 0.164 | ||
| Follow-up | 108 | 102 | 0.361 | 0.091 | 0.029 |
| 30-min | |||||
| Basal | 432 | 450 | 0.920 | ||
| Follow-up | 363 | 387 | 0.599 | 0.004 | <0.0001 |
| 120-min | |||||
| Basal | 636 | 558 | 0.021 | ||
| Follow-up | 510 | 510 | 0.776 | <0.0001 | 0.003 |
| HOMA-IR | |||||
| Basal | 4.8 | 4.5 | 0.115 | ||
| Follow-up | 4.6 | 4.5 | 0.130 | 0.194 | 0.293 |
| ΔI/G | |||||
| Basal | 36.3 | 33.7 | 0.632 | ||
| Follow-up | 24.0 | 27.2 | 0.220 | <0.0001 | <0.0001 |
Data are means ± SD (values were compared using paired or unpaired t tests) or medians (comparisons were done using median tests).
Outcomes of glucose tolerance at the 3rd year were analyzed in relation to the biochemical abnormalities categorized on the basis of the baseline values of HOMA-IR (abnormal ≥4.1) and ΔI/G (abnormal <28) (Table 2). The prevalence of diabetes was the highest in group 4 with both abnormalities (54.9%, χ2 = 7.53, P = 0.006 vs. normal group [group 1]) followed by group 3 with a β-cell defect (51.2%, χ2 = 4.87, P = 0.027 vs. group 1). Prevalence of NGT was higher in the normal group, but the intergroup differences were not statistically significant.
Table 2.
Outcome of glucose tolerance at 3rd year in relation to the status of baseline HOMA-IR and ΔI/G
| Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|
| Status of IR, ΔI/G | Both normal | IR abnormal | ΔI/G abnormal | Both abnormal |
| n | 92 | 168 | 86 | 91 |
| HOMA-IR | 3.1 | 6.1 | 2.6 | 5.7 |
| ΔI/G | 45.8 | 51.2 | 15.2 | 17.3 |
| Outcome at 3rd year | ||||
| NGT | 25 (27.2) | 34 (20.2) | 17 (19.8) | 17 (18.6) |
| IGT | 36 (39.1) | 65 (38.7) | 25 (29.1) | 24 (26.3) |
| Diabetes | 31 (33.7) | 69 (41.1) | 44 (51.2)* | 50 (54.9)†‡ |
Data are medians or n (%).
*χ2 = 4.87, P = 0.027 vs. group 1;
†χ2 = 7.53, P = 0.006 vs. group 1;
‡χ2=4.03, P = 0.045 vs. group 2. IR, insulin resistance.
Table 3 shows the baseline and follow-up values of HOMA-IR and ΔI/G in the control and in the total intervention group in relation to glucose tolerance status at the follow-up. The pattern of changes in insulin resistance and ΔI/G were similar in individual intervention groups, but minor statistical variations were seen due to smaller sample sizes (data not shown). Development of diabetes occurred mostly in individuals who had higher values of insulin resistance and lower values of ΔI/G at the baseline. On follow-up, both functions deteriorated further. On the other hand, subjects who reverted to NGT showed improvement in insulin sensitivity, and secretion of insulin remained in normal ranges.
Table 3.
Status of insulin resistance and insulinogenic index in relation to the glucose tolerance status in the control and intervention groups
| Control (n = 116) |
Intervention (n = 321) |
|||
|---|---|---|---|---|
| HOMA-IR | ΔI/G | HOMA-IR | ΔI/G | |
| NGT (n = 93) | ||||
| n | 15 | 15 | 78 | 78 |
| Basal | 4.4 | 32.6 | 4.5 | 40.9 |
| Follow-up | 3.2 (0.078) | 41.1 | 3.5 (0.021) | 35.6 |
| IGT (n = 150) | ||||
| n | 39 | 39 | 111 | 111 |
| Basal | 4.5 | 39.6 | 4.4 | 38.7 |
| Follow-up | 4.1 | 39.5 | 4.0 | 31.9 (0.002) |
| Diabetes (n = 94) | ||||
| n | 62 | 62 | 132 | 132 |
| Basal | 5.4 | 34.7*† | 4.6 | 27.2 |
| Follow-up | 6.9 (0.007)*† | 15.7 (<0.0001)*† | 5.4 (<0.0001)*† | 17.9 (<0.0001)*† |
Data are medians. Significant P values shown are for intragroup comparisons of follow-up vs. baseline (Wilcoxon rank test). Intergroup diabetes comparisons were done with a Mann-Whitney U test.
*P <0.001 vs. NGT;
†P <0.001 vs. IGT.
Cox regression analyses adjusted for baseline age, BMI, and waist circumference showed that lower 120-min plasma glucose (β = −0.029, hazard ratio [HR] 0.972, P = 0.011), lower HOMA-IR (β = −0.167, HR 0.846, P = 0.008), and higher ΔI/G (β = 0.016, HR 1.016, P = 0.002) at baseline and reduction in HOMA-IR (β = −0.152, HR 0.859, P = 0.005) and improved ΔI/G (β = 0.015, HR 1.015, P = 0.001) improved glucose tolerance from IGT to NGT. For diabetes, the risk was higher in those with lower 120-min insulin values (β = −0.006, HR 0.994, P = 0.007), higher HOMA-IR (β = 0.172, HR 1.187, P < 0.0001), and lower ΔI/G (β = −0.015, HR 0.985, P = 0.003) at baseline and an increase in HOMA-IR (β = 0.118, HR 1.126, P < 0.0001), and a reduction in ΔI/G (β = −0.016, HR 0.984, P = 0.001) increased the hazard for diabetes.
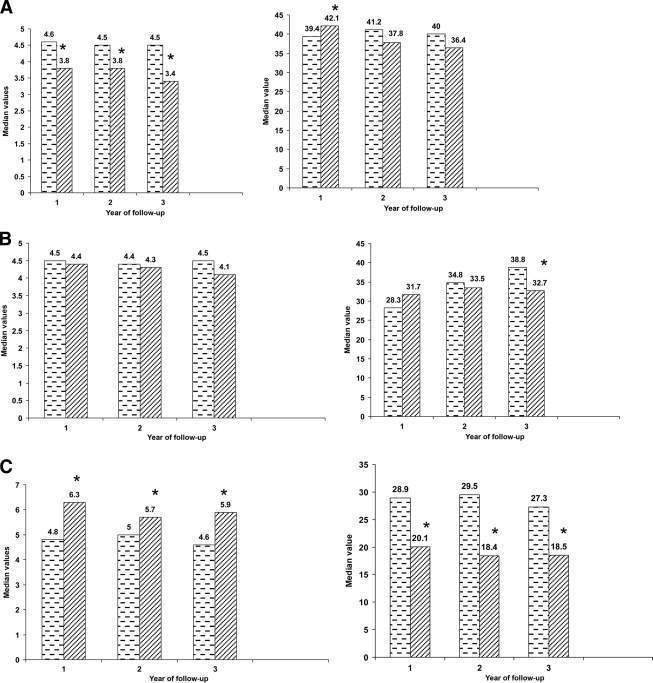
Figure 1 shows the changes in HOMA-IR and ΔI/G in relation to the corresponding baseline values in subjects who had NGT, IGT, and diabetes, at each annual follow-up. In the NGT group, insulin resistance decreased significantly at all follow-up visits, ΔI/G showed improvement in year 1 and remained normal at other periods. Those who continued to have IGT showed no significant changes in insulin resistance. Value of ΔI/G showed a significant reduction in the 3rd year. Subjects with diabetes showed increased insulin resistance and decreased ΔI/G at all time periods.
Figure 1.
Median values of HOMA-IR (A, left panel) and ΔI/G (A, right panel) were calculated in relation to glycemic status (NGT, IGT [B], and diabetes [C]) at annual follow-up. The median values at the 1st-, 2nd-, and 3rd-year follow-up in comparison with the corresponding baseline values in these participants are shown in each category of glucose tolerance. A: 1st year n = 160, 2nd year n = 155, 3rd year n = 93. B: 1st year n = 201, 2nd year n = 152, 3rd year n = 150. C: 1st year n = 70, 2nd year n = 60, 3rd year n = 64. ▩, baseline; ▨, follow-up. *P < 0.05 vs. baseline values.
CONCLUSIONS
Significant reductions in incident diabetes with interventions in the IDPP-1 were due to improvement in β-cell function and in insulin sensitivity. Diabetes developed when insulin resistance and β-cell function deteriorated. Reversal to NGT occurred when insulin sensitivity improved and β-cell function remained normal. In the intervention group (total or each), a higher proportion of subjects reverted to NGT and a smaller proportion developed diabetes compared with the control group.
Although the biochemical mechanisms causing diabetes and reversal to NGT were similar in the control and intervention groups, the benefits seen with inventions were due to augmented beneficial changes in insulin sensitivity and insulin secretion. Subjects with impaired fasting glucose or IGT have impaired phase I insulin secretion, which may explain their high risk for conversion to diabetes (17).
In subjects with IGT, a time-related deterioration in the ΔI/G was noted. Normal β-cell function with an improved sensitivity of insulin had favored reversal to NGT, whereas a deterioration in both functions resulted in diabetes. Individuals with higher insulin resistance and/or lower β-cell capacity at the baseline had a predisposition to the adverse outcome. These findings seen in the univariate and multivariate analyses agree with the sequence of changes described by Festa et al. (18) in the development of diabetes from NGT to diabetes in a longitudinal study. The Diabetes Prevention Programme (DPP) also demonstrated that the better preventive effect of intensive lifestyle was due to improved insulin sensitivity concomitant with preservation of β-cell function (19). Treatment with metformin also demonstrated similar changes, although to a lesser degree than with intensive lifestyle changes. The Finnish Diabetes Prevention Study (DPS) had shown similar effects of lifestyle modification (20). In our study, a moderate, but sustained, lifestyle modification and/or metformin produced similar beneficial changes. These effects were not associated with weight loss, unlike in the DPP and DPS studies (19,20). Probably a redistribution of body fat, which is shown to occur with enhanced physical activity (21), could have improved insulin sensitivity in our study subjects. At follow-up, BMI increased only in the control group, whereas waist circumference increased in both groups. The increase was significantly less in the intervention groups.
Compromised β-cell function is detectable in pre-diabetic individuals long before the onset of type 2 diabetes (11,18). The importance of declining β-cell function in the transition of NGT to IGT and IGT to diabetes has been demonstrated in a longitudinal study in Pima Indians (22). The Insulin Resistance Atherosclerosis Study addressed the longitudinal changes in β-cell function in a period of 5.2 years in subjects with NGT, IGT, and diabetes in a multiethnic population (18). The results showed that the mean insulin sensitivity, measured as an index (SI) from a frequently sampled intravenous glucose tolerance test, declined in all glucose tolerance categories with time. Importantly, it was demonstrated that on follow-up, the glucose tolerance status was principally maintained by the change in acute insulin response. A compensatory increase in insulin secretion maintained NGT; IGT status was due to a failure to increase insulin secretion and decreased insulin secretion led to diabetes (18). The results of the DPP (19) and the Troglitazone in Prevention of Diabetes (TRIPOD) study (7) had also demonstrated the pivotal role of β-cell dysfunction in the conversion of IGT to diabetes. β-Cell function is enhanced by improved insulin sensitivity, and the preservation of β-cell function decreases the conversion of IGT to diabetes.
Subjects with lower baseline 120-min plasma glucose were found to have a higher probability of reversal to NGT, which could be explained by the absence of the above pathophysiological components. A negative association between baseline 120-min plasma insulin response and development of diabetes in this study was in agreement with similar findings by Saad et al. (23).
The HOMA-IR index derived from the product of fasting glucose and insulin concentrations primarily reflects hepatic insulin resistance. It has been demonstrated that there is a 70% concordance between muscle insulin resistance and liver insulin resistance in the same subjects (24).
A limitation of this study is that the estimates of insulin secretion and action have been made by calculations based on the OGTT and not by a “gold standard” test (euglycemic clamp study). The indexes are being used in epidemiological studies because the clamp studies are not feasible in large numbers. The early-phase insulin secretion calculated as the ΔI/G correlates with the first-phase insulin release measured during hyperglycemic insulin clamp (25).
In summary, the IDPP-1 study showed that a decrease in insulin secretion combined with reduced insulin sensitivity resulted in diabetes and an improvement in these functions facilitated reversal to NGT in subjects with IGT. The rate of conversion to diabetes was significantly lower and reversal to NGT was higher in subjects who received interventions than in the control group. This result indicated that beneficial effects of interventions work through mechanisms of preserving β-cell function and improving insulin sensitivity even in subjects not having a reduction in body weight.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
We thank Catherin Seeli and Manian Muruganantham for helping in the field work for the study and Vijaya Balaji for helping in statistical analysis and secretarial help. The secretarial assistance of Bobby Alex is also acknowledged.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Tumilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Loutheranta A, Rastas M, Salminen V, Uusitupa M: the Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 2. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. Diabetes Prevention Program Research Group. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV: Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 4. Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Li H, Jiang Y, An Y, Shuai Y, Zhang B, Zhang J, Thompson TJ, Gerzoff RB, Roglic G, Hu Y, Bennett PH: The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 5. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V: The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49:289–297 [DOI] [PubMed] [Google Scholar]
- 6. The DREAM (Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication) Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomized controlled trial. Lancet 2006;368:1096–1105 [DOI] [PubMed] [Google Scholar]
- 7. Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP: Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 2002;51:2769–2803 [DOI] [PubMed] [Google Scholar]
- 8. Walker M, Mari A, Jayapaul MK, Bennett SM, Ferrannini E: Impaired β-cell glucose sensitivity and whole-body insulin sensitivity as predictors of hyperglycaemia in non-diabetic subjects. Diabetologia 2005;48:2470–2476 [DOI] [PubMed] [Google Scholar]
- 9. Abate N, Chandalia M: Ethnicity, type 2 diabetes and migrant Asian Indians. Indian J Med Res 2007;125:251–258 [PubMed] [Google Scholar]
- 10. Snehalatha C, Ramachandran A: Insulin resistance in Asians Indians. Prac Diabetes Int 1999;16:19–22 [Google Scholar]
- 11. UK Prospective Diabetes Study Group. UK Prospective Diabetes Study 16: overview of 6 years' therapy of type 2 diabetes: a progressive disease. Diabetes 1995;44:1249–1258 [PubMed] [Google Scholar]
- 12. World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Organization, 1999. (WHO/NCD/NCS/99.2) [Google Scholar]
- 13. Mathews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DR, Turner RL: Homeostatis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 14. Wareham NJ, Philips DIW, Byrne CD, Hales CN: The 30 minute insulin incremental response in an oral glucose tolerance test as a measure of insulin secretion. Diabet Med 1995;12:931 [DOI] [PubMed] [Google Scholar]
- 15. Ramachandran A, Snehalatha C, Satyavani K, Sivasankari S, Vijay V: Metabolic syndrome in urban Asian Indian adults—a population study using modified ATP III criteria. Diabetes Res Clin Pract 2003;60:199–204 [DOI] [PubMed] [Google Scholar]
- 16. Snehalatha C, Satyavani K, Sivasankari S, Vijay V, Ramachandran A: Insulin secretion and action in different stages of glucose tolerance in Asian Indians. Diabet Med 1999;16:408–414 [DOI] [PubMed] [Google Scholar]
- 17. Unwin N, Shaw J, Zimmet P, Alberti KGMM: Impaired glucose tolerance and impaired fasting glycemia: the current status on definition and intervention. Diabet Med 2002;19:708–723 [DOI] [PubMed] [Google Scholar]
- 18. Festa A, Williams K, D'Agostino R, Jr, Wagenknecht LE, Haffner SM: The natural course of β-cell function in nondiabetic and diabetic individuals: the Insulin Resistance Atherosclerosis Study. Diabetes 2006;55:1114–1120 [DOI] [PubMed] [Google Scholar]
- 19. The Diabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes 2005;54:2404-2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uusuitupa M, Lindi V, Loutheranta A, Salopuro T, Lindstrom J, Tumilehto J: Finnish Diabetes Prevention Study Group. Long-term improvement in insulin sensitivity by changing lifestyles of people with impaired glucose tolerance: 4-year results from the Finnish Diabetes Prevention Study. Diabetes 2003;52:2532–2538 [DOI] [PubMed] [Google Scholar]
- 21. Despres JP, Lamarche B: Effects of diet and physical activity on adiposity and body fat distribution: implications for the prevention of cardiovascular disease. Nutr Res Rev 1993;6:137–159 [DOI] [PubMed] [Google Scholar]
- 22. Weyer C, Bogardus C, Mott DM, Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 1999;104:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saad MF, Knowler WC, Pettitt DJ, Nelson RG, Mott DM, Bennett PH: The natural history of impaired glucose tolerance in the Pima Indians. N Engl J Med 1988;319:1500–1506 [DOI] [PubMed] [Google Scholar]
- 24. Matsuda M, DeFronzo RA: Relationship between insulin sensitivity in adipose tissue, liver, muscle and components of the insulin resistance syndrome (Abstract). Diabetes 1997;46(Suppl. 1):68A [Google Scholar]
- 25. Korytkowski MT, Berga SL, Horwitz MJ: Comparison of the minimal model and the hyperglycemic clamp for measuring insulin sensitivity and acute insulin response to glucose. Metabolism 1995;44:1121–1125 [DOI] [PubMed] [Google Scholar]