Abstract
OBJECTIVE
Diabetic large–nerve fiber dysfunction, as measured by vibration perception threshold (VPT), predicts foot ulceration, amputation, and mortality. Thus, determination of modifiable risk factors is of great clinical importance.
RESEARCH DESIGN AND METHODS
We assessed 1,407 patients with type 1 diabetes and a normal VPT participating in the EURODIAB Prospective Complications Study, at baseline mean ± SD age of 32.7 ± 10.2 years with diabetes duration of 14.7 ± 9.3 years and follow-up of 7.3 ± 0.6 years. VPT was measured using biothesiometry on the right big toe and medial malleolus. An abnormal result was defined as >2 SD from the predicted mean for the patient s age.
RESULTS
An abnormal VPT was associated with an increased incidence of gangrene, amputation, foot ulceration, leg bypass or angioplasty, and mortality (P ≤ 0.02). The incidence of abnormal VPT was 24% over the 7.3-year follow-up. Duration of diabetes and A1C significantly influenced the incidence of abnormal VPT (P < 0.0001). After correction for these, established risk factors for cardiovascular disease (CVD), including male sex (P = 0.0004), hypertension (P < 0.0001), total cholesterol (P = 0.002), LDL cholesterol (P = 0.01), smoking (P < 0.0001), weight (P < 0.0001), and diabetes complications (retinopathy [P = 0.0001], nephropathy [P = 0.01], and autonomic neuropathy [P = 0.001]), were all found to be significant risk factors. A previous history of CVD doubled the incidence of abnormal VPT.
CONCLUSIONS
This prospective study indicates that cardiovascular risk factors predict development of large-fiber dysfunction, which may account for the high mortality rate in patients with an abnormal VPT, and emphasizes the importance of early determination of VPT to detect subclinical neuropathy and to address cardiovascular risk factors.
Chronic diabetic peripheral neuropathy (DPN) is a slowly progressive process, the pathogenesis of which is poorly understood. However, we do know that large-fiber dysfunction, as measured by vibration perception threshold (VPT), predicts foot ulceration, lower-limb amputation, and mortality (1–3). One consequence of these clinical complications is a massive economic burden, estimated in 2001 to be $10.9 billion in the U.S. (4,5). Early in the natural history of DPN, patients are usually asymptomatic. Thus, reliable identification of individuals in the early stages of the neuropathic process is required so that more rigorous modification of risk factors and foot care education can be implemented. The best method to identify such patients is still a matter of some debate (6–9).
In patients with type 2 diabetes, the prevalence of an abnormal VPT has been shown to be 11.4% (10), and the incidence of an abnormal VPT was 19.9% over a 12-year period (11). Some investigators have used absolute values of VPT as predictors of foot ulceration. In one study, a VPT >25 V was associated with a sevenfold increase in ulcer risk, compared with that of individuals with a VPT <15 V (12). Another study showed an incidence of 7.2% of first foot ulceration within 1 year in patients with a VPT ≥25 (13). In addition, a cutoff of 25 V has been shown to be a more sensitive way of detecting patients at risk of foot ulceration than the 10-g monofilament (14). Small-fiber dysfunction may be determined by measuring thermal thresholds, but in terms of discriminating between patients with and without ulceration, it does not seem to provide any additional value above measurement of VPT (15).
Thus, ample evidence exists to highlight the clinical importance of an abnormally high VPT, but as yet there is little evidence to identify the risk factors involved in its development. Poor glycemic control is associated with an abnormal VPT even at diagnosis in patients with type 2 diabetes (16). Evidence also suggests that height is a determinant of VPT (10,17). Importantly, VPT is known to increase with age (18,19). The majority of research regarding large-fiber dysfunction, as measured by VPT, has been conducted in patients with type 2 diabetes. In this study, we have examined the incidence of abnormal VPT in a large cohort of patients with type 1 diabetes to identify possible modifiable risk factors.
RESEARCH DESIGN AND METHODS
The EURODIAB Prospective Complications Study recruited 3,250 patients (1,668 men and 1,582 women; aged [mean ± SD] 32.7 ± 10.2 years) with duration of diabetes of 14.7 ± 9.3 years. Subjects with type 1 diabetes were randomly selected from 31 diabetes clinics across Europe. The selection criteria and methods have been described in detail previously (20,21). The study was approved by the ethics committee at each center, and written informed consent was provided by all subjects. All measurements were obtained by trained physicians following standardized procedures. The baseline examinations were conducted from 1989 to 1991, and a subsequent follow-up visit occurred between 1997 and 1999.
VPT was measured by centrally calibrated biothesiometers (Bio-Medical Instrument Company, Newbury, OH). Three readings on the right big toe and right medial malleolus were obtained and averaged. Results were classified according to age-related criteria (19).
Neuropathy was defined as the presence of two or more of the following criteria: the presence of one or more symptoms, the absence of two or more reflexes of the ankle or knee tendons (with reinforcement if necessary), an abnormal VPT value, and the presence of cardiovascular autonomic neuropathy, as described previously (21). Symptoms of neuropathy were present if the subject had experienced any of the following in the preceding 6 months: “asleep” numbness or “dead feeling” in the feet, a prickling sensation in the feet, deep aching or burning pains in the legs, unusual difficulty in climbing stairs, difficulty with bladder control, and nocturnal diarrhea. Symptoms were assessed carefully to exclude nondiabetic causes.
Blood samples, taken after an overnight fast, if possible, were sent to central laboratories. To compare the results with those of the Diabetes Control and Complications Trial, measured A1C values were converted as reported previously (22). Lipid measurements included total cholesterol, HDL cholesterol, and triglycerides. Levels of LDL cholesterol were calculated.
Urinary albumin excretion rate was obtained from a single 24-h urine collection. An albumin excretion rate between 20 and 200 μg/min was defined as microalbuminuria, whereas a rate of >200 μg/min was defined as macroalbuminuria. Diabetic retinopathy was determined from centrally graded retinal photographs and was classified as either nonproliferative (background) or proliferative.
Cardiovascular disease (CVD) was defined as either a history of physician-diagnosed CVD (e.g., previous myocardial infarction, angina, coronary artery bypass grafting, or stroke) or ischemic changes on a 12-lead electrocardiogram (classified by two observers according to the Minnesota Code).
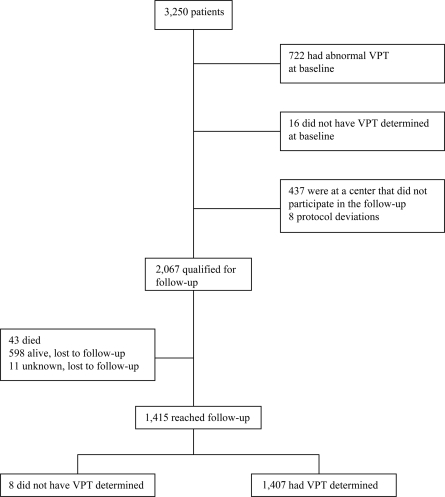
Of the 3,250 patients examined at baseline, 722 had abnormal VPT, whereas 1,407 with normal VPT were assessed at follow-up (Fig. 1). Baseline investigations and determination of VPT were repeated after 7.3 ± 0.6 years of follow-up.
Figure 1.
Patients examined for progression to abnormal VPT in the EURODIAB Study.
Statistical analysis
The data were analyzed using the statistical package SAS (version 8). P < 0.05 was considered statistically significant. For variables that were continuous and normally distributed, a t test was used to compare group means. If, however, the variable had a skewed distribution, the Mann-Whitney U test was used to compare group medians and the variable was log-transformed for any subsequent analysis. To compare group percentages, a χ2 test was used.
To assess which risk factors were associated with the development of VPT, multiple logistic regression was used to calculate odds ratios (ORs) adjusted for A1C and duration. So that the ORs could be compared, for each risk factor standardized ORs were calculated. For a continuous variable, this was the OR associated with an increase of 1 SD, and for a dichotomous variable, the reference group was those patients without the respective risk factor. Similarly, multiple logistic regression was used to assess which variables were associated with the incidence of VPT while adjusting for all other key risk factors.
To assess the effect of glycemic exposure on the incidence of abnormal VPT, linear regression was performed by the center to compare the results of A1C measured locally and centrally at the same time, both at baseline. This comparison provided a formula to convert locally measured A1C to the centralized assay at each center. An average of all local A1C values that had been measured for each patient, before the baseline visit, was calculated and converted to the central measure.
A breakpoint or threshold effect for the relation between A1C and progression to abnormal VPT was tested by a two-phase segmented weighted regression, where two straight lines are fitted through a series of defined points. These points were calculated by logistic regression adjusted for diabetes duration. This segmented regression was compared with the line of best fit using weighted linear regression. Logistic regression was also used to test for a threshold.
RESULTS
Baseline characteristics according to whether VPT was assessed at follow-up
The baseline characteristics of the 1,407 patients examined in this study were compared with those of patients who qualified for follow-up (n = 660) but did not have a follow-up assessment. Those who died or did not have VPT assessed at follow-up had higher baseline A1C (8.5 vs. 8.1%, P < 0.0001), waist-to-hip ratios (0.85 vs. 0.84, P = 0.04), systolic blood pressure (118 vs. 116 mmHg, P = 0.05), total cholesterol (5.35 vs. 5.21 mmol/l, P = 0.005), LDL cholesterol (3.37 vs. 3.27 mmol/l, P = 0.05), and triglyceride levels (0.96 vs. 0.87 mmol/l, P < 0.0001). In addition, they were more likely to have a history of smoking (51 vs. 46%, P = 0.03) and proliferative retinopathy (8 vs. 5%, P = 0.02) and had lower von Willebrand factor levels (1.09 vs. 1.14 units/ml, P = 0.02).
Incidence of clinical outcomes
An abnormal VPT at baseline predicted the incidence of several lower-limb complications, i.e., foot ulcers (7.7 vs. 1.7%, P < 0.0001), leg bypass or angioplasty (1.6 vs. 0.4%, P = 0.02), gangrene (2.2 vs. 0.6%, P = 0.004), amputation (2.4 vs. 0.4%, P = 0.0004), and, indeed, mortality (8.0 vs. 2.1%, P < 0.0001).
Risk factors for the development of an abnormal VPT
Of the 1,407 patients with normal a VPT at baseline, an abnormal VPT developed in 333 at follow-up, an incidence of 23.7%. Table 1 shows baseline characteristics of the study group divided into those who had an abnormal versus a normal VPT at follow-up. Patients who developed an abnormal VPT were significantly older (2.5 years), with longer duration of diabetes (2.2 years) and poorer blood glucose control (A1C 0.6% higher) and were taller (2 cm) at baseline than those patients who maintained a normal VPT. Furthermore, patients who developed an abnormal VPT had significantly higher levels of traditional cardiovascular risk factors, i.e., male sex, history of smoking, weight, BMI, waist-to-hip ratio, hypertension, total cholesterol, LDL cholesterol, triglycerides, history of CVD, and lower HDL cholesterol levels. They were also more likely to have established complications of diabetes, i.e., micro- or microalbuminuria, higher albumin excretion rates, any retinopathy, proliferative retinopathy, and cardiac autonomic neuropathy.
Table 1.
Baseline characteristics of 1,407 patients according to the incidence of abnormal VPT
| Progression to abnormal VPT | Maintenance of normal VPT | P * | |
|---|---|---|---|
| No. patients | 333 | 1,074 | |
| Age (years) | 33.4 ± 10.2 | 30.9 ± 8.8 | <0.0001 |
| Duration of diabetes (years) | 14.8 ± 9.0 | 12.6 ± 8.1 | <0.0001 |
| Male sex (%) | 56 | 46 | 0.001 |
| History of smoking (%) | 56 | 42 | <0.0001 |
| Height (cm) | 170 ± 9 | 168 ± 9 | 0.0004 |
| Weight (kg) | 70.1 ± 10.8 | 65.5 ± 10.1 | <0.0001 |
| BMI (kg/m2) | 24.3 ± 3.0 | 23.2 ± 2.6 | <0.0001 |
| Waist-to-hip ratio | 0.85 ± 0.11 | 0.83 ± 0.10 | 0.0053 |
| A1C (%) | 8.6 ± 2.0 | 8.0 ± 1.8 | <0.0001 |
| Insulin dose/kg body weight (IU) | 0.67 ± 0.21 | 0.69 ± 0.22 | 0.1 |
| Total cholesterol (mmol/l) | 5.45 ± 1.17 | 5.14 ± 1.02 | <0.0001 |
| LDL cholesterol (mmol/l) | 3.48 ± 1.05 | 3.20 ± 0.91 | 0.0005 |
| HDL cholesterol (mmol/l) | 1.46 ± 0.44 | 1.52 ± 0.42 | 0.04 |
| Triglycerides (mmol/l) | 0.95 (0.54, 2.55) | 0.84 (0.48, 2.06) | <0.0001 |
| Fibrinogen (g/l) | 3.26 ± 0.95 | 3.15 ± 0.87 | 0.1 |
| von Willebrand factor (units/ml) | 1.25 (0.64, 2.32) | 1.10 (0.54, 2.11) | 0.0005 |
| Systolic blood pressure (mmHg)† | 117 (98, 152) | 116 (96, 140) | 0.06 |
| Diastolic blood pressure (mmHg)† | 75 (57, 93) | 73 (57, 92) | 0.09 |
| Hypertension (%) | 27 | 15 | <0.0001 |
| History of CVD (%) | 11 | 6 | 0.005 |
| Albumin excretion rate (μg/min) | 12.6 (3.7, 447.4) | 9.5 (3.2, 122.5) | <0.0001 |
| Macroalbuminuria (%) | 9.5 | 3.3 | <0.0001 |
| Micro- or macroalbuminuria (%) | 33 | 22 | <0.0001 |
| Any retinopathy (%) | 52 | 33 | <0.0001 |
| Proliferative retinopathy (%) | 9 | 4 | 0.0001 |
| Cardiac autonomic neuropathy (%) | 39 | 28 | 0.0001 |
Data are means ± SD or medians (5th percentile, 95th percentile) in cases of skewed distributions.
*P values are derived from Student's t test or from a Mann-Whitney U test in cases of skewed distributions and/or from a χ2 test in cases of percentages.
†The blood pressure data exclude patients who were undergoing antihypertensive therapy.
By adjusting for the established confounding factors of duration of diabetes and A1C, risk factors for the development of an abnormal VPT were determined using regression analysis (Table 2). Virtually all of the traditional risk factors of CVD and established complications of diabetes remained significantly associated with the development of abnormal VPT, the only exception being HDL cholesterol. The risk factors with the highest ORs were macroalbuminuria and proliferative retinopathy, i.e., 2.48 and 2.17, respectively. Furthermore, as expected, taller patients were more likely to develop an abnormal VPT, and this association also remained statistically significant after adjustment for male sex (OR 1.38 [95% CI 1.16–1.64], P = 0.003).
Table 2.
Risk factors for the incidence of abnormal VPT after adjustment for A1C and duration of diabetes
| OR (95% CI) | P | |
|---|---|---|
| Male sex (%) | 1.58 (1.23–2.04) | 0.0004 |
| History of smoking (%) | 1.69 (1.31–2.18) | <0.0001 |
| Height (cm) | 1.40 (1.22–1.59) | <0.0001 |
| Weight (kg) | 1.64 (1.44–1.86) | <0.0001 |
| BMI (kg/m2) | 1.43 (1.26–1.62) | <0.0001 |
| Waist-to-hip ratio | 1.17 (1.04–1.32) | 0.009 |
| Insulin dose/kg body weight (IU) | 0.88 (0.77–0.99) | 0.05 |
| Total cholesterol (mmol/l) | 1.22 (1.08–1.39) | 0.002 |
| LDL cholesterol (mmol/l) | 1.22 (1.04–1.42) | 0.01 |
| HDL cholesterol (mmol/l) | 0.90 (0.79–1.02) | 0.10 |
| Triglycerides (mmol/l) | 1.35 (1.15–1.57) | 0.002 |
| von Willebrand factor (units/ml) | 1.27 (1.08–1.51) | 0.005 |
| Hypertension (%) | 1.88 (1.38–2.56) | <0.0001 |
| History of CVD (%) | 1.62 (1.04–2.52) | 0.03 |
| Albumin excretion rate (μg/min)* | 1.29 (1.14–1.45) | <0.0001 |
| Macroalbuminuria (%) | 2.48 (1.47–4.19) | 0.0006 |
| Micro- or macroalbuminuria (%) | 1.44 (1.08–1.93) | 0.01 |
| Any retinopathy (%) | 1.88 (1.36–2.61) | 0.0001 |
| Proliferative retinopathy (%) | 2.17 (1.22–3.86) | 0.008 |
| Cardiac autonomic neuropathy (%) | 1.58 (1.20–2.07) | 0.001 |
Standardized ORs are expressed per SD increase in each continuous risk factor. ORs for dichotomous variables have as a reference group those patients without the respective risk factor.
*Log transformation was used.
Multivariate models
To examine independent associations between key risk factors and the incidence of abnormal VPT, multiple logistic regression analysis was performed (Table 3). In model 1, the analysis mutually adjusted for the presence of all other risk factors. It was found that duration of diabetes, A1C value, triglyceride level, BMI, history of smoking, and presence of hypertension at baseline remained significantly associated with the incidence of abnormal VPT. The relation between total cholesterol level and the incidence of abnormal VPT did not reach statistical significance and neither did that of albumin excretion rate. The strongest relation was with history of smoking, which carried an OR of 1.71, and the second strongest relation was with hypertension with an OR of 1.65.
Table 3.
ORs for associations between key risk factors and the incidence of abnormal VPT with the use of two logistic-regression models
| Variable | Model 1 |
Model 2 |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Duration of diabetes (years) | 1.21 (1.03–1.43) | 0.02 | 1.12 (0.90–1.39) | 0.3 |
| A1C (%) | 1.26 (1.06–1.49) | 0.008 | 1.30 (1.07–1.68) | 0.009 |
| History of smoking (%) | 1.71 (1.23–2.37) | 0.001 | 1.85 (1.27–2.70) | 0.01 |
| Hypertension (%) | 1.65 (1.08–2.50) | 0.02 | 1.82 (1.12–2.94) | 0.01 |
| BMI (kg/m2) | 1.32 (1.12–1.56) | 0.001 | 1.26 (1.04–1.53) | 0.002 |
| Total cholesterol (mmol/l) | 1.06 (0.88–1.27) | 0.6 | 0.94 (0.76–1.17) | 0.6 |
| Triglycerides (mmol/l) | 1.21 (1.01–1.45) | 0.04 | 1.27 (1.03–1.58) | 0.03 |
| Albumin excretion rate (μg/min)* | 1.15 (0.98–1.35) | 0.09 | 1.20 (1.00–1.44) | 0.05 |
| History of CVD (%) | 2.13 (1.10–4.12) | 0.03 | ||
| Any retinopathy (%) | 1.44 (0.92–2.26) | 0.1 | ||
Standardized ORs are expressed per SD increase in each continuous risk factor. ORs for dichotomous variables have as a reference group those patients without the respective risk factor. Model 1 was mutually adjusted for all other risk factors. Model 2 was mutually adjusted for all other risk factors and complications of diabetes.
*Log transformation was used.
When complications of diabetes were added to the model (model 2, Table 3) duration of diabetes, as expected, was not statistically significant. A1C value, triglyceride level, BMI, history of smoking, and presence of hypertension at baseline remained significantly related to the incidence of abnormal VPT. In addition, the presence of CVD at baseline was independently related to the incidence of abnormal VPT, with an OR of 2.13. The relation between any retinopathy and abnormal VPT was not significant (P = 0.1).
As expected, chronic glycemic exposure was higher in the group who developed an abnormal VPT (A1C 0.3% higher, P = 0.001). When the above analyses were repeated using the adjusted mean A1C, the findings were virtually identical. Furthermore, when the relationship between A1C and progression to abnormal VPT was tested, there was no threshold effect.
CONCLUSIONS
Determination of VPT using a biothesiometer is a quick and easy way of detecting large-fiber dysfunction and therefore identifies patients at risk of foot ulceration, lower-limb amputation, and mortality (1,2). During a period of 12 years, the incidence of abnormal VPT in patients with type 2 diabetes is estimated to be 19.9% (11). In this larger prospective study of patients with type 1 diabetes, we observed an incidence of 23.7% over 7.3 years of follow-up. Apart from glycemic control and duration of diabetes, we have demonstrated that cardiovascular risk factors such as hypertension, smoking, obesity, elevated triglyceride levels, and the presence of CVD at baseline seem to predict the development of abnormal VPT. The patients who qualified for follow-up but were not assessed had worse glycemic and cardiovascular profiles, and hence our results may, if anything, have underestimated the associations. The same independent risk factors have previously been shown to be related to newly diagnosed DPN (21). Both hypertension and a history of smoking were strong risk factors for development of neuropathy and an abnormal VPT. As expected, a higher mean A1C value predicted the development of an abnormal VPT, but no threshold effect was identified. A similar outcome was found in a previous study of a mixed cohort of type 1 and type 2 diabetic patients (9).
An abnormal VPT at baseline predicted the development of foot ulcers, leg bypass or angioplasty, gangrene, amputation, and mortality. This finding is consistent with previous studies (12,13). In a general diabetes clinic, it is probably more important to routinely use a test that is highly sensitive, as opposed to highly specific, because the potential risk of DPN being underdiagnosed is greater than the risk of preventative measures being implemented in patients with false-positive results. Such measures include foot-care education and more frequent follow-up, for instance, in podiatrist-led neuropathy clinics (23). Studies have shown that measurement of VPT is more sensitive than the 10-g monofilament in assessing foot ulcer risk (14). Despite this evidence, at present monofilaments are routinely used in outpatient clinics. Although the loss of the ability to feel a 10-g monofilament is known to predict ulceration (13), it usually occurs at a late stage in the disease process. Conversely, an abnormal VPT is often evident earlier in the natural history of the disease (24). Another advantage of the VPT method is that it provides a quantitative result, and for each unit increase, the risk of foot ulceration increases (13).
It is well recognized that patients with diabetes have an increased risk for CVD. Within the EURODIAB cohort, when the cause of death was known, CVD was the cause in ∼40% of subjects (25). This present study shows that even in a young cohort of patients with type 1 diabetes (average age 32.7 years at baseline) an abnormal VPT identifies a subset of patients with adverse cardiovascular risk parameters. This knowledge enables identification of those patients in need of more aggressive treatment of their CVD risk factors. The all-cause mortality rate of patients with an abnormal VPT at baseline was four times higher than in those with a normal value (8 vs. 2% over 7.3 years of follow-up).
In summary, determination of an abnormal VPT is important for two reasons. First, it identifies a cohort of patients with large-fiber dysfunction and its inherent clinical risks, and second, it identifies a population with increased cardiovascular risk. Therefore, intensive management of cardiovascular risk factors could reduce not only cardiovascular events but also the clinical sequelae of neuropathy.
Supplementary Material
Acknowledgments
The EURODIAB Prospective Complications Study was supported by grants from the Wellcome Trust, the European Union, and Diabetes U.K.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Carrington AL, Shaw JE, Van Schie CHM, Abbott CA, Vileikyte L, Boulton AJM: Can motor nerve conduction velocity predict foot problems in diabetic subjects over a 6-year outcome period? Diabetes Care 2002;25:2010–2015 [DOI] [PubMed] [Google Scholar]
- 2. Adler AI, Boyko EJ, Ahroni JH, Smith DG: Lower-extremity amputation in diabetes: the independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care 1999;22:1029–1035 [DOI] [PubMed] [Google Scholar]
- 3. Crawford F, Inkster M, Kleijen J, Fahey T: Predicting foot ulcers in patients with diabetes: a systematic review and meta-analysis. Q J Med 2007;100:65–86 [DOI] [PubMed] [Google Scholar]
- 4. Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian J: The health care costs of peripheral neuropathy for people with diabetes in the U.S. Diabetes Care 2003;26:1790–1795 [DOI] [PubMed] [Google Scholar]
- 5. Shearer A, Scuffham P, Gordios A, Oglesby A: Predicted costs and outcomes from reduced vibration detection in people with diabetes in the U.S. Diabetes Care 2003;26:2305–2310 [DOI] [PubMed] [Google Scholar]
- 6. Perkins BA, Olaleye D, Zinman B, Bril V: Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care 2001;24:250–256 [DOI] [PubMed] [Google Scholar]
- 7. Meijer JWG, Smit AJ, Lefrandt JD, Van De Hoeven JH, Hoogenberg K, Links TP: Back to basics in diagnosing with diabetic polyneuropathy with the tuning fork. Diabetes Care 2005;28:2201–2205 [DOI] [PubMed] [Google Scholar]
- 8. Dyck PJ, Davies JL, Wilson DM, Service FJ, Melton LJ, O'Brien PC: Risk factors for severity of diabetic polyneuropathy. Diabetes Care 22:1479–1486 [DOI] [PubMed] [Google Scholar]
- 9. Dyck PJ, Davies JL, Clark VM, Litchy WJ, Dyck PJB, Klein CJ, Rizza RA, Pach JM, Klein R, Larson TS, Melton LJ, O'Brien PC: Modeling chronic glycemic exposure variables as correlates and predictors of microvascular complications of diabetes. Diabetes Care 2006;29:2282–2288 [DOI] [PubMed] [Google Scholar]
- 10. Sorensen L, Molyneaux L, Yue DK: Insensate versus painful diabetic neuropathy: the effects of height, gender, ethnicity and glycaemic control. Diabetes Res Clin Pract 2002;57:45–51 [DOI] [PubMed] [Google Scholar]
- 11. Coppini DV, Young PJ, Weng C, Macleod AF, Sonksen PH: Outcome on diabetic foot complications in relation to clinical examination and quantitative sensory testing: a case-control study. Diabet Med 1998;15:765–771 [DOI] [PubMed] [Google Scholar]
- 12. Young MJ, Breddy JL, Veves A, Boulton A: The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds: a prospective study. Diabetes Care 1994;17:557–560 [DOI] [PubMed] [Google Scholar]
- 13. Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJM: Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care 1998;21:1071–1075 [DOI] [PubMed] [Google Scholar]
- 14. Miranda-Palma B, Sosenko JM, Bowker JH, Mizel MS, Boulton AJ: A comparison of the monofilament with other testing modalities for foot ulcer susceptibility. Diabetes Res Clin Prac 2005;70:8–12 [DOI] [PubMed] [Google Scholar]
- 15. Krishnan STM, Baker NR, Carrington AL, Rayman G: Comparative roles of microvascular and nerve function in foot ulceration in type 2 diabetes. Diabetes Care 2004;27:1343–1348 [DOI] [PubMed] [Google Scholar]
- 16. Sosenko JM, Kato M, Soto R, Goldberg RB: Sensory function at diagnosis and in early stages of NIDDM in patients detected through screening. Diabetes Care 1993;16:847–852 [DOI] [PubMed] [Google Scholar]
- 17. Gadia MT, Nator N, Ramos LM, Ayyar DR, Skyler JS, Sosenko JM: Influence of height on quantitative sensory, nerve-conduction and clinical indices of diabetic peripheral neuropathy. Diabetes Care 1987;10:613–616 [DOI] [PubMed] [Google Scholar]
- 18. Bloom S, Till S, Sonksen P, Smith S: Use of a biothesiometer to measure individual vibration thresholds and their variation in 519 non-diabetic subjects. Br Med J 1984;288:1793–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wiles PG, Pearce SM, Rice PJS, Mitchell JMO: Vibration perception threshold: influence of age, height, sex, and smoking, and calculation of accurate centile values. Diabet Med 1991;8:157–161 [DOI] [PubMed] [Google Scholar]
- 20. The EURODIAB Prospective Complications Study Group. Microvascular and acute complications in IDDM patients. Diabetologia 1994;37:278–285 [DOI] [PubMed] [Google Scholar]
- 21. Tesfaye S, Chaturvedi N, Eaton SEM, Ward JD, Manes C, Ionescu-Tirgoviste C, Witte DR, Fuller JH: Vascular risk factors and diabetic neuropathy. N Engl J Med 2005;352:341–350 [DOI] [PubMed] [Google Scholar]
- 22. Chaturvedi N, Sjoelie AK, Porter M, Aldington SJ, Fuller JH, Songini M, Kohner EM: Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care 2001;24:284–289 [DOI] [PubMed] [Google Scholar]
- 23. Elliott J, Hallsworth J, Sutton MR, Tesfaye S: Podiatry-led neuropathy ulcer clinics allow effective management of limited time and resources (Abstract). Diabetologia 2002;45(Suppl. 2):A3 [Google Scholar]
- 24. McGill M, Molyneaux L, Yue DK: Which patients should receive podiatry care? An objective analysis. Intern Med J 2005;35:451–456 [DOI] [PubMed] [Google Scholar]
- 25. Van Hecke M, Dekker J, Stehouwer C, Polak B, Fuller J, Sjolie A, Kofinis A, Rottiers R, Porta M, Chaturvedi N: Diabetic retinopathy is associated with mortality and cardiovascular disease incidence: the EURODIAB Prospective Study. Diabetes Care 2005;28:1383–1389 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.