Abstract
OBJECTIVE
Insulin resistance and β-cell dysfunction both are important contributors to the pathogenesis of type 2 diabetes. Exercise training improves insulin sensitivity, but its effects on β-cell function are less well studied.
RESEARCH DESIGN AND METHODS
Sedentary, overweight adults were randomized to control or one of three 8-month exercise programs: 1) low amount/moderate intensity, 2) low amount/vigorous intensity, or 3) high amount/vigorous intensity. Of 387 randomized, 260 completed the study and 237 had complete data. Insulin sensitivity (Si), acute insulin response to glucose (AIRg), and the disposition index (DI = Si × AIRg) were modeled from an intravenous glucose tolerance test.
RESULTS
Compared with control subjects, all three training programs led to increases in DI. However, the moderate-intensity group experienced a significantly larger increase in DI than either of the vigorous-intensity groups and through a different mechanism. The high-amount/vigorous-intensity group improved Si and had a compensatory reduction in AIRg, whereas the moderate-intensity group had a similar improvement in Si but almost no reduction in AIRg. Importantly, the inactive control group experienced a significant increase in fasting glucose.
CONCLUSIONS
To the extent that the DI accurately reflects β-cell function, we observed that both moderate- and vigorous-intensity exercise training improved β-cell function, albeit through distinct mechanisms. It is not clear which of these mechanisms is preferable for maintenance of metabolic health. While moderate-intensity exercise led to a larger improvement in DI, which may reflect a transition toward a more normal DI, longer-term investigations would be necessary to determine which was more effective at reducing diabetes risk.
Insulin resistance and pancreatic β-cell dysfunction are important contributors to the pathogenesis of type 2 diabetes (1–3). Sedentary, overweight, and obese individuals are generally insulin resistant but often are able to maintain normal glucose tolerance through compensatory increases in pancreatic insulin secretion. Therefore, in order to fully assess and understand the progression to diabetes, the degree of insulin resistance should be interpreted relative to insulin secretion.
The relationship between insulin resistance and insulin secretion is hyperbolic and is sometimes referred to as the hyperbolic law of glucose tolerance (1–3,4). This relationship is represented by the disposition index (DI), the product of the insulin sensitivity index (Si) and acute insulin response to intravenous glucose (AIRg), both of which are modeled as parameters in the intravenous glucose tolerance test through the minimal model of Bergman (5). DI physiologically represents the degree to which the pancreatic β-cells are able to fully or partially compensate for changes in insulin sensitivity and is an accepted measure of pancreatic β-cell function. In normal individuals, DI is relatively high; however, in those along the progression from normal to type 2 diabetes, DI becomes progressively lower, reflecting a decreased ability of the pancreas to fully compensate for increases in insulin resistance (6–10).
The beneficial effect of exercise on insulin sensitivity is well known. However, the effects of exercise on AIRg and DI have not been well studied. We are not aware of any studies that have investigated the effects of different amounts or intensities of exercise on AIRg or DI. The purpose of Studies of a Targeted Risk Reduction Intervention through Defined Exercise (STRRIDE), a large, randomized, controlled clinical trial, was to investigate the effects of different amounts and intensities of exercise training on numerous cardiometabolic risk factors. This report focuses on the STRRIDE findings pertinent to β-cell function.
RESEARCH DESIGN AND METHODS
These data are part of the STRRIDE project described in detail previously (11,12). Subjects were aged 40–65 years, sedentary, overweight, or mildly obese (BMI 25–35 kg/m2) and moderately dyslipidemic (either LDL cholesterol 130–190 mg/dl and/or HDL cholesterol <40 mg/dl for men or <45 mg/dl for women). Women were postmenopausal. Exclusion criteria included medications that alter carbohydrate metabolism, diabetes, inability to exercise, and history of hypertension or heart disease. The protocol was approved by the relevant institutional review boards, and subjects provided written informed consent.
Exercise training
All subjects were randomly assigned to one of three training groups or a control group. The exercise groups were 1) high amount/vigorous intensity, 2) low amount/vigorous intensity, and 3) low amount/moderate intensity. The high-amount/vigorous-intensity group prescription was to expend 23 kcal · kg body wt−1 · week−1 exercising at 65–80% peak Vo2 (approximately calorically equivalent to 20 miles per week of walking or jogging). For the low-amount/vigorous-intensity group, the prescription was to expend 14 kcal · kg body wt−1 · week−1 (approximately calorically equivalent to 12 miles per week). For the low-amount/moderate-intensity group, subjects were to expend 14 kcal · kg body wt−1 · week−1 at 40–55% peak Vo2 (Table 1). Exercise modes included treadmill and elliptical trainers. All exercise sessions were verified by direct supervision or recordable heart rate monitors.
Table 1.
Exercise prescription adherence by group and substrate use
| Low amount/moderate intensity | Low amount/vigorous intensity | High amount/vigorous intensity | |
|---|---|---|---|
| n | 57 | 58 | 64 |
| Prescription and actual exercise dose | |||
| Intensity (% peak oxygen consumption) | 40–55% | 65–80% | 65–80% |
| Prescription amount (miles/week)* | 12 | 12 | 20 |
| Prescription amount (kcal/week) | 1,220 ± 212 | 1,230 ± 177 | 2,020 ± 307 |
| Prescription time (min/week) | 201 ± 37 | 125 ± 28 | 207 ± 44 |
| Adherence (%) | 88 ± 14 | 90 ± 12 | 84 ± 15 |
| Actual amount (miles/week)† | 10.6 | 10.8 | 16.8 |
| Actual time (min/week)‡ | 176 ± 36 | 113 ± 28 | 172 ± 41 |
| Frequency (sessions/week) | 3.5 ± 0.8 | 2.9 ± 0.5 | 3.6 ± 0.8 |
| Respiratory exchange ratio | 0.907 ± 0.05§ | 0.960 ± 0.06 | 0.961 ± 0.04 |
| Fat utilization | |||
| Energy from fat (%) | 30.2% | 12.6% | 12.6% |
| kcals from fat (kcal) | 368 | 155 | 255 |
Data are means ±SD.
*Prescription amount is presented as the approximate number of miles/week that are calorically equivalent to the prescribed kcal/week of 14 kcal · kg body wt−1 · week−1 for the low-dose groups and 23 kcal · kg body wt−1 · week−1 for the high-dose group.
†Actual amount = prescription amount × adherence for each group (therefore no SD).
‡Actual time = prescription time × adherence for each subject. Respiratory exchange ratios were obtained during the submaximal exercise bout that was performed to determine the correct exercise intensity (i.e., 40–55% for the moderate-intensity group and 65–80% for the vigorous-intensity groups).
§Respiratory exchange ratio for the moderate-intensity group was significantly different from both vigorous groups (P < 0.0005).
Crossover control subjects
Participants were assured that if they were assigned to the control group, after this period, they would be randomized into one of the exercise groups. This was important for recruiting subjects to an exercise study. Subjects who both finished the control period and then were randomized to and finished one of the exercise interventions had data for both control changes and exercise changes. To maintain the independent relation between the control changes and the exercise intervention changes, we have not used both control and exercise data from the same subject when comparing exercise response to control subjects. However, when exercise-only questions were of primary interest, as in the present study, we have used the exercise change data but not the control data from these subjects. This approach increases the statistical power for comparisons between exercise interventions because it increases the number of subjects in each of the exercise groups. All data from subjects that completed exercise training are included in these analyses; the control data are included only for nonstatistical comparisons.
Insulin action measures
Insulin action was determined with a 3-h intravenous glucose tolerance test (5). Glucose (50%) was injected through a catheter at 0.3 g/kg body mass. Insulin (0.025 units/kg body mass) was injected at min 20. Twenty-six blood samples were obtained, centrifuged, and stored at −80°C. Insulin was measured by immunoassay (Access Immunoassay System; Beckman Coulter, Fullerton, CA) and glucose with an oxidation reaction (YSI 2300; Yellow Springs, OH). Si, AIRg, and DI were calculated using Bergman's minimal model (5).
Statistical methods
Baseline differences between the exercise groups were evaluated via ANOVA with Fisher's post hoc test. Pre- to posttraining changes were determined independently for each of the four groups by two-tailed t tests. To determine differential training effects between the exercise-only groups for DI, Si, or AIRg, ANOVA with Fisher's post hoc tests were used. P values <0.05 for individual t tests and for post hoc tests were considered statistically significant.
RESULTS
Of 387 subjects randomized in STRRIDE, 260 completed the study and 127 (32.8%) dropped out. Of 260, 237 had complete pre- and postintervention data for the glucose tolerance test. Table 1 describes the exercise interventions in detail.
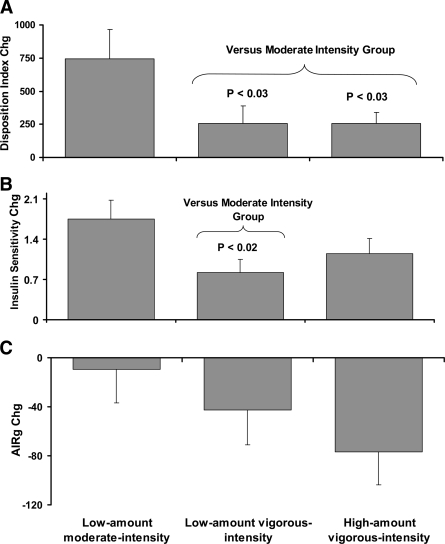
The results for DI, AIRg, and Si are presented in Table 2 and also in Fig. 1. In Table 2, the data show that DI was significantly improved for the low-amount/moderate-intensity and for the high-amount/vigorous-intensity groups. The low-amount/vigorous-intensity group was on the border of significance (P = 0.063). Surprisingly, the improvement in DI that occurred in the moderate-intensity group was significantly greater than in both of the vigorous-intensity groups (Fig. 1). All three groups experienced significant improvements in Si. The magnitude of the improvement for the moderate-intensity group was significantly greater than that for the same amount of exercise at a vigorous intensity, indicating a clear beneficial effect of moderate intensity over vigorous intensity for Si. Interestingly, only the high-amount/vigorous-intensity group showed the expected compensatory decrease in insulin response to the glucose challenge (AIRg decreased by 15.2% in this group). There was virtually no compensatory decrease in AIRg in the group that had the greatest improvement in Si (i.e., the moderate-intensity group; AIRg decreased only 2.2%). When sex was added to the model, there were no significant sex differences or sex by group interactions for DI, AIRg, or Si.
Table 2.
Baseline and change scores for DI, AIRg, Si, metabolic variables, body weight, and body composition variables by group
| n (men/women) | Control |
Low amount/moderate intensity |
Low amount/vigorous intensity |
High amount/vigorous intensity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 58 (30/28) |
57 (30/27) |
58 (33/25) |
64 (38/26) |
|||||||||
| Baseline | Change | P | Baseline | Change | P | Baseline | Change | P | Baseline | Change | P | |
| DI (× 10−5 min−1) | 1,562 ± 1,444 | −65 ± 893 | NS | 1,270 ± 1,147 | 742 ± 1,680 | 0.002 | 1,533 ± 1,362 | 255 ± 1,023 | 0.063 | 1,305 ± 1,121 | 255 ± 688 | 0.004 |
| AIRg (pmol/l) | 490.6 ± 431 | 22 ± 233 | NS | 457.3 ± 334 | −10 ± 207 | NS | 445.5 ± 353 | −43 ± 216 | 0.14 | 505.6 ± 427 | −77 ± 218 | 0.007 |
| Si (× 10−5 min/pmol) | 3.5 ± 2.1 | −0.35 ± 1.7 | 0.12 | 3.1 ± 2.2 | 1.7 ± 2.5 | <0.0001 | 3.7 ± 2.1 | 0.8 ± 1.8 | 0.001 | 3.2 ± 2.5 | 1.1 ± 2.1 | <0.0001 |
| Fasting glucose (mg/dl) | 91 ± 11 | 3.2 ± 8 | 0.005 | 94 ± 9 | −0.1 ± 9 | NS | 93 ± 8 | 1.2 ± 9 | NS | 94 ± 10 | 0.5 ± 10 | NS |
| Fasting insulin (μU/ml) | 8.5 ± 4.3 | 0.9 ± 3.3 | 0.04 | 10.3 ± 8.1 | −2.5 ± 5.6 | 0.002 | 8.8 ± 5.9 | −1.5 ± 4.1 | 0.008 | 9.6 ± 5.6 | −1.3 ± 3.3 | 0.002 |
| HOMA (mg/dl × μU/ml) | 2.0 ± 1.2 | 0.28 ± 0.8 | 0.011 | 2.5 ± 1.9 | −0.58 ± 1.4 | 0.003 | 2.1 ± 1.6 | −0.36 ± 1.1 | 0.014 | 2.3 ± 1.4 | −0.30 ± 0.9 | 0.009 |
| Triglycerides (mg/dl) | 147 ± 71 | 5 ± 43 | NS | 171 ± 108 | −35 ± 76 | 0.002 | 143 ± 67 | −16 ± 49 | 0.02 | 155 ± 84 | −21 ± 50 | 0.002 |
| Body mass (kg) | 88 ± 14 | 0.88 ± 2.4 | 0.007 | 87 ± 15 | −0.81 ± 2.4 | 0.012 | 88 ± 13 | −0.96 ± 2.3 | 0.003 | 88 ± 13 | −0.15 ± 2.7 | <0.0001 |
| Visceral fat (cm2) | 163 ± 66 | 13 ± 29 | 0.003 | 174 ± 77 | −2.1 ± 30 | NS | 158 ± 56 | −4.6 ± 36 | NS | 168 ± 72 | −12 ± 35 | 0.018 |
| Vo2 peak (ml · kg−1 · min−1) | 27 ± 6 | −0.7 ± 2 | 0.03 | 27 ± 6 | 1.8 ± 2 | <0.0001 | 29 ± 6 | 3.4 ± 3 | <0.0001 | 28 ± 5 | 5.0 ± 3 | <0.0001 |
Data are means ± SD. Homeostasis model assessment (HOMA) [(fasting glucose × fasting insulin)/22.5] (indicator of insulin sensitivity during fasting conditions). NS, P > 0.30 and therefore not significant. All other P values (< 0.30) are shown.
Figure 1.
The effects of exercise amount and intensity on changes in DI (A), Si (B), and AIRg (C) are shown. Data are means ± SE. All P <0.10 for group comparisons are reported. For low amount/moderate intensity (n = 57); low amount/vigorous intensity (n = 58); high amount/vigorous intensity (n = 64).
The inactive control group experienced a significant increase in fasting glucose, indicating a progression toward diabetes (Table 2). We observed significant deterioration in several other variables including fasting insulin, homeostasis model assessment, body mass, visceral fat, and cardiovascular fitness in the inactive control group. All exercise groups experienced significantly reduced triglycerides, but the low-amount/moderate-intensity group had the greatest decrease, significantly greater than the same amount of vigorous exercise (P < 0.035) and trending toward a greater decrease than even the high-amount group (P < 0.085). The moderate-intensity group had the greatest decrease in fasting insulin; however, this difference was not significant (P < 0.19). Only the high amount of exercise was sufficient to reduce visceral fat. All exercise programs resulted in significant improvements in peak oxygen consumption.
All data were investigated for outliers. We identified two outliers for DI change (>4 SDs above the mean). Removal of these two individuals had no effect on the significance of any analyses or on the interpretation of any of the findings, and as a result they were retained in all analyses.
CONCLUSIONS
The DI (DI = AIRg × Si) is an accepted measure of pancreatic β-cell function and predicts the development of type 2 diabetes (13). The major finding of the present study was that moderate-intensity exercise training improved DI significantly better than did vigorous-intensity exercise in sedentary, overweight, moderately dyslipidemic subjects. It is important to note that all three exercise-training regimens resulted in improved DI, although the low-amount/vigorous-intensity group was just on the border of statistical significance (P = 0.063). Although DI has been shown to predict the development of type 2 diabetes (13), additional studies would be necessary to determine whether the greater effect of moderate-intensity exercise on DI actually translated into a greater reduction in diabetes risk.
A second observation related to the early-phase pancreatic β-cell responses to the intravenous glucose challenge. While moderate intensity exercise resulted in the largest improvement in Si, this group experienced almost no compensatory decrease in first-phase insulin secretion (AIRg decreased 2%, nonsignificant); together, these effects resulted in a larger improvement in DI in this group versus the others. High-amount/vigorous-intensity exercise resulted in a similar increase in Si; however, this group did have a compensatory decrease in insulin secretion (AIRg decreased 15%, P = 0.007); together, this resulted in a smaller improvement in DI in this group versus the moderate-intensity group.
It is not altogether clear which of these is a preferable response for metabolic health. The subjects in this trial had DI levels that were well below those observed in healthy individuals (our average baseline DI was ∼1,400 compared with other reports for normal individuals ranging from 2,000 to 2,800) (6,8,9). Also, in overweight/obese subjects, AIRg decreases progressively across levels of glucose tolerance (from normal to impaired to diabetic) (8,9). Our own baseline data show a clear and progressive decrease in AIRg (and also DI and Si) even across tertiles of normal glucose (<100 mg/dl; data not shown). Therefore, the response experienced by the moderate-intensity intervention might reflect a transition toward a more normal and perhaps healthier DI. On the other hand, consistent findings of exercise-induced reductions in insulin levels (area under the curve) in response to an oral glucose challenge (14), and decreased insulin secretion with hyperglycemic clamps (15), have been a hallmark finding in exercise research, although the majority of these studies have been in response to vigorous exercise only.
The specific mechanisms responsible for these observations are not obvious. In previous publications in this study cohort, we have observed that the high-amount group, compared with both low-amount exercise groups, experienced greater reductions in body weight, body fat, waist circumference (16), visceral and abdominal subcutaneous fat (17), and greater and more widespread improvements in lipids and lipoproteins (11), as well as greater improvements in cardiovascular fitness (18). With the exception of fitness, exercise intensity was not a factor contributing to these differences; rather, the effect was due to a greater amount of exercise. Therefore, it seems clear, at least in the present study, that the greater improvement in DI resulting from moderate-intensity exercise cannot be explained by the factors more favorably modulated by vigorous exercise. In contrast, we have also previously reported that the moderate-intensity group experienced a number of unexpected metabolic benefits including 1) a greater reduction in plasma triglycerides (both acute and chronic), compared with both vigorous-intensity groups (11,19); 2) a greater increase in insulin sensitivity at both 24 h after the last bout and after 14 days after exercise withdrawal (20,21), which is significantly different from the same amount of vigorous exercise; and 3) a greater improvement in metabolic syndrome score compared with the same amount of vigorous exercise training (22).
It is well known that expending the same number of calories while exercising at a moderate intensity compared with vigorous intensity results in a lower respiratory exchange ratio, the ratio of CO2 production to O2 consumption (23,24), and thus reflects a greater percentage of fat oxidation for energy when compared with more vigorous exercise. Recently, in a study designed to investigate exercise training at an intensity that optimizes fat oxidation (FATmax), Venables and Jeukendrup (24) reported that moderate-intensity training improved both Si and fat oxidation more than the same amount of interval exercise training.
The robust effects observed with moderate-intensity exercise in these studies may be the result of improved fat oxidation leading to a reduction in lipotoxicity in skeletal muscle, liver, and/or pancreas. Schenk and Horowitz (25) recently observed that acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid–induced insulin resistance. Although they only studied one exercise intensity (moderate), they reported numerous responses in skeletal muscle that likely explain the acute effects, including enhanced key lipogenic enzymes, an increase in muscle triglyceride synthesis, reduced partitioning of fatty acids toward ceramide and diacylglycerol (both hypothesized to be causally related to insulin resistance), and a suppression of proinflammatory response. It seems likely that moderate-intensity exercise, which relies more heavily on fat oxidation, may be more effective than vigorous exercise in creating these specific adaptations. Our observation of greater acute and chronic reductions in plasma triglycerides with moderate compared with vigorous exercise (19) supports this hypothesis.
Finally, another important finding of the present study was the clinically and statistically significant increase of nearly 3 mg/dl in fasting glucose that occurred in the control group. There were no significant changes in fasting glucose in any exercise group emphasizing the important preventive effects of regular exercise on the deterioration of glucose control that occurs with physical inactivity, positive energy balance, and weight gain.
In summary, 8 months of continued physical inactivity in sedentary overweight and obese individuals with moderate dyslipidemia led to a significant increase in fasting glucose levels, indicating a progression toward type 2 diabetes. Further, to the extent that the DI accurately reflects β-cell function, we observed that both moderate- and vigorous-intensity exercise improved β-cell function but through distinct mechanisms. The high amount of vigorous-intensity exercise was associated with an improvement in insulin sensitivity and a compensatory decrease in insulin secretion. The low amount of moderate-intensity exercise was associated with a similar improvement in insulin sensitivity but with almost no reduction in insulin secretion. While the moderate-intensity intervention might reflect a transition toward a more normal DI in sedentary, moderately dyslipidemic individuals, longer-term investigations would be necessary to confirm a superior improvement in diabetes risk.
Acknowledgments
This study was supported by National Institutes of Health Grant HL-57354.
No potential conflicts of interest relevant to this article were reported.
Parts of this study were presented in abstract form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
Footnotes
Clinical trial reg. no. NCT0020093, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Kahn S, Hull R, Utzschneider K: Mechanisms linking obesity to insulin resistance and to type 2 diabetes. Nature 2006; 444: 840– 846 [DOI] [PubMed] [Google Scholar]
- 2.Ferrannini E, Gastaldelli A, Miayzaki Y, Matsuda M, Pettiti M, Natali A, Mari A, DeFronzo R: Predominant role of reduced beta-cell sensitivity to glucose over insulin resistance in impaired glucose tolerance. Diabetologia 2003; 46: 1211– 1219 [DOI] [PubMed] [Google Scholar]
- 3.Bergman R: Banting Lecture 2006: Orchestration of glucose homeostasis: from a small acorn to the California Oak. Diabetes 2007; 56: 1489– 1500 [DOI] [PubMed] [Google Scholar]
- 4.Stumvoll M, Tataranni P, Bogardus C: The hyperbolic law: a 25-year perspective. Diabetologia 2005; 48: 207– 209 [DOI] [PubMed] [Google Scholar]
- 5.Bergman R, Finegood D, Ader M: Assessment of insulin sensitivity in vivo. Endocr Rev 1985; 6: 45– 86 [DOI] [PubMed] [Google Scholar]
- 6.Elder DA, Prigeon RL, Wadwa RP, Dolan LM, D'Alessio DA: Beta-cell function, insulin sensitivity, and glucose tolerance in obese diabetic and nondiabetic adolescents and young adults. J Clin Endocrinol Metab 2006; 91: 185– 191 [DOI] [PubMed] [Google Scholar]
- 7.Cnop M, Vidal J, Hull R, Utzschneider K, Carr D, Schraw T, Scherer P, Boyko E, Fujimoto W, Kahn S: Progressive loss of β-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care 2007; 30: 677– 682 [DOI] [PubMed] [Google Scholar]
- 8.Hong J, Gu W, Zhang Y, Yang Y, Shen C, Xu M, Li X, Wang W, Ning G: The interplay of insulin resistance and beta-cell dysfunction involves the development of type 2 diabetes in Chinese obeses. Endocrine 2007; 31: 93– 99 [DOI] [PubMed] [Google Scholar]
- 9.Utzschneider K, Prigeon R, Carr D, Hull R, Tong J, Shofer J, Retzlaff B, Knopp R, Kahn S: Impact of differences in fasting glucose and glucose tolerance on the hyperbolic relationship between insulin sensitivity and insulin responses. Diabetes Care 2006; 29: 356– 362 [DOI] [PubMed] [Google Scholar]
- 10.Kahn S: Clinical Review 135: the importance of beta cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab 2001; 86: 4047– 4058 [DOI] [PubMed] [Google Scholar]
- 11.Kraus W, Houmard J, Duscha B, Knetgzer K, Wharton M, McCartney J, Bales C, Henes S, Samsa G, Otvos J, Kulkarni K, Slentz C: Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 2002; 347: 1483– 1492 [DOI] [PubMed] [Google Scholar]
- 12.Kraus W, Torgan C, Duscha B, Norris J, Brown S, Cobb F, Bales C, Annex B, Samsa G, Houmard J, Slentz C: Studies of a targeted risk reduction intervention through defined exercise (STRRIDE). Med Sci Sports Exerc 2001; 33: 1774– 1784 [DOI] [PubMed] [Google Scholar]
- 13.Utzschneider K, Prigeon R, Faulenbach M, Tong J, Carr D, Boyko E, Leonetti D, McNeely M, Fujimoto W, Kahn S: Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009; 32: 335– 341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holloszy J, Schultz J, Kusnierkiewicz J, Hagberg J, Ehsani A: Effects of exercise on glucose tolerance and insulin resistance. Brief review and some preliminary results. Acta Medica Scandinavica Supplementum 1986; 711: 55– 65 [DOI] [PubMed] [Google Scholar]
- 15.Arciero P, Vukovich M, Holloszy J, Racette S, Kohrt W: Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol 1999; 86: 1930– 1935 [DOI] [PubMed] [Google Scholar]
- 16.Slentz C, Duscha B, Johnson J, Ketchum K, Aiken L, Samsa G, Houmard J, Bales C, Kraus W: Effects of the amount of exercise on body weight, body composition, and measures of central obesity: STRRIDE, a randomized controlled study. Arch Intern Med 2004; 164: 31– 39 [DOI] [PubMed] [Google Scholar]
- 17.Slentz C, Aiken L, Houmard J, Bales C, Johnson J, Tanner C, Duscha B, Kraus W: Inactivity, exercise and visceral fat. STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol 2005; 99: 1613– 1618 [DOI] [PubMed] [Google Scholar]
- 18.Duscha B, Slentz C, Johnson J, Houmard J, Bensimhon D, Knetgzer K, Kraus W: Effects of exercise training amount and intensity on peak oxygen comsumption in middle-aged men and women at risk for cardiovascular disease. Chest 2005; 128: 2788– 2793 [DOI] [PubMed] [Google Scholar]
- 19.Slentz C, Houmard J, Johnson J, Bateman L, Tanner C, McCartney J, Duscha B, Kraus W: Inactivity, exercise training and detraining, and plasma lipoproteins: STRRIDE: a randomized, controlled study of exercise intensity and amount. J Appl Physiol 2007; 103: 432– 442 [DOI] [PubMed] [Google Scholar]
- 20.Houmard J, Tanner C, Slentz C, Duscha B, McCartney J, Kraus W: Effect of the volume and intensity of exercise training on insulin sensitivity. J Appl Physiol 2004; 96: 101– 106 [DOI] [PubMed] [Google Scholar]
- 21.Bajpeyi S, Tanner C, Slentz C, Duscha B, McCartney J, Hickner R, Kraus W, Houmard J: Effect of exercise intensity and volume on the persistence of insulin sensitivity during training cessation. J Appl Physiol 2009; 106: 1079– 1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson J, Slentz C, Houmard J, Samsa G, Duscha B, Aiken L, McCartney J, Tanner C, Kraus W: Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise). Am J Cardiol 2007; 100: 1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolfrey K, Doggett A, Boyd C, Pinner S, Sharples A, Barrett L: Postprandial Triacylglycerol in adolescent boys: a case for moderate exercise. Med Sci Sports Exerc 2008; 40: 1049– 1057 [DOI] [PubMed] [Google Scholar]
- 24.Venables M, Jeukendrup A: Endurance training and obesity: effect on substrate metabolism and insulin sensitivity. Med Sci Sports Exerc 2008; 40: 495– 502 [DOI] [PubMed] [Google Scholar]
- 25.Schenk S, Horowitz J: Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 2007; 117: 1690– 1698 [DOI] [PMC free article] [PubMed] [Google Scholar]