Abstract
OBJECTIVE
To evaluate the efficacy, safety, and tolerability of incremental doses of albiglutide, a long-acting glucagon-like peptide-1 receptor agonist, administered with three dosing schedules in patients with type 2 diabetes inadequately controlled with diet and exercise or metformin monotherapy.
RESEARCH DESIGN AND METHODS
In this randomized multicenter double-blind parallel-group study, 356 type 2 diabetic subjects with similar mean baseline characteristics (age 54 years, diabetes duration 4.9 years, BMI 32.1 kg/m2, A1C 8.0%) received subcutaneous placebo or albiglutide (weekly [4, 15, or 30 mg], biweekly [15, 30, or 50 mg], or monthly [50 or 100 mg]) or exenatide twice daily as an open-label active reference (per labeling in metformin subjects only) over 16 weeks followed by an 11-week washout period. The main outcome measure was change from baseline A1C of albiglutide groups versus placebo at week 16.
RESULTS
Dose-dependent reductions in A1C were observed within all albiglutide schedules. Mean A1C was similarly reduced from baseline by albiglutide 30 mg weekly, 50 mg biweekly (every 2 weeks), and 100 mg monthly (−0.87, −0.79, and −0.87%, respectively) versus placebo (−0.17%, P < 0.004) and exenatide (−0.54%). Weight loss (−1.1 to −1.7 kg) was observed with these three albiglutide doses with no significant between-group effects. The incidence of gastrointestinal adverse events in subjects receiving albiglutide 30 mg weekly was less than that observed for the highest biweekly and monthly doses of albiglutide or exenatide.
CONCLUSIONS
Weekly albiglutide administration significantly improved glycemic control and elicited weight loss in type 2 diabetic patients, with a favorable safety and tolerability profile.
Early intervention to improve glycemic control reduces microvascular complications in type 2 diabetes (1–4) and may provide long-term macrovascular benefits (5). Despite numerous available therapies, over half of patients with type 2 diabetes are unable to achieve the American Diabetes Association (ADA) target A1C level (<7%) (6–8). Moreover, weight gain and treatment-induced hypoglycemic episodes (9,10) are major barriers to achieving glycemic control (10).
Antidiabetic therapies based on glucagon-like peptide-1 (GLP-1) retain the ability of native GLP-1 to stimulate glucose-dependent insulin secretion and suppress inappropriately elevated glucagon secretion (11,12). Native GLP-1 also slows gastric emptying and reduces food intake, which leads to modest weight loss (11). However, native GLP-1 is rapidly inactivated (half-life 1–2 min) by dipeptidyl peptidase-4 (DPP-4), limiting its therapeutic potential (13). Exenatide (half-life 2.4 h) improves glycemic control in combination with metformin, a sulfonylurea, or a thiazolidinedione (14–18). Despite modest weight loss and improved glycemic control, gastrointestinal (GI) intolerability and twice-daily administration may lead to discontinuation (19).
Albiglutide (formerly known as albugon) is a GLP-1 receptor agonist developed through the fusion of two repeats of human GLP-1 (7–36) molecules to recombinant human albumin (20). The GLP-1 dimer was used to avoid potential reductions of the interaction of the GLP-1 moiety of the monomer with its receptor in the presence of albumin. A single amino acid substitution (ala8→gly) renders the molecule resistant to DPP-4. The structure of albiglutide provides an extended half-life (∼5 days), which may allow weekly or less frequent dosing. Furthermore, albiglutide is relatively impermeant to the central nervous system (21), which may have implications for GI tolerability. In nonclinical studies, albiglutide stimulated cAMP production through the GLP-1 receptor and induced insulin secretion from INS-1 cells in vitro and in animal models (21–22). It also delayed gastric emptying and reduced food intake in rodents (21–23).
This study was designed to explore a wide range of doses (4–100 mg) and schedules (weekly to monthly) to assess glycemic control and adverse event profiles for albiglutide. Exenatide was included as an open-label reference to provide clinical perspective for a GLP-1 receptor agonist.
RESEARCH DESIGN AND METHODS
This phase II trial was a prospective randomized double-blind placebo-controlled parallel-group study conducted between April 2007 and May 2008 at 118 sites in the U.S. (n = 106), Mexico (n = 9), Chile (n = 2), and the Dominican Republic (n = 1) (online appendix 1, available at http://care.diabetesjournals.org/cgi/content/full/dc09-0366/DC1). Men and women of non-childbearing potential (age 18–75 years) were eligible for inclusion if diagnosed with type 2 diabetes ≥3 months before screening. Subjects were drug naïve (diet and exercise) or treated with metformin monotherapy and stable for >3 months before prescreening (1 week prior to screening visit). Only subjects treated with metformin monotherapy were eligible for the exenatide arm (consistent with labeling). Additional inclusion criteria included BMI ≥20 and ≤40 kg/m2 and A1C at screening ≥7 and ≤10%.
Exclusion criteria included any oral antidiabetic monotherapy (except metformin) ≤3 months prior to screening or insulin <1 month prior to screening and not used for >7 days; pancreatitis within 5 years; significant cardiovascular, cerebrovascular, renal, or hepatobiliary diseases; fasting serum triglycerides ≥800 mg/dl (9 mmol/l) at screening; or hematological profiles considered to be clinically significant. Lipid-lowering medications must have been maintained at the same dose for 3 months prior to enrollment, and no prescription or over-the-counter weight-loss drugs were permitted.
The study protocol was approved by an institutional review board and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects at prescreening. A data safety monitoring committee of independent experts assessed safety data on an ongoing basis.
Randomization
Subjects were randomized into 1 of 10 treatment arms: double-blind placebo (matched to albiglutide arms); albiglutide weekly (4, 15, or 30 mg), every 2 weeks (biweekly; 15, 30, or 50 mg), or monthly (50 or 100 mg); or open-label exenatide (5 μg twice daily for 4 weeks followed by 12 weeks of 10 μg twice daily). Albiglutide (formulated in 10 mmol/l sodium phosphate, 4.2% trehalose, 2.8% mannitol, 0.01% polysorbate-80) and placebo (same formulation without active principle) were administered in the physician's office over the course of 16 weeks. Subjects receiving 4 mg albiglutide were given 1.0 ml (4 mg/ml solution). Subjects receiving 15, 30, or 50 mg albiglutide were given 0.32, 0.65, or 1.0 ml (50 mg/ml solution). Subjects receiving 100 mg albiglutide were given two 1.0-ml injections (50 mg/ml solution, >1 inch apart). Placebo volumes were matched to active treatment. Albiglutide/placebo injections were subcutaneous to the abdomen using 30-G needles. Subjects were observed for ≥30 min to monitor for injection site reactions. Subjects receiving exenatide initiated treatment in the physician's office and subsequently self-administered according to the package insert. After 16 weeks, subjects entered an 11-week washout phase to assess safety and immunogenicity.
Assessments
On therapy.
A1C and fasting plasma glucose (FPG) measurements were performed at screening, baseline, and weeks 2 (FPG only), 4, 5, 7, 8, 9, 12, 15, and 16. Fasting fructosamine, C-peptide, glucagon, insulin, and lipids were measured at baseline and weeks 5, 8, 12, and 16. β-Cell function was calculated using homeostasis model assessment (24).
Adverse event assessments and safety analyses were conducted throughout the study. Nausea and vomiting were monitored for occurrence and duration. Immunogenicity samples were taken at baseline and weeks 1, 4, 8, 12, and 16 and were screened for anti-albiglutide antibodies via ELISA (20). Plasma samples were collected to characterize the pharmacokinetics of albiglutide, quantified by ELISA (20) at baseline and at weeks 4, 5, 7, 8, 9, 12, 15, and 16. Population pharmacokinetics analysis was performed using a nonlinear mixed-effect modeling approach with NONMEM software (ICON Development Solutions, Ellicott City, MD).
Eleven-week washout.
A1C, FPG, and albiglutide concentrations were obtained at weeks 17, 18, 20, 23, and 27; fasting fructosamine, C-peptide, glucagon, insulin, and lipid profiles were obtained at weeks 20 and 27; and immunogenicity assessments were examined at weeks 20, 23, and 27.
Statistical analysis
The primary objective was to evaluate the dose response of albiglutide for safety and efficacy. With 30 subjects planned in each arm, a two-sided 95% CI for each group mean response had a half-width of 0.36% on the A1C scale, assuming a standard deviation of 1.00%.
The primary efficacy end point was change from baseline A1C at week 16 versus placebo across different doses within each schedule (weekly, biweekly, and monthly). The primary analysis was an ANCOVA model with main effects for group and prior metformin therapy, adjusting for baseline A1C. Dose response was evaluated using contrasts within the ANCOVA model framework. Pairwise comparisons were performed in the same ANCOVA model. Secondary end points were similarly analyzed. Responder analysis and incidence of hypoglycemia were summarized by group statistics. No formal statistical comparisons versus exenatide were conducted. Safety and tolerability data were categorically collected.
Comparisons were made on the intent-to-treat population, defined as all randomly assigned subjects with at least one postbaseline assessment of the primary end point, using last observation carried forward. The safety population included all randomized subjects who received at least one dose of any medication. An interim analysis was conducted at 8 weeks for administrative purposes by an independent statistical analysis group; blinding was retained for study investigators and study personnel with daily operational responsibility. No formal interim inferential hypothesis testing was conducted; the study conduct was unaltered based on the results of the interim analysis.
RESULTS
Subject disposition and baseline characteristics
A total of 774 subjects were screened. Of 361 subjects randomized, 356 received treatment and were included in the safety analysis, 345 were included in the efficacy analysis, and 255 completed the 16-week trial. Withdrawal rates were similar across groups (online appendix 2); the most frequent reason for withdrawal was adverse events. Adverse events occurring in at least one subject leading to withdrawal included hyperglycemia (0–11.8%, n = 24, mostly in placebo and lower-dose groups), GI events (0–11.4%, n = 10, across groups), injection site events (0–9.7%, n = 11, mostly in higher-dose groups), and hypertriglyceridemia (0–5.7%, n = 2 of 35 receiving 50 mg albiglutide biweekly, deemed unrelated to study drug; 0% in other groups). Other reasons for withdrawal included loss to follow-up, protocol violations, and voluntary withdrawal.
Baseline demographics and characteristics were comparable across groups (online appendix 3). Mean duration of diabetes was 4.9 years. Baseline A1C levels (mean 8.0%) were evenly distributed across arms. A similar proportion of subjects receiving placebo or albiglutide were drug naïe (25.7–34.4%) or receiving metformin monotherapy. All subjects receiving exenatide were on metformin monotherapy. The groups were similar in terms of race/ethnicity (43.8–71.0% Caucasian; 87.1 and 12.9% of subjects were from U.S. and Latin American clinics, respectively), and the rates of dyslipidemia, hypertension, and coronary artery disease were 50.0–80.0, 47.1–67.6, and 0–15.2%, respectively.
Efficacy
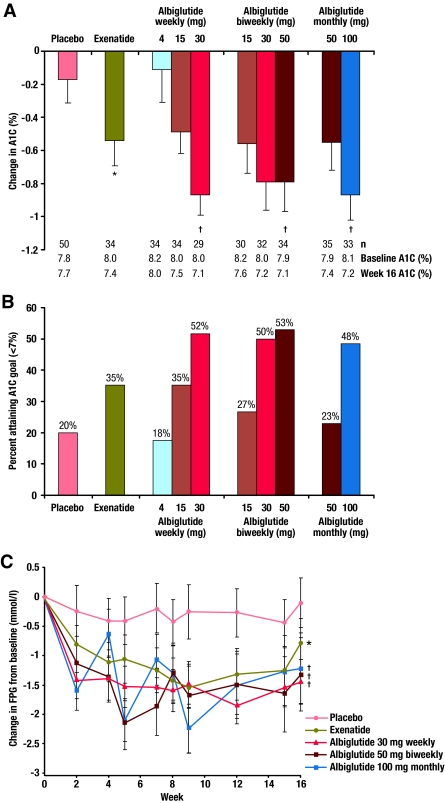
After 16 weeks, albiglutide significantly reduced A1C in a generally dose-dependent manner within each dose schedule (Table 1) (Fig. 1A). Mean A1C reductions from baseline in subjects receiving the highest dose in each treatment schedule were −0.87, −0.79, and −0.87% for 30 mg weekly, 50 mg biweekly, and 100 mg monthly, respectively, versus placebo (−0.17%) or exenatide (−0.54%). A1C reductions (based on ANCOVA model) for the highest doses compared with placebo were statistically significant: 30 mg weekly, −0.62% (95% CI −1.03 to −0.22), P < 0.003; 50 mg biweekly, −0.57% (−0.96 to −0.19), P < 0.003; and 100 mg monthly, −0.60% (−0.99 to −0.22), P < 0.002. Numerically greater reductions in A1C were observed in subjects with baseline A1C ≥8.5%.
Table 1.
Change from baseline in glycemic parameters at 16 weeks
| Placebo | Exenatide* twice daily, 5–10 μg | Albiglutide |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Weekly |
Biweekly |
Monthly |
||||||||
| 4 mg | 15 mg | 30 mg | 15 mg | 30 mg | 50 mg | 50 mg | 100 mg | |||
| n (A1C data) | 50 | 34 | 34 | 34 | 29 | 30 | 32 | 34 | 35 | 33 |
| Baseline A1C (%) | 7.8 ± 0.9 | 8.0 ± 0.9 | 8.2 ± 1.0 | 8.0 ± 0.9 | 8.0 ± 0.9 | 8.2 ± 1.0 | 8.0 ± 1.0 | 7.9 ± 0.7 | 7.9 ± 0.8 | 8.1 ± 1.0 |
| ΔA1C at 16 weeks vs. baseline | −0.17 ± 1.01 | −0.54 ± 0.91 | −0.11 ± 1.16 | −0.49 ± 0.74 | −0.87 ± 0.65† | −0.56 ± 0.97 | −0.79 ± 0.98† | −0.79 ± 1.04† | −0.55 ± 1.01 | −0.87 ± 0.87† |
| n (FPG data) | 47 | 33 | 32 | 32 | 29 | 28 | 32 | 32 | 34 | 33 |
| Baseline FPG (mmol/l) | 9.9 ± 3.7 | 9.5 ± 2.4 | 10.9 ± 3.9 | 9.5 ± 2.9 | 9.6 ± 3.1 | 10.2 ± 2.7 | 9.5 ± 3.3 | 10.0 ± 3.2 | 9.3 ± 2.7 | 9.8 ± 3.8 |
| ΔFPG at 16 weeks vs. baseline (%) | −0.10 ± 2.90 | −0.80 ± 2.48 | −0.47 ± 3.12 | −0.72 ± 1.68 | −1.44 ± 2.03† | −1.28 ± 2.43 | −1.58 ± 2.06† | −1.32 ± 3.52† | −0.72 ± 2.77 | −1.22 ± 3.50† |
Data are means ± SD for the intent-to-treat population, last observation carried forward.
*Exenatide was used to provide clinical reference; no statistical analyses were conducted.
†P < 0.05 vs. placebo.
Figure 1.
Effects of albiglutide on glycemic parameters. A: The reduction in A1C from baseline at 16 weeks. Data are presented as mean change from baseline (–SE). B: The proportion of subjects achieving ADA A1C target of <7%. Data are presented as % at goal. C: Change in fasting plasma glucose over time. Data are presented as mean change from baseline (±SE). *No formal statistical comparisons versus exenatide (open label) were conducted. †P < 0.05 vs. placebo.
The proportion of subjects achieving the ADA target A1C (<7.0%) at week 16 increased with increasing doses of albiglutide within each schedule; similar proportions of subjects achieved target A1C at the highest albiglutide dose among the three schedules. More subjects receiving albiglutide 30 mg weekly (52%), 50 mg biweekly (53%), and 100 mg monthly (48%) achieved A1C <7.0% compared with subjects receiving placebo (20%) and exenatide (35%) (Fig. 1B).
The time course of albiglutide-induced changes in FPG demonstrated that each schedule elicited a dose-dependent FPG reduction over 16 weeks, with no changes in FPG observed with placebo. Rapid reductions in FPG were observed, with similar FPG reductions at the 16-week end point for each of the highest doses (Fig. 1C). Statistically significant reductions were seen for FPG compared with placebo at week 16 (−1.38 mmol/l [P < 0.02], −1.16 mmol/l [P < 0.03], and −1.17 mmol/l [P < 0.03] for 30 mg weekly, 50 mg biweekly, and 100 mg monthly albiglutide doses, respectively). The 4- and 15-mg weekly dose regimens of albiglutide reduced FPG but were less effective. The greatest fluctuations in FPG over time were observed in subjects receiving albiglutide 100 mg monthly (Fig. 1C). Exenatide was associated with a relatively consistent FPG profile over time that was numerically less than FPG reductions seen with the highest doses of albiglutide (Fig. 1C).
Neither fasting insulin nor glucagon levels were consistently or significantly altered. Small improvements in β-cell function (assessed by homeostasis model assessment of β-cell function) were noted in subjects receiving albiglutide (online appendix 4).
There was no significant difference in weight reduction among groups. A consistent trend in weight reduction was noted with average weight loss ranging from −1.1 to −1.7 kg in subjects receiving the highest albiglutide dose in each schedule. These reductions were numerically greater than those for subjects receiving placebo (−0.7 kg) but less than those for subjects receiving exenatide (−2.4 kg). Albiglutide and exenatide tended to reduce mean systolic and diastolic blood pressure but did not significantly change the plasma lipoprotein profile (online appendix 4).
Safety and tolerability
The percentage of subjects reporting at least one adverse event was similar across groups (67–85%). The most frequently reported adverse events included nausea (11.8–54.3%), vomiting (0–41.2%), headache (5.9–23.5%), dizziness (5.7–14.3%), nasopharyngitis (5.7–11.4%), back pain (0–14.3%), influenza (0–9.7%), upper respiratory tract infections (0–15.2%), and local skin reactions (2.9–28.6%) (online appendix 5).
The proportion of subjects who experienced nausea and/or vomiting was lower with administration of ≤30 mg albiglutide compared with the proportion of subjects receiving higher doses. In the 30-mg weekly arm, 29.0% of subjects experienced nausea and/or vomiting, compared with 54.3% in the 50-mg biweekly group and 55.9% in the 100-mg monthly group. The percentage of exenatide subjects who experienced nausea and/or vomiting also was higher (45.7%) than in the 30-mg weekly albiglutide group.
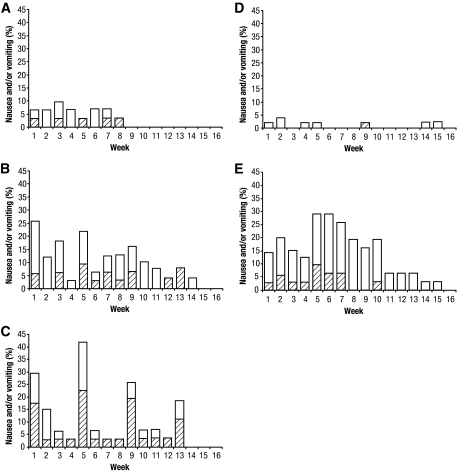
Examination of the time course (Fig. 2) of nausea and/or vomiting revealed that the proportion of subjects experiencing these adverse events each week was low in the 30-mg weekly arm (<10%) and declined over the course of the study, with no reports of nausea or vomiting after 8 weeks (Fig. 2A). Although the proportion of nausea and/or vomiting for the 50-mg biweekly dose was greater than for the 30-mg weekly dose, it also declined over the study period (Fig. 2B). Subjects receiving the 100-mg monthly dose also experienced higher rates of nausea and/or vomiting, with peak incidence occurring following each monthly dose administration. The overall rate was higher for 100-mg monthly than for the other albiglutide groups (Fig. 2C). The incidence of nausea and/or vomiting with exenatide reached 20% by week 2, increased at week 5 to a peak incidence of 29% (due to label-based titration), and also declined over the study period (Fig. 2E).
Figure 2.
Time course of nausea and vomiting as adverse events. The percentage of subjects experiencing vomiting with or without nausea (▨) or nausea (□) adverse events during each week of the 16-week trial for albiglutide 30 mg weekly (A), albiglutide 50 mg biweekly (B), albiglutide 100 mg monthly (C), placebo (D), and exenatide (E).
Other adverse events were less common than GI-related events and were similar across groups with no dose-dependent trends. Documented hypoglycemia was not increased with albiglutide (0–3.1%) relative to placebo (3.9%) and exenatide (2.9%). Cardiac-related adverse events (eight subjects; six assessed as severe) were distributed across groups, with no dose-dependent trends (online appendix 5). No episodes of pancreatitis were reported.
Eight subjects (2.5%) had confirmed positive anti-albiglutide antibodies at least once in the placebo and albiglutide arms after baseline measurement. However, two subjects tested positive prior to albiglutide treatment, and one subject who tested positive received placebo. The remaining five albiglutide-positive subjects were detected in the weekly and biweekly arms. The appearance of anti-albiglutide antibodies was largely transient: one subject remained positive at week 27. Antibodies were non-neutralizing and low titer and showed cross-reactivity with GLP-1 in four of five subjects. There was no obvious association between the presence of anti-albiglutide antibodies and either efficacy or safety.
Injection site local skin reactions, most of which were small, were observed in the study and were more common in the albiglutide groups (2.9–28.6%) compared with placebo (5.9%) and exenatide (2.9%). Injection site reactions tended to occur once per person in subjects receiving 30 mg albiglutide and approximately twice per person in subjects receiving higher albiglutide doses. Skin reactions were not associated with positive IgE antibodies or neutralizing antibodies and did not worsen upon repeated dosing or exhibit dose dependency. No systemic allergic reactions attributable to albiglutide were observed.
Pharmacokinetics
Albiglutide exhibited a plasma half-life of ∼5 days. Steady-state albiglutide levels were reached within ∼4 to 5 weeks of the first dose (online appendix 6). Greater peak/trough fluctuations in circulating albiglutide concentrations were observed with less frequent administration of higher doses.
CONCLUSIONS
In this study, the dose- and time-dependent effects of albiglutide, a long-acting GLP-1 receptor agonist, was evaluated to identify potential dose regimens for future studies. Within each dose schedule, albiglutide was associated with generally dose-dependent A1C reductions that were significantly different from placebo. Maximum doses in each schedule (albiglutide 30 mg weekly, 50 mg biweekly, and 100 mg monthly) elicited similar responses in A1C, providing meaningful reductions within the range of ∼0.8–0.9% from a mean baseline A1C of 8.0%.
Albiglutide also significantly reduced FPG at week 16 compared with placebo, with reductions observed at the time of the first assessment (2 weeks postdose). In a previous study, FPG reductions were observed as early as 2 days following a single dose (20).
Variability in glycemic response appeared to be related to circulating concentrations of albiglutide. With a plasma half-life of ∼5 days and at doses sufficient to achieve consistent therapeutic response (i.e., 30 mg), weekly dosing provided consistent FPG reduction. Greater FPG fluctuations were observed following biweekly or monthly dosing, despite similar A1C reductions.
Albiglutide 30-mg weekly dosing elicited steady and consistent improvements in FPG reductions, with a more favorable nausea/vomiting profile than exenatide. When dosed biweekly, 50 mg albiglutide also improved glycemic indexes but with higher GI adverse event rates, possibly related to the higher initial dose. In all dosing schedules, rates of nausea and vomiting declined over time. However, when dosed monthly, albiglutide did not produce stable FPG reductions between dosing and was associated with higher GI event rates. The increase in FPG fluctuation and GI events in the biweekly and monthly regimens is likely due to fluctuations in plasma albiglutide concentrations resulting from less frequent dosing. An escalating-dose titration for the biweekly regimen might have resulted in a lower frequency of GI events. This approach may be tested in future studies to explore the possibility of using biweekly dosing as a maintenance option in patients who respond well and tolerate the initial weekly regimen.
Mechanistically, the reasons for differences in the tolerability of albiglutide and exenatide are unknown but may be due to differences in pharmacokinetics, including gradual absorption (Tmax is ∼3 days vs. 2.1 h for albiglutide and exenatide, respectively) and a long plasma half-life of ∼5 days resulting in a steady state achieved after 4–5 doses for 30 mg albiglutide weekly. The slow accumulation of albiglutide may ameliorate GI intolerability often observed with exenatide, and albiglutide's relative impermeance to the central nervous system may result in a more benign profile with respect to nausea and vomiting than exenatide (21).
Weight loss was similar across albiglutide arms and numerically less than with exenatide, which had a higher baseline BMI. Exploratory post hoc analyses indicated that there was no obvious correlation between reduction in A1C and weight loss for all albiglutide doses (data not shown). However, larger and longer-term studies will determine the true effect on weight and cardiometabolic parameters.
Immunogenicity was closely monitored owing to the possible appearance of neutralizing antibodies or the development of immediate hypersensitivity reactions. In this study, anti-albiglutide antibodies were detected in 2.5% (n = 8) of subjects. However, the observation that positive titers of anti-albiglutide antibodies were detected in three subjects at baseline suggests that the immunogenicity rate may be overestimated. Exenatide, which has 53% homology to human GLP-1 (14), is associated with antibody development following administration with twice-daily (15–18) and weekly (25) formulations (>40%). Antibody formation may attenuate efficacy, especially among patients developing high levels of anti-exenatide antibodies (25).
There are limitations to this phase II study. First, the number of subjects in each arm is relatively small compared with phase III studies. Second, relative to the number of subjects, the dropout rate was high owing to adverse events, loss to follow-up, and voluntary withdrawals. Third, the duration of active treatment was 16 weeks, so the magnitude or durability of response cannot be fully appreciated. Fourth, no escalating doses were tested for biweekly and monthly dosing, which may have attenuated GI adverse events and FPG fluctuations. Finally, because exenatide was administered open label, formal statistical comparisons were not conducted between exenatide and albiglutide.
In conclusion, albiglutide improved glucose control in a dose-dependent manner when given weekly and biweekly. Higher monthly doses of albiglutide were efficacious, but their use was constrained by the higher frequency of GI-related adverse events. Weekly albiglutide significantly improved glycemic control with an acceptable safety and tolerability profile and modest weight loss without increasing the risk of hypoglycemia or immunological response in subjects with type 2 diabetes. Future studies may elucidate whether titration dosing or biweekly scheduling could be options for patients who respond to and tolerate the initial weekly regimen.
Supplementary Material
Acknowledgments
This study was sponsored by GlaxoSmithKline, Middlesex, U.K.
J.Ro. has received research grants and consulting honoraria for serving on scientific advisory boards from GlaxoSmithKline. J.Re. has received research grants from and acted as a consultant for GlaxoSmithKline. M.B., F.Y., and M.S. are employees/stockholders of GlaxoSmithKline. No other potential conflicts of interest relevant to this article were reported.
J.Ro. and J.Re. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Caroline Perry (GlaxoSmithKline) for technical support through clinical operations, Claire Holland (GlaxoSmithKline) for performing the immunogenicity assays, and Kenneth Pomerantz (GlaxoSmithKline), Sandra Harris and Joelle Escoffery (MediTech Media) for editorial support for the manuscript.
Footnotes
Clinical trial reg. no. NCT00518115, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 2. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F: Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 4. Abraira C, Duckworth WC, Moritz T: VADT Group. Glycaemic separation and risk factor control in the Veterans Affairs Diabetes Trial: an interim report. Diabetes Obes Metab 2009;11:150–156 [DOI] [PubMed] [Google Scholar]
- 5. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 6. American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care 2009;32(Suppl. 1):S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saaddine JB, Cadwell B, Gregg EW, Engelgau MM, Vinicor F, Imperatore G, Narayan KM: Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med 2006;144:465–474 [DOI] [PubMed] [Google Scholar]
- 8. Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS: Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol 2008;18:222–229 [DOI] [PubMed] [Google Scholar]
- 9. Carver C: Insulin treatment and the problem of weight gain in type 2 diabetes. Diabetes Educ 2006;32:910–917 [DOI] [PubMed] [Google Scholar]
- 10. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G: ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 11. Deacon CF, Pridal L, Klarskov L, Olesen M, Holst JJ: Glucagon-like peptide 1 undergoes differential tissue-specific metabolism in the anesthetized pig. Am J Physiol 1996;271:E458–E464 [DOI] [PubMed] [Google Scholar]
- 12. Holst JJ, Deacon CF, Vilsboll T, Krarup T, Masbad S: Glucagon-like peptide-1, glucose homeostasis, and diabetes. Trends Molec Med 2008;14:161–168 [DOI] [PubMed] [Google Scholar]
- 13. Kreymann B, Williams G, Ghatei MA, Bloom SR: Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 1987;2:1300–1304 [DOI] [PubMed] [Google Scholar]
- 14. Bray GM: Exenatide. Am J Health Syst Pharm 2006;63:411–418 [DOI] [PubMed] [Google Scholar]
- 15. DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092–1100 [DOI] [PubMed] [Google Scholar]
- 16. Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD: Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005;28:1083–1091 [DOI] [PubMed] [Google Scholar]
- 17. Zinman B, Hoogwerf BJ, Durán García S, Milton DR, Giaconia JM, Kim DD, Trautmann ME, Brodows RG: The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2007;146:477–485 [DOI] [PubMed] [Google Scholar]
- 18. Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD: Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628–2635 [DOI] [PubMed] [Google Scholar]
- 19. Fineman MS, Shen LZ, Taylor K, Kim DD, Baron AD: Effectiveness of progressive dose-escalation of exenatide (exendin-4) in reducing dose-limiting side effects in subjects with type 2 diabetes. Diabetes Metab Res Rev 2004;20:411–417 [DOI] [PubMed] [Google Scholar]
- 20. Bush MA, Matthews JE, De Boever EH, Dobbins RL, Hodge RJ, Walker SE, Holland MC, Gutierrez M, Stewart MW: Safety, tolerability, pharmacodynamics and pharmacokinetics of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in healthy subjects. Diabetes Obes Metab 2009;11:498–505 [DOI] [PubMed] [Google Scholar]
- 21. Baggio LL, Huang Q, Brown TJ, Drucker DJ: A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes 2004;53:2492–2500 [DOI] [PubMed] [Google Scholar]
- 22. Bloom M, Bock J, Duttaroy A, Grzegorzewski K, Moore P, Ou Y, Wojcik S, Zhou X, Bell AC: Albugon fusion protein: a long-acting analog of GLP-1 that provides lasting antidiabetic effect in animals (Abstract). Diabetes 2003;52(Suppl. 1):A112 [Google Scholar]
- 23. Ou YC, Bloom M, Grzegorzewski KJ, Bock J, Duttaroy A, Moore P, Wojcik S, Zhou JX, Sung C, Bell AC: Pharmacokinetic and pharmacodynamic analysis of albugon, a long-acting analog of glucagon-like peptide-1, in mice and monkeys (Abstract). AAPS PharmSci 2003;5:5263 [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 25. Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L: DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet 2008;372:1240–1250 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.