In the past 15 years, multiple clinical trials have attempted to find prevention for type 1 diabetes. The accompanying article by Bresson and von Herrath (1) reviews basic mechanisms underlying immunoprevention and immunotherapy of type 1 diabetes as well as selected human trials in the context of data from animal models. The second part of this mini-symposium provides an overview of the recent or ongoing human trials. Immunotherapy for prevention of type 1 diabetes or to ameliorate the course of the disease after clinical diagnosis is currently restricted to research studies. References are provided to the clinical.trials.gov database or other sources where the reader can find additional information.
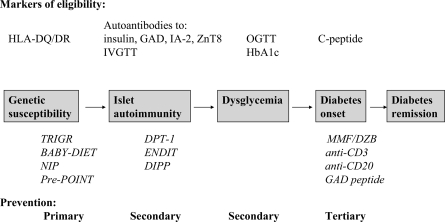
Type 1 diabetes is an autoimmune disease caused by interplay of genetic and environmental factors. Figure 1 summarizes the main stages in the development of type 1 diabetes and examples of prevention trials at these stages. The initial step—development of islet autoimmunity marked by the presence of autoantibodies to insulin, GAD (GAD65), insulinoma-associated protein 2 (IA-2), and tyrosine phosphatase or zinc transporter (ZnT8)—is believed to be driven by environmental trigger(s) (2). Over the past 40 years, the incidence of childhood type 1 diabetes worldwide has increased by 3–5% annually (3–6). Elimination of the environmental trigger(s) responsible for this epidemic would be the most efficient approach to primary prevention. However, there is lack of consensus regarding which environmental factor(s) initiates islet autoimmunity. The National Institutes of Health have established The Environmental Determinants of type 1 Diabetes in the Young (TEDDY) consortium to evaluate the leading candidates (7,8).
Figure 1.
Natural history of type 1 diabetes and prevention opportunities.
After initiation of islet autoimmunity, most patients have a long preclinical period (9–12) that offers opportunity for secondary prevention—halting progression to clinical diabetes (Fig. 1). Large randomized trials initiated in the 1990s, including the Diabetes Prevention Trial Type 1 (DPT-1), the European Nicotinamide Diabetes Intervention Trial (ENDIT), and the Diabetes Prediction and Prevention (DIPP) project (13–16), have targeted this stage of pre–type 1 diabetes. The trials (17,18) and a cohort study (19) have shown that mild asymptomatic hyperglycemia, detected by oral glucose tolerance test (OGTT) or A1C, may precede by months or years overt insulin dependence among individuals with islet autoantibodies. Intervention at this “dysglycemic” stage of pre–type 1 diabetes may theoretically preserve endogenous insulin secretion and prevent acute and long-term complications of type 1 diabetes (20–22).
For the same reasons, preservation or restoration of insulin secretion after diagnosis of diabetes continues to be an attractive goal. Such tertiary prevention trials have used a number of immunomodulatory agents. These agents are often considered first in patients with established diabetes and, when proven safe, may be applied to patients with dysglycemic pre–type 1 diabetes and eventually those with normoglycemic pre–type 1 diabetes. However, efficacy in preserving C-peptide after diagnosis of type 1 diabetes should not be a precondition to applying an intervention to patients with pre–type 1 diabetes, as there may be a “point of no return” in the autoimmune destruction of the islets, rendering some interventions effective only at the earlier stages of the process.
Current approaches to prevent type 1 diabetes include:
Avoidance of environmental triggers of islet autoimmunity such as cow's milk or gluten. Celiac disease provides an encouraging example of autoimmune disease that can be prevented in this way. Alternatively, diet is supplemented with nutrients for which deficiency presumably promotes islet autoimmunity, e.g., n-3 fatty acids or vitamin D.
Antigen-specific “vaccination” using islet autoantigens, e.g., intact insulin, altered insulin or proinsulin peptides, GAD65, or heat shock protein 60 (HSP60) peptide. The goal is to induce autoantigen-specific tolerance by induction of regulatory T-cells that downregulate immunity to a specific autoantigen as well as promote tolerance to additional autoantigens.
Non–antigen-specific systemic therapies that range from mild modulation with oral nicotinamide or bacille Calmette–Guerin (BCG) vaccination to immunosuppression and cellular therapies.
Stimulation of β-cell regeneration in conjunction with suppression of apoptosis that is increased in islet autoimmunity to overcome the relapsing-remitting course of pre-diabetes.
Metabolic modifications, such as weight loss and maintenance, increased physical activity, and β-cell rest.
PRIMARY PREVENTION OF ISLET AUTOIMMUNITY
The target population for primary prevention trials are young children who carry high-risk HLA-DR,DQ genotypes. Finding such children in the general population is hampered by low specificity of these genotypes; the specificity is much higher in populations with higher a priori risk of type 1 diabetes, i.e., first-degree relatives. While a number of non-HLA susceptibility gene markers have been reported, they have yet to be included in the design of primary prevention trials. The young age of potential trial participants and that most of them will never develop diabetes sets the safety bar high for prevention trials in this population. Ongoing primary prevention trials, summarized in Table 1, include largely low-risk dietary modifications: elimination of cow's milk (23) or gluten (24) and supplementation of diet with n-3 fatty acids (25) or vitamin D. A pilot trial of antigen-specific immunomodulation is using insulin (26) found previously to be safe in large secondary prevention trials.
Table 1.
Primary or secondary prevention trials prior to clinical diagnosis of diabetes
| Study (ref.) | Drug/phase | Sponsor/contact | Age | Eligibility | Dosing | Placebo | Follow-up duration/primary end point | Status/target size |
|---|---|---|---|---|---|---|---|---|
| TRIGR (23) | Cow's milk hydrolyzate/phase III | NIH, NICHD, et al./www.trigr.org | 0–7 days | FDRs, high-risk HLA | Supplementation of breast-feeding up to the age of 8 months | Yes | 10 years/type 1 diabetes | Enrollment closed/n = 2,160 |
| BABY DIET (24) | Gluten-free diet/phase II pilot | German Research Foundation/anziegler@lrz.uni-munchen.de | <3 months | Relatives, high-risk HLA DR, DQ | Gluten-free diet until age 12 months | No | 3 years/islet autoantibodies | Enrollment closed/n = 50 |
| TrialNet NIP (27) | DHA/phase II pilot | NIH, NIDDK/www.diabetestrialnet.org | >24 weeks gestation/newborn | Relatives, HLA-DR3 or DR4 | Oral DHA once daily | Yes | 2 years/20% higher plasma levels of DHA | Enrollment closed/n = 119 |
| Vitamin D (31,82) | Vitamin D3/phase I pilot | Canadian Diabetes Association/dcatte@mich.ca | 0–4 weeks | High-risk HLA DR, DQ | Oral vitamin D 2,000 IU once daily | No | 1 year/25(OH) vitamin D levels, serum/urine Ca, islet autoantibodies | Enrolling/n = 20 |
| TrialNet Oral Insulin (33) | Human insulin/phase III | NIH, NIDDK/www.diabetestrialnet.org | 1–45 years | Relatives, 2+ islet antibodies including to insulin | Oral insulin 7.5 mg once daily | Yes | 7–8 years/type 1 diabetes | Enrolling/n ≈ 400 |
| INIT II (34) | Human insulin/phase II | Melbourne Health/harrison@wehi.edu.au | 4–30 years | Relatives, 2+ islet antibodies HLA not DR2, DQ6 | Intranasal 1.6 mg and 16 mg/day | Yes | 5 years/type 1 diabetes | Enrolling/n = 262 |
| Pre-POINT (26) | Human insulin/phase I/II | JDRF/prevent.diabetes@crt-dresden.de | 1.5–7 years | FDRs/>50% risk of type 1 diabetes | Insulin daily for the first 10 days, after that twice a week. Increasing dose: oral 2.5–67.5 mg/day, intranasal 0.28–7.5 mg/day* | Yes | 3–18 months/islet autoantibodies | Enrolling/n = 40 |
| FINDIA | Insulin-free whey-based formula/phase I/II | National Public Health Institute, Helsinki, Finland/outi.vaarala@ktl.fi | Infants | General population, high-risk HLA DQ | Yes | 2 years/islet autoantibodies, type 1 diabetes | Enrollment closed/n = 982 |
*Staggered enrollment. DHA, docosahexaenoic acid; FDR, first-degree relative; JDRF, Juvenile Diabetes Research Foundation; NICHD, National Institute of Child Health and Human Development; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIH, National Institutes of Health; NIP, Nutritional Intervention to Prevent type 1 diabetes.
Dietary modifications
The cow's milk hypothesis is being tested by the Trial to Reduce type 1 diabetes in the Genetically at Risk (TRIGR) (23). This randomized, double-masked trial is evaluating the effect of hydrolyzed infant formula, where protein fragments are too small to stimulate the immune system, compared with cow's milk–based formula. Eligible to participate were newborns who had a first-degree relative with type 1 diabetes and one of the high-risk HLA-DQ genotypes. The recruitment of 2,160 children from 77 centers in 15 countries was completed at the end of 2006. All participant mothers received the recommendation to breast-feed for at least the first 6 months of life. If a mother was unable to exclusively breast-feed before the baby was 8 months of age, her child was randomly assigned to either a formula of extensively hydrolyzed protein (Nutramigen) or a formula based on nonhydrolyzed cow's milk (Enfamil) containing a small amount of Nutramigen (for masking purpose).The main end point of the trial is development of diabetes by the age of 10 years.
The Finnish Intervention Trial for the Prevention of Type I Diabetes (FINDIA) tests an extension of the cow's milk hypothesis, i.e., that bovine insulin present in cow's milk triggers islet autoimmunity. FINDIA includes two arms similar to TRIGR, and, in addition, one-third of high-risk infants receive insulin-free whey-based formula. In contrast to TRIGR, none of the 982 high-risk children participating in FINDIA has a first-degree relative with type 1 diabetes (O.Vaarala, unpublished data). The interim results of the 3-year follow-up of study participants is expected in early 2009.
BABY DIET is another example of an elimination diet trial (24). This randomized, unmasked feasibility study is evaluating the effect of delaying exposure to gluten until the age of 1 year.
The TrialNet Nutritional Intervention to Prevent type 1 diabetes (NIP) (27) is a pilot study that enrolled pregnant women in their 3rd trimester expecting high-risk babies based on family history. In addition, newborn first-degree relatives with high-risk HLA-DR,DQ genotypes were enrolled. The trial was not powered to formally test efficacy of dietary supplementation with docosahexaenoic acid (DHA) before 6 months of age but rather to pilot the feasibility of a definitive trial.
Vitamin D supplementation in early childhood has attracted attention as a possible primary preventive measure (28,29). However, this interest has been mitigated by potential nephrotoxicity of vitamin D (30). At least one phase I clinical trial is testing the feasibility of this approach (31).
Antigen-specific vaccines
The Primary Oral/intranasal INsulin Trial (POINT) investigators (26), encouraged by the excellent safety profile in secondary prevention trials of oral (15) and intranasal insulin (16), have initiated a feasibility trial of primary prevention with oral or intranasal insulin vaccination. The pre-POINT phase I trial will determine the dose and route of insulin administration that is safe and is bioavailable to the immune system. The study will determine whether administration of insulin leads to both B- and T-cell responses that have characteristics consistent with protection. Protective B-cell responses may potentially include production of IgA-insulin antibodies, lower-affinity insulin antibodies, and insulin antibodies that do not react with proinsulin. T-cell responses to insulin will be evaluated by ELISpots, with increased production Th2-type cytokines such as interleukin (IL)-4, IL-10, and transforming growth factor-β suggesting protection. Eligible are children who have multiple first-degree relatives with type 1 diabetes or those who have the HLA-DR3/DR4-DQ8 genotype inherited identical by descent with a sibling proband; such siblings have type 1 diabetes risk as high as 80% (32). Children will be monitored for the development of islet autoantibodies, diabetes, and protective immune responses to insulin. Depending upon the outcome of pre-POINT, the study will continue to the phase II POINT study, which will determine the efficacy of mucosal insulin administration in primary prevention.
SECONDARY PREVENTION OF CLINICAL DIABETES
Approximately 1 in 20 first-degree relatives and 1 in 300 people without type 1 diabetes in the immediate family has multiple islet autoantibodies. That is, 1 million people in the U.S. alone are currently at an increased risk of developing type 1 diabetes. Most young individuals with multiple islet autoantibody positivity progress to diabetes in 5–10 years; however, the rate of progression decreases with age. Preventing insulin dependency in a significant proportion of this population would be a major public health achievement. However, it is expensive to identify these high-risk subjects, about $1,200 per subject, according to the DPT-1, and four large prevention trials have found no effect on the rate of progression to clinical type 1 diabetes for insulin administered parenterally (13), orally (15), or intranasally (16), as well as for oral nicotinamide (14). Smaller studies have evaluated other agents, also with little success. We will not review details of these studies, as they have been widely publicized. Two large randomized double-masked secondary prevention trials using oral and intranasal insulin are still underway (33,34) (Table 1). Interestingly, a post hoc analysis of data from the DPT-1 trial of oral insulin suggested that relatives with high levels of insulin autoantibodies appeared to experience a delay in progression to diabetes by ∼4 years (15). This observation led to a second oral insulin trial, conducted by the Type 1 Diabetes TrialNet consortium. The study is enrolling first-degree relatives age 1–45 years and second-degree relatives age 1–20 years (the relative with diabetes must have been diagnosed before the age of 40 years and started on insulin within the 1st year of diagnosis). Eligible subjects must be positive for insulin autoantibodies on two samples within a 6-month period and meet additional criteria for other islet autoantibodies. As of February 2009, an initial 121 of the anticipated 400 subjects have been randomized.
The DPT-1 and ENDIT data have provided a wealth of information concerning prediction of type 1 diabetes and trial design. In October 2001, the DPT-1 trial centers and several new centers formed the Type 1 Diabetes TrialNet consortium (www.diabetestrialnet.org) for the prevention of type 1 diabetes. TrialNet systematically evaluates therapies in new-onset patients, as well as in pre-diabetic subjects, and invites proposals from the research community at large. The Immune Tolerance Network (ITN) (www.immunetolerance.org) is also accepting applications to support therapies aimed at tolerance induction and assays of tolerance.
Although the secondary prevention trials have failed to prevent or delay the onset of diabetes thus far, a growing body of evidence suggests that prevention of diabetic ketoacidosis (DKA) and hospitalization in newly diagnosed children is possible and should be a major goal of diabetes care systems. The DPT-1 demonstrated that DKA can be prevented by testing for islet autoantibodies and close biochemical monitoring (13), and the Diabetes Autoimmunity Study in Youth (DAISY) has confirmed this observation in the setting of an observational study (35). Early diagnosis and treatment not only eliminates mortality and greatly reduces the cost of initial treatment but may also help preserve endogenous insulin secretion and prevent acute and long-term complications of the disease (20–22). In the near future, we will likely see a resurgence of secondary prevention trials translating the most successful findings from tertiary prevention trials in patients with established type 1 diabetes.
TERTIARY PREVENTION AFTER DIAGNOSIS OF DIABETES
In the past several years, trials in patients with newly diagnosed type 1 diabetes became the main focus of the research community. This shift away from secondary to tertiary prevention trials has been partially due to the ease of finding and retaining trial participants as well as the realization of how difficult and expensive trials of the magnitude of TRIGR, DIPP, ENDIT, or DPT-1 are to perform. The goal is preservation of remaining islet β-cells to induce and prolong partial remission. Unfortunately, most islets have already been destroyed by the time diabetes is diagnosed (36). Autoimmune β-cell destruction continues after the diagnosis of diabetes. A spontaneous temporary remission from insulin dependency may occur in up to 27% of patients, soon after diagnosis (37), and may be related to β-cell rest caused by insulin treatment (22). Younger age at onset, male sex, high titer of islet autoantibodies, severe DKA at diagnosis, and a short duration of symptoms prior to diagnosis are associated with a more rapid loss of C-peptide secretion (38). There are conflicting reports concerning the effect of the HLA-DR,DQ genotypes (37–39). Residual β-cell function can be retained for decades after the onset of diabetes in a subset of patients; however, for most patients very little normal function is retained, β-cell apoptosis continues, and there is little spontaneous β-cell regeneration (40). Complete spontaneous remission of type 1 diabetes is rare (41).
A realistic outcome of tertiary prevention trials is prolongation of residual insulin secretion, rather than complete reversal of diabetes. Benefits may include simpler insulin regimen, lower A1C, and reduced risk of hypoglycemia and microvascular complications. Success is usually measured by higher fasting and stimulated C-peptide secretion in the treatment versus placebo arm, with both groups of patients maintaining good glycemic control. Preserved C-peptide is associated with better glycemic control despite use of less insulin. Lower insulin dose, lower A1C, decreased glycemic variability, and decreased incidence of hypoglycemia have been used as secondary end points. On behalf of the Immunology of Diabetes Society, Greenbaum and Harrison (42) have developed useful guidelines for intervention trials in subjects with newly diagnosed type 1 diabetes.
Antigen-specific vaccines
Antigen-specific therapies are summarized in Table 2. In the past couple of years, perhaps the most exciting development in the area of tolerance induction has been apparent efficacy of Diamyd vaccine based on the whole recombinant human GAD65 (rhGAD65) molecule suspended in alum. Clinical trials in late-onset autoimmune diabetes in adults (LADA) (43,44) and adolescents with newly diagnosed type 1 diabetes (45) have suggested benefit. In the latter study, patients receiving just two subcutaneous injections of the vaccine experienced a decline in stimulated C-peptide secretion approximately one-half that in the placebo group. Maximum stimulated C-peptide at 15 months also decreased less in the GAD-alum group compared with the placebo group. The protective effect was most pronounced in patients treated within 3 months of diagnosis; these patients preserved their endogenous insulin secretion over 15 months, in contrast to the placebo group. The apparent beneficial effects were not explained by changes in the GAD65 epitope pattern. GAD65 autoantibody levels increased in some patients; however, no serious side effects were observed, and there has been no evidence of the stiff person syndrome. Treatment with GAD65 seemed to induce a deviation of the GAD65-specific T-cell response toward a protective immune profile. Three phase III trials of the rhGAD65-alum vaccine are underway in the U.S. and Europe (Table 2), and a secondary prevention trial is under consideration.
Table 2.
Tertiary prevention trials of antigen-specific vaccines after diagnosis of diabetes
| Study (ref.) | Drug/phase | Sponsor/contact | Age (years) | Time from diagnosis/eligibility | Route | Dosing | Treated:placebo | Follow-up duration/primary end point | Status/target size |
|---|---|---|---|---|---|---|---|---|---|
| rhGAD65 (45) | rhGAD65-alum/phase II | Diamyd | 10–18 | ≤18 months/C-peptide ≥0.1 pmol/ml, GAD autoantibody positive | s.c. | 20 μg twice in 30 days | 1:1 | 15 months/fasting C-peptide, change in fasting and MMTT C-peptide | Published/n = 70 |
| rhGAD65 (83) | rhGAD65-alum/phase II/III | NIH, NIDDK, TrialNet/diabetestrialnet.org | 3–45* | ≤12 weeks/C-peptide ≥0.2 pmol/ml, GAD autoantibody positive | s.c. | 20 μg at 0, 4, and 12 weeks vs. 20 μg at 0 and 4 weeks vs. alum | 2:1 | 2 + 2 years/MMTT C-peptide (4-h AUC) | Enrolling/n = 126 |
| rhGAD65 (84) | rhGAD65-alum/phase III | Diamyd Therapeutics/swolf@tklreserach.com | 10–20* | ≤12 weeks/C-peptide ≥0.1 pmol/ml, GAD autoantibody positive | s.c. | 20 μg at 1, 30, 90, and 270 days vs. 20 μg at 0 and 30 days vs. alum alone | 2:1 | 15 months/MMTT C-peptide | Enrolling/n = 320 |
| rhGAD65 (85) | rhGAD65-alum/phase III | Diamyd Therapeutics/ulf.parkhede@trialformsupport.com | 10–20 | ≤12 weeks/C-peptide ≥0.1 pmol/ml, GAD autoantibody positive | s.c. | 20 μg at 1, 30, 90, and 270 days vs. 20 μg at 0 and 30 days vs. alum alone | 2:1 | 15 months/MMTT C-peptide | Enrolling/n = 320 |
| Proinsulin peptide (48) | Proinsulin C19-A3/phase I | Diabetes Vaccine Development Centre, JDRF, NHMRC Australia | 21–53 | >5 years/C-peptide <0.2 pmol/ml | i.d. | Intradermal 30 or 300 μg in 3 monthly doses | 3:1 | 6 months/adverse events | Published/n = 48 |
| IBC-VS01 (86) | Insulin peptide + IFA/phase I | NIAID ITN/Tihamer Orban, MD | 18–35 | ≤30 days | One injection | 1:1 | 2 years/adverse events MMTT C-peptide | Enrollment closed/n = 12 | |
| BHT-3021 (87) | plasmid encoding proinsulin/phase I | Bayhill Therapeutics/kwoody@bayhilltx.com | ≥18 | ≤5 years/diagnosed ≤40 years, C-peptide ≥0.066 pmol/ml | i.m. | One of four dose levels (0.3, 1, 3, or 6 mg) weekly for 12 weeks | 2:1 | 25–37 months/crossover optional, adverse events | Enrolling/n = 72 |
| DIA-AID (88) | DiaPep277/phase III | Andromeda Biotech/merana@andromedabio.com | 16–45 | ≤12 weeks/C-peptide ≥0.22 pmol/ml | s.c. | 1 mg nine times in 21 months | 1:1 | 2 years/MMTT C-peptide | Enrolling/n = 500 |
*Staggered enrollment. AUC, area under the curve; IFA, incomplete Freund's adjuvant; MMTT, mixed-meal tolerance test; NIAID ITN, National Institute of Allergy and Infectious Diseases Immune Tolerance Network; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NHMRC, National Health and Medical Research Council; NIH, National Institutes of Health; JDRF, Juvenile Diabetes Research Foundation.
Insulin-related molecules continue to attract great interest in vaccine development. An altered peptide ligand of the immunodominant insulin peptide B:9-23 (NBI-6024; Neurocrine Biosciences) completed phase I trials with a suggestion of immunologic efficacy (46) but was not shown to be effective in phase II B trials (47). Phase I studies have been completed or are nearing completion for a proinsulin peptide 19-A3 (48), an insulin peptide with incomplete Freund adjuvant and a plasmid encoding proinsulin (Table 2).
The DiaPep277 peptide of HSP60 has been reported to preserve C-peptide in a small trial of LADA patients with relatively short follow-up (49). Phase II trials in children showed no (50) or little (51) effect. A large phase III trial is currently enrolling patients in Europe and South Africa (Table 2).
Systemic immunomodulators
Numerous non–antigen-specific immunomodulators have been tried in newly diagnosed patients. In early 2007, an excellent review by Staeva-Vieira, Peakman, and von Herrath (52) summarized previously completed interventions, now largely of historical value. Some interventions, e.g., cyclosporine A, azatiopirine, and anti-thymocyte globulin (ATG) plus prednisolone, had unattractive side effects, including weakening of immunity to infections, renal and pancreatic toxicity, and potential long-term risk of malignancies. Others, such as nicotinamide, BCG vaccine, vitamin D supplementation, or elimination of dietary gluten, while safer, have shown no efficacy. Cyclosporine A was efficacious in prolonging insulin production (53,54); however, the treatment had to be continued for at least 6–12 months to show benefit, and the effect was lost when the drug was discontinued. In addition, the patients would progress to insulin dependency within 3 years, even if treatment was continued and C-peptide secretion was maintained (55). Renal and pancreatic β-cell toxicity as well as the costs of the drug and the monitoring of its levels in the blood led to a consensus that the risks outweigh the benefits. Nevertheless, cyclosporine trials provided a proof of principle that immunosupression can slow the destruction of the β-cells, even if it cannot stop it. The trials also showed that the effect of immunosupression was greatest if the intervention was started within 6 weeks of diabetes diagnosis, suggesting that β-cell mass at the initiation of immunotherapy may by a key predictor of success. Table 3 summarizes currently registered trials.
Table 3.
Tertiary prevention trials of systemic immunomodulation after diagnosis of diabetes
| Study (ref.) | Drug/phase | Sponsor/contact | Age (years) | Time from diagnosis/eligibility | Route | Dosing | Treated:placebo | Follow-up duration/primary end point | Status/target size |
|---|---|---|---|---|---|---|---|---|---|
| TTEDD (89) | Anti-CD3 (TRX4)/phase II | TolerX Inc., JDRF/http://www.tolerrx.com and clinicaltrials@tolerx.com | 18–45 | Any duration/C-peptide detectable | i.v. | 8 daily injections | All:0 | 4 years/define highest tolerated dose | Enrolling/n = 100 |
| DEFEND (59) | Anti-CD3 (otelixizumab)/phase III | TolerX Inc., JDRF/http://www.tolerrx.com and defend@tolerx.com | 18–35 | ≤12 weeks/C-peptide 0.2–3.5 pmol/ml | i.v. | 8 daily injections | 2:1 | 2 years/MMTT C-peptide | Enrolling/n = 240 |
| AbATE (90) | Anti-CD3 (teplizumab)/phase II | NIH, NIAID ITN/info@abatetrial.org | 8–30 | <8 weeks | i.v. | 14 daily injections/escalation dose; 2nd course after 12 months | 2:1/open label | 2 years/MMTT C-peptide (4-h AUC) | Enrolling/n = 81 |
| Delay (91) | Anti-CD3 (teplizumab)/phase II | NIH, NIDDK, JDRF/kevan.herold@yale.edu | 8–30 | 4–12 months/C-peptide* ≥0.2 pmol/ml | i.v. | 14 daily injections/escalation dose; 2nd course after 12 months | 1:1 | 1 year/MMTT C-peptide (4-h AUC) | Enrolling/n = 60 |
| Protégé (92) | Anti-CD3 (teplizumab)/phase II | MacroGenics, Inc., JDRF/aknesel@mmgct.com | 8–35: 12–17†, 8–11† | ≤12 weeks/C-peptide detectable | i.v. | 14 daily injections/2nd course after 6 months | 3:1 | 2 years/insulin dose + A1C MMTT C-peptide | Enrolling/n = 530 |
| TrialNet Rituximab (93) | Anti-CD20 (rituximab)/phase II | NIH, NIDDK, TrialNet, et al./diabetestrialnet.org | 8–45 | ≤12 weeks/C-peptide* ≥0.2 pmol/ml | i.v. | 4 weekly injections of 375 mg/m2 each | 2:1 | 2 years/MMTT C-peptide | Enrollment closed/n = 87 |
| START (94) | ATG/phase II | NIH, NIAID ITN/info@type1diabetestrial.org | 12–35 | ≤6 weeks/C-peptide* >0.4 pmol/ml | i.v. | 4 daily injections/escalation dose | 2:1 | 2 years/MMTT C-peptide | Enrolling/n = 66 |
| ATG (95,96) | ATG/phase II | Ministry of Health Czech Republic/frsa@medicon.cz | 15–35 | ≤6 weeks/C-peptide* ≥0.3 pmol/ml | i.v. | 4 daily injections | 1:1 | 3 years/C-peptide | Enrollment closed/n = 28 |
| TrialNet Abatacept (97) | Anti–CTLA-4 | NIH, NIDDK, TrialNet, et al./diabetestrialnet.org | 6–45 | ≤12 weeks/C-peptide* ≥0.2 pmol/ml | i.v. | 10 mg/kg monthly injections for 2 years (27 doses) | 2:1 | 2 + 2 years/MMTT C-peptide | Enrollment closed/n = 111 |
| Interferon (62) | hrIFN-α/phase II | NIH, NIDDK/kr58q@nih.gov | 3–25 | ≤6 weeks | p.o. | 5,000 or 30,000 units once daily for 1 year | 2:1 | 1 year/MMTT C-peptide | Enrollment closed/n = 81 |
| Neulasta (98) | Pegylated GCSF (pegfilgrastim)/phase I/II | JDRF, University of Florida/hallemj@peds.ufl.edu | 12–45 | ≤6 months/C-peptide ≥0.2 pmol/ml | s.c. | 6 mg weekly for 12 weeks | 1:1 | 2 years/adverse events, MMTT C-peptide | Enrolling/n = 21 |
| Anakinra (99) | IL-1r antagonist (anakinra)/phase I/II | University of Texas Southwestern Med Center/Soumya Adhikarti, MD | 6–18 | ≤1 week | s.c | Daily for 28 days | Open label | Change in EGR2 expression by PBMC, C-peptide | Enrolling/n = 15 |
| AIDA (100) | IL-1r antagonist (anakinra)/phase II/III | JDRF, Steno Diabetes Center, Oeresund Diabetes Academy/tmpo@steno.dk | 18–35 | ≤12 weeks/C-peptide* ≥0.2 pmol/ml | s.c. | 100 mg once daily for 2 years | 1:1? | 2 years/adverse events, MMTT C-peptide | Enrolling/n = 160 |
| Etanercept (61) | TNF-α inhibitor (etanercept)/phase I/II | University of Buffalo, Immunex, Amgen/tquattrin@upa.chob.edu | 7–18 | ≤4 weeks/positive islet autoantibody | s.c. | 0.4 mg/kg up to 25 mg twice weekly for 24 weeks | 1:1 | 24 weeks/change in A1C, MMTT C-peptide | Enrollment closed/n = 18 |
| Cord blood (63) | Autologous umbilical cord blood transfusion/phase I/II | JDRF, NIH, University of Florida/hallemj@peds.ufl.edu | >1 | Autologous cord blood stored | i.v. | One infusion | Open label | 2 years/MMTT C-peptide, A1C, insulin dose | Enrolling/n = 23 |
| Prochymal (101) | Adult human mesenchymal stem cells/phase II | Osiris Therapeutics, JDRF/osiris@osiris.com | 18–30 | 2–16 weeks/C-peptide detectable | i.v. | Infusion once per month for 3 months | 1:1? | 2 years/MMTT C-peptide | Enrolling/n = 60 |
| AdiStem (102) | Autologous adipose-derived stem cells/phase I/II | AdiStem Ltd./lettielucero@yahoo.com | 16–60 | ≤2 years | i.v. | One infusion | Open label | Insulin dependence/insulin dose | Enrolling/n = 30 |
| Dendritic cells (68) | Autologous dendritic cells/phase I | NIH, NIDDK, University of Pittsburgh/brian.copeman@chp.edu | 18–35 | >5 years | i.d. | Intradermal injection of cells treated ex vivo with antisense oligonucleotides | 1:1 | Adverse events | Enrolling/n = 15 |
MMTT C-peptide = area under the curve for C-peptide in response to a 2-h mixed meal tolerance test.
*Stimulated.
†Pending approval by data monitoring committee. AbATE, Autoimmunity-Blocking Antibody for Tolerance in Recently Diagnosed Type 1 Diabetes; AIDA, Anti–Interleukin-1 in Diabetes Action; ATG, anti–T-cell globulin; AUC, area under the curve; IFN, interferon; GCSF, granulocyte colony–stimulating factor; MMTT, mixed-meal tolerance test; NIAID ITN, National Institute of Allergy and Infectious Diseases Immune Tolerance Network; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIH, National Institutes of Health; PBMC, peripheral blood mononuclear cell; JDRF, Juvenile Diabetes Research Foundation; START, Study of Thymoglobulin to Arrest Newly Diagnosed Type 1 Diabetes; TNF-α, tumor necrosis factor-α; TTEDD, TRX4 Monoclonal Antibody in Type 1 Diabetes.
Monoclonal anti-CD3 antibody.
Monoclonal anti-CD3 antibody treatment has received a lot of attention. The antibody transiently activates the CD3 receptor, causes cytokine release, and ultimately blocks T-cell proliferation and differentiation; the longer-term benefits may be due to the induction of regulatory T-cells. Humanized Ortho-Kung T-cell antibody hOKT3γ1(Ala-Ala) or CHAglyCD3 anti-CD3 monoclonals, engineered to abrogate complement Fc binding, do not induce severe cytokine release syndromes, in contrast to the standard OKT3, but have been associated with fever, rash, and in some patients adenopathy, depending upon the dose (56). Reactivation of Epstein-Barr virus infection observed in some patients appears to be self-limiting with a single course of therapy (57). Two randomized, placebo-controlled phase I/II trials with hOKT3 have suggested slower decline in stimulated C-peptide, lower A1C levels, and lower insulin requirements in patients receiving hOKT3 compared with placebo (56,57). The C-peptide levels held for at least 12 months, especially in patients with higher baseline C-peptide levels, followed by a recurrence of progressive loss of C-peptide. Significant but smaller benefits in C-peptide levels persist up to 4 years after treatment with a single course of the antibody (58). To achieve better effects, this therapy will likely require repeated administration of the drug, which is being tested in several trials (Table 3) or in combination with another therapeutic agent. While the short-term follow-up results are encouraging, anti-CD3 therapy represents one of the more aggressive approaches to tertiary prevention of type 1 diabetes today, with a significant burden on patients. The typical protocol includes 2–3 screening visits, 8–12 outpatient visits on consecutive days, where patients may spend 4 h each day undergoing treatment and observation, and about 10 additional visits during the 2-year follow-up (59). With two dosing cycles 6–12 months apart, the number of visits increases to 30–40 during the initial 2 years postdiagnosis, clearly more than the 8–10 visits for insulin therapy required by current clinical standards of care.
Rituximab.
Rituximab is a monoclonal antibody that targets the CD20 receptor unique to B-cells. Rituximab inhibits the B-cell function, thus reducing presentation of autoantigen to T-cells and theoretically secondarily preventing B-cell expansion and islet autoantibody production. This medication is approved for the treatment of non-Hodgkin's lymphoma and has shown success in treatment of patients with rheumatoid arthritis. TrialNet has completed a phase II trial including 4 weekly injections of rituximab (Table 3), and the results were presented at the American Diabetes Association's Scientific Sessions in June 2009. Newly diagnosed patients with type 1 diabetes (age 8–40 years) treated with rituximab had higher C-peptide 2-h area under the curve after a mixed meal and lower A1C and insulin doses compared with the placebo group. The full results of this trial should be available shortly.
Anti–CTLA-4 Ig.
A trial of monthly infusions of anti–CTLA-4 Ig (abatacept) over a 2-year period has finished recruitment, and the results from TrialNet should be available in 2011. A high-affinity variant of CTLA-4 Ig (LEA29Y, belatacept) has been tested in islet transplantation studies and may be next in line.
Antithymocyte globulin.
Antithymocyte globulin (ATG) has been used in organ transplantation but has not yet been shown to be effective in inducing immune tolerance. ATG is produced by taking human thymus cells and injecting them into an animal such as a rabbit or horse. The animal makes multiple antibodies to the thymic antigens that are primarily but not only T-cell in origin, and then they are purified to ATG. Injected back into the subject, ATG binds to T-cells and other immune cells, causing the host to see these as foreign because of the attached antibodies and eliminate them. A small clinical trial previously showed a reduction of A1C levels and lower insulin requirements (60). However, two patients developed severe thrombocytopenia. A European trial has recently been completed using a newer form of ATG, and a phase II trial of ATG through the ITN is enrolling type 1 diabetic patients (Table 3).
Tumor necrosis factor-α, IL-1 receptor antagonist, pegylated granulocyte colony–stimulating factor, and human recombinant interferon-α.
A number of agents previously proven effective in other autoimmune diseases are being evaluated in phase I/II tertiary prevention trails of type 1 diabetes (Table 3). Tumor necrosis factor-α (TNF-α) inhibitor (etanercept) has been previously used in treatment of arthritis and Crohn's disease. A small pilot study has found increased C-peptide area under the curve and lower A1C and insulin doses in type 1 diabetic patients after 24 weeks of etanercept therapy started not more than 4 weeks after diagnosis (61). Trials of IL-1 receptor antagonist (IL-1ra) (anakinra) approved for rheumatoid arthritis and pegylated granulocyte colony–stimulating factor (GCSF) (pegfilgrasim) used for neutropenia are enrolling participants. These agents are administered subcutaneously. Oral human recombinant interferon-α (hrINF-α) has been found safe in doses of 5,000 and 30,000 units/day but slowed C-peptide loss only in the 5,000 units/day arm, a finding that requires replication (62).
Regulatory T-cells.
Cell therapy targeting regulatory T-cells (Tregs) in vivo using certain drugs can be potentially hazardous, resulting in significant side effects and “off-target” effects. Therefore, novel approaches to isolate and expand polyclonal and antigen-specific Tregs in vitro have been developed for immunotherapy. While the efficacy of Treg transfer is well established in animal models, clinical trials in new-onset type 1 diabetic patients have just begun (Table 3). Umbilical cord blood may contain higher numbers of functional populations of Tregs. An open-label trial of autologous cord blood transfusion in children with newly diagnosed type 1 diabetes is underway (63). In the future, cord blood may turn out to be a reliable source of pluripotent hematopoetic stem cells applicable to trials of islet regeneration. Two trials of adult stem cell infusions are registered in the clinicaltrials.gov database (Table 3), and more are likely to be added in the near future. Autologous stem cell transplantation, particularly from bone marrow, has been successfully used in cancer patients and is intensively discussed as a treatment option for autoimmune disorders. Autologous nonmyeloablative hematopoietic stem cell transplantation, with concomitant high-dose immunosuppression, has been reported in new-onset type 1 diabetes (64,65). During a mean follow-up of 19 months, 14 of 15 patients became insulin free, their β-cell function increased significantly, their anti-GAD antibody levels decreased, and their A1C levels were maintained at <7%. A longer follow-up (30 months) has suggested increased C-peptide levels in some of these patients (66). Nevertheless, nearly all patients suffered from transplantation-related complications, which may compromise the application of this approach to type 1 diabetes. Trials to replicate this report have been registered in several countries (67).
Dendritic cells.
Cell therapy usingdendritic cells is based on the hypothesis that these antigen-presenting cells can be modified to favor a protective phenotype rather than one favoring disease development. These strategies use“immature” dendritic cells that are in vitro derived from monocyte precursors isolated from diabetic subjects and then either modified with siRNA or an insulin peptide and then reinjected into the same individuals with the hope of resetting the immune response to islet antigens. One protocol is completing phase I safety studies (68), and the other is about to begin this year.
Islet regeneration
This topic has been recently covered by excellent reviews (e.g., 69) and is beyond the focus of the current article. However, we list current clinical trials of monotheraphy (Table 4) or combination therapy (Table 5) that include exenatide, sitaglipin, islet neogenesis–associated protein (INGAP) peptide, and pioglitazone. The promising E1-I.N.T. trial, a combination therapy containing gastrin and epidermal growth factor, will be followed by a trial of proton pump inhibitor to elevated gastrin levels combined with a glucagon-like peptide analog in one arm and further combined with GAD in another arm and will be starting soon.
Table 4.
Tertiary prevention trials after diagnosis of diabetes: islet regeneration and β-cell rest
| Study (ref.) | Drug/phase | Sponsor/contact | Age (years) | Time from diagnosis/eligibility | Route | Dosing | Treated: placebo | Follow-up duration/primary end point | Status/target size |
|---|---|---|---|---|---|---|---|---|---|
| Islet regeneration (103) | Exenatide/phase IV | NIH, Baylor College of Medicine/Rubina Heptulla, MD | 12–21 | ≥1 year | s.c. | Each patient to receive 3 different doses | 1:1 | AUC glucose | Enrollment closed/n = 17 |
| SPIRIT1 (104) | INGAP peptide/phase II | Procter & Gamble/kathleen.dungan@osumc.edu | 18–65 | Age of diagnosis <20 years/fasting C-peptide ≤0.1 pmol/ml | s.c. | 300 or 600 mg/day for 90 days | 1:1 | 6 months/Arg-stimulated C-peptide | Enrollment closed/n = 63 |
| Islet regeneration and metabolic control (105) | Pioglitazone/phase I | Stony Brook University/thomas.a.wilson@sunysb.edu | 6–18 | ≤12 week | p.o. | 1:1? | 4 months/adverse events, MMTT C-peptide | Enrolling/n = ? | |
| TrialNet metabolic control (74) | Near normoglycemia/phase II | NIH, NIDDK, TrialNet/diabetestrialnet.org | 3–20 | 1–7 days | NA | Insulin pump therapy and CGM | Open label | 2 years/MMTT C-peptide | Not yet enrolling/n = 108 |
| β-Cell rest (106) | Diazoxide/phase IV | University of Trondheim, Norway/valdemar.grill@ntnu.no | 18–40 | ≤12 weeks/C-peptide* >0.2 pmol/ml | p.o. | Daily at bedtime for 6 months | 1:1 | At least 1 year/C-peptide, A1C | Enrollment closed/n = 35 |
*Stimulated. AUC, area under the curve; CGM, continuous glucose monitoring; INGAP, islet neogenesis-associated protein; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; NIH, National Institutes of Health; SPIRIT1, Stimulation of Pancreatic Islet Regeneration In Type 1 and Type 2 diabetes.
Table 5.
Tertiary prevention trials of combination immunotherapy after diagnosis of diabetes
| Study (ref.) | Drug/phase | Sponsor/contact | Age (years) | Time from diagnosis/eligibility | Route | Dosing | Treated: placebo | Follow-up duration/primary end point | Status/target size |
|---|---|---|---|---|---|---|---|---|---|
| TrialNet MMF/DZB (107) | Mycophenolate mofetil & daclizumab/phase III | NIH, NIDDK, TrialNet/diabetestrialnet.org | 8–45 | ≤12 weeks/C-peptide* ≥0.2 pmol/ml | MMF p.o., DZB i.v. | MMF twice daily 600 mg/m2 for 2 years, DZB twice in 2 weeks 1 mg/kg up to 100 mg | 2:1 | 4 years/MMTT C-peptide | Enrollment closed/n = 126 |
| 03-DK-0245 (108) | exenatide & daclizumab/phase II | NIH, NIDDK, Amylin Pharmaceuticals/1-800-411-1222 (Amylin), prpl@mail.cc.nih.gov | 18–60 | >5 years/C-peptide* 0.3–1.2 ng/ml | Exendin-4 s.c., DZB i.v. | 2 × 2 factorial | 20 weeks | Enrollment closed/n = 16 | |
| Proleukin + rapamune (109) | hrIL-2 (aldesleukin) & sirolimus/phase I | NIH, NIAID ITN/diabetes@benaroyaresearch.org | 18–45 | 3–48 months | hrIL-2 s.c., sirolimus p.o. | hrIL-2 4.5 × 106 IU/day three times weekly for 4 weeks, sirolimus escalating dose for 12 weeks | Open label | 2 years/adverse events MMTT C-peptide | Enrolling/n = 10 |
| 09-DK-0056 (75) | Sitagliptin/lansoprazole rhGAD65 (Diamyd)/phase II | NIH, NIDDK, Diamyd Therapeutics/1-800-411-1222 (Diamyd), prpl@mail.cc.nih.gov, davidmh@intra.niddk.nih.gov | 16–30 | ≤4 months/C-peptide ≥0.2 pmol/ml | Sitagliptin p.o., rhGAD65 s.c. | MMTT C-peptide | Enrolling/n = 164 | ||
| E1-INT (110) | EGF and gastrin/phase II | Transition therapeutics/Aleksandra Pastrak, MD | 18–40 | >1 year | s.c. | Daily for 4 weeks | 3:1 | 6 months/Arg-stimulated C-peptide | Enrollment closed/n = 20 |
| Sao Paulo (64,65) | Autologous stem cell transplantation and cyclophosphamide + rabbit ATG/phase II | University of Sao Paulo, Northwestern University, Genzyme/jvoltar@fmrp.usp.br | 14–31 | ≤6 weeks | i.v. | Cyclophosphamide 200 mg/kg, rabbit ATG 4.5 mg/kg | Open label | 3 years/adverse events, insulin dose | Published/n = 20 |
| Shanghai (67) | Autologous stem cell transplantation and cyclophosphamide + rabbit ATG/phase II | Shanghai JiaoTong University/guangning@medmail.com.cn | 14–35 | ≤6 months | i.v. | Cyclophosphamide 200 mg/kg, rabbit ATG 4.5 mg/kg | Open label | 3 years/insulin dose | Enrolling/n = 30 |
*Stimulated. ATG, anti–T-cell globulin; DZB, daclizumab; EGF, epidermal growth factor; MMF, mycophenolate mofetil; NIAID ITN, National Institute of Allergy and Infectious Diseases Immune Tolerance Network.
Metabolic control and β-cell rest
Weight loss and increased physical activity (70) can neutralize the powerful effect of insulin resistance on progression to type 1 diabetes (71,72). Meticulous blood glucose control after diabetes onset resulting in β-cell rest is also believed to help preserve residual insulin secretion (73), and the TrialNet Metabolic Control Trial (74) is about to test this hypothesis.
Combination treatments
Many in the field of immunotherapy today feel that combination therapies may enhance efficacy while lowering risk and predict that one day multidrug immunotherapy will become the standard of care for newly diagnosed type 1 diabetes. Although combination treatments may be more likely to increase the risk of adverse events if chosen within the same therapeutic family, using therapies from different treatment pathways may reduce these risks. Current trials of combination immunotherapy are summarized in Table 5. Initial systemic immunosuppression followed by antigen-specific induction of tolerance or islet regeneration seems to be a logical approach and is about to be tested by a recently opened National Institute of Diabetes and Digestive and Kidney Diseases trial (108).
CONCLUSIONS AND A LOOK INTO THE FUTURE
Development of safe and effective prevention of type 1 diabetes is a major public health goal in industrialized countries today, as evidenced by strong legislative support in the U.S. in the form of the Special Statutory Funding Program (http://www.t1diabetes.nih.gov). While hundreds of preventive modalities have succeeded in animal models of type 1 diabetes (76,77), prevention of human type 1 diabetes remains elusive as of early 2009. Genetic and environmental factors that determine the relapsing-remitting course of β-cell destruction, culminating in full insulin dependence, are being discovered. In the long run, primary prevention of islet autoimmunity will likely be the optimal approach to the prevention of type 1 diabetes, especially in high-risk groups, such as first-degree relatives. However, environmental triggers of islet autoimmunity need to be better defined. Poor predictive value of the existing genetic screening tools also means that the number of children needing intervention will remain high in relation to the number of type 1 diabetes cases prevented. If a primary prevention is not feasible in the general population, mass screening for islet autoantibodies and secondary prevention may be the next option.
Once more than one islet autoantibody is present, most individuals progress to diabetes in 5–10 years. The presence of more than one islet autoantibody, combined with susceptibility HLA-DR,DQ and protein tyrosine phosphatase N22 (PTPN22) genotypes (78), helps to identify individuals with sufficiently high risk of disease to attempt prevention. However, these screening tools need further improvement to exclude individuals, particularly adults, with loss of β-cells so slow that overt diabetes will not occur during the person's lifetime. Furthermore, prediction algorithms need to be sharpened before being applied to the general population, where the majority of type 1 diabetes cases occur, yet the predictive value of genetic markers is lower than among relatives.
As patients develop autoimmunity, β-cell function declines and so does the potential therapeutic benefit of intervention. Additionally, once the autoimmune process has begun it might become progressively more difficult to alter, as suggested by animal models where antigen-specific therapies used prior to the onset of disease can be far more effective than the same treatments given at the time of disease onset. In retrospect, the DPT-1 and ENDIT trials seem somewhat speculative when viewed in the more complete context of the complexity of immunoregulation and autoimmunity we have now defined. It is something to keep in mind while extrapolating to pre-diabetes, the promising findings from prevention trials in patients with established type 1 diabetes.
Technological advantages of insulin pumps and continuous glucose monitoring influence perceived benefits of immunotherapy after diagnosis of diabetes. Multiple logistic issues remain, e.g., the anticipated duration, toxicity, and complexity of immunotherapy. Unless tolerance can be established or restored permanently in a limited time period, intervention may need to be life-long akin to gluten-free diet for celiac disease. It is currently impossible to compare the cost-to-benefit ratio of such efforts with those of the established and emerging insulin treatment regimens. It is, however, important to keep in mind that insulin therapy, while not easy or complication free, has led to a dramatic improvement of the mortality and morbidity associated with type 1 diabetes over the past 20–30 years (79–81).
Although more targeted antibody therapies are being used, these agents are still relatively nonspecific and potentially toxic to some trial participants. Currently used systemic immunomodulators may carry a risk of long-term complications that is unacceptable for type 1 diabetes prevention. However, this work is important because even with successful primary or secondary prevention programs there will always be patients who develop clinical type 1 diabetes.
Diabetes prevention research is expanding at an unprecedented rate. The history of diabetes is filled with many groundbreaking discoveries. If the past performance does predict future returns, the prevention of type 1 diabetes has a bright future.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 1753.
References
- 1. Bresson D, von Herrath M: Immunotherapy for the prevention and treatment of type 1 diabetes: optimizing the path from bench to bedside. Diabetes Care 2009;32:1753–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rewers M, Norris J, Kretowski A: Epidemiology of type 1 diabetes mellitus. In Type 1 Diabetes: Cellular, Molecular & Clinical Immunology. Eisenbarth GS. Ed. Online edition, version 3.0. Available from http://www.uchsc.edu/misc/diabetes/books/type1/type1_ch9.html. Accessed 15 January 2009 [Google Scholar]
- 3. Diabetes Epidemiology Research International Group. Secular trends in incidence of childhood IDDM in 10 countries. Diabetes 1990;39:858–864 [PubMed] [Google Scholar]
- 4. Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, Laporte R, Tuomilehto J: Incidence of childhood type 1 diabetes worldwide: Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000;23:1516–1526 [DOI] [PubMed] [Google Scholar]
- 5. Harjutsalo V, Sjoberg L, Tuomilehto J: Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 2008;371:1777–1782 [DOI] [PubMed] [Google Scholar]
- 6. Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith G, Bloch C, Rewers M, Dabelea D: Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care 2007;30:503–509 [DOI] [PubMed] [Google Scholar]
- 7. TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007;8:286–298 [DOI] [PubMed] [Google Scholar]
- 8. TEDDY Study Group. The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann N Y Acad Sci 2008;1150:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bingley PJ, Bonifacio E, Williams AJ, Genovese S, Bottazzo GF, Gale EA: Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes 1997;46:1701–1710 [DOI] [PubMed] [Google Scholar]
- 10. Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, Rewers M: Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA 2003;290:1713–1720 [DOI] [PubMed] [Google Scholar]
- 11. Achenbach P, Warncke K, Reiter J, Williams AJ, Ziegler AG, Bingley PJ, Bonifacio E: Type 1 diabetes risk assessment: improvement by follow-up measurements in young islet autoantibody-positive relatives. Diabetologia 2006;49:2969–2976 [DOI] [PubMed] [Google Scholar]
- 12. Kupila A, Muona P, Simell T, Arvilommi P, Savolainen H, Hamalainen AM, Korhonen S, Kimpimaki T, Sjoroos M, Ilonen J, Knip M, Simell O: Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia 2001;44:290–297 [DOI] [PubMed] [Google Scholar]
- 13. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med 2002;346:1685–1691 [DOI] [PubMed] [Google Scholar]
- 14. Gale EA, Bingley PJ, Emmett CL, Collier T: European Nicotinamide Diabetes Intervention Trial (ENDIT): a randomised controlled trial of intervention before the onset of type 1 diabetes. Lancet 2004;363:925–931 [DOI] [PubMed] [Google Scholar]
- 15. Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E: Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care 2005;28:1068–1076 [DOI] [PubMed] [Google Scholar]
- 16. Nanto-Salonen K, Kupila A, Simell S, Siljander H, Salonsaari T, Hekkala A, Korhonen S, Erkkola R, Sipila JI, Haavisto L, Siltala M, Tuominen J, Hakalax J, Hyoty H, Ilonen J, Veijola R, Simell T, Knip M, Simell O: Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 2008;372:1746–1755 [DOI] [PubMed] [Google Scholar]
- 17. Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS: Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2006;29:643–649 [DOI] [PubMed] [Google Scholar]
- 18. Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS: Increasing the accuracy of oral glucose tolerance testing and extending its application to individuals with normal glucose tolerance for the prediction of type 1 diabetes: the Diabetes Prevention Trial-Type 1. Diabetes Care 2007;30:38–42 [DOI] [PubMed] [Google Scholar]
- 19. Stene LC, Barriga K, Hoffman M, Kean J, Klingensmith G, Norris JM, Erlich HA, Eisenbarth GS, Rewers M: Normal but increasing hemoglobin A1c levels predict progression from islet autoimmunity to overt type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). Pediatr Diabetes 2006;7:247–253 [DOI] [PubMed] [Google Scholar]
- 20. Steffes MW, Sibley S, Jackson M, Thomas W: Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–836 [DOI] [PubMed] [Google Scholar]
- 21. Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW: C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve β-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 2004;53:250–264 [DOI] [PubMed] [Google Scholar]
- 22. Sherry NA, Tsai EB, Herold KC: Natural history of beta-cell function in type 1 diabetes. Diabetes 2005;54(Suppl. 2):S32–S39 [DOI] [PubMed] [Google Scholar]
- 23. Study design of the Trial to Reduce IDDM in the Genetically at Risk (TRIGR). Pediatr Diabetes 2007;8:117–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmid S, Buuck D, Knopff A, Bonifacio E, Ziegler AG: BABYDIET, a feasibility study to prevent the appearance of islet autoantibodies in relatives of patients with type 1 diabetes by delaying exposure to gluten. Diabetologia 2004;47:1130–1131 [DOI] [PubMed] [Google Scholar]
- 25. Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, Orton HD, Baron AE, Clare-Salzler M, Chase HP, Szabo NJ, Erlich H, Eisenbarth GS, Rewers M: Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA 2007;298:1420–1428 [DOI] [PubMed] [Google Scholar]
- 26. Pre-POINT [clinical trial registry]. Available at http://www.diabetes-point.org/nav2uk.html. Accessed 1 February 2009
- 27. The Nutritional Intervention to Prevent Type 1 Diabetes (NIP) Pilot Study [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00333554. Accessed 1 February 2009
- 28. Wasserfall C, Atkinson MA: Taking a daily vitamin to prevent type 1 diabetes? Diabetes 2009;58:24–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baeke F, van EE, Gysemans C, Overbergh L, Mathieu C: Vitamin D signaling in immune-mediated disorders: evolving insights and therapeutic opportunities. Mol Aspects Med 2008;29:376–387 [DOI] [PubMed] [Google Scholar]
- 30. Paterson CR: Vitamin-D poisoning: survey of causes in 21 patients with hypercalcaemia. Lancet 1980;1:1164–1165 [DOI] [PubMed] [Google Scholar]
- 31. Feasibility study of 2000 IU per day of vitamin D for the primary prevention of type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/record/NCT00141986. Accessed 1 February 2009
- 32. Aly TA, Ide A, Jahromi MM, Barker JM, Fernando MS, Babu SR, Yu L, Miao D, Erlich HA, Fain PR, Barriga KJ, Norris JM, Rewers MJ, Eisenbarth GS: Extreme genetic risk for type 1A diabetes. Proc Natl Acad Sci U S A 2006;103:14074–14079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oral insulin for prevention of diabetes in relatives at risk for type 1 diabetes mellitus [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/record/NCT00419562. Accessed 1 February 2009
- 34. Trial of intranasal insulin in children and young adults at risk of type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/record/NCT00336674. Accessed 1 February 2009
- 35. Barker JM, Goehrig SH, Barriga K, Hoffman M, Slover R, Eisenbarth GS, Norris JM, Klingensmith GJ, Rewers M: Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care 2004;27:1399–1404 [DOI] [PubMed] [Google Scholar]
- 36. Gepts W, De MJ: Islet cell survival determined by morphology: an immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes 1978;27(Suppl. 1):251–261 [DOI] [PubMed] [Google Scholar]
- 37. Martin S, Pawlowski B, Greulich B, Ziegler AG, Mandrup-Poulsen T, Mahon J: Natural course of remission in IDDM during 1st yr after diagnosis. Diabetes Care 1992;15:66–74 [DOI] [PubMed] [Google Scholar]
- 38. Schiffrin A, Suissa S, Weitzner G, Poussier P, Lalla D: Factors predicting course of beta-cell function in IDDM. Diabetes Care 1992;15:997–1001 [DOI] [PubMed] [Google Scholar]
- 39. Knip M, Ilonen J, Mustonen A, Akerblom HK: Evidence of an accelerated B-cell destruction in HLA-Dw3/Dw4 heterozygous children with type 1 (insulin-dependent) diabetes. Diabetologia 1986;29:347–351 [DOI] [PubMed] [Google Scholar]
- 40. Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC: Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 2005;48:2221–2228 [DOI] [PubMed] [Google Scholar]
- 41. Karges B, Durinovic-Bello I, Heinze E, Boehm BO, Debatin KM, Karges W: Complete long-term recovery of β-cell function in autoimmune type 1 diabetes after insulin treatment. Diabetes Care 2004;27:1207–1208 [DOI] [PubMed] [Google Scholar]
- 42. Greenbaum CJ, Harrison LC: Guidelines for intervention trials in subjects with newly diagnosed type 1 diabetes. Diabetes 2003;52:1059–1065 [DOI] [PubMed] [Google Scholar]
- 43. Bekris LM, Jensen RA, Lagerquist E, Hall TR, Agardh CD, Cilio CM, Lethagen AL, Lernmark A, Robertson JA, Hampe CS: GAD65 autoantibody epitopes in adult patients with latent autoimmune diabetes following GAD65 vaccination. Diabet Med 2007;24:521–526 [DOI] [PubMed] [Google Scholar]
- 44. Agardh CD, Lynch KF, Palmer M, Link K, Lernmark A: GAD65 vaccination: 5 years of follow-up in a randomised dose-escalating study in adult-onset autoimmune diabetes. Diabetologia 2009;52:1363–1368 [DOI] [PubMed] [Google Scholar]
- 45. Ludvigsson J, Faresjo M, Hjorth M, Axelsson S, Cheramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R: GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 46. Alleva DG, Maki RA, Putnam AL, Robinson JM, Kipnes MS, Dandona P, Marks JB, Simmons DL, Greenbaum CJ, Jimenez RG, Conlon PJ, Gottlieb PA: Immunomodulation in type 1 diabetes by NBI-6024, an altered peptide ligand of the insulin B epitope. Scand J Immunol 2006;63:59–69 [DOI] [PubMed] [Google Scholar]
- 47. Walter M, Philotheou A, Bonnici F, Ziegler AG, Jimenez R: No effect of the altered-peptide ligand NBI-6024 on β-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thrower SL, James L, Hall W, Green KM, Arif S, Allen JS, Van-Krinks C, Lozanoska-Ochser B, Marquesini L, Brown S, Wong FS, Dayan CM, Peakman M: Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man phase I safety study. Clin Exp Immunol 2009;155:156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR: Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet 2001;358:1749–1753 [DOI] [PubMed] [Google Scholar]
- 50. Lazar L, Ofan R, Weintrob N, Avron A, Tamir M, Elias D, Phillip M, Josefsberg Z: Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: a randomised, double-blind phase II study. Diabete Metab Res Rev 2007;23:286–291 [DOI] [PubMed] [Google Scholar]
- 51. Schloot NC, Meierhoff G, Lengyel C, Vandorfi G, Takacs J, Panczel P, Barkai L, Madacsy L, Oroszlan T, Kovacs P, Suto G, Battelino T, Hosszufalusi N, Jermendy G: Effect of heat shock protein peptide DiaPep277 on beta-cell function in paediatric and adult patients with recent-onset diabetes mellitus type 1: two prospective, randomized, double-blind phase II trials. Diabetes Metab Res Rev 2007;23:276–285 [DOI] [PubMed] [Google Scholar]
- 52. Staeva-Vieira T, Peakman M, von Herrath M: Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol 2007;148:17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Feutren G, Papoz L, Assan R, Vialettes B, Karsenty G, Vexiau P, Du RH, Rodier M, Sirmai J, Lallemand A: Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset: results of a multicentre double-blind trial. Lancet 1986;2:119–124 [DOI] [PubMed] [Google Scholar]
- 54. Canadian-European Randomized Control Trial Group. Cyclosporin-induced remission of IDDM after early intervention: association of 1 yr of cyclosporin treatment with enhanced insulin secretion. Diabetes 1988;37:1574–1582 [PubMed] [Google Scholar]
- 55. Bougneres PF, Landais P, Boisson C, Carel JC, Frament N, Boitard C, Chaussain JL, Bach JF: Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes 1990;39:1264–1272 [DOI] [PubMed] [Google Scholar]
- 56. Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA: Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 57. Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De BC, Seigneurin JM, De PP, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L: Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 58. Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA: A single course of anti-CD3 monoclonal antibody hOKT3γ1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 2005;54:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Phase 3 trial of Otelixizumab for adults with newly diagnosed type 1 (autoimmune) diabetes mellitus: DEFEND-1 [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00678886. Accessed 1 February 2009
- 60. Eisenbarth GS, Srikanta S, Jackson R, Rabinowe S, Dolinar R, Aoki T, Morris MA: Anti-thymocyte globulin and prednisone immunotherapy of recent onset type 1 diabetes mellitus. Diabetes Res 1985;2:271–276 [PubMed] [Google Scholar]
- 61. Mastrandrea L, Yu J, Behrens T, Buchlis J, Albini C, Fourtner S, Quattrin T: Etanercept treatment in children with new onset type 1 diabetes: pilot randomized, placebo-controlled, double blind study. Diabetes Care 2009;32:1244–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rother KI, Brown RJ, Morales MM, Wright E, Duan Z, Campbell C, Harlan DM, Orlander PR, Brod SA: Effect of ingested interferon-α on β-cell function in children with new-onset type 1 diabetes. Diabetes Care 2009;32:1250–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Haller MJ, Wasserfall C, McGrail K, Viener HL, Cintron M, Gaitan S, Brusko T, Wingard J, Cogle C, Kelly S, Slayton W, Atkinson MA, Schatz DA: Autologous umbilical cord blood transfusion in very young children with T1D: 1 year follow-up (Abstract). Diabetes 2009;58(Suppl. 1):A7 [Google Scholar]
- 64. Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simoes BP, Foss MC, Squiers E, Burt RK: Autologous hematopoietic stem cell transplantation for type 1 diabetes. Ann N Y Acad Sci 2008;1150:220–229 [DOI] [PubMed] [Google Scholar]
- 65. Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, Coutinho M, Malmegrim KC, Foss-Freitas MC, Simoes BP, Foss MC, Squiers E, Burt RK: Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2007;297:1568–1576 [DOI] [PubMed] [Google Scholar]
- 66. Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, Madeira MI, Malmegrim KC, Foss-Freitas MC, Simoes BP, Martinez EZ, Foss MC, Burt RK, Voltarelli JC: C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009;301:1573–1579 [DOI] [PubMed] [Google Scholar]
- 67. Autologous hematopoietic stem cell transplantation for early onset type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00807651. Accessed 16 February 2009
- 68. Autologous dendritic cell therapy for type 1 diabetes suppression: a safety study [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00445913. Accessed 1 February 2009
- 69. Akirav E, Kushner JA, Herold KC: β-Cell mass and type 1 diabetes: going, going, gone? Diabetes 2008;57:2883–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baughcum AE, Johnson SB, Carmichael SK, Lewin AB, She JX, Schatz DA: Maternal efforts to prevent type 1 diabetes in at-risk children. Diabetes Care 2005;28:916–921 [DOI] [PubMed] [Google Scholar]
- 71. Fourlanos S, Narendran P, Byrnes GB, Colman PG, Harrison LC: Insulin resistance is a risk factor for progression to type 1 diabetes. Diabetologia 2004;47:1661–1667 [DOI] [PubMed] [Google Scholar]
- 72. Xu P, Cuthbertson D, Greenbaum C, Palmer JP, Krischer JP: Role of insulin resistance in predicting progression to type 1 diabetes. Diabetes Care 2007;30:2314–2320 [DOI] [PubMed] [Google Scholar]
- 73. Brown RJ, Rother KI: Effects of beta-cell rest on beta-cell function: a review of clinical and preclinical data. Pediatr Diabetes 2008;9:14–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Effect of metabolic control at onset of diabetes on progression of type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00505206. Accessed 16 February 2009
- 75. Novel therapy combining regenerative stimuli immunomodulation to preserve beta cell function in new onset type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00837759. Accessed 16 February 2009
- 76. Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, Eisenbarth GS, Mathis D, Rossini AA, Campbell SE, Kahn R, Kreuwel HT: A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity 2005;23:115–126 [DOI] [PubMed] [Google Scholar]
- 77. Atkinson MA: Thirty years of investigating the autoimmune basis for type 1 diabetes: why can't we prevent or reverse this disease? Diabetes 2005;54:1253–1263 [DOI] [PubMed] [Google Scholar]
- 78. Steck AK, Zhang W, Bugawan TL, Barriga KJ, Blair A, Erlich HA, Eisenbarth GS, Norris JM, Rewers MJ: Do non-HLA genes influence development of persistent islet autoimmunity and type 1 diabetes in children with high-risk HLA-DR,DQ genotypes? Diabetes 2009;58:1028–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ: The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469 [DOI] [PubMed] [Google Scholar]
- 80. Nishimura R, LaPorte RE, Dorman JS, Tajima N, Becker D, Orchard TJ: Mortality trends in type 1 diabetes: the Allegheny County (Pennsylvania) Registry 1965–1999. Diabetes Care 2001;24:823–827 [DOI] [PubMed] [Google Scholar]
- 81. Hovind P, Tarnow L, Rossing K, Rossing P, Eising S, Larsen N, Binder C, Parving HH: Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care 2003;26:1258–1264 [DOI] [PubMed] [Google Scholar]
- 82. Wicklow BA, Taback SP: Feasibility of a type 1 diabetes primary prevention trial using 2000 IU vitamin D3 in infants from the general population with increased HLA-associated risk. Ann N Y Acad Sci 2006;1079:310–312 [DOI] [PubMed] [Google Scholar]
- 83. Effects of recombinant human glutamic acid decarboxylase (rhGAD65) formulated in Alum (GAD-Alum) on the progression of type 1 diabetes in new onset subjects [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00529399. Accessed 16 February 2009
- 84. A phase III study to investigate the impact of diamyd in patients newly diagnosed with type 1 diabetes (USA) [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00751842. Accessed 16 February 2009
- 85. A phase III study to investigate the impact of diamyd in patients newly diagnosed with type 1 diabetes (EU) [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00723411. Accessed 16 February 2009
- 86. Evaluation of a diabetes vaccine in newly diagnosed diabetics [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00057499. Accessed 16 February 2009
- 87. Phase 1 study of BHT-3021 in subjects with type 1 diabetes mellitus [clinical trial registry]. Available from http://clinicaltrials.gov/ct2/show/NCT00453375. Accessed 16 February 2009
- 88. Efficacy study of DiaPep277 in newly diagnosed type 1 diabetes patients (DIA-AID) [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00615264. Accessed 16 February 2009
- 89. TRX4 monoclonal antibody in type 1 diabetes (T1 DM) (TTEDD) [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00451321. Accessed 1 February 2009
- 90. Autoimmunity-blocking antibody for tolerance in recently diagnosed type 1 diabetes (AbATE) [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00129259. Accessed 1 February 2009
- 91. Anti-CD3 mAb treatment of recent onset type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00378508. Accessed 1 February 2009
- 92. The Protégé Study: clinical trial of MGA031 in children and adults with recent-onset type 1 diabetes mellitus [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00385697. Accessed 1 February 2009
- 93. Effects of rituximab on the progression of type 1 diabetes in new onset subjects [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00279305. Accessed 1 February 2009
- 94. Study of Thymoglobulin to Arrest Newly Diagnosed Type 1 Diabetes (START) [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00515099. Accessed 1 February 2009
- 95. Saudek F, Havrdova T, Boucek P, Karasova L, Novota P, Skibova J: Polyclonal anti-T-cell therapy for type 1 diabetes mellitus of recent onset. Rev Diabet Stud 2004;1:80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Polyclonal anti-T-lymphocyte globulin (ATG) in type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00190502. Accessed 1 February 2009
- 97. Intravenous CTLA4-lg treatment in recent onset type 1 diabetes mellitus [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00505375. Accessed 1 February 2009
- 98. Neulasta in type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00662519. Accessed 1 February 2009
- 99. Anti-inflammatory therapy with anakinra in newly diagnosed type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00645840. Accessed 1 February 2009
- 100. Anti-Interleukin-1 in Diabetes Action (AIDA) [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00711503. 16 February 2009
- 101. Prochymal (human adult stem cells) for the treatment of recently diagnosed type 1 diabetes mellitus (T1DM) [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00690066. Accessed 16 February 2009
- 102. Safety and efficacy of autologous adipose-derived stem cell transplantation in patients with type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00703599. 16 February 2009
- 103. Role of exenatide in type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00456300. Accessed 16 February 2009
- 104. Safety and efficacy of INGAP-peptide in patients with type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00071409. Accessed 16 February 2009
- 105. Effect of pioglitazone on the course of new onset type 1 diabetes mellitus [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00545857. Accessed 16 February 2009
- 106. Efficacy of diazoxide in type 1 diabetes [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00131755. Accessed 16 February 2009
- 107. New onset of type 1 diabetes mycophenolate mofetil-daclizumab clinical trial [clinical trial registry]. Available at http://clinicaltrials.gov/ct2/show/NCT00100178. Accessed 16 February 2009
- 108. Effect of AC2993 With or Without Immunosuppression on Beta Cell Function in Patients With Type I Diabetes. http://clinicaltrials.gov/ct2/show/NCT00064714. 2–16-2009
- 109. Proleukin and Rapamune in Type 1 Diabetes. http://clinicaltrials.gov/ct2/show/NCT00525889. 2–16-2009
- 110. A Study in Type 1 Diabetic Patients With Repeated Doses of E1 in Combination with G1. http://clinicaltrials.gov/ct2/show/NCT00239148. 2-16-2009