Abstract
OBJECTIVE
Albuminuria and impaired glomerular filtration rate (GFR) are each associated with poor health outcomes among individuals with diabetes. Joint associations of albuminuria and impaired GFR with mortality have not been comprehensively evaluated in this population.
RESEARCH DESIGN AND METHODS
This is a cohort study among Cardiovascular Health Study participants with diabetes, mean age 78 years. GFR was estimated using serum cystatin C and serum creatinine. Albumin-to-creatinine ratio (ACR) was measured in single-voided urine samples.
RESULTS
Of 691 participants, 378 died over 10 years of follow-up. Cystatin C–estimated GFR <60 ml/min per 1.73 m2, creatinine-based estimated GFR <60 ml/min per 1.73 m2, and urine ACR ≥30 mg/g were each associated with increased mortality risk with hazard ratios of 1.73 (95% CI 1.37–2.18), 1.54 (1.21–1.97), and 1.73 (1.39–2.17), respectively, adjusting for age, sex, race, diabetes duration, hypoglycemic medications, hypertension, BMI, smoking, cholesterol, lipid-lowering medications, prevalent cardiovascular disease (CVD), and prevalent heart failure. Cystatin C–estimated GFR and urine ACR were additive in terms of mortality risk. Cystatin C–estimated GFR predicted mortality more strongly than creatinine-based estimated GFR.
CONCLUSIONS
Albuminuria and impaired GFR were independent, additive risk factors for mortality among older adults with diabetes. These findings support current recommendations to regularly assess both albuminuria and GFR in the clinical care of patients with diabetes; a focus on interventions to prevent or treat CVD in the presence of albuminuria, impaired GFR, or both; and further consideration of cystatin C use in clinical care.
Kidney disease is a major complication of diabetes that markedly increases risk of cardiovascular disease (CVD) and mortality. Albuminuria, which is believed to reflect hemodynamic disturbances within the glomerulus, has long been identified as a major prognostic indicator in individuals with diabetes (1,2). Impaired glomerular filtration rate (GFR), a complementary sign of kidney damage, is also associated with increased risk (2,3). As a result, screening for kidney disease using urinary albumin and serological markers of kidney function has become a cornerstone of diabetes care, facilitating targeted interventions to prevent CVD and kidney disease progression (4).
Albuminuria and impaired GFR may provide additive prognostic information regarding health outcomes in diabetes. However, the joint association of albuminuria and impaired GFR with mortality has not been defined in this setting, because few studies have evaluated albuminuria and GFR simultaneously, studies that have reached different conclusions (3,5), and GFR has only been estimated using serum creatinine. GFR estimated from serum cystatin C may reflect kidney function more precisely than GFR estimated using traditional serum creatinine-based methods and is more strongly linked with adverse health outcomes in community-based, mostly nondiabetic populations (6,7).
We examined associations of cystatin C–estimated GFR, creatinine-estimated GFR, change in estimated GFR over time, and albuminuria with mortality among older adults with diabetes. The relationship of kidney disease with mortality may be particularly important among older adults. In the general population, traditional cardiovascular risk factors predict CVD weakly in older compared with younger adults, and the prognostic value of kidney disease is increased (8). Our aim was to assess whether cystatin C–estimated GFR would provide additive prognostic information to urine albumin excretion and to compare the strength of the association with mortality with that of creatinine-estimated GFR.
RESEARCH DESIGN AND METHODS
The Cardiovascular Health Study (CHS) is a prospective, community-based cohort designed to study risk factors for the development and progression of CVD in individuals aged ≥65 years (9). Participants were recruited from four U.S. communities: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. Eligible participants were sampled using Medicare eligibility lists, were not institutionalized, and were expected to remain in the area for at least 3 years. Individuals who were wheelchair-bound in the home or receiving hospice treatment, radiation therapy, or chemotherapy for cancer were excluded. The original CHS cohort of 5,201 participants was enrolled in 1989–1990, with an additional 687 predominantly African American participants enrolled in 1992–1993. Urine albumin was first measured in 1996–1997.
The current study includes CHS participants who in 1996–1997 had diabetes as well as measurements of serum cystatin C, serum creatinine, and urine albumin. At this examination, blood glucose was measured after a 12-h fast and again 2 h after a 75-g oral glucose load. Diabetes was defined as use of insulin or oral hypoglycemic agents, fasting blood glucose ≥126 mg/dl, and/or 2-h postchallenge blood glucose ≥200 mg/dl (4). Measurements of serum cystatin C, serum creatinine, and urine albumin were available for 691 of 814 participants meeting the criteria for diabetes (85%). (A full list of the principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.)
Measurements of kidney disease
Serum cystatin C was measured using a BNII nephelometer (N Latex Cystatin C; Dade Behring, Deerfield, IL). Coefficients of variation ranged from 2.0 to 2.8% (intra-assay) and from 2.3 to 3.1% (interassay). GFR was estimated from cystatin C (eGFRCYS) using 1996–1997 (baseline) measurements and the following equation: eGFRCYS = 76.7 × [cystatin C]−1.18 (10). Previous change in eGFRCYS over time was calculated for each participant using additional measurements from 1989 to 1990 and/or 1992 to 1993 and linear regression (11). Serum creatinine was measured in 1996–1997 (baseline) with the Kodak Ektachem 700 Analyzer (Eastman Kodak, Rochester, NY), which uses a colorimetric method. The mean coefficient of variation was 1.94% (range 1.16–3.60). Serum creatinine was calibrated to Cleveland Clinic values using indirect calibration to National Health and Nutrition Examination Survey (NHANES) III data and then was used to estimate GFR with the four-variable Modification of Diet in Renal Disease (MDRD) formula (12). Estimated GFR was evaluated as both a continuous and a dichotomous variable (< or >60 ml/min per 1.73 m2) (13). Urine was collected as a single morning void. Urine albumin was measured by rate nephelometry using the Array 360 CE Protein Analyzer (Beckman Instruments, Fullerton, CA). Urine creatinine was measured using a Kodak Ektachem 700 Analyzer. Albuminuria was quantified as urine albumin-to-creatinine ratio (ACR), expressed in milligrams per gram and evaluated as both a continuous (log-transformed) and a categorical variable, defined using a threshold of 30 mg/g (4,13).
Ascertainment of mortality
All-cause mortality was ascertained by examination of death certificates, inpatient records, nursing home or hospice records, physician questionnaires, and autopsy reports (6,7,11). For this study, participants were followed from their 1996–1997 visit through 30 June 2006. CVD mortality, defined as death from coronary heart disease, myocardial infarction, sudden cardiac death, or stroke, was evaluated as a secondary outcome.
Covariates
Covariates were measured at the 1996–1997 CHS study visit. Race (Caucasian or African American) and smoking status were defined by self-report. Hypoglycemic medication use was classified as none, oral medications only, or insulin (with or without oral medications) (14). Prevalent CVD was defined as diagnoses of coronary heart disease, stroke, or transient ischemic attack. Hypertension was defined as use of antihypertensive medications, systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg (15). Total cholesterol was measured using standard enzymatic methods. C-reactive protein was measured with an enzyme-linked immunosorbent assay developed in the CHS laboratory. Duration of diabetes was defined as <4 years if the definition of diabetes above was first met at the 1996–1997 CHS study visit (4). Among participants who entered the CHS in 1989–1990, duration of diabetes was classified as ≥7 years if the definition of diabetes above was met at the 1989–1990 CHS study visit and 4–7 years if it was first met at the 1992–93 CHS visit. Among participants who entered in 1992–1993 and had diabetes at that time, duration was classified as ≥7 years if duration was self-reported as >3 years, and otherwise classified as 4–7 years.
Statistical analysis
We examined the distribution of demographic characteristics, traditional cardiovascular risk factors, prevalent CVD, and laboratory measures by level of eGFRCYS (< or >60 ml/min per 1.73 m2) (13). We generated descriptive summary statistics for each category using means ± SD (or median and interquartile range) for continuous variables and proportions for dichotomous variables. Within categories of kidney disease defined by estimated GFR or urine ACR, we examined mortality rates per 100 person-years. Cox proportional hazards models were used to assess associations of kidney disease with all-cause and cardiovascular mortality in unadjusted and adjusted models. Covariates were selected on the basis of their biological plausibility to confound associations of kidney disease and mortality. The fully adjusted model included age, sex, race, diabetes duration, hypoglycemia medications, hypertension, BMI, smoking, cholesterol, lipid-lowering medications, prevalent CVD, and prevalent heart failure. Interaction of estimated GFR with urine ACR was tested on the multiplicative scale using the likelihood ratio test and on the additive scale using the synergy index. Cumulative survival by eGFRCYS and urine ACR was plotted using the Kaplan-Meier method and compared using the log-rank test.
The ability of the models to discriminate between participants with and without all-cause mortality was estimated by computing the c-index, a generalization of the c-statistic or the area under the curve, which allows for censored data. Because prediction models commonly show overly optimistic performance (i.e., overfitting) the c-index was computed with bootstrap sampling using the Design library in S-PLUS. We also estimated the relative contribution to global risk by each individual measure of kidney function using the variable and model likelihood ratio χ2 statistic. All analyses were performed using S-Plus (release 8.0; Insightful, Seattle, WA) and SPSS statistical software (release 15.0.1.1; SPSS, Chicago, IL).
RESULTS
Baseline characteristics
In 1996–1997 (baseline for this study), mean age was 77 years, 58% of participants were female, and 19% were African American (Table 1). Participants with lower GFR estimated from cystatin C (eGFRCYS) were slightly older and were more likely to be male, to have CVD, to smoke, and to have hypertension (Table 1). eGFRCYS was weakly correlated with urine ACR (Pearson ρ = −0.267). Of the participants, 27% with eGFRCYS ≥ 60 ml/min per 1.73 m2 and 47% of those with eGFRCYS < 60 ml/min per 1.73 m2 had urine ACR ≥30 mg/g. eGFRCYS was highly correlated with MDRD-estimated GFR (eGFRMDRD, ρ = 0.762). eGFRMDRD was <60 ml/min per 1.73 m2 for 151 participants (22%).
Table 1.
Baseline characteristics of 691 CHS participants with diabetes by serum cystatin C–estimated GFR
| Estimated GFR |
||
|---|---|---|
| ≥60 ml/min per 1.73 m2 | <60 ml/min per 1.73 m2 | |
| n | 467 | 224 |
| Cystatin C–estimated GFR (ml/min per 1.73 m2) | 80 ± 16 | 45 ± 10 |
| Medical history | ||
| Age (years) | 77 ± 4 | 79 ± 5 |
| Sex (female) | 284 (61) | 116 (52) |
| Race (African American) | 97 (21) | 37 (17) |
| Duration of diabetes | ||
| <4 years | 189 (41) | 88 (39) |
| 4–7 years | 60 (13) | 21 (9) |
| ≥7 years | 218 (47) | 115 (51) |
| CVD | 150 (32) | 101 (45) |
| Coronary heart disease | 124 (27) | 83 (37) |
| Stroke | 31 (7) | 24 (11) |
| Transient ischemic attack | 20 (4) | 15 (7) |
| Congestive heart failure | 43 (9) | 49 (22) |
| Smoking | 232 (50) | 122 (56) |
| Hypertension | 280 (60) | 156 (70) |
| Medications | ||
| Hypoglycemic medications | ||
| None | 235 (50) | 118 (53) |
| Oral medications only | 183 (39) | 65 (29) |
| Insulin | 49 (11) | 41 (18) |
| Antihypertensive medications | 321 (69) | 186 (83) |
| ACE inhibitors | 94 (20) | 78 (35) |
| Lipid-lowering medications | 61 (13) | 40 (18) |
| Physical measurements | ||
| BMI (kg/m2) | 28.2 ± 5.0 | 28.9 ± 4.9 |
| Systolic blood pressure (mmHg) | 137 ± 20 | 139 ± 20 |
| Diastolic blood pressure (mmHg) | 69 ± 12 | 68 ± 11 |
| Laboratory measurements | ||
| Total cholesterol (mg/dl) | 201 ± 41 | 198 ± 43 |
| C-reactive protein (mg/l) | 3.31 (1.48–6.64) | 4.18 (2.00–7.88) |
| MDRD estimated GFR | ||
| Mean ± SD (ml/min per 1.73 m2) | 91 ± 22 | 59 ± 17 |
| <60 ml/min per 1.73 m2 | 27 (6) | 124 (55) |
| Change in cystatin C–estimated GFR (ml/min per 1.73 m2/year) | −1.25 ± 2.27 | −2.85 ± 2.71 |
| Urine ACR (mg/g) | ||
| Median (interquartile range) (mg/g)* | 11 (6–32) | 24 (10–111) |
| ≥30 mg/g | 124 (27) | 105 (47) |
Data are means ±SD, n (%), or median (interquartile range).
Mortality
Over 10 years of follow-up, 378 participants (55%) died. The mortality rate was 7.7 per 100 person-years, and median survival was 8.8 years.
Cystatin C versus creatinine
Lower eGFRCYS and lower eGFRMDRD were each associated with increased risk of mortality (Table 2). The association of each GFR estimate with mortality was monotonic and roughly linear (supplementary Fig. 1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-0191/DC1). However, the magnitude of association with mortality was modestly greater for eGFRCYS compared with eGFRMDRD, whether estimated GFR was evaluated as a categorical or continuous variable (Table 2). Results were similar for all-cause and cardiovascular mortality. GFR <60 ml/min per 1.73 m2 estimated from cystatin C was associated with a 73% increased risk of mortality after full adjustment (P < 0.001). When both eGFRCYS and eGFRMDRD were included simultaneously in the fully adjusted model, eGFRCYS but not eGFRMDRD was associated with increased risk of mortality: hazard ratios (HRs) 1.21 (95% CI 1.10–1.33) and 1.00 (0.94–1.08) per 10 ml/min per 1.73 m2, respectively. Results were not altered by inclusion of ACE inhibitor use as a covariate or evaluation of systolic and diastolic blood pressures as continuous variables.
Table 2.
Associations of kidney disease with mortality among 691 CHS participants with diabetes
| Total mortality |
CV mortality |
|||||||
|---|---|---|---|---|---|---|---|---|
| Events (n) | Mortality rate* | Relative risk |
Events (n) | Mortality rate* | Relative risk |
|||
| Model 1† | Model 2‡ | Model 1† | Model 2‡ | |||||
| Cystatin C–estimated GFR | ||||||||
| ≥60 ml/min per 1.73 m2 | 217 | 6.0 | 1.00 (ref) | 1.00 (ref) | 94 | 2.6 | 1.00 (ref) | 1.00 (ref) |
| <60 ml/min per 1.73 m2 | 161 | 12.2 | 1.81 (1.45–2.25) | 1.73 (1.37–2.18) | 75 | 5.7 | 2.00 (1.45–2.77) | 1.71 (1.21–2.42) |
| Per 10 ml/min per 1.73 m2 lower estimated GFR | 1.23 (1.15–1.30) | 1.22 (1.14–1.30) | 1.26 (1.15–1.38) | 1.21 (1.10–1.33) | ||||
| MDRD-estimated GFR | ||||||||
| ≥60 ml/min per 1.73 m2 | 276 | 6.9 | 1.00 (ref) | 1.00 (ref) | 125 | 3.1 | 1.00 (ref) | 1.00 (ref) |
| <60 ml/min per 1.73 m2 | 102 | 11.0 | 1.62 (1.28–2.04) | 1.54 (1.21–1.97) | 44 | 4.7 | 1.56 (1.10–2.22) | 1.36 (0.94–1.96) |
| Per 10 ml/min per 1.73 m2 lower estimated GFR | 1.13 (1.08–1.18) | 1.12 (1.07–1.17) | 1.14 (1.06–1.22) | 1.11 (1.03–1.18) | ||||
| Loss of cystatin C–estimated GFR§ | ||||||||
| <3 ml/min per 1.73 m2/year | 271 | 7.0 | 1.00 (ref) | 1.00 (ref) | 113 | 2.9 | 1.00 (ref) | 1.00 (ref) |
| ≥3 ml/min per 1.73 m2/year | 107 | 10.4 | 1.66 (1.30–2.11) | 1.57 (1.22–2.01) | 56 | 5.5 | 2.07 (1.46–2.92) | 1.85 (1.29–2.65) |
| Per ml/min per 1.73 m2/year decrease in estimated GFR | 1.07 (1.02–1.12) | 1.06 (1.01–1.10) | 1.10 (1.04–1.17) | 1.07 (1.01–1.13) | ||||
| Urine ACR | ||||||||
| <30 mg/g | 214 | 6.0 | 1.00 (ref) | 1.00 (ref) | 86 | 2.4 | 1.00 (ref) | 1.00 (ref) |
| ≥30 mg/g | 164 | 12.0 | 1.88 (1.52–2.32) | 1.73 (1.39–2.17) | 83 | 6.1 | 2.32 (1.70–3.18) | 1.96 (1.40–2.73) |
| Per doubling | 1.22 (1.15–1.30) | 1.18 (1.10–1.26) | 1.32 (1.20–1.45) | 1.24 (1.12–1.37) | ||||
*Per 100 person-years.
†Model 1 adjusted for age, sex, and race.
‡Model 2 adjusted for age, sex, race, diabetes duration, hypoglycemic medications, hypertension, BMI, smoking, total cholesterol, lipid-lowering medications, prevalent cardiovascular disease, and prevalent congestive heart failure.
§Loss of glomerular filtration rate estimated from serum cystatin C over the 7 years preceding ascertainment of mortality. ref, referent.
Table 3 represents a comparison of the relative contributions to global mortality risk made by each of the four measures of kidney disease, after accounting for multiple covariates. eGFRCYS was the strongest predictor of risk among these measures, leading to a high likelihood ratio and a c-index of 0.7645. Adding eGFRCYS to a fully adjusted model that included eGFRMDRD improved both the model likelihood ratio and the c-statistic, whereas adding eGFRMDRD to a fully adjusted model that included eGFRCYS resulted in no improvement in fit.
Table 3.
Contributions of kidney disease to prediction of total mortality among 691 CHS participants with diabetes
| Likelihood ratio test |
c-statistic (95% CI) | |||
|---|---|---|---|---|
| Model χ2 | Variable χ2 | P* | ||
| Base model† | 175.24 | 0.7385 (0.7046–0.7783) | ||
| + Cystatin C–estimated GFR | 212.14 | 36.90 | <0.001 | 0.7654 (0.7319–0.8026) |
| + MDRD-estimated GFR | 198.50 | 23.26 | <0.001 | 0.7550 (0.7221–0.7939) |
| + Change in cystatin C–estimated GFR | 180.12 | 4.88 | 0.019 | 0.7421 (0.7082–0.7814) |
| + Urine ACR | 189.82 | 14.58 | 0.009 | 0.7525 (0.7114–0.7844) |
| Base model† + MDRD-estimated GFR | 198.50 | 0.7550 (0.7221–0.7939) | ||
| + Cystatin C–estimated GFR | 212.83 | 14.33 | <0.001 | 0.7674 (0.7319–0.8026) |
| Base model† + cystatin C–estimated GFR | 212.14 | 0.7654 (0.7319–0.8026) | ||
| + MDRD-estimated GFR | 212.83 | 0.69 | 0.916 | 0.7674 (0.7319–0.8026) |
| + Change in cystatin C–estimated GFR | 212.24 | 0.10 | 0.834 | 0.7676 (0.7319–0.8026) |
| + Urine ACR | 219.28 | 7.14 | 0.274 | 0.7746 (0.7336–0.8041) |
*P value tests whether model with variable differs from corresponding base model in bold.
†Adjusted as per Table 2, model 2, without estimated GFR or urine ACR.
Change in cystatin C versus baseline cystatin C
Loss of eGFRCYS before 1996–1997 was also associated with increased risk of mortality, with a strength of association somewhat less than that for a single 1996–1997 eGFRCYS measurement (Table 2). When both eGFRCYS and loss of GFR were included simultaneously in the fully adjusted model, eGFRCYS but not loss of GFR was associated with increased risk of mortality with HRs of 1.22 (95% CI 1.14–1.31) per 10 ml/min per 1.73 m2 and 1.00 (0.95–1.04) per ml/min per 1.73 m2/year, respectively. Prediction models suggested that loss of GFR added substantially less to mortality prediction than a single eGFRCYS measurement, and loss of GFR did not predict mortality when included in a model with eGFRCYS (Table 3). Cystatin C concentrations were highly correlated within individuals over time, as evidenced by a regression dilution ratio of 0.74 (95% CI 0.70–0.79).
Cystatin C and albuminuria
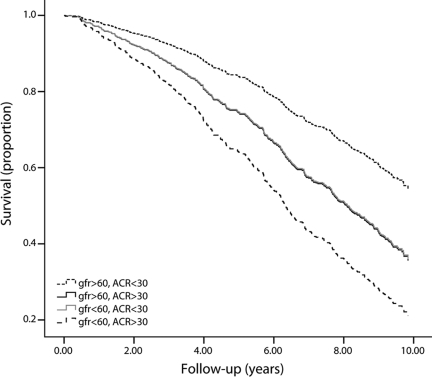
Elevated urine ACR was a strong predictor of mortality, particularly cardiovascular mortality (Table 2). When both eGFRCYS and urine ACR were included simultaneously in the fully adjusted model, each was independently associated with increased risk of mortality with HRs of 1.18 (95% CI 1.11–1.26) per 10 ml/min per 1.73 m2 and 1.11 (1.04–1.20) per doubling, respectively. Urine ACR added to prediction of mortality with or without simultaneous inclusion of eGFRCYS, as demonstrated by likelihood ratios and a change in the c-statistic (Table 3). Urine ACR and eGFRCYS were additive in regard to mortality risk. Specifically, a reduction in cumulative survival among participants with low eGFRCYS was nearly identical to that for participants with elevated urine ACR, and this reduction was approximately doubled among participants with both low eGFRCYS and elevated urine ACR (Fig. 1). Compared with participants with normal eGFRCYS (≥60 ml/min per 1.73 m2) and normal urine ACR (<30 mg/g), fully adjusted HRs for mortality were 1.69 (95% CI 1.26–2.27) for impaired eGFRCYS (<60 ml/min per 1.73 m2) and normal urine ACR, 1.67 (1.23–2.28) for normal low eGFRCYS and elevated urine ACR (≥30 mg/g), and 2.54 (1.88–3.44) for both low eGFRCYS and elevated urine ACR. There was no interaction on the multiplicative scale (interaction P = 0.634) or the additive scale (synergy index 1.14 [95% CI 0.48–1.79]).
Figure 1.
Cumulative survival by GFR estimated from serum cystatin C (milliliters per minute per 1.73 m2) and urine ACR (milligrams per gram) among 691 CHS participants with diabetes.
CONCLUSIONS
Albuminuria and impaired GFR were independent, additive risk factors for mortality among older adults with diabetes. GFR estimated using serum cystatin C was more strongly associated with mortality than GFR estimated using serum creatinine. These findings support current recommendations to regularly assess both albuminuria and GFR in the clinical care of patients with diabetes (4) with a sharp focus on interventions to prevent or treat CVD in the presence of albuminuria, impaired GFR, or both and further consideration of cystatin C use in clinical care.
The additive prognostic utilities of albuminuria and impaired GFR are consistent with prior studies in populations without diabetes or with low diabetes prevalence (8,16–20). Demonstration of this relationship in a diabetic population is particularly useful, because this population has a high cardiovascular risk and is well suited for regular health screening. Our results are similar and complementary to those from a Hong Kong cohort in which associations of albuminuria and estimated GFR with incident cardiovascular events were assessed (3). In contrast, an Italian study of type 2 diabetes observed albuminuria to be the primary renal risk factor for mortality, with estimated GFR being an independent risk factor only at levels <30 ml/min per 1.73 m2 (5). Our results may differ from results for the Italian study owing to older participant age or to use of serum cystatin C to estimate GFR.
In our study, 53% of participants with impaired GFR (eGFRCYS <60 ml/min per 1.73 m2) had urine ACR <30 mg/g. This proportion is higher than that observed in NHANES III, (21) possibly because of 1) the added effects of diabetes and advanced age on GFR and 2) more sensitive detection of impaired GFR using serum cystatin C. This large proportion of participants with impaired GFR but normal urine ACR is similar to that observed in the UK Prospective Diabetes Study (22), and, when combined with the independent and additive associations of each kidney disease measure with mortality, supports the general concept that albuminuria and impaired GFR are separate but overlapping, complementary manifestations of diabetic kidney disease. This concept has recently emerged as an alternative to an earlier paradigm describing albuminuria and impaired GFR as serial manifestations of diabetic kidney disease along a single, linear disease pathway (23,24).
Thus, albuminuria and impaired GFR may reflect different pathways of kidney damage. Albuminuria may reflect widespread vascular damage and endothelial dysfunction, thus identifying susceptibility to disease in nonrenal vascular beds (25). Impaired GFR may reflect loss of nephrons and parenchymal fibrosis that leads to CVD through accumulation of uremic toxins, impaired volume and blood pressure regulation, and multiple metabolic abnormalities. Whether albuminuria and impaired GFR lead to CVD through distinct mechanisms, thus requiring distinct interventions, has not been established.
The association of cystatin C–estimated GFR with mortality was modestly stronger than that for GFR estimated from serum creatinine. This finding is consistent with prior studies using the full CHS population (6,7). These studies demonstrated 1) that cystatin C–estimated GFR was linearly associated with mortality risk, whereas MDRD-estimated GFR demonstrated a U-shaped association with mortality, and 2) that elevated serum cystatin C was associated with mortality when MDRD-estimated GFR was in the “normal” range. It is possible that cystatin C estimates true GFR more precisely in the near-normal range or that cystatin C is less susceptible to confounding by body size among older adults. We evaluated only one formula estimating GFR from cystatin C, which was developed among individuals with impaired kidney function, and other cystatin C–based formulas may perform differently.
In our study, a change in cystatin C–estimated GFR within individuals over time was not associated with mortality more strongly than a single baseline cystatin C measurement. This result may be explained in part by the relatively high correlation of cystatin C concentration within individuals over time and suggests that single cystatin C measurements carry important prognostic information on their own, even without prior context. However, the value of longitudinal measurements may be increased when more measurements per individual are available.
Limitations of this study include the availability of only a single urine ACR measurement and lack of data assessing current glycemic control. Notably, however, we were able to account for diabetes duration and the use of hypoglycemic medications, which reflect cumulative exposure to hyperglycemia and disease severity and have been associated with mortality in CHS (14). In addition, our findings may not extrapolate to younger diabetic populations. Study strengths include the diverse, community-based diabetic population, multiple measurements of kidney disease, long-term follow-up for mortality and adjudicated CVD events, and large numbers of events to facilitate study of kidney disease interactions.
Supplementary Material
Acknowledgments
The research reported in this article was supported by the National Heart, Lung, and Blood Institute (contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133 and Grant U01-HL-080295), with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support came from National Institutes of Health grants R01-AG-027002 and 1KL2- RR-025015-01.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Borch-Johnsen K, Kreiner S: Proteinuria: value as predictor of cardiovascular mortality in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed) 1987;294:1651–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR: Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63:225–232 [DOI] [PubMed] [Google Scholar]
- 3.So WY, Kong AP, Ma RC, Ozaki R, Szeto CC, Chan NN, Ng V, Ho CS, Lam CW, Chow CC, Cockram CS, Chan JC, Tong PC: Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care 2006;29:2046–2052 [DOI] [PubMed] [Google Scholar]
- 4.Standards of medical care in diabetes—2007. Diabetes Care 2007;30(Suppl. 1):S4–S41 [DOI] [PubMed] [Google Scholar]
- 5.Bruno G, Merletti F, Bargero G, Novelli G, Melis D, Soddu A, Perotto M, Pagano G, Cavallo-Perin P: Estimated glomerular filtration rate, albuminuria and mortality in type 2 diabetes: the Casale Monferrato study. Diabetologia 2007;50:941–948 [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS: Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 2006;145:237–246 [DOI] [PubMed] [Google Scholar]
- 7.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005;352:2049–2060 [DOI] [PubMed] [Google Scholar]
- 8.Hallan S, Astor B, Romundstad S, Aasarød K, Kvenild K, Coresh J: Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med 2007;167:2490–2496 [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. : The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1991;1:263–276 [DOI] [PubMed] [Google Scholar]
- 10.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 2008;51:395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rifkin D, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman A, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 2008;168:2212–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Greene T, Kusek JW, Beck GJ: A simplified equation to predict glomerular filtration rate from serum creatinine (Abstract). J Am Soc Nephrol 2000;11:A0828 [Google Scholar]
- 13.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl. 1):S1–S266 [PubMed] [Google Scholar]
- 14.Kronmal RA, Barzilay JI, Smith NL, Psaty BM, Kuller LH, Burke GL, Furberg C: Mortality in pharmacologically treated older adults with diabetes: the Cardiovascular Health Study, 1989–2001. PLoS Med 2006;3:e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Seventh Report of the Joint National Committee in Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7). Bethesda, MD, U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute, 2004 [PubMed] [Google Scholar]
- 16.Irie F, Iso H, Sairenchi T, Fukasawa N, Yamagishi K, Ikehara S, Kanashiki M, Saito Y, Ota H, Nose T: The relationships of proteinuria, serum creatinine, glomerular filtration rate with cardiovascular disease mortality in Japanese general population. Kidney Int 2006;69:1264–1271 [DOI] [PubMed] [Google Scholar]
- 17.Foster MC, Hwang SJ, Larson MG, Parikh NI, Meigs JB, Vasan RS, Wang TJ, Levy D, Fox CS: Cross-classification of microalbuminuria and reduced glomerular filtration rate: associations between cardiovascular disease risk factors and clinical outcomes. Arch Intern Med 2007;167:1386–1392 [DOI] [PubMed] [Google Scholar]
- 18.Tonelli M, Jose P, Curhan G, Sacks F, Braunwald E, Pfeffer M: Proteinuria, impaired kidney function, and adverse outcomes in people with coronary disease: analysis of a previously conducted randomised trial. BMJ 2006;332:1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astor BC, Hallan SI, Miller ER, 3rd, Yeung E, Coresh J: Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol 2008;167:1226–1234 [DOI] [PubMed] [Google Scholar]
- 20.Cirillo M, Lanti MP, Menotti A, Laurenzi M, Mancini M, Zanchetti A, De Santo NG: Definition of kidney dysfunction as a cardiovascular risk factor: use of urinary albumin excretion and estimated glomerular filtration rate. Arch Intern Med 2008;168:617–624 [DOI] [PubMed] [Google Scholar]
- 21.Kramer HJ, Nguyen QD, Curhan G, Hsu CY: Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003;289:3273–3277 [DOI] [PubMed] [Google Scholar]
- 22.Retnakaran R, Cull CA, Thorne KI, Adler AI, Holman RR: Risk factors for renal dysfunction in type 2 diabetes: UK Prospective diabetes study 74. Diabetes 2006;55:1832–1839 [DOI] [PubMed] [Google Scholar]
- 23.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS: Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol 2007;18:1353–1361 [DOI] [PubMed] [Google Scholar]
- 24.de Boer IH, Steffes MW: Glomerular filtration rate and albuminuria: twin manifestations of nephropathy in diabetes. J Am Soc Nephrol 2007;18:1036–1037 [DOI] [PubMed] [Google Scholar]
- 25.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A: Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia 1989;32:219–226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.