Abstract
OBJECTIVE
We investigated whether the antiproteinuric effect of the direct renin inhibitor aliskiren is comparable to that of irbesartan and the effect of the combination.
RESEARCH DESIGN AND METHODS
This was a double-blind, randomized, crossover trial. After a 1-month washout period, 26 patients with type 2 diabetes, hypertension, and albuminuria (>100 mg/day) were randomly assigned to four 2-month treatment periods in random order with placebo, 300 mg aliskiren once daily, 300 mg irbesartan once daily, or the combination using identical doses. Patients received furosemide in a stable dose throughout the study. The primary end point was a change in albuminuria. Secondary measures included change in 24-h blood pressure and glomerular filtration rate (GFR).
RESULTS
Placebo geometric mean albuminuria was 258 mg/day (range 84–2,361), mean ± SD 24-h blood pressure was 140/73 ± 15/8 mmHg, and GFR was 89 ± 27 ml/min per 1.73 m2. Aliskiren treatment reduced albuminuria by 48% (95% CI 27–62) compared with placebo (P < 0.001), not significantly different from the 58% (42–79) reduction with irbesartan treatment (P < 0.001 vs. placebo). Combination treatment reduced albuminuria by 71% (59–79), more than either monotherapy (P < 0.001 and P = 0.028). Fractional clearances of albumin were significantly reduced (46, 56, and 67% reduction vs. placebo). Twenty-four-hour blood pressure was reduced 3/4 mmHg by aliskiren (NS/P = 0.009), 12/5 mmHg by irbesartan (P < 0.001/P = 0.002), and 10/6 mmHg by the combination (P = 0.001/P < 0.001). GFR was significantly reduced 4.6 (95% CI 0.3–8.8) ml/min per 1.73 m2 by aliskiren, 8.0 (3.6–12.3) ml/min per 1.73 m2 by irbesartan, and 11.7 (7.4–15.9) ml/min per 1.73 m2 by the combination.
CONCLUSIONS
The combination of aliskiren and irbesartan is more antiproteinuric in type 2 diabetic patients with albuminuria than monotherapy.
Albuminuria is the best available surrogate parameter in the treatment of diabetic nephropathy. Degree of proteinuria is associated with risk of renal and cardiovascular events (1). Proteinuria reduction is associated with a slowing of the decline in renal function (2). Blockade of the renin-angiotensin-aldosterone system (RAAS) is the cornerstone treatment of incipient and overt diabetic nephropathy, and in type 2 diabetes angiotensin II receptor blockers (ARBs) such as irbesartan are considered standard treatment after the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA) 2 Study (3) and Irbesartan Diabetic Nephropathy Trial (IDNT) (1).
Aliskiren represents a new principle of blocking the RAAS, inhibiting renin directly and acting at the rate-limiting step. The drug is approved for treatment of hypertension but has also shown renoprotective potential in patients with type 2 diabetes and albuminuria (4,5).
Combining an ARB and a direct renin inhibitor could offer improved RAAS blockade by acting both at the receptor level and at the first step of the cascade. We compared the antiproteinuric effect of maximal recommended doses of aliskiren, irbesartan, and the combination in patients with type 2 diabetes and albuminuria. We also assessed the impact of the treatments on RAAS components and biomarkers of inflammation, endothelial dysfunction, and cardiovascular risk.
RESEARCH DESIGN AND METHODS
This was a double-blind, randomized, crossover trial in accordance with the Declaration of Helsinki and good clinical practice. The primary objective was to assess albuminuria during different treatments compared with that with placebo; secondary objectives were to assess effect on 24-h blood pressure, glomerular filtration rate (GFR), biomarkers, and RAAS components. Patients were recruited from the Steno Diabetes Center, Gentofte, Denmark. The protocol was approved by the local ethics committee and the Danish Medicine Agency. After informed consent, patients attended a screening visit comprising laboratory tests and evaluations of inclusion/exclusion criteria. A 1-month washout followed, in which all antihypertensive treatment was stopped. Slow-release furosemide in a fixed dose (mean dose 109 mg/24 h, range 60–360 mg/24 h) was prescribed to prevent blood pressure elevation and fluid retention. Patients used an electronic blood pressure device (UA-779; A&D Instruments, Abingdon, U.K.) to measure home blood pressure throughout the study. Home blood pressure exceeding 170/105 mmHg led to exclusion.
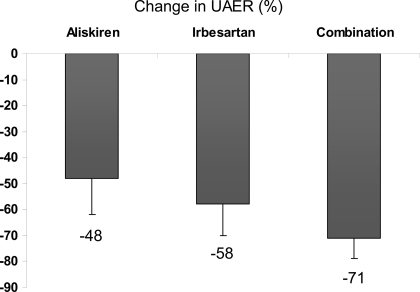
Figure 1.
Change in UAER (percentage) versus placebo during treatment with 300 mg aliskiren daily, 300 mg irbesartan daily, or the combination (P < 0.001 vs. placebo for all treatments).
From a list of prescreened candidates, 41 patients were screened for study participation (supplementary Fig. 1, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-0168/DC1). Nine of these were screen failures mainly due to albuminuria levels below the randomization requirement. Thirty-two patients were randomly assigned, and 22 patients completed the study. Of the 10 randomly assigned patients who left the study before completion, 2 died, 1 was lost to follow-up, 4 had an adverse event that led to exclusion (diarrhea, severe hypertension, recurrent urinary tract infection, and dizziness), and 3 withdrew consent. Twenty-six patients had the primary end point, albuminuria assessed after randomization, and were included in the final analysis; the remaining six patients dropped out shortly after random assignment and were not included in the final analysis. After washout, patients attended a randomization visit before 2 months of treatment with placebo, 300 mg aliskiren once daily, 300 mg irbesartan daily, or the combination of the two, in random order. Patients with type 2 diabetes (World Health Organization criteria) aged 30–80 years were eligible for randomization with baseline urinary albumin excretion rate (UAER) > 100 mg/24 h, hypertension (baseline office blood pressure >135/85 mmHg), and baseline GFR >40 ml/min per 1.73 m2. Exclusion criteria included major cardiovascular disease (within 6 months), heart failure (New York Heart Association class II–IV), A1C >11%, and history of malignancy. Cardiovascular history was assessed at screening using medical records and an electrocardiogram. Seven patients had previously been exposed to aliskiren.
There was no washout of study medication between the treatment periods. Rather, we used active washout: during the first 14 days of all treatments, every patient received 150 mg aliskiren daily to avoid risk of hypotension or a drastic increase in blood pressure in the switch from placebo to combination treatment or vice versa.
During the last 3 days of each treatment, patients collected three consecutive 24-h urine samples for assessment of geometric mean UAER. At the last day of each treatment period, patients attended our clinic for assessment of GFR and mounting of standard Takeda 24-h blood pressure devices (TM2421, version 7; A&D Medical, Tokyo, Japan). Measurements were performed every 15 min from 7 to 23 h (daytime) and every 30 min from 23 to 7 h (nighttime). Nondipping was defined as >10% difference between daytime and nighttime blood pressure. GFR was measured as plasma clearance of 51Cr-EDTA (6).
Urinary albumin and creatinine concentrations were determined on a turbidimetric Hitachi 912 system (Roche Diagnostics, Mannheim, Germany). Samples for prorenin, plasma renin activity (PRA), high-sensitivity PRA (hs-PRA), immunoreactive plasma renin concentration (ir-PRC), angiotensinogen, ANG I, ACE activity, ANG II, and aldosterone levels were determined after 30 min of supine rest, and the plasma was frozen after centrifugation (−80°C).
RAAS components and biomarkers were measured at baseline and at the end of treatment periods. Biomarkers of inflammation, endothelial dysfunction, and cardiovascular risk were measured: high-sensitivity C-reactive protein (hs-CRP) (enzyme immunosorbent assay [EIA]; Dako, Glostrup, Denmark); serum soluble vascular adhesion molecule-1 and serum soluble intercellular adhesion molecule-1 (EIA; Diaclone, Besançon, France); plasma plasminogen activator inhibitor-1 (HYPHEN BioMed kit; Andresy, France); serum NH2-terminal-probrain natriuretic peptide (EIA kit; Biomedica, Wien, Austria); fibrinogen (immunoturbidimetry); and plasma asymmetrical dimethyl arginine (high-performance liquid chromatography). Total renin concentration, prorenin concentration, plasma angiotensinogen, PRA, and biomarkers of inflammation and endothelial dysfunction were measured using methodology described previously (4). ir-PRC was measured with an immunoradiometric kit (Renin III; CisBio, Gif-sur-Yvette, France). hs-PRA was measured by in-house radioimmunoassay of ANG I formed during incubation of 25 μl plasma and 50 pmol sheep angiotensinogen for 3 h at 37°C in a total reaction volume of 100 μl. The assay was double calibrated against ANG I and the international reference preparation of renin, 68/356, from NIBSC (Hertfordshire, U.K.). Plasma ANG I and ANG II were measured using in-house radioimmunoassays and ethanol extraction of plasma samples. Antibodies were raised in rabbits, and calibrators were purchased from NIBSC. ACE activity was determined using a commercial radioenzymatic assay (ACE direct; Bühlmann-Laboratories, Schönenbuch, Switzerland).
Randomization was blinded to all investigators, and the study drugs were packed and labeled before delivery to the site. The treatment code was revealed only after database lock.
Statistical analysis
It was estimated that 20 patients completing the study could provide 80% power to demonstrate a significant difference between two treatments in antiproteinuric effect (UAER) if the true difference was 15%. This was based on the assumption that intrasubject coefficient of variation for the UAER was 13%. The log-transformed values of UAER were analyzed by a PROC MIXED model with sequence, treatment, and period as fixed factors and subject (nested in sequence) as a random factor. For 24-h blood pressure data, daytime average, nighttime average, and 24-h average values for systolic blood pressure and diastolic blood pressure were analyzed using a PROC MIXED model with sequence, treatment, and period as fixed factors and subject (nested in sequence) as a random factor. Extra analyses were performed to test the assumption of no carryover effect by fitting a carryover effect term into the model. GFR results and all other laboratory assessment data were analyzed similarly to blood pressure data. A two-sided P value < 0.05 was considered significant.
The correlation between changes in albuminuria and changes in hs-PRA or ANG II were assessed by a nonparametric Spearman correlation coefficient. Correlations between changes in albuminuria and changes in ir-PRC were assessed by linear regression analysis within each active treatment (aliskiren, irbesartan, and aliskiren/irbesartan combination) and for all active treatments combined. Fractional clearance was calculated using urine samples collected during GFR measurements (urinary albumin excretion/serum albumin concentration × GFR), and log-transformed levels were compared using a paired t test. Statistical analyses were performed using SAS (version 8.2 or higher; SAS Institute, Cary, NC) and SPSS (version 14.0; SPSS, Chicago, IL).
RESULTS
Baseline demographic data are shown in Table 1. Primary and secondary objectives were met. During placebo treatment geometric mean UAER was 258 (range 84–2,361) mg/day, mean ± SD 24-h blood pressure was 140/73 ± 15/8 mmHg, daytime blood pressure was 144/76 ± 17/9, and nighttime blood pressure was 130/68 ± 17/9. Eleven patients were defined as nondippers. GFR was 89 ± 27 ml/min per 1.73 m2 and mean serum creatinine was 88 μmol/l.
Table 1.
Demographics of the 32 randomly assigned patients with type 2 diabetes, hypertension, and albuminuria and the 26 included in the final analysis
| Total randomized population | Included in analysis | |
|---|---|---|
| n | 32 | 26 |
| Age (years) | 60.3 ± 9.0 | 59.8 ± 9.2 |
| Male (%) | 25 (78) | 20 (77) |
| Caucasian (%) | 32 (100) | 26 (100) |
| Height (cm) | 175 ± 9 | 175 ± 10 |
| Weight (kg) | 99.9 ± 20.6 | 100.9 ± 21.4 |
| BMI (kg/m2) | 32.3 ± 5.4 | 32.7 ± 5.5 |
| A1C (%) | 8.1 ± 1.3 | 8.2 ± 1.3 |
| Total cholesterol (mmol/l) | 3.7 ± 0.8 | 3.8 ± 0.8 |
| Smoking | 10 | 8 |
| CVD history | 4 | 1 |
| Blood pressure medications before study inclusion (n) | 2.5 ± 0.8 | 2.3 ± 0.8 |
| RAAS blocking treatment prior to study inclusion (n) | 30 | 24 |
| Baseline 24-h blood pressure (mmHg)* | 142/74 ± 12/8 | 141/74 ± 12/7 |
| Baseline UAER (mg/day)* | 307 (87–1,378) | 275 (103–1,088) |
Data are means ±SD, n, or mean (range).
*Baseline was defined as day of randomization. Values from the placebo treatment period were used in end point analysis.
Aliskiren treatment led to a significant reduction in albuminuria by 48% (95% CI 27–62) compared with placebo (P < 0.001) but not significantly different from irbesartan, lowering UAER by 58% (42–70) (P < 0.001 vs. placebo). Combination treatment reduced albuminuria by 71% (59–79) (P < 0.001) compared with placebo, significantly more than with either monotherapy (P < 0.001 and P = 0.028). The relative difference between aliskiren and combination treatment was 31%. To adjust for treatment-induced changes in GFR and the potential influence on albuminuria reduction, we calculated fractional clearance, which was reduced by 46% versus placebo during aliskiren treatment (P = 0.021), by 56% versus placebo during irbesartan treatment (P = 0.002), and by 67% versus placebo during combination treatment (P = 0.001). There were no indications of carryover effects on the results.
Systolic/diastolic 24-h blood pressure was reduced 3/4 mmHg by aliskiren (NS/P = 0.009), 12/5 mmHg by irbesartan (P < 0.001/P = 0.002), and 10/6 mmHg by the combination (P = 0.001/P < 0.001) versus placebo. There was no significant change in 24-h blood pressure from irbesartan to combination therapy. A correlation was found between change in albuminuria and change in 24-h diastolic blood pressure during all treatments (P = 0.039). Seated office systolic/diastolic blood pressure was reduced 7/4 mmHg by aliskiren, 6/4 mmHg by irbesartan, and 12/8 mmHg by the combination, all statistically significant compared with placebo, except for diastolic blood pressure during irbesartan treatment.
GFR was significantly reduced 4.6 (95% CI 8.8–0.3) ml/min per 1.73 m2 by aliskiren (P = 0.037), 8.0 (12.3–3.6) ml/min per 1.73 m2 by irbesartan (P < 0.001), and 11.7 (15.9–7.4) ml/min per 1.73 m2 by the combination (P < 0.001) compared with placebo. Aliskiren significantly reduced hs-PRA, ANG I, and ANG II by 87, 75, and 52%, respectively, compared with placebo; irbesartan had the opposite effect (Table 2). When combined, the activating effect of irbesartan was counteracted by aliskiren, reducing hs-PRA, ANG I, and ANG II by 88, 78, and 56%, respectively, compared with irbesartan monotherapy. Whereas combination treatment caused a 1,068% increase in ir-PRC versus a 279% increase during aliskiren monotherapy and a 178% increase during irbesartan monotherapy, hs-PRA was reduced 47% compared with placebo after combination therapy. PRA measured by a conventional method was affected similarly to hs-PRA, although the changes were smaller (Table 2). The renin-specific activity (renin bioactivity/total renin mass) was 3 and 4% after aliskiren and combination therapy compared with placebo, respectively, thereby confirming that 96–97% of renin was aliskiren bound. During irbesartan monotherapy, renin-specific activity increased 52% compared with placebo.
Table 2.
Changes in RAAS components and cardiovascular biomarkers versus placebo
| Placebo: geometric mean (range) | Aliskiren |
Irbesartan |
Combination |
||||
|---|---|---|---|---|---|---|---|
| Geometric mean (range) | Ratio (95% CI) vs. placebo | Geometric mean (range) | Ratio (95% CI) vs. placebo | Geometric mean (range) | Ratio (95% CI) vs. placebo | ||
| Angiotensinogen (nmol/l) | 969 (726–2,686) | 956 (500–2,505) | 0.99 (0.90–1.08)) | 719 (317–2,480) | 0.74 (0.67–0.81)† | 899 (605–2,564) | 0.92 (0.84–1.01) |
| Plasma prorenin concentration (ng/l) | 295 (75–1,646) | 336 (85–1,600) | 1.14 (0.99–1.30) | 406 (107–2,386) | 1.39 (1.21–1.59)† | 392 (69–2,764) | 1.34 (1.17–1.53)† |
| Plasma renin concentration (ng/l) | 29 (6–180) | 110 (18–1,024) | 3.79 (2.79–5.17)† | 80 (9–868) | 2.78 (2.04–3.79)† | 335 (42–1,926) | 11.68 (8.58–15.91)† |
| PRA (ng · ml−1 · h−1) | 1.44 (0.63–3.18) | 0.40 (0.03–1.24) | 0.36 (0.26–0.49)† | 2.69 (0.43–13.67) | 2.43 (1.75–3.36)† | 1.19 (0.00–6.17) | 1.00 (0.72–1.40) |
| hs-PRA (ng · ml−1 · h−1) | 4.5 (1.1–15.3) | 0.6 (0.2–3) | 0.13 (0.09–0.21)† | 18.7 (2.5–113) | 4.21 (2.71–6.54)† | 2.3 (0.2–23.3) | 0.53 (0.34–0.82)‡ |
| ACE activity (units) | 43 (32–57) | 44 (32–57) | 1.02 (0.97–1.07) | 45 (29–61) | 1.04 (0.99–1.10) | 42 (26–53) | 0.98 (0.93–1.03) |
| ANG I (pmol/l) | 19 (7.6–76) | 4.9 (0.9–19) | 0.25 (0.17–0.36)† | 60 (5.5–408) | 3.07 (2.12–4.44)† | 14 (0.5–100) | 0.70 (0.48–1.01) |
| ANG II (pmol/l) | 10 (3–33) | 4.8 (1.1–27) | 0.48 (0.32–0.72)† | 33 (3.7–199) | 3.37 (2.26–5.03)† | 15 (1.8–102) | 1.52 (1.02–2.27)* |
| hs-CRP (mg/l) | 2.5 (0.1–12) | 1.6 (0.1–19) | 0.65 (0.43–0.99)* | 1.6 (0.1–17) | 0.65 (0.42–0.99)* | 1.7 (0.1–15) | 0.68 (0.44–1.03) |
| Fibrinogen (g/l) | 3.9 (2.6–5.5) | 3.7 (2.4–5.2) | 0.93 (0.87–1.00)* | 3.7 (2.8–5.1) | 0.94 (0.88–1.00) | 3.9 (2.5–5.5) | 0.99 (0.93–1.06) |
| VWF (%) | 184 (98–289) | 172 (76–297) | 0.94 (0.81–1.09) | 175 (107–282) | 0.95 (0.82–1.10) | 177 (78–278) | 0.97 (0.83–1.12) |
| ICAM-1 (μg/l) | 636 (397–1,643) | 632 (408–1,825) | 1.00 (0.94–1.05) | 637 (425–2,137) | 1.00 (0.95–1.05) | 595 (398–1,232) | 0.94 (0.89–0.99)* |
| VCAM-1 (μg/l) | 937 (661–1,588) | 937 (693–1,890) | 1.00 (0.96–1.04) | 954 (685–1,747) | 1.02 (0.98–1.06) | 943 (662–1,520) | 1.01 (0.97–1.05) |
| Aldosterone (ng/l) | 52 (8–200) | 40 (1–150) | 0.77 (0.51–1.17) | 47 (1–656) | 0.90 (0.59–1.36) | 36 (6–147) | 0.70 (0.46–1.06) |
| ADMA (μmol/l) | 0.49 (0.40–0.60) | 0.50 (0.40–0.60) | 1.01 (0.96–1.07) | 0.50 (0.40–0.60) | 1.01 (0.96–1.07) | 0.50 (0.30–0.60) | 1.02 (0.96–1.08) |
| PAI-1 (μg/l) | 75 (26–267) | 80 (34–282) | 1.05 (0.81–1.37) | 76 (17–324) | 1.02 (0.78–1.33) | 59 (13–191) | 0.79 (0.60–1.02) |
| NT-proBNP (pmol/l) | 268 (129–995) | 258 (119–881) | 0.96 (0.84–1.10) | 251 (93–965) | 0.93 (0.81–1.07) | 239 (105–979) | 0.89 (0.78–1.02) |
All treatments were administered once daily.
*P < 0.05;
†P < 0.001;
‡P < 0.01. ADMA, asymmetrical dimethyl arginine; ICAM-1, intracellular adhesion molecule-1; NT-proBNP, NH2-terminal-probrain natriuretic peptide; PAI-1, plasminogen activator inhibitor-1; VCAM-1, vascular adhesion molecule-1; VWF, von Willebrand factor.
A significant correlation between reduction in albuminuria and increase in ir-PRC was observed for all active treatments combined (r2 = 0.597, P = 0.0001). There was a significant correlation between changes in albuminuria and increase in ANG II during irbesartan treatment (correlation coefficient −0.486, P = 0.022); no significant correlations were observed in the other treatment groups.
Table 2 depicts changes in cardiovascular biomarkers compared with placebo levels. hs-CRP was reduced 35% from the placebo level with aliskiren (P = 0.047) and 35% with irbesartan (P = 0.043). Other statistically significant changes from placebo levels were a 6% reduction in soluble intracellular adhesion molecule-1 (P = 0.017) observed with the combination treatment and a 7% reduction in fibrinogen during aliskiren treatment (P = 0.037). No treatment led to significant changes from placebo levels in any of the other cardiovascular biomarkers (Table 2).
The most frequent adverse events were urinary tract infection (four patients, one male), pneumonia (three patients), and cough (three patients), occurring during different treatments. Anemia and hypomagnesemia were detected in two patients during the combination treatment. Compared with each monotherapy, combination treatment showed an increase in plasma potassium by 0.2 mmol/l (P = 0.036). No patients developed hyperkalemia (defined as plasma potassium >5.5 mmol/l). There were no incidences of hypotension. One patient dropped out during the placebo period after several systolic blood pressure readings >180 mmHg.
Two patients died before the first measurement of albuminuria after the randomization and were not included in the final end point analysis. The first death was that of a 42-year-old obese man (BMI 42 kg/m2) with a history of ischemic heart disease, myocardial infarction, and hypertension 4 years before study entry. The patient experienced sudden cardiac arrest, seemingly after a myocardial infarction during aliskiren treatment. The second death was that of a hypertensive, obese, 73-year-old man with diabetes duration of 16 years. Sudden death was possibly caused by a pulmonary embolism during the placebo period. The deaths were instantly reported to relevant authorities and were not suspected as being related to any of the drugs studied. Subsequently, home blood pressure measurement frequency was increased from twice weekly to twice daily, and patients were instructed to contact the investigator by direct phone (available around the clock), if any measurement was >160/100 mmHg. Extra measurements of sodium, potassium, and creatinine were introduced 3 weeks into each treatment period.
CONCLUSIONS
In this exploratory study, we demonstrated that treatment with 300 mg aliskiren once daily was as efficient in reducing albuminuria as standard therapy with 300 mg irbesartan once daily. When we combined the two treatments at the same doses, the reduction in albuminuria was enhanced. The added antiproteinuric effect with combination treatment compared with aliskiren alone was ∼31%.
Given that the reductions in 24-h systolic blood pressure with aliskiren were unexpectedly small compared with those with placebo relative to 24-h diastolic blood pressure changes and were substantially lower than the office systolic blood pressure measurements in this study, we conducted a thorough review of potential flaws in data collection, storage, device calibration, reporting, and calculation. There was no evidence to indicate that the ambulatory data collection, storage, or reporting was flawed, and the unexpected results could be a chance occurrence.
Our study suggested that the combination of aliskiren and irbesartan had an additional RAAS blocking effect compared with monotherapy because a synergistic increase in ir-PRC was observed with the combination, which was related to the antiproteinuric effect, whereas hs-PRA was reduced 50% compared with the reduction with placebo.
As opposed to the Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID) study (5), which showed an additional 20% albuminuria reduction after 24 weeks of treatment with aliskiren compared with placebo added to the maximal recommended dose of losartan and optimal antihypertensive therapy, this is the first study with a head-to-head comparison between aliskiren and irbesartan treatment. No other antihypertensive drugs except for furosemide were allowed in our study, thereby offering a clearer picture of the effect of the two compounds used, compared with the AVOID study, in which aliskiren was combined with losartan and a mixture of other antihypertensive drugs. This study is different with regard to patient population, with a lower mean baseline UAER compared with that in the AVOID study. In addition, we assessed the effect of renin inhibition on GFR, a measurement that is more precise than the estimated GFR used in the AVOID study.
RAAS blockade is believed to reduce proteinuria through several different mechanisms: the mean transcapillary hydraulic pressure difference, the glomerular surface area, and the size and charge selectivity of the glomerular filter. In diabetic nephropathy several of these variables are abnormal, and RAAS blockade has been demonstrated to normalize directly measured or estimated glomerular hydraulic pressure (7–9), to reduce the shunt-like defects in the membrane, at least in part (10), and to restore the charge-selectivity properties of the glomerular membrane (11).
Aliskiren is thought to reduce albuminuria by the same mechanism as during treatment with ACE inhibitors or ARBs. Recently, Fisher et al. (12) have shown that aliskiren treatment increases renal plasma flow to a larger extent than the ACE inhibitor captopril. The increase in renal plasma flow may be a response to angiotensin AT1 receptor–dependent reduction of the vascular tone in the efferent arteriole. Reduced vascular tone in the efferent glomerular arteriole could be responsible for the decrease in intraglomerular pressure, leading to the reduction in albuminuria and GFR as demonstrated in our study. Combination treatment may reduce vascular tone to a greater extent than monotherapy. More research on the impact of renin inhibition on renal physiology is needed.
GFR changes seemed to be dependent on treatment during the study. Although the combination reduced GFR up to 12 ml/min (95% CI 15.9–7.4), we interpreted this as an reversible hemodynamic change and not as an indication of nephrotoxicity (13). In fact, it has been shown that an early hemodynamic reduction in GFR can translate into long-term renoprotection (13). When the albuminuria reduction was adjusted during combination treatment for changes in GFR (fractional clearance), it was 11% higher than during irbesartan treatment, a nonsignificant change that was possibly due to a small sample number.
Signs of more effective RAAS blockade were evident from the synergistic effect of combination treatment on ir-PRC. This conclusion is based on the fact that renin release into plasma is proportional to the interruption of the permanent negative feedback loop of ANG II on renin secretion (14). Combining aliskiren with irbesartan provided a 12-fold increase in ir-PRC, but still with a 50% reduction in hs-PRA compared with changes during the placebo period. This renin rise could reflect a high degree of intrarenal RAAS blockade during combination treatment as compared with that for the monotherapies, as has been suggested in nondiabetic patients (15). The reductions in albuminuria in our study were correlated with the rise in ir-PRC, supporting the concept of increased intrarenal RAAS blockade underlying the additional effects observed during combination treatment. Compared with other studies of dual RAAS blockade, the rise in ir-PRC is higher in dual RAAS blockade using aliskiren than in dual RAAS blockade with an ACE inhibitor and an ARB (5,16,17). Such marked increases in renin during aliskiren treatment have been noted before (18). Apart from reflecting more complete (intrarenal) RAAS blockade, they may also be due to the detection of prorenin as renin (19) or a change in the renin half-life after its binding to aliskiren (20).
PRA was measured both by a conventional method and by a new high-sensitivity assay (hs-PRA), which is independent of endogenous substrate variation (21). Because high PRA levels confer the risk of cardiovascular disease (22), it will be interesting to evaluate long-term effects of direct renin inhibition in the ongoing Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE), providing data on hard cardiovascular and renal end points (23).
The antihypertensive effect of aliskiren was smaller than that found in previous larger studies (16), although the office blood pressure reduction did not differ from that caused by irbesartan. More research on the possible differential dosing of aliskiren treatment is warranted.
The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) investigators (24) concluded that in a cardiovascular risk population, dual RAAS blockade with the ARB telmisartan and the ACE inhibitor ramipril is equivalent in reducing cardiovascular events compared with either as monotherapy, although with more frequent adverse events, including renal adverse events. Almost 3,000 of the participating 25,260 patients had microalbuminuria at baseline, and substudies of albuminuria effects are expected. Several short-term studies using dual RAAS blockade in diabetic nephropathy have shown promising antiproteinuric effects, as reviewed by Rossing (17), but the largest study so far (25) did not show additional benefits of a combination of ramipril and irbesartan, compared with ramipril monotherapy, in terms of albuminuria reduction after 20 weeks.
The only biomarker showing a systematic reduction during treatment was hs-CRP.
The sample size and the short treatment periods are obvious limitations of the study. The size was, however, sufficient to demonstrate the likely beneficial effect of combination therapy with aliskiren and irbesartan, although we evaluated a surrogate end point. In addition, the discrepancy between 24 h and office blood pressure readings complicates interpretation of the results. Studies evaluating mortality and morbidity are ongoing and will provide further information on dual RAAS blockade with aliskiren.
In summary, we demonstrate an antiproteinuric effect of dual RAAS blockade with aliskiren and irbesartan in combination compared with either treatment alone in patients with type 2 diabetes, hypertension, and albuminuria. The synergistic effect on ir-PRC illustrates a higher degree of intrarenal RAAS blockade during combination treatment.
Supplementary Material
Acknowledgments
This study was sponsored by Novartis.
F.P. has received lecture fees from Novartis. P.R. has received lecture fees and a research grant from Novartis. A.H.J.D. has received research grants from Novartis and has served as a consultant to Novartis. H.-H.P. has served as a consultant for Novartis and Sanofi-aventis, has received lecture fees from Novartis and Sanofi-aventis, and has received grant support from Novartis and Sanofi-aventis. No other potential conflicts of interest relevant to this article were reported.
We thank Ching-Ming Yeh for statistical contributions and William P. Dole, Hans-Armin Dieterich, Lisa Sandford, and Margaret Prescott for assistance through all study phases. We acknowledge the work of laboratory technicians Ulla M. Smidt, Lotte Pietraszeck, Anne G. Lundgaard, and Berit R. Jensen.
Footnotes
Clinical trial reg. no. NCT00464880, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851–860 [DOI] [PubMed] [Google Scholar]
- 2. Rossing P, Hommel E, Smidt UM, Parving H-H: Reduction in albuminuria predicts a beneficial effect on progression in diabetic nephropathy during antihypertensive treatment. Diabetologia 1994;37:511–516 [DOI] [PubMed] [Google Scholar]
- 3. Parving H-H, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P: The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001;345:870–878 [DOI] [PubMed] [Google Scholar]
- 4. Persson F, Rossing P, Schjoedt KJ, Juhl T, Tarnow L, Stehouwer CDA, Schalkwijk C, Boomsma F, Frandsen E, Parving H-H: Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney Int 2008;73:1419–1425 [DOI] [PubMed] [Google Scholar]
- 5. Parving H-H, Persson F, Lewis JB, Lewis EJ, Hollenberg NK: Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008;358:2433–2446 [DOI] [PubMed] [Google Scholar]
- 6. Bröchner-Mortensen J: A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 1972;30:271–274 [DOI] [PubMed] [Google Scholar]
- 7. Hostetter TH, Troy JL, Brenner BM: Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int 1981;19:410–415 [DOI] [PubMed] [Google Scholar]
- 8. Trevisan R, Tiengo A: Effect of low-dose ramipril on microalbuminuria in normotensive or mild hypertensive non-insulin-dependent diabetic patients. North-East Italy Microalbuminuria Study Group. Am J Hypertens 1995;8:876–883 [DOI] [PubMed] [Google Scholar]
- 9. Imanishi M, Yoshioka K, Konishi Y, Okumura M, Okada N, Sato T, Tanaka S, Fujii S, Kimura G: Glomerular hypertension as one cause of albuminuria in type II diabetic patients. Diabetologia 1999;42:999–1005 [DOI] [PubMed] [Google Scholar]
- 10. Andersen S, Blouch K, Bialek J, Deckert M, Parving H-H, Myers BD: Glomerular permselectivity in early stages of overt diabetic nephropathy. Kidney Int 2000;58:2129–2137 [DOI] [PubMed] [Google Scholar]
- 11. Langham RG, Kelly DJ, Cox AJ, Thomson NM, Holthofer H, Zaoui P, Pinel N, Cordonnier DJ, Gilbert RE: Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: effects of angiotensin converting enzyme inhibition. Diabetologia 2002;45:1572–1576 [DOI] [PubMed] [Google Scholar]
- 12. Fisher NDL, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK: Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation 2008;117:3199–3205 [DOI] [PubMed] [Google Scholar]
- 13. Palmer BF: Renal dysfunction complicating the treatment of hypertension. N Engl J Med 2002;347:1256–1261 [DOI] [PubMed] [Google Scholar]
- 14. Azizi M, Bissery A, Lamarre-Cliche M, Menard J: Integrating drug pharmacokinetics for phenotyping individual renin response to angiotensin II blockade in humans. Hypertension 2004;43:785-790 [DOI] [PubMed] [Google Scholar]
- 15. Azizi M, Menard J, Bissery A, Guyenne TT, Bura-Riviere A, Vaidyanathan S, Camisasca RP: Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol 2004;15:3126–3133 [DOI] [PubMed] [Google Scholar]
- 16. Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A: Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet 2007;370:221–229 [DOI] [PubMed] [Google Scholar]
- 17. Rossing K: Progression and remission of nephropathy in type 2 diabetes: new strategies of treatment and monitoring. Dan Med Bull 2007;54:79–98 [PubMed] [Google Scholar]
- 18. Sealey JE, Laragh JH: Aliskiren, the first renin inhibitor for treating hypertension: reactive renin secretion may limit its effectiveness. Am J Hypertens 2007;20:587–597 [DOI] [PubMed] [Google Scholar]
- 19. Danser AHJ, Deinum J: Renin, prorenin and the putative (pro)renin receptor. Hypertension 2005;46:1069–1076 [DOI] [PubMed] [Google Scholar]
- 20. Batenburg WW, de Bruin RJA, van Gool JMG, Muller DN, Bader M, Nguyen G, Danser AHJ: Aliskiren-binding increases the half life of renin and prorenin in rat aortic vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2008;28:1151–1157 [DOI] [PubMed] [Google Scholar]
- 21. Frandsen E, Boomsma F, Persson F, Dieterich HA, Yeh CM, Dole WP, Lund ED, Prescott M: Renin measurements in the presence of the oral direct renin inhibitor aliskiren: development and validation of a novel high-sensitivity plasma renin activity (hsPRA) assay for measurement of bioactive renin. J Hypertens 2008;26:S383 [Google Scholar]
- 22. Alderman MH, Madhavan S, Ooi WL, Cohen H, Sealey JE, Laragh JH: Association of the renin-sodium profile with the risk of myocardial infarction in patients with hypertension. N Engl J Med 1991;324:1098–1104 [DOI] [PubMed] [Google Scholar]
- 23. Parving H-H, Brenner BM, McMurray J, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Ghadanfar M, Weissbach N, Xiang Z, Armbrecht J, Pfeffer MA: Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE): rationale and study design. Nephrol Dial Transplant 2009;24:1663–1671 [DOI] [PubMed] [Google Scholar]
- 24. Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsärinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S: Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008;372:547–553 [DOI] [PubMed] [Google Scholar]
- 25. Bakris GL, Ruilope L, Locatelli F, Ptaszynska A, Pieske B, de Champlain J, Weber MA, Raz I: Treatment of microalbuminuria in hypertensive subjects with elevated cardiovascular risk: Results of the IMPROVE trial. Kidney Int 2007;72:879–885 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.