Abstract
OBJECTIVE
To determine the extent of β-cell function in youth with diabetes and GAD65 and/or IA2 autoantibodies.
RESEARCH DESIGN AND METHODS
Fasting C-peptide levels from 2,789 GAD65- and/or IA2 autoantibody-positive youth aged 1–23 years from the SEARCH for Diabetes in Youth study were used. Preserved β-cell function was defined on the basis of cut points derived from the Diabetes Control and Complications Trial (DCCT) (fasting C-peptide ≥0.23 ng/ml) and from the U.S. adolescent population of the National Health and Nutrition Examination Survey (NHANES) 5th percentile for fasting C-peptide (≥1.0 ng/ml). We compared the clinical characteristics between those with and without preserved β-cell function.
RESULTS
Within the first year of diagnosis, 82.9% of youth had a fasting C-peptide ≥0.23 ng/ml and 31.2% had values ≥1.0 ng/ml. Among those with ≥5 years of diabetes duration, 10.7% had preserved β-cell function based on the DCCT cutoff and 1.0% were above the 5th percentile of the NHANES population.
CONCLUSIONS
Within the 1st year of diagnosis, four of five youth with autoantibody-positive diabetes have clinically significant amounts of residual β-cell function and about one-third have fasting C-peptide levels above the 5th percentile of a healthy adolescent population. Even 5 years after diagnosis, 1 of 10 has fasting C-peptide above a clinically significant threshold. These findings have implications for clinical classification of youth with diabetes as well as clinical trials aimed to preserve β-cell function after diabetes onset.
Immune-mediated β-cell destruction, marked by the presence of diabetes autoantibodies, occurs before and continues after the clinical diagnosis of type 1 diabetes. This model has served as the foundation of pathophysiological studies of the disease process and clinical studies designed to identify future risk for diabetes and to modify clinical course. The resultant perception is that most individuals with type 1 diabetes will have complete destruction of β-cells within a few years after diagnosis without a targeted intervention to sustain β-cell function.
Despite frequent use of this model in research and patient care, it represents only part of the picture. Data from placebo-controlled populations in clinical intervention trials suggest that some individuals with type 1 diabetes will have persistent β-cell function years after diagnosis (1,2). The screening phase of the Diabetes Control and Complications Trial (DCCT) demonstrated that 48% of adults had significant C-peptide levels within 5 years of diagnosis, and 8% had significant C-peptide 5–15 years after diagnosis (3). Another study reported similar findings in 15% of individuals with measurable C-peptide levels 8–15 years after diagnosis (4). Multiple studies have reported that the loss of β-cell function after diagnosis is related to age of onset as well as to factors linked to autoimmunity such as autoantibodies (5–11). Nonetheless, there is a common belief that persistence of β-cell function is rare in young children with type 1 diabetes.
The SEARCH for Diabetes in Youth (SEARCH) study, designed to determine the prevalence, incidence, and characteristics of diabetes in U.S. youth, provides an opportunity to examine the frequency of residual β-cell function in a population-based sample of GAD65+ or IA2+ youth with diabetes.
RESEARCH DESIGN AND METHODS
Data for this analysis derive from the SEARCH study as described previously (12). SEARCH is a population-based study conducted at six centers in the U.S., including existing (prevalent) and newly diagnosed (incident) cases of diabetes in youth aged <20 years. Participants were asked to complete an initial survey and then were invited to an in-person study visit. After informed consent was provided, a brief examination was performed and blood samples were obtained.
Study population
Youth with prevalent cases in 2001 and incident cases in 2002–2005 who participated in the in-person visit and had fasting C-peptide measured were eligible for this study (n = 4,529). The present analysis includes 2,789 SEARCH participants who are also GAD65 and/or IA2 antibody positive. DNA samples were available for HLA analysis for 1,968 (70.6%) of these individuals.
Measurements
BMI was normalized as standard deviation score (SDS or Z score) based on age and sex. Weight was classified according to sex-specific percentiles on Centers for Disease Control and Prevention BMI-for-age growth charts as follows: underweight, <15th percentile; healthy weight, 15th–<85th percentile; overweight, 85th–<95th percentile; and obese, ≥95th percentile.
Laboratory methods
Fasting samples were obtained under conditions of metabolic stability, defined as no episode of diabetic ketoacidosis during the previous month. Assays were performed at the Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington.
Autoantibody testing was performed using a standardized assay protocol and a common serum calibrator developed by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-sponsored standardization group. Results are expressed as NIDDK units (NIDDKU) per milliliter. Based on analysis of 550 samples, the cutoff values for positivity/negativity are 33 NIDDKU/ml for GAD65 and 5 NIDDKU/ml for IA2. The calculated specificity and sensitivity are 97 and 76, respectively, for GAD65 and 99 and 64, respectively, for IA2.
C-peptide was measured by a two-site immunoenzymetric assay (Tosoh Bioscience, San Francisco, CA). The assay sensitivity is 0.05 ng/ml.
A1C levels were determined by an automated nonporous ion-exchange high-performance liquid chromatography system (G-7 Tosoh Biosciences) with reference ranges of 4.2–5.9% and HLA class II genotyping was performed by a commercially available LABType SSO method (OneLambda, Los Angeles, CA).
C-peptide categories
DCCT.
Post hoc analysis of DCCT data demonstrated that those with preserved C-peptide, defined as stimulated C-peptide, value >0.6 ng/ml, had superior clinical outcomes, including less hypoglycemia and retinopathy, than those with lower C-peptide (13). Re-analysis of these data indicated that the corresponding fasting C-peptide was 0.23 ng/ml (J. Lachin, P.F. McGee, personal communication). Fasting C-peptide values ≥0.23 ng/ml are therefore considered clinically significant.
Healthy adolescents.
The 5th and 50th percentiles of fasting C-peptide in healthy adolescents, aged 12–19 years, who participated in the National Health and Nutrition Survey 1999–2002 (NHANES), were, respectively, 1.0 and 1.9 ng/ml (14).
Statistics
SAS for Windows (version 9.1; SAS Institute, Cary, NC) was used for analysis. χ2 or t tests were used to evaluate relationships between preserved β-cell function status and characteristics of interest. Two sets of regression models were run. The first included logistic regression models examining associations between variables of interest and preserved β-cell function status, stratified by duration of diabetes (<1, 1–2, and >2 years). Next, among participants with preserved C-peptide levels based on the DCCT cutoff, multiple linear regression models were used to identify variables significantly associated with fasting C-peptide levels. All logistic and linear regression models included the following covariates: age at diagnosis, sex, race/ethnicity, BMI Z score at time of the visit, A1C, number of autoantibodies present, fasting plasma glucose, and HLA genotype. In addition, linear models were adjusted for duration of disease.
RESULTS
The average age at diagnosis was 9.0 years: in 16% (n = 434) of the individuals diabetes was diagnosed when they were <5 years of age, 38% (n = 1,057) were between ages 5 and 10, 37% (n = 1,028) were between ages 11 and 15, and 10% (n = 270) were between 15 and 19 years of age at diagnosis. Average duration of diabetes was 3 years, with 33% (n = 925) having duration of <1 year, 24% (n = 661) having duration of 1–2 years, and 43% (n = 1,202) having duration of ≥years.
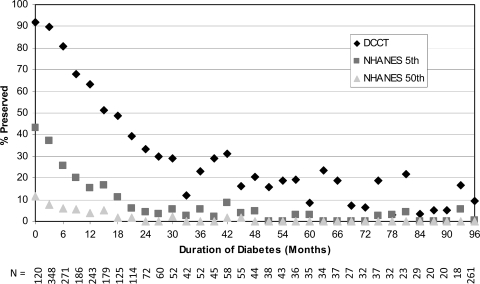
Among individuals with duration of diabetes of <1 year, 82.9% had a fasting C-peptide level ≥0.23 ng/ml, 31.2% had fasting C-peptide at or above the 5th percentile of the NHANES population and 7.2% had fasting C-peptide at or above the NHANES 50th percentile level. As expected, the proportion of individuals with preserved C-peptide diminished with increasing disease duration. This finding is illustrated in Fig. 1, which shows the proportion with preserved C-peptide in quarterly intervals from time from diagnosis. Among those with duration of ≥5 years, 10.7% had preserved C-peptide at the DCCT cut point, 1.0% at NHANES 5th percentile, and 0% at NHANES 50th percentile.
Figure 1.
Percentage of participants with preserved C-peptide by duration of diabetes in 3-month intervals according to the DCCT definition (fasting C-peptide <0.23 ng/ml) (♦), NHANES 5th percentile definition (fasting C-peptide <1.0 ng/ml) (■), and NHANES 50th percentile definition (fasting C-peptide <1.9 ng/ml) (▴). N, number of participants in each 3-month interval.
We then explored various characteristics of participants with and without preserved fasting C-peptide (Table 1). In the unadjusted analysis, the proportion of non-Hispanic whites who had preserved fasting C-peptide was smaller than that of other race/ethnicities. More individuals with preserved C-peptide had two antibodies and a significantly higher percentage of HLA DR15. In addition, those with preserved fasting C-peptide were older at diagnosis, had a shorter duration of diabetes, and had a lower A1C at the time of the study visit. Those with C-peptide above the NHANES 5th percentile also had a higher BMI Z score than those with C-peptide below this level.
Table 1.
Characteristics of 2,789 SEARCH antibody-positive participants by preserved β-cell function status as defined by fasting C-peptide levels
| Entire cohort | β-Cell function |
||||
|---|---|---|---|---|---|
| DCCT |
NHANES 5th percentile |
||||
| Preserved (FCP ≥0.23 ng/ml) | Not preserved (FCP <0.23 ng/ml) | Preserved (FCP ≥1.0 ng/ml) | Not preserved (FCP <1.0 ng/ml) | ||
| n (%) | 2,789 | 1,329 (47.7) | 1,460 (52.4) | 406 (14.6) | 2,383 (85.4) |
| Sex | |||||
| Male | 1,385 (49.7) | 682 (49.2) | 703 (50.8) | 210 (15.2) | 1,175 (84.4) |
| Female | 1,404 (50.3) | 647 (46.1) | 757 (53.9) | 196 (14.0) | 1,208 (86.0) |
| Race/ethnicity | |||||
| Non-Hispanic white | 2,103 (75.4) | 9,781 (46.5) | 1,125 (53.5) | 288 (13.7) | 1,815 (86.3) |
| Other | 686 (24.6) | 351 (51.2) | 335 (48.8) | 118 (17.2) | 568 (82.8) |
| HLA DR* | |||||
| 03/04 | 1,781 (90.5) | 716 (40.2) | 1,065 (59.8) | 221 (12.4) | 1,560 (87.6) |
| 15 | 35 (1.8) | 23 (65.7) | 12 (34.3) | 10 (28.6) | 25 (71.4) |
| Other | 152 (7.7) | 62 (40.8) | 90 (59.2) | 18 (11.8) | 134 (88.2) |
| No. of autoantibodies | |||||
| 2 | 1,172 (42.0) | 647 (55.2) | 525 (44.8) | 210 (17.9) | 962 (82.1) |
| 1 | 1,617 (58.0) | 682 (42.2) | 935 (57.8) | 196 (12.1) | 1,421 (87.9) |
| Age at diagnosis (years) | 9.0 (0–19) | 10.5 (1–19) | 7.6 (0–18) | 12.6 (3.0–18.0) | 8.9 (0–19.0) |
| Age at visit (years) | 12.4 (1.5–22.7) | 12.3 (1.9–22.5) | 12.5 (1.5–22.7) | 13.8 (4.0–21.8) | 12.4 (1.5–22.7) |
| Duration of diabetes (months) | 35.8 (0–213) | 16.1 (0–210) | 53.7 (0–213) | 8.8 (0–47) | 36.8 (0–213) |
| BMI Z score | 0.6 (−4.2 to 4.7) | 0.7 (−4.2 to 3.1) | 0.6 (−3.3 to 4.7) | 1.6 (−2.0 to 3.0) | 0.6 (−4.2 to 4.7) |
| A1C (%) | 8.1 (3.1–17.9) | 7.7 (3.1–17.3) | 8.5 (5.3–17.9) | 6.9 (4.8–13.0) | 8.1 (3.1–17.9) |
| Fasting glucose (mg/dl) | 191.2 (42–658) | 170.8 (42–518) | 209.8 (43–658) | 141.3 (79–330) | 192 (42–658) |
Data are n (%) or mean (range). For the column “Entire cohort,” percentages total to 100 vertically within each variable (sex, race/ethnicity, HLA, and autoantibody number). For the remaining columns, the percentages total to 100 horizontally between preserved and not preserved for each definition. All continuous variables were different between those with preserved and not preserved C-peptide (t test; P < 0.05), except age at visit and BMI Z score for DCCT cutoff. Race/ethnicity, number of autoantibodies, and HLA DR showed a significant association with preserved status (χ2, at P < 0.05).
*HLA was tested on a subset of subjects (n = 1,968). FCP, fasting C-peptide.
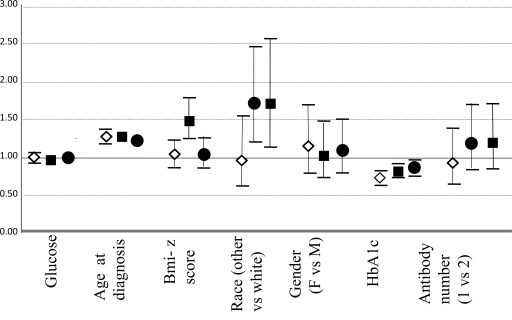
Figure 2 presents the results of the multiple logistic regression models with the dichotomous outcome of preserved fasting C-peptide status based on the DCCT definition stratified by diabetes duration. Variables found to be independently associated with preserved β-cell function in all the models included older age at diagnosis and lower A1C at time of study visit. Race/ethnicity other than non-Hispanic white was also significant for those participants with diabetes duration of 1–2 years and ≥2 years; higher current BMI Z score was also significant only for those participants with duration of 1–2 years (Fig. 2).
Figure 2.
Odds ratios for having preserved C-peptide according to the DCCT definition for individuals <1 year (♢), 1–2 years (■), and >2 years (●) from diagnosis. Incremental units are 1 mg/dl glucose, age 1 year, BMI 1 kg/m2, and A1C 1%. F, female; M, male.
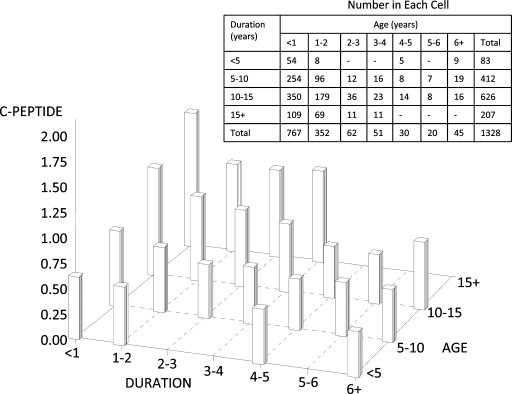
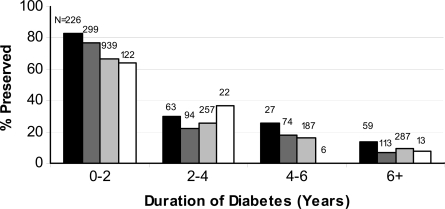
Among those with preserved fasting C-peptide (DCCT definition), older age at diagnosis, higher current BMI Z score, race/ethnicity other than non-Hispanic white, female sex, lower A1C, and shorter duration of diabetes were independently related to higher fasting C-peptide levels. The association of age at diagnosis and diabetes duration with fasting C-peptide is illustrated in Fig. 3, demonstrating that lower fasting C-peptide is seen in participants with diagnosis of diabetes at a younger age and those with longer duration of disease. The relationship between weight categories at the time of the study visit and prevalence of preserved β-cell function at various times from diagnosis is shown in Fig. 4. Although a greater percentage of obese/overweight compared with underweight subjects had preserved function, the association was significant (P < 0.0001) only in those with duration of diabetes of <2 years (P = 0.48, 0.41, and 0.58 for 2–4, 4–6, and >6 years from diagnosis, respectively).
Figure 3.
Fasting C-peptide by duration and age at diagnosis among those with preserved C-peptide by DCCT definition. Cells with less than five subjects are not reported.
Figure 4.
Percentage of participants with preserved C-peptide by duration of diabetes according to DCCT definition (fasting C-peptide <0.23 ng/ml) stratified by BMI classification (black bar, obese; dark gray bar, overweight; light gray bar, normal weight; white bar, underweight).
CONCLUSIONS
In this study we found that approximately four of five youth with antibody-positive diabetes have clinically significant amounts of residual β-cell function within the 1st year after diagnosis. In adjusted regression analyses of individuals within 1 year of diagnosis only age at diagnosis and A1C were identified as significant variables in whether or not C-peptide was preserved. In addition, race/ethnicity was important in those further from diagnosis. Although it is known that β-cell function continues to decline after diabetes diagnosis, our data also indicate that as many as 1 of 10 youth have preserved β-cell function even 5 years after diagnosis.
Despite evidence to the contrary, the belief persists that youth with type 1 diabetes have little insulin secretion at diagnosis and that insulin secretion rapidly disappears after diagnosis. Classification schemes define type 1 diabetes as a state of absolute insulin deficiency and type 2 diabetes as a state of insulin resistance combined with inadequate insulin secretion (15). Thus, health care providers have used C-peptide measurements clinically to establish type of diabetes and to select therapies. Although this analysis does not address the question of whether there is a C-peptide cut point value that could distinguish different types of diabetes, the data demonstrate that many youth with antibody-positive diabetes have C-peptide levels within the range of those of the normal population. Specifically, within 1 year of diagnosis, almost one-third of subjects had C-peptide values that exceeded the 5th percentile and ∼1 in 14 (7%) exceeded the 50th percentile for healthy adolescents.
Most researchers (3,13,16–20), but not all (21), documented that even a small amount of residual β-cell function is of clinical significance. Using a level of C-peptide associated with clinical significance in the DCCT, we found that 82.9% of participants with diabetes duration of <1 year exceed this threshold. The frequency of clinically significant β-cell function is lower among youth with longer duration of disease (53.3% for duration of 1–2 years and 18.6% for 4–5 years). Because the DCCT cut point has been suggested as an end point for clinical trials designed to preserve β-cell function, we suggest caution in interpreting data from nonrandomized trials or short-term pilot studies because our data show that endogenous C-peptide is present years after diagnosis in a substantial portion of children.
The data in the present study are similar to those reported by the DCCT, in which 33% of the 466 adolescents tested <5 years from diagnosis had residual β-cell function. Because the DCCT population only included individuals aged >13 years, many pediatricians felt that preservation of C-peptide was unlikely in younger children. Among SEARCH participants, we found a profound impact of advancing age at diagnosis on preservation of C-peptide production. The association was linear throughout the complete range of age of diagnosis (from 1 to 20 years), suggesting that insulin resistance associated with puberty does not account for this finding. Others have demonstrated an effect of age on C-peptide in individuals at risk for type 1 diabetes (22).
Several reasons have been suggested for preserved β-cell function. First, increased awareness of the symptoms of type 1 diabetes and improved screening and diagnostic tools may have resulted in diagnosis at an earlier point in the autoimmune destruction of the β-cells. Thus, the individuals may have more β-cell reserve at diagnosis. Second, aggressive treatment at diagnosis, with rapid and tight control of hyperglycemia, may result in improved β-cell function (23). Third, increased insulin resistance associated with the epidemic of obesity may have created a greater strain on the declining β-cell function resulting in diagnosis at a time when the individuals have more β-cell function than individuals in the past (24). Consistent with this concept, among those with preserved β-cell function, BMI Z score was an important variable in determining the fasting C-peptide level. However, the impact of BMI on whether or not C-peptide was preserved was less clear. As evident in Fig. 4, even many years from diagnosis, about 10% of normal-weight individuals had preserved function, suggesting that other, as yet, uncharacterized factors contribute to heterogeneity in disease progression.
This study also emphasizes the relationship between A1C and C-peptide but provides no insight as to whether better glucose control results in preserved function or whether preserved function allows for better A1C. Prospectively conducted trials with clinical data such as insulin use, carbohydrate consumption, and exercise may be helpful to address this question.
This article documents the frequency of preserved β-cell function in a population-based racially/ethnically diverse cohort of antibody-positive youth with diabetes. Although the fasting C-peptide concentration in antibody-positive youth with diabetes is often below normal, these data suggest that clinically significant amounts may persist in some individuals for some time even among subjects with more than one autoantibody. Furthermore, these data indicate that there is a marked relationship between age at diagnosis, race/ethnicity, and residual C-peptide. Differences in pathophysiology between non-Hispanic white youth and youth of other races/ethnicities may be present. Incorporating age and race/ethnicity into assessments of residual β-cell function may provide a better picture of the natural history of disease both before and after diagnosis and a more accurate assessment of the effectiveness of interventions designed to prevent β-cell destruction.
Acknowledgments
SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097 and DP-05-069) and supported by the National Institutes of Health: Kaiser Permanente Southern California (U01 DP000246); University of Colorado Health Sciences Center (U01 DP000247); Pacific Health Research Institute (U01 DP000245); Children's Hospital Medical Center (Cincinnati) (U01 DP000248); University of North Carolina (U01 DP000254); University of Washington School of Medicine (U01 DP000244); and Wake Forest University School of Medicine (U01 DP000250).
No potential conflicts of interest relevant to this article were reported.
We thank John Lachin and Paula Friedenberg McGee of the George Washington University Biostatistics Center for their analysis of the DCCT data to determine the fasting C-peptide cut point used to define preserved C-peptide.
APPENDIX
We acknowledge the involvement of General Clinical Research Centers at the following institutions: Medical Center of South Carolina (Grant M01 RR01070), Cincinnati Children's Hospital (Grant M01 RR08084), Children's Hospital and Regional Medical Center and the University of Washington School of Medicine (Grant M01RR00037 and Grant M01RR001271), and Colorado Pediatric General Clinical Research Center (Grant M01 RR00069).
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Chaillous L, Lefevre H, Thivolet C, Boitard C, Lahlou N, Atlan-Gepner C, Bouhanick B, Mogenet A, Nicolino M, Carel JC, Lecomte P, Marechaud R, Bougneres P, Charbonnel B, Sai P: Oral insulin administration and residual β-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabete Insuline Orale group. Lancet 2000;356:545–549 [DOI] [PubMed] [Google Scholar]
- 2. Pozzilli P, Pitocco D, Visalli N, Cavallo MG, Buzzetti R, Crino A, Spera S, Suraci C, Multari G, Cervoni M, Manca Bitti ML, Matteoli MC, Marietti G, Ferrazzoli F, Cassone Faldetta MR, Giordano C, Sbriglia M, Sarugeri E, Ghirlanda G: No effect of oral insulin on residual β-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia 2000;43:1000–1004 [DOI] [PubMed] [Google Scholar]
- 3. Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW: C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve β-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 2004;53:250–264 [DOI] [PubMed] [Google Scholar]
- 4. Scholin A, Bjorklund L, Borg H, Arnqvist H, Bjork E, Blohme G, Bolinder J, Eriksson JW, Gudbjornsdottir S, Nystrom L, Ostman J, Karlsson AF, Sundkvist G: Islet antibodies and remaining β-cell function 8 years after diagnosis of diabetes in young adults: a prospective follow-up of the nationwide Diabetes Incidence Study in Sweden. J Intern Med 2004;255:384–391 [DOI] [PubMed] [Google Scholar]
- 5. Karjalainen J, Salmela P, Ilonen J, Surcel H-M, Knip M: A comparison of children and adult type I diabetes. N Engl J Med 1989;881–886 [DOI] [PubMed] [Google Scholar]
- 6. Bonfanti R, Bazzigaluppi E, Calori G, Riva MC, Viscardi M, Bognetti E, Meschi F, Bosi E, Chiumello G, Bonifacio E: Parameters associated with residual insulin secretion during the first year of disease in children and adolescents with type 1 diabetes mellitus. Diabet Med 1998;15:844–850 [DOI] [PubMed] [Google Scholar]
- 7. Montanya E, Fernandez-Castaner M, Rosel P, Gomez J, Soler J: Age, sex and ICA influence on β-cell secretion during the first year after the diagnosis of type 1 diabetes mellitus. Diabetes Metab 1991;17:460–468 [PubMed] [Google Scholar]
- 8. Peig M, Gomis R, Ercilla G, Casamitjana R, Bottazzo GF, Pujol-Borrell R: Correlation between residual β-cell function and islet cell antibodies in newly diagnosed type I diabetes: follow-up study. Diabetes 1989;38:1396–1401 [DOI] [PubMed] [Google Scholar]
- 9. Sochett EB, Daneman D, Clarson C, Ehrlich RM: Factors affecting and patterns of residual insulin secretion during the first year of type 1 (insulin-dependent) diabetes mellitus in children. Diabetologia 1987;30:453–459 [DOI] [PubMed] [Google Scholar]
- 10. Steele C, Hagopian WA, Gitelman S, Masharani U, Cavaghan M, Rother KI, Donaldson D, Harlan DM, Bluestone J, Herold KC: Insulin secretion in type 1 diabetes. Diabetes 2004;53:426–433 [DOI] [PubMed] [Google Scholar]
- 11. Torn C, Landin-Olsson M, Lernmark A, Palmer JP, Arnqvist HJ, Blohme G, Lithner F, Littorin B, Nystrom L, Schersten B, Sundkvist G, Wibell L, Ostman J: Prognostic factors for the course of beta cell function in autoimmune diabetes. J Clin Endocrinol Metab 2000;85:4619–4623 [DOI] [PubMed] [Google Scholar]
- 12. SEARCH for Diabetes in Youth: a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials 2004;25:458–471 [DOI] [PubMed] [Google Scholar]
- 13. Steffes MW, Sibley S, Jackson M, Thomas W: β-Cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–836 [DOI] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Third National Health and Nutrition Examination Survey (NHANES III) Public-Use Data Files [article online], 2008. Available from http://www.cdc.gov/nchs/products/elec_prods/subject/nhanes3.htm. Accessed July 2008
- 15. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 16. Assan R, Feutren G, Sirmai J, Laborie C, Boitard C, Vexiau P, Du Rostu H, Rodier M, Figoni M, Vague P: Plasma C-peptide levels and clinical remissions in recent-onset type I diabetic patients treated with cyclosporin A and insulin. Diabetes 1990;39:768–774 [DOI] [PubMed] [Google Scholar]
- 17. Fukuda M, Tanaka A, Tahara Y, Ikegami H, Yamamoto Y, Kumahara Y, Shima K: Correlation between minimal secretory capacity of pancreatic β-cells and stability of diabetic control. Diabetes 1988;37:81–88 [DOI] [PubMed] [Google Scholar]
- 18. Madsbad S, Alberti K, Binder C, Burrin J, Faber O, Krarup T, Regeur L: Role of residual insulin secretion in protecting against ketoacidosis in insulin-dependent diabetes. Br Med J 1979;2:1257–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin S, Pawlowski B, Greulich B, Ziegler AG, Mandrup-Poulsen T, Mahon J: Natural course of remission in IDDM during 1st yr after diagnosis. Diabetes Care 1992;15:66–74 [DOI] [PubMed] [Google Scholar]
- 20. Sjöberg S, Gjötterberg M, Berglund L, Möller E, Ostman J: Residual C-peptide excretion is associated with a better long-term glycemic control and slower progress of retinopathy in type I (insulin-dependent) diabetes mellitus. J Diabet Complications 1991;5:18–22 [DOI] [PubMed] [Google Scholar]
- 21. Madsbad S, Lauritzen E, Faber O, Binder C: The effect of residual β-cell function on the development of diabetic retinopathy. Diabet Med 1986;3:42–45 [DOI] [PubMed] [Google Scholar]
- 22. Tsai EB, Sherry NA, Palmer JP, Herold KC: The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia 2006;49:261–270 [DOI] [PubMed] [Google Scholar]
- 23. The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual β-cell function in patients with type 1 diabetes in the Diabetes Control and Complications Trial: a randomized, controlled trial. Ann Intern Med 1998;128:517–523 [DOI] [PubMed] [Google Scholar]
- 24. Wilkin TJ: Diabetes mellitus: type 1 or type 2? The accelerator hypothesis. J Pediatr 2002;141:449–450 [DOI] [PubMed] [Google Scholar]