Abstract
OBJECTIVE
Bariatric surgery is gaining acceptance as an efficient treatment modality for obese patients. Mechanistic explanations regarding the effects of bariatric surgery on body composition and fat distribution are still limited.
RESEARCH DESIGN AND METHODS
Intra-abdominal and subcutaneous fat depots were evaluated using computed tomography in 27 obese patients prior to and 6 months following bariatric surgery. Associations with anthropometric and clinical changes were evaluated.
RESULTS
Excess weight loss 6 months following surgery was 47% in male and 42.6% in female subjects. Visceral fat and subcutaneous fat were reduced by 35% and 32%, respectively, in both sexes, thus the visceral-to-subcutaneous fat ratio remained stable. The strongest relation between absolute and relative changes in visceral and subcutaneous fat was demonstrated for the excess weight loss following the operations (r ∼0.6–0.7), and these relations were strengthened further following adjustments for sex, baseline BMI, and fat mass. Changes in waist circumference and fat mass had no relation to changes in abdominal fat depots. All participants met the criteria of the metabolic syndrome at baseline, and 18 lost the diagnosis on follow-up. A lower baseline visceral-to-subcutaneous fat ratio (0.43 ± 0.15 vs. 0.61 ± 0.21, P = 0.02) was associated with clinical resolution of metabolic syndrome parameters.
CONCLUSIONS
The ratio between visceral and subcutaneous abdominal fat remains fairly constant 6 months following bariatric procedures regardless of sex, procedure performed, or presence of metabolic complications. A lower baseline visceral-to-abdominal fat ratio is associated with improvement in metabolic parameters.
Bariatric surgery is gaining acceptance as an efficient treatment modality for patients with class 2 and class 3 obesity. Various bariatric procedures have been reported to lead not only to significant weight reduction but also to improvement or disappearance of the typical comorbidities of obese individuals (altered glucose metabolism, hypertension, and dyslipidemia) (1). These results, especially for long-term follow-up (2), seem very promising in comparison with lifestyle modifications and pharmacological interventions, which have limited long-term success against obesity (3,4). Despite these observations, mechanistic explanations regarding the effects of such procedures on body composition, fat distribution, and hormonal alterations are still limited.
Abdominal fat is composed of subcutaneous fat and intra-abdominal fat (5). These two depots have a major influence on the metabolic phenotype of obese individuals and differ in their hormonal and cytokine secretion profile as well in their anatomical vascular drainage (6). Increased intra-abdominal fat is associated with an adverse metabolic profile and predicts the development of type 2 diabetes (7) and cardiovascular disease (8). The effects of weight loss induced by lifestyle modifications (9) and pharmacotherapy (10) on abdominal fat depots have been described, yet the short-term impact of bariatric procedures on these depots is unknown. Because bariatric procedures are characterized by a significant rapid weight loss that includes a substantial amount of fat during the first months after surgery, our aim was to test the impact of such surgery on abdominal fat depots 6 months after surgery. We further tested the relation of changes in the content of fat in the abdominal depots and improvement of the metabolic phenotype.
RESEARCH DESIGN AND METHODS
Twenty-seven morbidly obese adults undergoing bariatric surgery at the Hadassah Medical Center were recruited for this study. Procedures included laparoscopic gastric banding, sleeve gastrectomy, laparoscopic Roux en Y gastric bypass, and duodenal switch procedures. All patients met the criteria for bariatric operations as recommended by the National Institutes of Health Consensus Conference (11). Pre- and postoperative anthropometric measures included height to the nearest centimeter, weight to the nearest 0.1 kg, and waist (measured at the midpoint between the costal margin and the iliac crest) and hip circumference. Patients were seen at the obesity clinic before surgery and then monthly after surgery in the first month. Blood tests performed before and after surgery at 6 months included a blood count, general chemistry analysis, liver and kidney function tests, and lipid profiles. Abdominal fat depots were evaluated using computed tomography scans before and at 6 months after surgery. Visceral and subcutaneous adipose tissue cross-section areas were calculated using computer software specifically designed for area measurement (12). Body composition was assessed using bioelectrical impedance analysis (Tanita 305 body fat analyzer; Tanita, Tokyo, Japan). Clinical parameters related to the metabolic syndrome were evaluated using the Adult Treatment Panel III criteria (13). Resolution of these parameters was considered as drug discontinuation with normal measurements of fasting glucose, systolic and diastolic blood pressure, and normalization of triglyceride or HDL cholesterol levels. The study was approved by the institutional review board at the Hadassah Hebrew University Medical Center and registered in the National Institutes of Health Protocol Registration System.
Statistical analysis
Data are presented as means ± SD. Group comparisons between men/women and between those who lost the diagnosis of metabolic syndrome were performed using Wilcoxon's rank-sum test. P < 0.05 was considered significant. All analyses were performed using SPSS 15.0 for Windows.
RESULTS
Twenty-seven subjects underwent laparoscopic bariatric surgery that included Roux en Y gastric bypass (n = 14), sleeve gastrectomy (n = 7), laparoscopic gastric banding (n = 2), and duodenal switch (n = 4). Baseline and follow-up anthropometric characteristics of the groups according to sex are shown in Table 1. Participants had an excess body weight of 52.2 ± 15.1 kg (women) and 66.0 ± 10.5 kg (men) at baseline. Absolute weight loss and an excess weight loss at 6 months were 31.3 ± 9.6 kg and 47 ± 12% in men and 22.0 ± 8.7 kg and 42 ± 13% in women. In women, fat mass represented 55% of total weight loss, whereas in men it represented 70% of the total weight loss. Visceral fat was ∼35% lower on follow-up compared with baseline in both sexes, whereas subcutaneous fat was ∼32% lower on follow-up compared with baseline in both sexes. Thus, the visceral-to-subcutaneous fat ratio remained stable after surgically induced weight loss despite the significant weight loss observed.
Table 1.
Anthropometric parameters and abdominal fat depots before and after surgery
| Men (n = 13) |
Women (n = 14) |
|||||
|---|---|---|---|---|---|---|
| Before | After | P | Before | After | P | |
| n | 13 | 14 | ||||
| Age (years) | 49 ± 10 | 49 ± 11 | 0.96 | |||
| Weight (kg) | 134 ± 13 | 103 ± 11 | <0.001 | 104 ± 18 | 82 ± 14 | <0.001 |
| Height (cm) | 175 ± 9 | 157 ± 7 | ||||
| BMI (kg/m2) | 43.7 ± 4.6 | 33.5 ± 3.2 | <0.001 | 41.9 ± 5.4 | 33.1 ± 4.6 | <0.001 |
| Excess weight (kg) | 66.0 ± 10.5 | 34.7 ± 8.8 | <0.001 | 52.2 ± 15.1 | 30.2 ± 12.1 | <0.001 |
| Excess weight loss (%) | 47 ± 13 | 42 ± 13 | 0.36 | |||
| Fat mass (kg) | 48.9 ± 6.1 | 31.6 ± 5.8 | <0.001 | 51.2 ± 11.7 | 35.7 ± 9.5 | <0.001 |
| Fat mass lost (kg) | 17.3 | 15.5 | 0.43 | |||
| Waist circumference (cm) | 135 ± 7 | 115 ± 12 | <0.001 | 123 ± 13 | 104 ± 11 | <0.001 |
| Visceral fat (cm) | 163 ± 40 | 102 ± 30 | <0.001 | 126 ± 36 | 81 ± 21 | <0.001 |
| Subcutaneous fat (cm) | 309 ± 52 | 206 ± 51 | <0.001 | 306 ± 79 | 218 ± 85 | <0.001 |
| Visceral-to-subcutaneous ratio | 0.54 ± 0.15 | 0.50 ± 0.12 | 0.37 | 0.44 ± 0.21 | 0.44 ± 0.27 | 0.99 |
Data are means ±SD.
Clinical characteristics of study participants associated with the metabolic syndrome before and 6 months after surgery are shown in Table 2. All participants met the waist circumference threshold before surgery, and all men kept it on follow-up, whereas two women reduced their waist circumference below the threshold of 88 cm. Diabetes was present in 10 of 13 men and in 11 of 14 women before surgery and only 2 men and 4 women continued to take antihyperglycemic medications on follow-up. Similarly, significant improvements were observed in triglyceride levels and in the presence of hypertension. The prevalence of a low HDL cholesterol level did not change after surgery.
Table 2.
Presence of metabolic syndrome criteria before and after surgery
| Before surgery |
After surgery |
|||||
|---|---|---|---|---|---|---|
| Men | Women | P | Men | Women | P | |
| Hypertension | 13/0 | 11/3 | 0.12 | 4/9 | 5/9 | 0.55 |
| Diabetes | 10/3 | 11/3 | 0.63 | 2/11 | 4/10 | 0.36 |
| High triglyceride | 11/2 | 10/4 | 0.36 | 2/11 | 3/11 | 0.53 |
| Low HDL cholesterol | 9/4 | 9/5 | 0.55 | 10/3 | 9/5 | 0.38 |
| High waist circumference | 13/13 | 14/14 | 1.0 | 13/13 | 12/2 | 0.25 |
Data are n.
Relations of anthropometric changes and fat depots changes in study participants
The strongest relation between absolute and relative changes in visceral and subcutaneous fat was demonstrated for the excess weight loss after the operations (r = ∼0.6–0.7), and this relation was strengthened further for subcutaneous fat after adjustment for sex, baseline BMI, and fat mass (Table 3). Changes in weight and BMI correlated with absolute changes in abdominal fat depots, and the relation with changes in subcutaneous fat were significantly strengthened after adjustment for sex, baseline BMI, and fat mass. Changes in waist circumference and in fat mass had no relation to changes in abdominal fat depots.
Table 3.
Correlations between changes in anthropometric indices and changes in abdominal fat depots
| Δ Weight (%) | Δ BMI (5) | Δ Waist circumference (cm) | Δ Fat mass (kg) | Δ Visceral (cm) | Δ Visceral (%) | Δ Subcutaneous (cm) | Δ Subcutaneous (%) | |
|---|---|---|---|---|---|---|---|---|
| Excess weight loss (%) | 0.75* | 0.71* | 0.31 | 0.51† | 0.62* | 0.63* | 0.62* | 0.71* |
| Adjusted | 0.96* | 0.99* | 0.46 | 0.71† | 0.58† | 0.57† | 0.73† | 0.80* |
| Δ weight (%) | 0.91* | 0.60† | 0.78* | 0.50† | 0.43† | 0.53† | 0.37 | |
| Adjusted | 0.97* | 0.48 | 0.69† | 0.51 | 0.47 | 0.70* | 0.78* | |
| Δ BMI (%) | 0.65 † | 0.77* | 0.45 † | 0.46† | 0.51* | 0.32 | ||
| Adjusted | 0.48 | 0.72† | 0.55† | 0.55† | 0.77* | 0.82* | ||
| Δ waist circumference (cm) | 0.38 | 0.03 | 0.03 | −0.39 | −0.11 | |||
| Adjusted | 0.12 | −0.01 | 0.01 | −0.38 | −0.43 | |||
| Δ fat mass (kg) | −0.18 | −0.07 | −0.05 | 0.13 | ||||
| Adjusted | −0.43 | −0.47 | −0.42 | −0.43 | ||||
| Δ visceral (cm) | 0.91* | 0.59* | 0.66* | |||||
| Adjusted | 0.88* | 0.61† | 0.47 | |||||
| Δ Visceral (%) | 0.59* | 0.64* | ||||||
| Adjusted | 0.41 | 0.50 | ||||||
| Δ subcutaneous (cm) | 0.87* | |||||||
| Adjusted | 0.96* |
All changes are in their absolute value of change. Adjusted correlations are adjusted for sex, baseline BMI, and baseline fat mass.
*P < 0.001;
†P < 0.01.
Relation of changes in abdominal fat depots and metabolic parameters
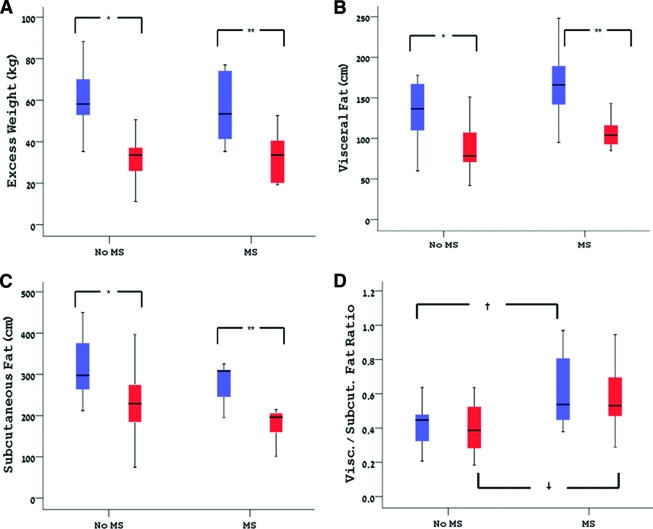
All study subjects met the criteria of the metabolic syndrome before surgery, and 18 of them did not meet these criteria at follow-up. When comparing those who lost the diagnosis on follow-up with those who did not (Table 4), we found no sex or procedure differences between the groups, yet those who did not meet the criteria of the syndrome after surgery were significantly younger (45 ± 9 vs. 57 ± 10 years, P = 0.01). The baseline numbers of metabolic syndrome criteria, BMI, fat mass, and excess weight were comparable between the groups. BMI, excess weight loss, and fat mass after the surgery were similar between the groups. As shown in Fig. 1A, excess weight loss on the follow-up was comparable between those who met the criteria of the metabolic syndrome on follow-up and those who did not. The only significant baseline difference between the groups was in the absolute amount of visceral fat (Fig. 1B), which was greater in those who did not lose the diagnosis (166 ± 49 vs. 132 ± 33 cm, P = 0.04). This difference translated to a significantly greater visceral-to-subcutaneous fat ratio (0.61 ± 0.21 vs. 0.43 ± 0.15, P = 0.02) (Fig. 1D). Similarly, both groups had similar amounts of subcutaneous (Fig. 1C) abdominal fat at baseline and a comparable reduction after surgery. Thus, all subjects lost a comparable significant amount of fat from both abdominal fat depots yet maintained a constant ratio between these depots.
Table 4.
Anthropometric parameters before surgery and their changes in those who lost the diagnosis of the metabolic syndrome compared with those who did not 6 months after surgery
| Lost MS | Remained MS | P | |
|---|---|---|---|
| n | 18 | 9 | |
| Age (years) | 45 ± 9 | 57 ± 10 | 0.01 |
| Sex (male/female) | 9/9 | 4/5 | 0.55 |
| Procedure (RYGB/LAGB/SG/DS) | 9/0/5/4 | 5/2/2/0 | 0.10 |
| MS score before | 3.9 ± 0.2 | 4.4 ± 0.2 | 0.11 |
| MS score after | 1.8 ± 0.3 | 3.33 ± 0.5 | <0.001 |
| Weight before (kg) | 120.7 ± 19.7 | 115.1 ± 25.7 | 0.46 |
| Weight after (kg) | 92.4 ± 15.1 | 92.3 ± 19.1 | 0.86 |
| BMI before (kg/m2) | 43.5 ± 5.3 | 41.2 ± 4.4 | 0.27 |
| BMI after (kg/m2) | 33.3 ± 4.0 | 33.1 ± 3.9 | 0.70 |
| Excess weight (kg) | 60.5 ± 13.6 | 55.6 ± 16.9 | 0.42 |
| Excess weight loss (%) | 46.6 ± 14.0 | 41.4 ± 10.8 | 0.99 |
| Fat mass before (kg) | 52.8 ± 10.2 | 46.2 ± 9.2 | 0.17 |
| Fat mass after (kg) | 34.9 ± 8.4 | 33.7 ± 9.3 | 0.71 |
Data are means ± SD. DS, duodenal switch; LAGB, laparoscopic gastric banding; MS, metabolic syndrome; RYGB, Roux en-Y gastric bypass; SG, sleeve gastrectomy.
Figure 1.
Comparison of excess weight lost (A), visceral fat change (B), subcutaneous fat change (C), and the visceral-to-subcutaneous fat ratio (D) between those who lost the diagnosis of the metabolic syndrome (No MS) and those who did not (MS). *P < 0.001; **P < 0.01; †P = 0.03.
CONCLUSIONS
This study demonstrates for the first time the early pattern of change in abdominal fat depots 6 months after bariatric procedures in severely obese subjects. The most consistent finding is that the ratio between visceral and subcutaneous abdominal fat remains fairly constant regardless of sex, procedure performed, or the presence of metabolic complications. Excess weight loss was associated with a reduction in fat in both abdominal fat depots more strongly than other anthropometric measures, whereas changes in waist circumference or fat mass had no relation with this reduction. A lower baseline visceral-to-abdominal fat ratio was the determinant of the improvement in metabolic parameters.
Lipid partitioning of specific fat depots in obese subjects is known to be associated with the overall metabolic phenotype (14,15). Specifically, intra-abdominal fat is associated with greater insulin resistance and has long been described as a culprit for accelerated atherogenesis and altered glucose metabolism. Standard interventions for obese individuals consist of lifestyle modifications that include dietary changes and physical activity. Diet-induced modest weight loss has been shown to affect visceral fat preferentially, whereas with larger degrees of weight loss the effect was shown to be similar for both visceral and subcutaneous fat (16). For every 1 kg of diet-induced weight loss, the corresponding reduction in visceral fat expressed in absolute terms is ∼3–4 cm2, and a 1-cm reduction in waist circumference corresponds to a 5-cm2 reduction in visceral fat area (17). In our subjects, mean visceral fat area reduction was 53.1 ± 35 cm2 (a reduction of 34.5 ± 17.1%). Mean weight loss in 6 months was 26.4 ± 10.2 kg (∼2 cm2 or 1.32% of visceral fat/kg weight lost), whereas mean waist circumference reduction was 18.5 ± 9.2 cm (∼ 3 cm2 or 1.85% of visceral fat per cm waist circumference reduction). Importantly, the strongest relation of visceral fat changes was with the excess weight lost, whereas changes in waist circumference had no relation whatsoever with the reduction in visceral fat or subcutaneous fat. Our subjects were more severely obese and had a greater absolute weight loss than those described in the previous references, which may provide some explanation for the seemingly lower visceral fat reduction in relation to weight loss. Whereas previous studies have shown a preferential loss of visceral fat in the early phases after bariatric surgery (8–10 weeks) (18,19), minimal changes in peripheral insulin sensitivity were observed. These results suggest that the ratio between abdominal and subcutaneous fat, which according to our data is rather stable, is one of the determinants of overall insulin sensitivity and of its clinical consequences.
Whether the absolute amount of visceral fat has a threshold above which metabolic derangements tend to occur or whether the ratio of visceral-to-subcutaneous fat is the main determinant of such derangements (or maybe both) is debatable. In both men and women, a value of 100 cm2 of visceral fat has been shown to be associated with significant alterations in cardiovascular disease risk profile and a further deterioration in the metabolic profile was observed with values >130 cm2 of visceral adipose tissue (20). Participants in our study had greater visceral fat before surgery than those described in previous studies, yet a significant number had an area of <100 cm2 after the bariatric procedure, correlating well with the significant improvement in metabolic parameters (the mean visceral fat area in the group who lost the diagnosis of metabolic syndrome was 86.6 ± 26.5 cm2).
On the other hand, a ratio of visceral-to-subcutaneous fat of 0.4 has been suggested to be a threshold value that signifies metabolic risk (21). Indeed, the patients who did not lose the diagnosis of the metabolic syndrome had a high visceral-to-subcutaneous fat ratio to begin with, and this ratio did not change despite significant weight loss. In comparison with exercise regimens, which have been reported to reduce the visceral-to-subcutaneous ratio by up to 33% (22), treatment with metformin showed no change in the relation of abdominal fat depots despite a significant weight loss (23) and thiazoladinediones reduced this ratio by way of increasing the amount of subcutaneous fat (24). It seems that rapid weight loss induced by bariatric surgical procedures, which can be considered a period of a very low calorie diet, induces a proportional decrease in both abdominal fat depots, resulting in a maintained ratio between them. Because both the absolute amount of visceral fat and the visceral-to-subcutaneous fat ratio are determinants of the clinical metabolic characteristics of obese individuals, a lower ratio at baseline probably raises the chances of clinical improvement because the ratio remains fairly constant due to proportional fat loss from both depots. However, the “visceral threshold” is probably crossed back to the tolerable range (25).
This study is limited by the modest sample size, short follow-up period, and the variability of procedures performed, which differ in their malabsorption component and may thus have a different impact on lipid partitioning after surgery. Therefore, although we were interested in the impact of surgically induced rapid weight loss on lipid depot distribution in general, specific procedures at different ages may differ in their overall impact. The findings raise the possibility of adding parameters in addition to BMI as indications and predictors of the metabolic response to bariatric procedures. Present indications for bariatric surgery are based (11) only on BMI and obesity-related comorbidity and disregard lipid partitioning patterns. Our findings suggest that baseline assessment of abdominal fat depots may add information regarding the expected early clinical response, whereas changes in waist circumference correlate poorly with visceral fat reduction. Longer follow-up is still needed to determine whether the observed changes remain stable over longer periods.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial reg. no. NCT00431587, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K: Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 2. Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lönroth H, Näslund I, Olbers T, Stenlöf K, Torgerson J, Agren G, Carlsson LM: Swedish Obese Subjects Study. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 3. Norris SL, Zhang X, Avenell A, Gregg E, Brown TJ, Schmid CH, Lau J: Long-term non-pharmacologic weight loss interventions for adults with type 2 diabetes. Cochrane Database Syst Rev 2005;2:CD004095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, Hilton L, Suttorp M, Solomon V, Shekelle PG, Morton SC: Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med 2005;142:532–546 [DOI] [PubMed] [Google Scholar]
- 5. Wajchenberg BL: Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21:697–738 [DOI] [PubMed] [Google Scholar]
- 6. Mårin P, Andersson B, Ottosson M, Olbe L, Chowdhury B, Kvist H, Holm G, Sjöström L, Björntorp P: The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism 1992;41:1242–1248 [DOI] [PubMed] [Google Scholar]
- 7. Bray GA, Jablonski KA, Fujimoto WY, Barrett-Connor E, Haffner S, Hanson RL, Hill JO, Hubbard V, Kriska A, Stamm E, Pi-Sunyer FX: Diabetes Prevention Program Research Group. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am J Clin Nutr 2008;87:1212–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, Sr, O'Donnell CJ: Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 2007;116:39–48 [DOI] [PubMed] [Google Scholar]
- 9. Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, Janssen I: Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men: a randomized, controlled trial. Ann Intern Med 2000;133:92–103 [DOI] [PubMed] [Google Scholar]
- 10. Després JP, Ross R, Boka G, Alméras N, Lemieux I: ADAGIO-Lipids Investigators. Effect of rimonabant on the high-triglyceride/low-HDL-cholesterol dyslipidemia, intraabdominal adiposity, and liver fat: the ADAGIO-Lipids trial. Arterioscler Thromb Vasc Biol 2009;29:416–423 [DOI] [PubMed] [Google Scholar]
- 11. NIH Consensus Development Panel. Gastrointestinal surgery for severe obesity. Ann Intern Med 1991;115:956–961 [PubMed] [Google Scholar]
- 12. Chowdhury B, Sjöström L, Alpsten M, Kostanty J, Kvist H, Löfgren R: A multicompartment body composition technique based on computerized tomography. Int J Obes Relat Metab Disord 1994;18:219–234 [PubMed] [Google Scholar]
- 13. National Institutes of Health. Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Bethesda, MD, National Institutes of Health; 2001. (NIH publ. no. 01-3670) [DOI] [PubMed] [Google Scholar]
- 14. Després JP, Lemieux I: Abdominal obesity and metabolic syndrome. Nature 2006;444:881–887 [DOI] [PubMed] [Google Scholar]
- 15. Weiss R: Fat distribution and storage: how much, where, and how? Eur J Endocrinol 2007;157(Suppl. 1):S39–S45 [DOI] [PubMed] [Google Scholar]
- 16. Chaston TB, Dixon JB: Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes (Lond) 2008;32:619–628 [DOI] [PubMed] [Google Scholar]
- 17. Ross R: Effects of diet- and exercise-induced weight loss on visceral adipose tissue in men and women. Sports Med 1997;24:55–64 [DOI] [PubMed] [Google Scholar]
- 18. Phillips ML, Lewis MC, Chew V, Kow L, Slavotinek JP, Daniels L, Valentine R, Toouli J, Thompson CH: The early effects of weight loss surgery on regional adiposity. Obes Surg 2005;15:1449–1455 [DOI] [PubMed] [Google Scholar]
- 19. Busetto L, Tregnaghi A, Bussolotto M, Sergi G, Benincà P, Ceccon A, Giantin V, Fiore D, Enzi G: Visceral fat loss evaluated by total body magnetic resonance imaging in obese women operated with laparoscopic adjustable silicone gastric banding. Int J Obes Relat Metab Disord 2000;24:60–69 [DOI] [PubMed] [Google Scholar]
- 20. Lamarche B: Effects of diet and physical activity on adiposity and body fat distribution: implications for the prevention of cardiovascular disease. Nutr Res Rev 1993;6:137–159 [DOI] [PubMed] [Google Scholar]
- 21. Fujioka S, Matsuzawa Y, Tokunaga K, Tarui S: Contribution of intra-abdominal fat accumulation to the impairment of glucose and lipid metabolism in human obesity. Metabolism 1987;36:54–59 [DOI] [PubMed] [Google Scholar]
- 22. Boudou P, de Kerviler E, Erlich D, Vexiau P, Gautier JF: Exercise training-induced triglyceride lowering negatively correlates with DHEA levels in men with type 2 diabetes. Int J Obes Relat Metab Disord 2001;25:1108–1112 [DOI] [PubMed] [Google Scholar]
- 23. Lord J, Thomas R, Fox B, Acharya U, Wilkin T: The effect of metformin on fat distribution and the metabolic syndrome in women with polycystic ovary syndrome—a randomised, double-blind, placebo-controlled trial. BJOG 2006;113:817–824 [DOI] [PubMed] [Google Scholar]
- 24. Yang X, Smith U: Adipose tissue distribution and risk of metabolic disease: does thiazolidinedione-induced adipose tissue redistribution provide a clue to the answer? Diabetologia 2007;50:1127–1139 [DOI] [PubMed] [Google Scholar]
- 25. Kral JG: Surgical treatment of regional adiposity: lipectomy versus surgically induced weight loss. Acta Med Scand Suppl 1988;723:225–231 [PubMed] [Google Scholar]