Abstract
OBJECTIVE
Cystic fibrosis–related diabetes (CFRD) without fasting hyperglycemia (CFRD FH−) is not associated with microvascular or macrovascular complications, leading to controversy about the need for treatment. The Cystic Fibrosis Related Diabetes Therapy (CFRDT) Trial sought to determine whether diabetes therapy improves BMI in these patients.
RESEARCH DESIGN AND METHODS
A three-arm multicenter randomized trial compared 1 year of therapy with premeal insulin aspart, repaglinide, or oral placebo in subjects with cystic fibrosis who had abnormal glucose tolerance.
RESULTS
One hundred adult patients were enrolled. Eighty-one completed the study, including 61 with CFRD FH− and 20 with severly impaired glucose tolerance (IGT). During the year before therapy, BMI declined in all groups. Among the group with CFRD FH−, insulin-treated patients lost 0.30 ± 0.21 BMI units the year before therapy. After 1 year of insulin therapy, this pattern reversed, and they gained 0.39 ± 21 BMI units (P = 0.02). No significant change in the rate of BMI decline was seen in placebo-treated patients (P = 0.45). Repaglinide-treated patients had an initial significant BMI gain (0.53 ± 0.19 BMI units, P = 0.01), but this effect was not sustained. After 6 months of therapy they lost weight so that by 12 months there was no difference in the rate of BMI change during the study year compared with the year before (P = 0.33). Among patients with IGT, neither insulin nor repaglinide affected the rate of BMI decline. No significant differences were seen in the rate of lung function decline or the number of hospitalizations in any group.
CONCLUSIONS
Insulin therapy safely reversed chronic weight loss in patients with CFRD FH−.
Approximately 30,000 individuals with cystic fibrosis live in the U.S. With steady advances in medical care, average life expectancy is now ∼38 years. Diabetes due to insulin insufficiency is the most common comorbidity in this population, occurring in 40–50% of adult patients with cystic fibrosis (1). Approximately 15% have diabetes with fasting hyperglycemia (CFRD FH+) and require insulin therapy to prevent classic diabetes symptoms and microvascular complications. The 25% of adult patients with cystic fibrosis who have diabetes without fasting hyperglycemia (CFRD FH−) pose a greater clinical dilemma. During a standard oral glucose tolerance test (OGTT), they have normal fasting glucose levels, but their 2-h glucose is ≥200 mg/dl (11.1 mmol/l). It has been argued that patients with CFRD FH− do not require diabetes therapy because they are asymptomatic, have relatively normal glucose levels when measured at home by self-monitoring of blood glucose, and have minimal A1C elevation (2). Unlike the general diabetic population, they do not appear to be at risk for microvascular or macrovascular complications (3). However, it has also been argued that although the metabolic complications of intermittent postprandial hyperglycemia may not be severe in these patients, the nutritional consequences of insulin deficiency may be life-threatening (4).
Survival of individuals with cystic fibrosis is intimately connected to nutritional status; underweight and protein catabolism are associated with poor pulmonary function and death. Insulin is a potent anabolic hormone, and insulin-insufficient cystic fibrosis patients have increased protein and fat breakdown (4–6). Pulmonary function decline is also related to the severity of insulin insufficiency (7). Thus, insulin insufficiency may increase morbidity and mortality by contributing to loss of weight and lean body mass. We hypothesized that insulin therapy would reverse nutritional deterioration in these patients.
The addition of an insulin regimen is a significant treatment burden for patients whose lives involve complex and time-consuming medical care, and prescription of such a regimen requires clear evidence of benefit. The question of whether CFRD FH− patients should receive diabetes therapy was given the highest research priority by a national consensus conference on CFRD (2). The Cystic Fibrosis Related Diabetes Therapy (CFRDT) Trial was undertaken to determine whether premeal therapy with either rapid-acting insulin or the oral insulin secretagogue repaglinide would improve BMI in this population.
RESEARCH DESIGN AND METHODS
Fourteen cystic fibrosis centers in the U.S., Canada, and U.K. participated. The University of Minnesota was the Coordinating Center, and the Data Management Center was located at the University of Massachusetts (Amherst, MA). Participating centers routinely performed annual OGTT screening. Fasting subjects were given 1.75 g/kg (maximum 75 g) of an oral glucose solution, and glucose levels were measured over 2 h. OGTTs were performed during stable baseline health.
Patients with cystic fibrosis who had CFRD FH− (fasting plasma glucose <126 mg/dl [7.0 mmol/l] and 2-h glucose ≥200 mg/dl [11.1 mmol/l]) or severe impaired glucose tolerance (IGT) (glucose level ≥200 mg/dl [11.1 mmol/l] during the OGTT and a 2-h glucose level of 180–199 mg/dl [10.0–11.1 mmol/l]) were recruited. Additional eligibility criteria included completion of linear growth, stable weight within 5% during the previous 3 months, and no evidence of acute infection in the previous 2 months. Exclusion criteria included fasting hyperglycemia in the previous year, oral or intravenous glucocorticoid therapy in the previous 6 months, liver dysfunction, and pregnancy. Block randomization, using a pseudo–random number generator with stratification by center, was used to ensure a more even distribution of treatments over time and across sites in the study. One-third of patients per block were assigned to each treatment arm. Institutional review board approval was obtained at the Coordinating Center and at each center; informed consent was obtained from all subjects.
Treatment protocol
Patients were randomly assigned to receive 0.5 unit insulin aspart/15 g dietary carbohydrate, 2.0 mg repaglinide orally, or oral placebo three times a day before meals. The oral agents were double-blinded; there was no injectable placebo because of ethical concerns. Study coordinators contacted patients weekly. If subjects experienced hypoglycemia, additional education was provided to ensure sufficient carbohydrate intake. If dietary measures were insufficient to prevent postprandial hypoglycemia, the medication dose was reduced. Persistent postprandial hyperglycemia led to a dosage increase. Subjects were followed for 1 year and were seen by the study team quarterly. Insulin vials and pill bottles were returned quarterly for medication adherence monitoring.
Ongoing diabetes education was provided. Dietary recommendations were the same as for all patients with cystic fibrosis, including consumption of a high-calorie diet with three meals and at least three snacks per day with no restriction on total daily carbohydrate intake. Patients were taught carbohydrate counting and instructed to consume a minimum of 60 g carbohydrate with each meal to reduce the risk of hypoglycemia. Those receiving rapid-acting insulin were taught to match the insulin dose to the carbohydrate content of the meal at a dose of 0.5 unit/15 g carbohydrate.
Patients with CFRD FH− are at risk for permanent progression to CFRD FH+. This was a planned stopping point because it required patients to switch to basal-bolus insulin therapy. However, patients with cystic fibrosis can develop transient fasting hyperglycemia during acute illness. Patients were withdrawn from the study only if they developed acute illness-associated fasting hyperglycemia that persisted for >1 month, at which point a chronic change in their diabetes condition was considered to have occurred. In addition, patients were withdrawn if they required systemic glucocorticoids for >1 month because of the effect of these drugs on BMI.
Study end point measurement
All patients were followed at accredited cystic fibrosis centers with standardized procedures for quarterly measurement of clinical outcomes including BMI and pulmonary function. BMI was chosen as the primary study end point because it is well correlated with survival in cystic fibrosis (8–10). BMI and lung function parameters measured 12 months before study entry were obtained from chart review. During the study they were prospectively measured quarterly. Additional measures obtained at baseline and 12 months included dual-energy X-ray absorptiometry for body composition, the National Institutes of Health (NIH) prognostic score (a measure of overall clinical health) (11,12), a validated cystic fibrosis quality-of-life (CFQOL) survey (13), 3-day dietary histories, A1C (measured centrally at University of Minnesota), and fasting and 90-min after main meal capillary glucose levels obtained at home by self-monitoring of blood glucose.
Data analysis
Summary statistics were constructed at each measurement occasion with the use of means, SDs, medians, and interquartile ranges for continuous factors and frequencies and percentages for categorical factors. χ2 or Fisher's exact tests were used to evaluate association of treatment arm and baseline OGTT status with patient baseline characteristics, study completion, and treatment adherence for each categorical factor; ANOVA models were used to evaluate differences for continuous-scale factors. To assess treatment effects, a series of ANCOVA models for change from baseline in BMI, forced vital capacity (FVC), and forced expiratory volume in time interval (FEV1) were evaluated for each measurement occasion, adjusting for patient baseline characteristics, adherence, and prior-year change. Initial models included all subjects with data available at each measurement occasion. Final models were stratified by OGTT class (FH− or IGT) at baseline and included the 81 patients who completed the full study year. Additional models were used to evaluate the change from baseline to 1 year in fat-free mass, NIH score, CFQOL, and A1C.
RESULTS
Study enrollment, dropouts, and adherence
One hundred patients were enrolled, 74 with CFRD FH− and 26 with severe IGT. Nineteen dropped out or were stopped early; the study was completed by 77% of subjects with IGT and 82% of subjects with CFRD FH− (P = 0.57). There was no difference by treatment group in the percentage of patients dropping out/stopping early (P = 0.87) (supplementary Appendix B, available at http://care.diabetesjournals.org/cgi/content/full/dc9-0585/DC1). Among all participants, eight chose to quit, including five (13%) receiving insulin and three (5%) receiving oral therapy (P = 0.25). Five patients were stopped early because they developed permanent fasting hyperglycemia, including two (5%) receiving insulin, three (10%) receiving placebo, and none receiving repaglinide (P = 0.27). Six additional patients (one receiving insulin and five receiving oral therapy) discontinued the study for other medical reasons, including chronic use of steroids (one receiving insulin and one receiving repaglinide), pregnancy (one receiving repaglinide), rash attributed by the patient to medication (placebo), and chronic hospitalization, too ill to take medication (two receiving repaglinide) (P = 0.12 for association of reason for discontinuation with treatment). Reasons for dropout are shown in supplementary Appendix C (available in an online appendix).
Study drug adherence was similar among the study arms. Compliance data were available at ∼75% of study visits. Patients were deemed compliant if they used ≥90% of the expected drug; ∼25% of those with adherence data were deemed noncompliant. This result is similar to the reported adherence to medication regimens in adults with cystic fibrosis (14). There was no difference in adherence between those with CFRD FH− and IGT (P = 0.91) and no difference by treatment arm (P = 0.91).
Baseline characteristics
There were no significant differences in baseline characteristics between patients who completed the study and those who stopped early, with the exception of CFQOL. Those who stopped early had lower scores for eating disturbance (P < 0.05) and social/marginalization scales (P < 0.05).
Baseline characteristics of the 81 study completers are presented in Table 1. Patients in the placebo group appeared slightly healthier at baseline, with somewhat higher BMI and better pulmonary function, but these did not achieve statistical significance. Age, baseline BMI, and BMI change during the preceding year, baseline lung function and change during the preceding year, body composition, and CFQOL did not differ between patients with CFRD FH− and IGT. However, a greater proportion of those with IGT were men (75 vs. 49%, P = 0.04). As expected, A1C was lower in the IGT group (5.5 ± 0.4 vs. 6.1 ± 0.6%, P = 0.0002).
Table 1.
Baseline characteristics of the 81 subjects who completed the CFRDT trial
| CFRD FH− |
IGT |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total cohort | Insulin | Repaglinide | Placebo | Total cohort | Insulin | Repaglinide | Placebo | |
| n | 61 | 23 | 22 | 16 | 20 | 7 | 4 | 9 |
| n (% female) | 31 (51)* | 12 (52) | 11 (50) | 8 (50) | 5 (25) | 3 (43) | 0 (0) | 2 (22) |
| Age (years) | 28 ± 9 | 29 ± 2 | 26 ± 2 | 28 ± 3 | 28 ± 7 | 27 ± 2 | 29 ± 5 | 28 ± 2 |
| BMI (kg/m2) | 21.3 ± 2.9 | 20.6 ± 0.6 | 21.3 ± 0.7 | 22.2 ± 0.6 | 22.0 ± 2.7 | 20.7 ± 0.7 | 22.9 ± 1.7 | 22.5 ± 0.9 |
| % Fat-free mass | 79 ± 9 | 79 ± 2 | 80 ± 2 | 77 ± 2 | 80 ± 8 | 81 ± 3 | 79 ± 5 | 80 ± 3 |
| FVC (% predicted) | 90 ± 22 | 87 ± 4 | 90 ± 6 | 94 ± 4 | 88 ± 17 | 79 ± 7 | 82 ± 10 | 98 ± 4 |
| FEV1 (% predicted) | 70 ± 25 | 63 ± 5 | 71 ± 6 | 78 ± 5 | 69 ± 21 | 61 ± 7 | 61 ± 13 | 78 ± 6 |
| NIH score | 83 ± 13 | 80 ± 3 | 84 ± 3 | 88 ± 3 | 82 ± 14 | 79 ± 6 | 74 ± 9 | 89 ± 2 |
| A1C (%) | 6.1 ± 0.6* | 6.2 ± (0.1) | 6.2 ± 0.1 | 6.0 ± 0.1 | 5.5 ± 0.4 | 5.5 ± 0.3 | 5.6 ± 0.1 | 5.5 ± 0.1 |
Data for the CFRD FH− and IGT total cohorts are means ± SD; data by treatment group as means ± SEM. There were no significant differences across treatment groups for CFRD FH− or IGT.
*Total cohort CFRD FH− vs. IGT, P < 0.05.
Treatment effect on body composition
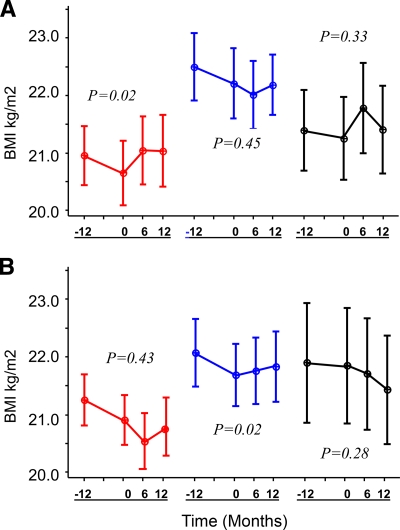
Change in BMI the year before study participation was compared with change during the study year (Table 2). The previous year, all groups showed a decline in BMI with no significant difference among treatment groups in the loss (Fig. 1). Insulin-treated patients with CFRD FH− lost an average of 0.30 ± 0.21 BMI unit in the year before therapy (∼ 2.5 pounds in women and 3 pounds in men). After 1 year of study participation, those who received premeal rapid-acting insulin reversed this chronic downward clinical course and gained 0.39 ± 21 BMI unit (P = 0.02) (Fig. 1). Weight gain distribution was about 80% fat and 20% fat-free mass. No significant difference in the rate of BMI loss relative to the prior year was seen in placebo-treated CFRD FH− patients (P = 0.45). Patients who received repaglinide had an initial significant gain of 0.53 ± 0.19 BMI units within the first 6 months of therapy (P = 0.01). This effect, however, was not sustained. After 6 months they lost weight so that by the end of 12 months there was no difference in the rate of BMI change during the study year compared with the year before (P = 0.33). Adjustment for baseline BMI, weight, or sex did not influence these conclusions. Changes in body composition were not related to dietary intake; there was no significant difference in daily calorie consumption from baseline to 12 months in any treatment group and no differences between the groups. The absolute change in BMI during the study year did not differ significantly between CFRD FH− groups (P = 0.36, insulin vs. placebo; P = 0.95, repaglinide vs. placebo; and P = 0.35, insulin vs. repaglinide).
Table 2.
Comparison of the change in BMI and lung function the year before and after 1 year of study participation
| CFRD FH− |
IGT |
|||||
|---|---|---|---|---|---|---|
| Insulin | Repaglinide | Placebo | Insulin | Repaglinide | Placebo | |
| n | 61 | 20 | ||||
| BMI | ||||||
| −12 months to baseline (kg/m2) | −0.30 ± 0.21 | −0.14 ± 0.21 | −0.29 ± 0.25 | −0.60 ± 0.30 | −0.08 ± 0.40 | −0.66 ± 0.27 |
| Baseline to +12 months (kg/m2) | 0.39 ± 0.21 | 0.15 ± 0.21 | −0.02 ± 0.25 | −0.26 ± 0.30 | −0.71 ± 0.40 | 0.24 ± 0.27 |
| P value | 0.02 | 0.33 | 0.45 | 0.43 | 0.28 | 0.02 |
| FVC | ||||||
| −12 months to baseline (% predicted) | −5.8 ± 2.0 | −5.5 ± 2.1 | −4.3 ± 2.5 | 2.0 ± 4.2 | −3.4 ± 5.6 | −2.8 ± 3.7 |
| Baseline to +12 months | −0.5 ± 2.0 | −2.1 ± 2.1 | −1.1 ± 2.5 | −10.3 ± 4.2 | −3.1 ± 5.6 | −5.1 ± 3.7 |
| P value | 0.21 | 0.25 | 0.37 | 0.05 | 0.96 | 0.6 |
| FEV1 | ||||||
| −12 months to baseline (% predicted) | −5.7 ± 2.2 | −6.5 ± 2.2 | −0.5 ± 2.7 | 0.6 ± 5.6 | −2.5 ± 7.4 | 2.8 ± 4.9 |
| Baseline to +12 months | −1.8 ± 2.2 | −1.3 ± 2.2 | −3.0 ± 2.7 | 12.1 ± 5.6 | −4.9 ± 7.4 | −11.5 ± 4.9 |
| P value | 0.21 | 0.10 | 0.51 | 0.12 | 0.82 | 0.05 |
Data are means ± SEM.
Figure 1.
BMI 12 months before and 6 and 12 months after treatment with insulin (red), placebo (blue), or repaglinide (black) in 61 subjects with CFRD FH− (A) and 20 subjects with IGT (B). Data are means ± SEM. P values are for study year (0–12 months) compared with previous year (−12 to 0 months) within each group.
Among patients with IGT, neither the insulin- nor the repaglinide-treated groups showed a significant difference in the rate of BMI decline during the study year compared with the previous year. A significant improvement was seen in the placebo group (P = 0.02). The absolute change in BMI during the study year was significantly worse for repaglinide-treated patients with IGT (P = 0.34, insulin vs. placebo; P = 0.03, repaglinide vs. placebo; and P = 0.18, insulin vs. repaglinide).
Treatment effect on clinical status
A1C levels did not significantly change in any group. After 1 year of therapy there was no difference in fasting glucose between treatment groups and no difference from baseline. Postprandial glucose, however, was somewhat lower in those receiving insulin therapy, although this did not achieve statistical significance. For CFRD FH− patients, the 90-min postprandial glucose was 116 ± 4 mg/dl (6.4 ± 0.2 mmol/l) in the insulin group, 138 ± 12 mg/dl (7.7 ± 0.7 mmol/l) in the placebo group, and 131 ± 7 mg/dl (7.3 ± 0.4 mmol/l) in the repaglinide group (P = 0.06, insulin vs. placebo and P = 0.81, repaglinide vs. placebo). Among those with IGT, the 90-min postprandial glucose was 114 ± 3 mg/dl (6.3 ± 0.2 mmol/l) in the insulin group, 122 ± 4 mg/dl (6.8 ± 0.2 mmol/l) in the placebo group, and 131 ± 9 mg/dl (7.3 ± 0.5 mmol/l) in the repaglinide group (P = 0.20, insulin vs. placebo; P = 0.81, repaglinide vs. placebo; and P = 0.03, insulin vs. repaglinide).
Although there appeared to be a trend toward less decline in FVC in all of the CFRD FH− study arms and less decline in FEV1 in the insulin and repaglinide arms, these changes were not statistically significant (Table 2). There were no differences in the number of episodes of acute illness during the study year by treatment arm or OGTT class. NIH and CFQOL scores did not differ between groups at baseline or over the treatment year in any group.
Adverse events
No serious adverse events related to study medication occurred. In the first 3 months, significantly more patients receiving active medication compared with those receiving placebo reported mild hypoglycemic events (insulin 16%, repaglinide 23%, and placebo none, P < 0.04). In most cases these resolved with patient education. After the first 3 months, there were no significant differences between groups in the frequency of hypoglycemia, and there were some episodes of mild hypoglycemia reported by patients receiving placebo. Of 30 subjects receiving insulin who completed the study, persistent postprandial glucose abnormalities led to an increased dose in 4 patients (13%) and a decreased dose in 2 patients (7%). Of 26 subjects receiving repaglinide who completed the study, persistent postprandial glucose abnormalities led to an increased dose in 3 patients (12%) and a decreased dose in 4 patients (15%).
CONCLUSIONS
It has been hypothesized that insulin insufficiency compromises health and survival in cystic fibrosis by producing a catabolic state with associated weight loss and reduced lean body mass (4). Previous reports have suggested that weight and/or pulmonary function may improve after institution of insulin therapy, but none of these studies were randomized controlled trials and most had mixed populations of CFRD patients with and without fasting hyperglycemia (15–20). In the current placebo-controlled multicenter trial, insulin replacement therapy significantly reversed the trajectory of chronic weight loss in CFRD FH− patients.
No significant changes were seen in the annual rate of decline in FEV1 or FVC or the number of acute illnesses, and there were no changes from baseline in NIH scores. These findings are not surprising given that an earlier study required 4 years of observation before significant trends in lung function could be documented between subjects with normal, impaired, and diabetic glucose tolerance (7). Patients with cystic fibrosis die of chronic inflammatory lung disease, and ultimately lung function is the most important end point. However, body weight is strongly related to cystic fibrosis lung function and survival (8–10).
In the current study, insulin-treated patients stopped losing weight and gained both fat and lean body mass. This result seemed to be mediated primarily via the anabolic rather than the glucose homeostatic effect of insulin because it was accomplished without a significant change in A1C or blood glucose levels. Although total caloric intake did not differ between groups and all patients received the same education with regard to carbohydrate counting, we cannot exclude the possibility that patients receiving insulin therapy were more attentive to carbohydrate intake because they were matching it to insulin dose and because patients receiving insulin were not blinded.
Repaglinide had a transient effect on weight gain in CFRD FH− patients. It was chosen for this study because sulfonylureas had previously been anecdotally associated with hypoglycemia in CFRD. In pilot studies, premeal treatment with either insulin or 1 mg repaglinide improved glucose and insulin excursion during a standardized liquid mixed meal, but insulin had a significantly greater effect (21). In the current study, the initial benefits of 2 mg repaglinide were not sustained, because after 6 months the rate of weight loss was similar to pretreatment values and early gains were lost. There was not a measurable change in medication adherence to explain this result. Concerns have been raised in other conditions of reduced β-cell mass that agents that stimulate insulin secretion may ultimately wear out stressed β-cells. In one study, a year of insulin therapy improved endogenous insulin secretion in subjects with early type 2 diabetes, whereas patients who received 1 year of sulfonylurea therapy had a decline in β-cell function (22). The proposed mechanism was “resting” versus “exhausting” the cells. Prolonged excessive stimulation with sulfonylureas has been shown to induce β-cell apoptosis in cultured human islets (23). Paradoxically, repaglinide therapy had a negative effect on weight in patients with IGT. Thus, use of this agent in patients with cystic fibrosis and IGT cannot be recommended at present.
Insulin therapy is not easy. Although the number of dropouts was similar among treatment groups, patients receiving insulin injections were more likely to choose to quit, whereas in the other groups dropout was more likely to be due to a medical problem that disqualified the patient from further study participation. However, the majority of subjects in all three groups completed the study, adherence with study drug was similar among the oral and injection treatment arms, and there was no difference in CFQOL among treatment groups. During the study, patients were told that it was not known whether diabetes therapy would have a positive health impact. Adherence may be better when there is a proven benefit.
Is premeal rapid-acting insulin the best regimen for patients with CFRD FH−? It was chosen for this trial on the basis of evidence that metabolic defects in cystic fibrosis are most pronounced in the postprandial state (4,5,23) and because of theoretical concerns that bedtime NPH insulin might result in hypoglycemia in these patients with normal fasting glucose levels. At the time the study began, the newer basal insulin analogs were not yet widely available. Recently, small pilot studies have suggested that low-dose insulin glargine may improve weight or pulmonary function in patients with cystic fibrosis who have abnormal glucose tolerance without causing significant hypoglycemia (19,20). Further work needs to be done to compare and/or combine basal and premeal insulin regimens in this population.
No apparent clinical improvement was seen with treatment of patients with cystic fibrosis who had severe IGT, although for reasons that are not clear, BMI improved in placebo-treated patients. These data need to be interpreted with caution. Glucose tolerance abnormalities in cystic fibrosis represent a spectrum. We originally believed that subjects with IGT and CFRD FH− were metabolically similar enough that their results could be pooled, but their responses to treatment proved to be different. There was greater variability in the IGT group compared with the CFRD FH− group, along with fewer participants; we would have needed a greater number of subjects with IGT to derive any definitive conclusions about the effect of treatment. Furthermore, there were significantly more men in the IGT group. This difference may be important because prognosis is worse in women with CFRD (24). The present data demonstrate that studies exploring carbohydrate metabolism in cystic fibrosis should consider subjects with CFRD separately from those with IGT and that further research is needed to determine the most appropriate treatment of IGT.
In the CFRDT trial, insulin therapy safely reversed chronic weight loss in patients with CFRD FH−. Insulin may be successful in breaking the cycle of protein catabolism, weight loss, and pulmonary function decline because of its anabolic effects. A significant percentage of the adult cystic fibrosis patient population could benefit from this therapy, with a substantial impact on morbidity and mortality.
Supplementary Material
Acknowledgments
This project was supported by NIH Grant R01-DK-58356 (to A.M.), NIH Grant M01-RR-00400 (General Clinical Research Center), and a grant from the Cystic Fibrosis Foundation.
Study drug and blood glucose testing supplies were generously donated by Novo Nordisk (Princeton, NJ) and Lifescan (Johnson & Johnson, Milpitas, CA), respectively. No other potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial reg. no. NCT00072904, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Moran A, Doherty L, Wang X, Thomas W: Abnormal glucose metabolism in cystic fibrosis. J Pediatr 1998;133:10–16 [DOI] [PubMed] [Google Scholar]
- 2. Moran A, Hardin D, Rodman D, Allen HF, Beall RJ, Borowitz D, Brunzell C, Campbell PW, Chesrown SE, Duchow C, Fink RJ, FitzSimmons SC, Hamilton N, Hirsch I, Howenstine MS, Klein DJ, Madhun Z, Pencharz PB, Quittner AL, Robbins MK, Schindler T, Schissel K, Schwarzenberg SJ, Stallings VA, Tullis DE, Zipf WB: Diagnosis, screening, and management of CFRD: a consensus conference report. J Diabetes Res Clin Pract 1999;45:55–71 [DOI] [PubMed] [Google Scholar]
- 3. Schwarzenberg SJ, Thomas W, Olsen TW, Grover T, Walk D, Milla CE, Moran A: Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007;30:1056–1061 [DOI] [PubMed] [Google Scholar]
- 4. Moran A, Milla C, DuCret R, Nair KS: Protein metabolism in clinically stable adult CF patients with abnormal glucose tolerance. Diabetes 2001;50:1336–1343 [DOI] [PubMed] [Google Scholar]
- 5. Moran A, Basu R, Milla C, Jensen M: Insulin regulation of free fatty acid kinetics in adult cystic fibrosis patients with impaired glucose tolerance. Metabolism 2004;53:1467–1472 [DOI] [PubMed] [Google Scholar]
- 6. Hardin DS, Leblanc A, Lukenbaugh S, Para L, Seilheimer DK: Proteolysis associated with insulin resistance in cystic fibrosis. Pediatrics 1998;101:433–437 [DOI] [PubMed] [Google Scholar]
- 7. Milla CE, Warwick WJ, Moran A: Trends in pulmonary function in cystic fibrosis patients correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med 2001;162:891–895 [DOI] [PubMed] [Google Scholar]
- 8. Corey M, McLaughlin FJ, Williams M, Levison H: A comparison of survival, growth and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol 1988;41:583–591 [DOI] [PubMed] [Google Scholar]
- 9. Snell GI, Bennetts K, Bartolo J, Levvey B, Griffiths A, Williams T, Rabinov M: Body mass index as a predictor of survival in adults with cystic fibrosis referred for lung transplantation. J Heart Lung Transplant 1998;17:1097–1103 [PubMed] [Google Scholar]
- 10. Kerem E, Reisman J, Corey M, Canny GC, Levison H: Prediction of mortality in patients with cystic fibrosis. N Engl J Med 1992;326:1187–1191 [DOI] [PubMed] [Google Scholar]
- 11. Taussig LM, Kattwinkel J, Friedewald WT, di Sant'Agnese PA: A new prognostic score and clinical evaluation system for cystic fibrosis. J Pediatr 1973;82:380–390 [DOI] [PubMed] [Google Scholar]
- 12. Sockrider MM, Swank MR, Seilheimer DK, Schidlow DV: Measuring clinical status in cystic fibrosis: internal validity and reliability of a modified NIH score. Pediatr Pulmonol 1994;17:86–96 [DOI] [PubMed] [Google Scholar]
- 13. Modi AC, Quittner AL: Validation of a disease-specific measure of health-related quality of life for children with cystic fibrosis. J Pediatr Psychol 2003;28:535–546 [DOI] [PubMed] [Google Scholar]
- 14. Kettler LJ, Sawyer SM, Winefield HR, Greville HW: Determinants for adherence in adults with cystic fibrosis. Thorax 2002;57:459–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lanng S, Thorsteinsson B, Nerup J, Koch C: Diabetes mellitus in cystic fibrosis: effect of insulin therapy on lung function and infections. Acta Paediatr 1994;83:849–853 [DOI] [PubMed] [Google Scholar]
- 16. Rolon MA, Benali K, Munck A, Navarro J, Clement A, Tubina-Rufi N, Paul C, Polzk M: CFRD: clinical impact of prediabetes and effects of insulin therapy. Acta Paediatr 2001;90:860–867 [PubMed] [Google Scholar]
- 17. Nousia-Arvanitakis S, Galli-Tsinopoulou A, Karamouzis M: Insulin improves clinical status of patients with cystic fibrosis-related diabetes mellitus. Acta Paediatr 2001;90:515–519 [PubMed] [Google Scholar]
- 18. Mohan K, Israel KL, Miller H, Grainger R, Ledson MJ, Walshaw MJ: Long-term effect of insulin treatment in cystic fibrosis-related diabetes. Respiration 2008;76:181-186 [DOI] [PubMed] [Google Scholar]
- 19. Mozzillo E, Franzese A, Valerio G, Sepe A, De Simone I, Mazzarella G, Ferri P, Raia V: One year glargine treatment can improve the course of lung disease in children and adolescents with cystic fibrosis and early glucose derangements. Pediatr Diabetes 2009;10:162–167 [DOI] [PubMed] [Google Scholar]
- 20. Bizzarri C, Lucidi V, Ciampalini P, Bella S, Russo B, Cappa M: Clinical effects of early treatment with insulin glargine in patients with cystic fibrosis and impaired glucose tolerance. J Endocrinol Invest 2006;29:1–4 [DOI] [PubMed] [Google Scholar]
- 21. Moran A, Phillips J, Milla C: Insulin and glucose excursion following pre-meal insulin lispro or repaglinide in cystic fibrosis related diabetes. Diabetes Care 2001;24:1706–1710 [DOI] [PubMed] [Google Scholar]
- 22. Alvarsson M, Sundkivst G, lager I, Hernricksson M, Berntorp K, Fernqvist-Forbes E, Steen L, Westermark G, Westermark P, Orn T, Grill V: Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care 2003;26:2231–2237 [DOI] [PubMed] [Google Scholar]
- 23. Maedler K, Carr R, Bosco D, R. Z., Berney T, Donath MY: Sulfonylurea induced β-cell apoptosis in cultured human islets. J Clin Endocrinol Metab 2005;90:501–506 [DOI] [PubMed] [Google Scholar]
- 24. Milla CE, Billings J, Moran A: Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care 2005;28:2141–2144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.