Abstract
Background
Age-related outcomes have become increasingly common in evaluating patients with melanoma. For instance, as age increases, sentinel node (SN) non-identification increases and SN positivity decreases. Furthermore, advanced age is a risk factor for in transit disease. We hypothesized that increasing age is accompanied by alterations in lymphatic function, possibly explaining these findings
Methods
Our center’s melanoma database was queried to identify patients who underwent successful sentinel node biopsy (SNB) after lymphoscintigraphy. Records of those treated between 2000 and 2005 were reviewed for age, sex, drainage basin, intraoperative radioactivity, and SN pathology.
Results
The 858 patients had a mean age of 55 years; 59% were male. Mean radioactivity in the hottest SN was 5232 counts/second; 179 patients (21%) had SN metastases. SN count-rates were significantly and inversely related to age (p<0.001 by Pearson correlation, ANOVA and chi-square). Mean counts/second were 6105, 5883 and 2720 for axillary, inguinal and cervical basins, respectively (p<0.01), and count-rates in these basins were consistently lower with increasing age (neck and axilla, p<0.001; groin, p=0.060; Pearson correlation). Multivariate analysis confirmed an independent inverse association between age and count-rates (p<0.001), overall and within each primary site.
Conclusions
Lymphatic function, as assessed by radiocolloid transit to and uptake within the SN, declines with age. Altered lymphatic function in older patients may modify metastatic patterns and help clarify findings of reduced nodal positivity and increased in-transit disease in this population.
Keywords: Melanoma, sentinel node, lymphatics, elderly
INTRODUCTION
Melanoma is the sixth most common malignancy in the United States; the estimated 62,480 new cases that will be diagnosed this year1 justify ongoing efforts to identify variables that will accurately predict outcome. In evaluating these variables, age has become an important factor. For instance, although melanoma mortality increases directly with age,2 increasing age has been correlated with decreased rates of metastasis to the first tumor-draining lymph node, i.e., the sentinel node (SN).3, 4 Researchers also have reported increased rates of SN non-visualization in older patients.5 Furthermore, advanced age was recently identified as a risk factor for in transit disease.6 Could an age-related alteration in the lymphatic environment affect metastatic processes?
Nodal sampling for staging melanoma was profoundly changed when Morton and colleagues7 introduced intraoperative lymphatic mapping and SN biopsy (SNB), which has since become standard for early-stage melanoma. SNB is preceded by lymphoscintigraphy to identify tumor-draining lymphatic channels leading to each SN. A radiotracer is injected into the interstitial space around the primary tumor site. This tracer diffuses into and travels through afferent lymphatics until it is trapped by the first tumor-draining lymph node (SN), which is identified and removed during SNB. While melanoma staging has been the primary use of SNB, this technique also allows an objective measurement of afferent lymphatic function, defined as the movement of fluid or particles from the interstitial space, along the lymphatic channel, and into the regional lymph node(s).8
In the current study, lymphoscintigraphic data from over 850 SNB procedures were collected and reviewed to test our hypothesis that older patients display altered lymphatic physiology that may be altering metastatic patterns. Our findings may help explain reports of increased SN non-visualization, reduced SN positivity, and increased in transit disease in aged melanoma patients.
METHODS
The John Wayne Cancer Institute (JWCI) prospectively enters medical records of melanoma patients into a computerized database containing nearly 14,000 entries. This database was queried to identify melanoma patients who underwent SNB for cutaneous melanoma from 2000–2005 at JWCI.
Technical details of preoperative lymphoscintigraphy and intraoperative lymphatic mapping at JWCI have been previously described.9 In brief, on the day of SNB, patients undergo lymphoscintigraphy with Tc-99m sulfur colloid. This radiopharmaceutical is injected into the peritumoral dermis, usually in a dose of 500 μCi; the exact dose administered is recorded prospectively by the nuclear medicine physician. Scintigraphic images are recorded to determine the path of lymphatic drainage to one or more SNs. The location of each SN is marked on the skin to facilitate intraoperative lymphatic mapping and SNB. All studies were done in the same nuclear medicine department, with minimizing environmental changes during the study period. Patients were kept at rest during acquisition of scintigraphic images.
In the operating room, shortly after the scintigraphy portion of the procedure, blue dye (Lymphazurin, Tyco Healthcare, Norwalk, CT) is injected into the peritumoral dermis. After a small skin incision is made over the scintigraphically identified area of the SN, gamma probe (Neoprobe, Dublin, OH) readings and blue-stained lymphatic channels are used to guide further dissection. Each SN is identified by its blue stain and elevated radioactive count-rate. One-second count-rates are measured intraoperatively over the primary tumor, the skin over the SN, the SN itself (in vivo and ex vivo), and a neutral background site. Each of these measurements is recorded in triplicate and averaged.
For this study, data retrieved for each patient included age, sex, primary tumor characteristics and location, surgical treatment of the primary lesion and regional lymph nodes, and nodal pathology. Count-rates were the mean of the triplicate ex vivo values, measured at the hottest part of the SN. If there was more than one SN or more than one tumor-draining basin, count-rates were based on the hottest SN in any basin.
Statistical analysis was performed by the JWCI biostatistics department to identify the relationship between SN count-rate and the following variables: patient age and sex, primary lesion location and characteristics, number of SNs, number and site of drainage basins, nodal status, and the amount of injected radiocolloid. The interval between scintigraphic injection and blue dye injection was also included to control for overall diffusion time. Age was examined at a cut-point of 60 years using chi-square analysis, by decile using ANOVA, and as a continuous variable using Pearson correlation.
Significance for all analyses was designated at p<0.05 a priori. This study was approved by the JWCI Institutional Review Board.
RESULTS
The mean and median age of our patients was 55 years (range, 3 – 99 years). Primary cutaneous melanomas were on the head/neck, trunk or extremities, with lymphatic drainage to the neck, axillary, and groin basins (Table 1). Of the 858 patients, 179 (20.9%) had SN metastases.
TABLE 1.
Demographic and Tumor-Related Characteristics of Study Population
| Characteristic | Number of Patients (%) |
|---|---|
| Total Number of Patients | 858 (100) |
| Age | |
| Mean | 55 years |
| Median | 55 years |
| Range | 3 to 99 years |
| Sex | |
| Male | 503 (59) |
| Female | 355 (41) |
| Primary Site | |
| Head and Neck | 173 (21.3) |
| Patients ≤ 60 years old | 84 |
| Patients > 60 years old | 89 |
| Upper Extremity | 161 (19.8) |
| Patients ≤ 60 years old | 96 |
| Patients > 60 years old | 65 |
| Trunk | 296 (36.5) |
| Patients ≤ 60 years old | 192 |
| Patients > 60 years old | 104 |
| Lower Extremity | 182 (22.4) |
| Patients ≤ 60 years old | 123 |
| Patients > 60 years old | 59 |
| Primary Thickness | |
| <1.0 mm | 381 (45.7) |
| 1.0–2.0 mm | 239 (28.7) |
| 2.0–4.0 mm | 139 (16.7) |
| >4.0 mm | 75(9) |
| Ulceration | 122 (14.7) |
| Nodal Metastasis | 179 (20.9) |
| Sentinel Node Basin | |
| Axilla | 434 (50.6) |
| Groin | 216 (25.2) |
| Neck | 208 (24.2) |
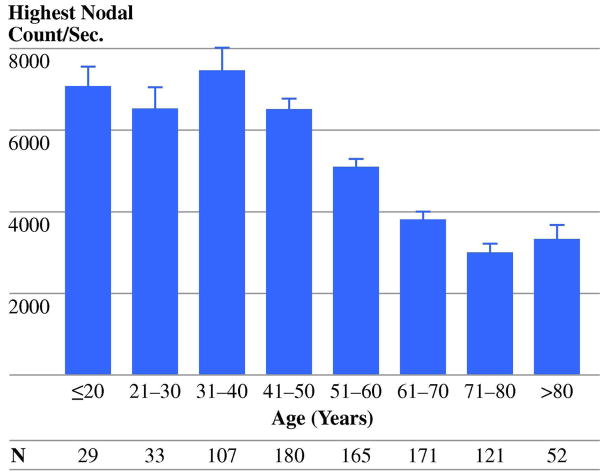
Increasing age was significantly associated with decreasing count-rates (Fig. 1). Age was the strongest predictor of count-rates when analyzed as a continuous variable (p<0.001, Pearson correlation), by decile (p<0.001, ANOVA), or by a cut-point of 60 years (p<0.001, chi-square). Multivariate analysis confirmed increasing age as an independent predictor of lower count-rates (p<0.001, Table 2).
Figure 1.
Lymphatic function declined with increasing age. This was true when looking at age as a continuous variable, by decile (shown here), and using a cut-off of ≤ 60 years (for all, p<0.001).
Table 2.
Multivariate Analysis of Predictors of Count-Rates
| Predictors of Count-Rates | Univariate P value | Multivariate P value |
|---|---|---|
| Age (continuous) | <0.001 | <0.001 |
| Volume of Radiocolloid (uCI) | <0.001 | <0.001 |
| Mean: 470 | ||
| Median: 500 | ||
| Range: 50 – 1500 | ||
| Sex | 0.51 | 0.01 |
| Primary Site | 0.001 | <0.001 |
| Basin (axilla, groin or neck) | 0.16 | 0.03 |
| Nodal Pathology (+/−) | 0.92 | 0.51 |
| Ulceration | 0.47 | 0.26 |
| Breslow (<1 mm vs. 1–2 vs. >2–4 vs. >4) | 0.11 | 0.14 |
| No. of Drainage Basins (1 vs. >1) | 0.58 | 0.26 |
Although count-rates were not significantly different among men and women (mean 5332 vs. 5040 counts/second, p=0.51), when controlling for other variables in multivariate analysis, men were more likely to have higher count-rates (Table 2, p=0.01). As expected, injecting more radiocolloid produced higher count-rates (Table 2, p<0.001).
Factors that did not influence the count-rates independently included nodal pathology, number of drainage basins (1 vs. >1), Breslow thickness of the primary tumor, and ulceration (Table 2). The interval between radiocolloid injection and blue dye injection was analyzed for the node-negative population. In this group, this diffusion time did not affect SN count-rates (p=0.58).
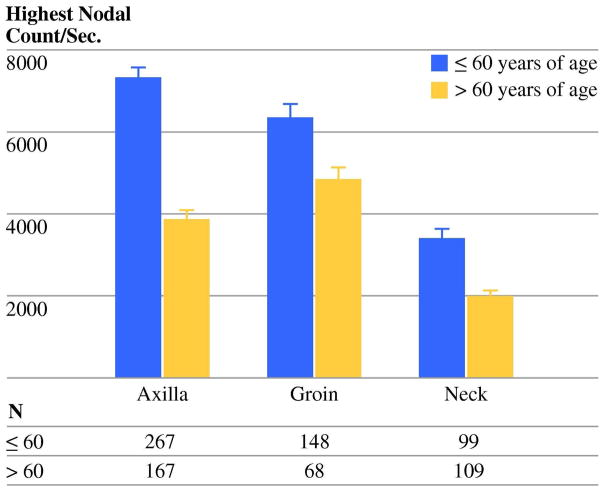
Tumor location and drainage basin were also predictive of count-rates in multivariate analysis (Table 2). We expected that counts might be higher in the neck area because a shorter distance is traveled by the radiocolloid. A recent report of reduced scintigraphic appearance time in this region would also suggest such a result.10 However, mean nodal count-rates were significantly lower in the neck (2726 ±15.8 counts/second) compared with the axilla (6164±15.2 counts/second) and groin (5883±32.2 counts/second, p<0.001, Fig. 2). The inverse relationship between age and count-rate was significant in neck (n=213, p<0.001) and axillary (n=442, p<0.001) basins and approached significance in the groin (n=226, p=0.06).
Figure 2.
SN count-rates were significantly lower for nodes in the neck basin (p<0.01). Within the basins, increasing age continued to be associated with reduced count-rates (neck, p<0.001; axilla, p<0.001; groin, p=0.06).
Since there was a significant difference in the count-rates among the drainage basins, as well as an increased proportion of head and neck melanomas in older versus younger patients (28% vs. 17%, p=0.001), the relationship between age and count-rate was examined for primary melanomas located in the head and neck, upper extremity, trunk, or lower extremity (Table 1). Increasing age continued to be predictive of decreasing counts at each site, independent of all other variables in Table 1 (head and neck, p<0.001; upper extremity, p=0.003; lower extremity, p=0.037; trunk, p<0.001).
DISCUSSION
For many years SNB has been used to stage melanoma, identify candidates for adjuvant therapy, and eliminate routine use of elective nodal dissection in patients with clinically normal drainage basins. With few exceptions,8 its potential for studying lymphatic function has not been exploited. Our SNB-based study of afferent lymphatic function found a significant age-related decline in the ability to move radiocolloid from the peritumoral dermis to the SN and retain it in that location. This relationship held true for age analyzed as a continuous variable, age analyzed by decile, and age analyzed by a cut-point of 60 years; the significance of age was independent of an exhaustive list of variables in a multivariate analysis. These results may help explain several important age-related findings associated with the lymphatic system in melanoma.
There are now several reports of a significant age-related effect on melanoma outcome, all seemingly related to the lymphatic system. For example, recent literature has identified an inverse relationship between patient age and rates of SN positivity.11 Chao et al4 observed that the rate of SN positivity was 23% for patients aged 18–30 years but only 12% for patients aged 61–70 years. Sondak et al3 reported nodal positivity rates of 26.3% in those younger than 35 years, and 11.8% in those older than 60; the predictive power of age was independent of tumor thickness and mitotic rate. This has been considered a paradoxical result because advanced age is also a marker for worse melanoma-specific outcome.2 Age also may influence scintigraphic identification of the SN: Birdwell et al12 and Chakera et al5 found increasing age to predict SN non-visualization in patients with breast cancer; however, other investigators have not found age to predict radiopharmaceutical transit time.10, 13 Pawlik et al6 noted age to be independently associated with an increased risk of in transit disease. An age-related decline in lymphatic function could explain these findings.
Age-related declines in lymphatic function have been described in animal models14, 15 but until now have not been objectively demonstrated in humans. The nuclear medicine literature does note an increase in SN non-visualization with increasing age.12 This data, however, relies on a subjective evaluation of scintigraphic images to determine when the node becomes “hotter” than the background activity. The subjectivity of this method may explain conflicting data.13 In our study, probe-measured count rates of the SN immediately after its excision (ex vivo), recorded in triplicate and averaged, were a more objective estimate of the amount of activity in the node and therefore a more accurate reflection of afferent lymphatic function.
Altered lymphatic function may be related to well-described anatomical changes that accompany aging. The skin, especially the upper dermis, shows significant changes with age. There is a destruction of elastic tissues,16 a reduction of skin blood flow,17 and alterations in its fatty composition.18 These changes may affect Starling forces, reduce tissue turgor and thus alter lymphatic flow. Reduced dermal fluid clearance and increased blistering in the elderly are likely related to such changes.19 Lymph nodes themselves also display alterations with aging, notably showing an increase in fat composition.20 Fatty-replacement may limit nodal trapping of the radiocolloid during lymphoscintigraphy, and possibly tumor cells during metastatic migration.
The associations between count-rates, age, and local recurrence add complexity to the role of the lymphatic system in the metastatic process. Recent research into this area has discovered important microenvironmental factors, including vascular endothelial growth factors C and D,21 which may be influencing this process. Macroenvironmental factors such as tissue turgor and dermal atrophy might also be playing a role. A tumor cell that migrates into the lymphatic system is more likely to reach and enter the SN if lymphatic flow rate is higher versus lower. Faster transit of tumor cells to the regional lymph nodes might even have a protective function by allowing an earlier, more robust antitumor immune response. Dysfunction of regional lymphatic function may increase the transit interval, giving the tumor more time to proliferate locally or within lymphatic channels, and eventually produce immune-suppressing factors.22
To be certain our finding was not simply a diffusion distance phenomenon, count-rate data were evaluated for inguinal, cervical and axillary basins. This allowed us to roughly control for the differing distances between the injection site and the drainage basin. In this subanalysis, the inverse relationship between age and count-rates was independent of axillary and cervical basin sites. Age and count-rates were not significantly correlated in the groin subset (p=0.06), probably because of the low number of elderly patients (70 patients >60 years old vs.156 patients ≤60 years old). Seemingly paradoxical was the observation that patients with melanomas draining to the neck basins displayed significantly lower mean count-rates than those with lesions draining to the axilla or groin. A similar finding was reported by Uren et al,23 who noted reduced lymphatic flow rates as compared with the trunk and extremities. This may indicate that count-rates were more affected by regional lymphatic function than overall lymphatic distance. The lymphatic drainage of the neck is a complicated system elegantly described by Pan et al.24 There can be multiple channels draining the same area, and even lymphaticovenous connections. These unique anatomic features may explain our finding of lower count-rates in the neck basin. The findings likely explain increased rates of SN non-identification in the head and neck region.9
In conclusion, older melanoma patients undergoing SNB display significantly reduced count-rates. This indicates an age-related alteration in lymphatic physiology with emphasis on changes in lymphatic flow and nodal trapping. Future studies will attempt to elucidate relationships between lymphatic function, nodal positivity, recurrence, and survival.
Acknowledgments
Supported by grant CA29605 from the National Cancer Institute and by funding from the Wayne and Gladys Valley Foundation (Oakland, CA), the Family of Robert Novick (Los Angeles, CA), Ruth and Martin H. Weil Fund (Los Angeles, CA), and the Wrather Family Foundation (Los Alamos, CA). Dr. Conway is the Carolyn Dirks Fellow.
Footnotes
Presented in part at the International Sentinel Node Society Annual Meeting, Sydney, Australia, February 20, 2008.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 3.Sondak VK, Taylor JM, Sabel MS, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic model. Ann Surg Oncol. 2004;11(3):247–58. doi: 10.1245/aso.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 4.Chao C, Martin RC, 2nd, Ross MI, et al. Correlation between prognostic factors and increasing age in melanoma. Ann Surg Oncol. 2004;11(3):259–64. doi: 10.1245/aso.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Chakera AH, Friis E, Hesse U, Al-Suliman N, Zerahn B, Hesse B. Factors of importance for scintigraphic non-visualisation of sentinel nodes in breast cancer. Eur J Nucl Med Mol Imaging. 2005;32(3):286–93. doi: 10.1007/s00259-004-1681-z. [DOI] [PubMed] [Google Scholar]
- 6.Pawlik TM, Ross MI, Johnson MM, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol. 2005;12(8):587–96. doi: 10.1245/ASO.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392–9. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 8.Faries MB, Bedrosian I, Reynolds C, Nguyen HQ, Alavi A, Czerniecki BJ. Active macromolecule uptake by lymph node antigen-presenting cells: a novel mechanism in determining sentinel lymph node status. Ann Surg Oncol. 2000;7(2):98–105. doi: 10.1007/s10434-000-0098-6. [DOI] [PubMed] [Google Scholar]
- 9.Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg. 1999;230(4):453–63. doi: 10.1097/00000658-199910000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahieu-Renard L, Cammilleri S, Giorgi R, et al. Slow Dynamics of Lymphoscintigraphic Mapping Is Associated to the Negativity of the Sentinel Node in Melanoma Patients. Ann Surg Oncol. 2008;15(10):2878–86. doi: 10.1245/s10434-008-0080-2. [DOI] [PubMed] [Google Scholar]
- 11.Bleicher RJ, Essner R, Foshag LJ, Wanek LA, Morton DL. Role of sentinel lymphadenectomy in thin invasive cutaneous melanomas. J Clin Oncol. 2003;21(7):1326–31. doi: 10.1200/JCO.2003.06.123. [DOI] [PubMed] [Google Scholar]
- 12.Birdwell RL, Smith KL, Betts BJ, Ikeda DM, Strauss HW, Jeffrey SS. Breast cancer: variables affecting sentinel lymph node visualization at preoperative lymphoscintigraphy. Radiology. 2001;220(1):47–53. doi: 10.1148/radiology.220.1.r01jn2347. [DOI] [PubMed] [Google Scholar]
- 13.Haigh PI, Hansen NM, Giuliano AE, Edwards GK, Ye W, Glass EC. Factors affecting sentinel node localization during preoperative breast lymphoscintigraphy. J Nucl Med. 2000;41(10):1682–8. [PubMed] [Google Scholar]
- 14.Gasheva OY, Knippa K, Nepiushchikh ZV, Muthuchamy M, Gashev AA. Age-related alterations of active pumping mechanisms in rat thoracic duct. Microcirculation. 2007;14(8):827–39. doi: 10.1080/10739680701444065. [DOI] [PubMed] [Google Scholar]
- 15.Yoon YS, Murayama T, Gravereaux E, et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest. 2003;111(5):717–25. doi: 10.1172/JCI15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy C, Bastiaens MT, Bajdik CD, Willemze R, Westendorp RG, Bouwes Bavinck JN. Effect of smoking and sun on the aging skin. J Invest Dermatol. 2003;120(4):548–54. doi: 10.1046/j.1523-1747.2003.12092.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchida Y, Fukuda O, Nakano M. The effect of aging on skin blood flow in human. In: Mesmer K, Kubler WM, editors. Sixth World Congress for Microcirculation. Bologna: Monduzzii Editore; 1996. [Google Scholar]
- 18.Ryan TJ, Curi S. The cutaneous adipose tissue. Philadelphia: Lippincott; 1989. [Google Scholar]
- 19.Fenske NA, Lober CW. Structural and functional changes of normal aging skin. J Am Acad Dermatol. 1986;15(4 Pt 1):571–85. doi: 10.1016/s0190-9622(86)70208-9. [DOI] [PubMed] [Google Scholar]
- 20.Luscieti P, Hubschmid T, Cottier H, Hess MW, Sobin LH. Human lymph node morphology as a function of age and site. J Clin Pathol. 1980;33(5):454–61. doi: 10.1136/jcp.33.5.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundar SS, Ganesan TS. Role of lymphangiogenesis in cancer. J Clin Oncol. 2007;25(27):4298–307. doi: 10.1200/JCO.2006.07.1092. [DOI] [PubMed] [Google Scholar]
- 22.Cochran AJ, Huang RR, Lee J, Itakura E, Leong SP, Essner R. Tumour-induced immune modulation of sentinel lymph nodes. Nat Rev Immunol. 2006;6(9):659–70. doi: 10.1038/nri1919. [DOI] [PubMed] [Google Scholar]
- 23.Uren RF, Howman-Giles RB, Thompson JF, Roberts J, Bernard E. Variability of cutaneous lymphatic flow rates in melanoma patients. Melanoma Res. 1998;8(3):279–82. doi: 10.1097/00008390-199806000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Pan WR, Suami H, Taylor GI. Lymphatic drainage of the superficial tissues of the head and neck: anatomical study and clinical implications. Plast Reconstr Surg. 2008;121(5):1614–24. doi: 10.1097/PRS.0b013e31816aa072. [DOI] [PubMed] [Google Scholar]