Abstract
Delay classical eyeblink conditioning (EBC) is an important model of associative, cerebellar dependent learning. Norepinephrine (NE) plays a significant modulatory role in the acquisition of learning; however, other neurotransmitters are also involved. The goal was to determine whether NE, gamma-aminobutyric acid (GABA) and glutamate (GLU) release are observed in cerebellar cortex during EBC, and whether such release was selectively associated with training. Further studies examined the role of the β-noradrenergic receptor in consolidation of the learned response by local infusion of propranolol at 5 – 120 minutes following training into the cerebellar cortex. In vivo microdialysis coupled to EBC was performed to examine neurotransmitter release. An increase in the extracellular level of NE was observed during EBC and was maximal on day 1 and diminished in amplitude with subsequent days of training. No changes in baseline NE release were observed in pseudo-conditioning indicating that NE release is directly related to the associative learning process. The extracellular levels of GABA were also increased selectively during paired training however the magnitude of GABA release increased over days of training. GLU release was observed to increase during both paired and unpaired training, suggesting that learning does not occur prior to the information arriving in the cerebellum. When propranolol was administered at either 5, 60, or 120 minutes post training, there was an inhibition of conditioned responses, these data support the hypothesis that NE is important for consolidation of learning.
In another set of experiments we demonstrate that the timing of release of NE, GABA and glutamate are significantly delayed in onset and lengthened in duration in the 22 month old F344 rats. Over days of training the timing of release becomes closer to the timing of training and this is associated with increased learning of conditioned responses in the aged rats.
Keywords: Norepinephrine, GABA, glutamate, cerebellum, classical conditioning, aging
INTRODUCTION
Delay eyeblink conditioning is a model of cerebellar motor learning. Norepinephrine (NE) is known to play a modulatory role in cerebellar-dependent learning. NE induces synaptic plasticity in Purkinje neurons by selectively improving the signal to noise ratio of evoked versus spontaneous activity, enhancing the sensitivity of cerebellar neurons to both excitatory and inhibitory afferent inputs (Freedman, Hoffer, Puro, and Woodward, 1976; Freedman, Hoffer, Woodward, and Puro, 1977; Hoffer, Freedman, Woodward, Puro, and Moises, 1978). Based upon the Marr-Albus theories of cerebellar learning Gilbert proposed that NE should have a role in the consolidation of memory within the cerebellum (Gilbert, 1975). Behavioral evidence which shows the involvement of NE in memory consolidation in the cerebellum is observed in several cerebellar dependent paradigms. For example in rod running motor learning, a cerebellar-dependent task, the ability of rats to learn is reduced following lesion of the locus coeruleus (LC) (Watson and McElligott, 1983). This effect is localized to cerebellar NE (Watson and McElligott, 1984a) and is specific to the β-adrenergic receptor (Bickford, Heron, Young, Gerhardt, and de la Garza, 1992). Adaptation of the vestibulo ocular reflex (VOR) is modulated by noradrenergic inputs (McElligott and Freedman, 1988) and also appears to be mediated by the β-noradrenergic receptor (Pompeiano, van, Collewijn, and van der, 1991). Cerebellar delay classical conditioning has been shown to be modulated by NE. Electrolytic lesions of the LC induce resistance to extinction in rabbits (McCormick and Thompson, 1982) and 6-hyroxydopamine (6-OHDA) has been shown to retard acquisition but not performance of conditioned eyelid responses in rabbits (Winsky and Harvey, 1992). Systemic blockade of the β-adrenergic receptor retards the acquisition of learned responses in delay conditioning tasks in rats (Cartford, Allgeier, and Bickford, 2002; Cartford, Samec, Fister, and Bickford, 2004b) The activation of adenylyl cyclase-AMP-protein kinase A (PKA) intracellular signaling cascade has been shown to be essential for long-term memory consolidation in diverse brain areas including the hippocampal formation and prefrontal cortex (Ardenghi, Barros, Izquierdo, Bevilaqua, Schroder, Quevedo, Rodrigues, Madruga, Medina, and Izquierdo, 1997; Huang, Li, and Kandel, 1994; Vianna, Izquierdo, Barros, Ardenghi, Pereira, Rodrigues, Moletta, Medina, and Izquierdo, 2000; Vianna, Szapiro, McGaugh, Medina, and Izquierdo, 2001). The time period post training is thought to be critical for memory consolidation (Bao, Chen, Kim, and Thompson, 2002; De Zeeuw and Yeo, 2005b; Nolan, Malleret, Lee, Gibbs, Dudman, Santoro, Yin, Thompson, Siegelbaum, Kandel, and Morozov, 2003; Yeo, 2004a). NE is also involved in non-cerebellar dependent tasks, for example LC neurons fire prior to a target cue in a vigilance task in monkeys and during reversal of task contingency, the LC response to the new stimuli precedes behavioral responding (Aston-Jones, Rajkowski, and Kubiak, 1997).
Other neurotransmitter systems are also implicated in delay eyeblink conditioning tasks. Administration of the glutamatergic AMPA receptor antagonist CNQX or the GABA-A receptor antagonist picrotoxin into the cerebellar cortex completely and reversibly impairs fully established conditioned response’s (CR’s), suggesting that GABAergic and glutamatergic transmission are involved in CR performance (Attwell, Ivarsson, Millar, and Yeo, 2002). It has been suggested that the basal GABAergic output from the cortex onto the interpositus nucleus modulates CR expression, whereas timing of CR's is modulated by the stimulus activated inhibition (Bao et al, 2002). The expression of CR’s can be interrupted by both GABA mediated inactivation of the interpositus neurons with muscimol as well as up-regulation of activity with picrotoxin (Aksenov, Serdyukova, Irwin, and Bracha, 2004).
To date work examining the role of neurotransmitters in delay eyeblink conditioning has used either the application of agonists or antagonists to investigate the role of these neurotransmitters. However, questions remain regarding presynaptic mechanisms that operate during performance of the delay conditioning task. The present study uses in vivo microdialysis to examine the temporal patterns of release of NE, GABA, and GLU during eyeblink conditioning training to examine critical presynaptic events in the neurochemical orchestration within the cerebellum during the eyeblink conditioning. In this study we also manipulated the activity of the β-adrenergic receptors by administering microinjections of propranolol directly into the cerebellar cortex at specific post training periods, a time during which we observed that NE overflow remains elevated. It has been shown that NE, applied iontophoretically or via activation of the locus coeruleus, potentiates GABA-induced inhibition of cerebellar Purkinje neurons. Using both extracellular recordings from the intact cerebellum and whole cell patch recordings from in vitro preparations it has been demonstrated that this modulatory effect of NE on GABA-induced inhibition is specific for the GABAA receptor and involves the signal transduction system associated with the βAR and in whole cell patch clamp recordings NE increases GABA activated chloride currents (IGABA) (Cheun and Yeh, 1992; Parfitt, Hoffer, and Bickford-Wimer, 1990; Yeh and Woodward, 1983).. The ability of NE to augment GABAergic inhibition is down-regulated in aged rats (Bickford, Hoffer, and Freedman, 1985; Lin, Freund, and Palmer, 1993).
The necessity of cerebellar norepinephrine for motor learning has been demonstrated in our labs and others (Bickford, 1993b; Bickford, 1995a; Bickford et al, 1992). Depletion of cerebellar NE or blockade of βARs impairs the ability of rats to improve performance on a runway task where the rats must learn to walk on varying patterns of pegs that protrude from the runway walls (Bickford et al, 1992; Bickford, 1995b; Watson et al, 1983; Watson and McElligott, 1984b). Aged rats show impairments on this task that are correlated with the loss of βAR sensitivity (Bickford 1995b). A second motor learning task that has been tied to cerebellar function is classical eyelid conditioning. Classical conditioning of the eyelid response is disrupted with lesions of cerebellar lobule HVI in rabbits (Perrett, Ruiz, and Mauk, 1993; Yeo, Hardiman, and Glickstein, 1985) and neurons within lobule HVI show conditioning related activity (Berthier and Moore, 1986; Gould and Steinmetz, 1994). Both rat and rabbits show age-related declines in performance on this task. (Weiss and Thompson, 1992; Woodruff-Pak and Thompson, 1985). And it has been shown in both rats and rabbits that NE is critical for learning of these tasks (Gould, 1998; Heron, Gould, and Bickford, 1996). Thus, both the rod walking task and classical conditioning show age-related declines in performance that are reflective of altered cerebellar physiology. Furthermore it has been demonstrated that the blockade of βAR in the cerebellum disrupts learning (Cartford et al, 2004b) and the performance of aged rats is similar to young rats during blockade of βAR’s.
The role of post-synaptic β-AR function has been demonstrated as discussed above, however the effect of aging on the pre-synaptic afferents to cerebellar cortex and how this might impact acquisition of motor learning tasks is less well established. It was the goal of this investigation to examine the release of neurotransmitters in the cerebellar cortex during acquisition of delay eyeblink conditioning in aged rats.
Materials and Methods
Subjects
Male F344 rats four and twenty two months of age were used in this study. Room temperature was kept at 72 °F and the dark/light circle was 12-h (lights were on from 7:00 AM to 7:00 PM). Each animal was used for only one experimental condition. All procedures were carried out in accordance with the institutional guidelines (IACUC) and with USA National Institute of Health Guide for the Care and Use of Laboratory Animals.
Surgery
Rats were anesthetized with isoflurane and placed in a stereotaxic instrument. A 10 mm long guide shaft (cannula) made of 21-gauge stainless-steel tubing (Plastics One) was inserted into the cerebellum. The guide cannula was implanted to the skull and cemented with dental acrylic, two jeweler screws were attached to provide support for the dental cement. The guide cannulae was implanted aimed at the cerebellar lobule HVI (simplex, and interpositus nucleus) using the coordinates AP-11.3, ML +2.5 and DV −2.5 mm with reference to bregma. Guide cannulae placement was the same for microdialysis and microinjection procedures. In the same surgical session rats were prepared for eyelid training by fixing a small ITT/Cannon connector strip to their skull to hold gold pin connectors to EMG wires that were run under the left eyelid. This method has been previously published by our lab (Cartford, Gemma, and Bickford, 2002b). Rats were allowed to recover for at minimum of one week after surgery before starting the eyeblink conditioning training.
Training
The rats were habituated to the training chamber and headstage cable for three days prior to the training sessions (10 minutes handling plus 20 minutes in the chamber). The training consisted of 50 trials each training trial consisted of a 250 ms baseline, a 500 ms CS, and a 100 ms US period that co-terminated with the CS, the intertrial interval was variable and random between 5 and 20 seconds. The training tone (CS) was 3 kHz and the airpuff 10 psi. Hardware and software used to train and analyze data were manufactured by J. Tracy, J. Green and Joe Steinmetz, (Bloomington, Indiana). Eyelid EMG data were collected, amplified, rectified, and integrated. Learned responses were determined using a 10 standard deviation criterion for eyelid amplitude elevated during the CS period when compared to the baseline. Alpha responses to the tone are excluded from learned response analysis by using a 70 ms discrimination/exclusion window. Learning was measured as the percentage of learned (conditioned) responses (CR’s) made in each training session. For study 1 with microdialysis, rats were given 1 training session per day. For study 2 with drug infusions, rats were given 2 training sessions per day separated by 6 hours.
Microdialysis and Eye-Blink Conditioning procedure
Independent groups of rats were used for training coupled to microdialysis for each day of training, thus each group had microdialysis performed only once. Microdialysis probes were lab-made with cellulose hollow fiber (MW 13,000) attached to stainless steel tubing, with a 45 cm length of fused silica capillary (internal diameter [I.D.] 76 µm; outside diameter [O.D.] 150 µm) inserted into the cellulose tube (Hernandez et al., 1986). The effective length of the dialysis piece was 3 mm. The night prior to the experiment the probe was inserted into the cerebellum of rats via the guide cannula. The inlet of the probe was connected to a syringe pump filled with Ringer's solution (134.9 mM NaCl, 3.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, and 10 mM NaHCO3 at pH 7.4) and the perfusion flow rate was set at 0.1µl/min during 12 hours (overnight) to allow recovery from the probe insertion. The following day in the morning the flow rate was set a 2 µl/min for 1 h and the head stage was coupled to the rat head stage, then microdialysates (flow rate = 2µl per minute) were collected every 10 minutes. Microdialysis were collected at 10 min intervals for 1 hour prior to training to obtain the basal levels of extracellular neurotransmitters from the dialysate during training (~18 min) and for 2 hrs post-training.
Microinjection of propranolol
For this experiment, the rats received training twice a day, 8–9 am and 2–3 pm Six hours were allowed in between sessions. Rats received (each training session) a microinjection of 1 µL of propranolol (100 µM) over five minutes through the guide cannula with an infusion pump, the infusion syringe was then left in place for an additional 5 minutes. The depth of the tip of the infusion syringe was −2.6 DV from brain surface placing the infusion into the cerebellar cortex only. Infusions were initiated at intervals of 5, 60 or 120 minutes after the training session. The control group received artificial cerebral spinal fluid at pH: 7.4.
Neurochemical analysis
Immediately after each sample collection, one microliter of sample was taken from the vial and placed into another vial for GLU and GABA analysis, the rest of the sample (nineteen microliters) was acidified with 2 microliters of HCl (0.1 M) and all the samples immediately frozen at −80 °C for high performance liquid chromatography (HPLC) and capillary zone electrophoresis (CZE) analysis.
HPLC
Norepinephrine
The frozen microdialysis samples were unfrozen for analysis in the HPLC. This method has been published and routinely used by our laboratory (Bowenkamp, Lapchak, Hoffer, Miller, and Bickford, 1997; Hall, Hoffer, and Gerhardt, 1986). The detection of NE was performed using an isocratic HPLC system (Beckman, Inc., Fullerton, CA), at a flow rate of 1 ml/min. This is coupled to a dual-channel electrochemical array detector (model 5100A, ESA, Inc., Chelmsford, MA), E1 = +0.35 mV and E2 = −0.25 mV, using an ESA model 5011 dual analytical cell. The compounds of interest are separated with reverse-phase chromatography, using a C18 column (4.6 mm × 100 mm, 3 µm particles, ODS Thermo Hypersil; Keystone Scientific, Bellefonte, PA) with a pH 4.1 citrate-acetate mobile phase, containing 10% methanol and 0.45 mM 1-octane-sulfonic acid. Data were quantified using Totalchrom software V6.2 (Perkin Elmer) based on peak area, in comparison with an external standard calibration curve.
Capillary electrophoresis
Glutamate (Glu) and GABA
This method has been previously published (Rada, Mendialdua, Hernandez, and Hoebel, 2003). A capillary electrophoresis system equipped with an argon laser tuned to 488 nm was used (Model R2D2, Meridialysis Co., Merida, Venezuela). Fluorescein isothiocyanate (FITC) was conjugated with glutamate and GABA as the fluorescent chromophore. Optimal concentrations of FITC and the calibration curves for both amino acids have been reported previously. One microliter of dialysates and standards respectively were derivatized with 1µL of FITC (1 mM) and carbonate buffer (25 mM) mixture (1:1). A syringe loaded with FITC–carbonate mixture was placed in a precision pump, and 1 µL of the mixture was delivered into a tube containing a 1 µL microdialysis sample. The samples reacted overnight (14 hr) at room temperature in a water-saturated chamber that minimized evaporation. Homoglutamine (10–5 M) was used as an internal standard and was mixed in the carbonate buffer used to derivatize samples and standards. A carbonate buffer (20 mM carbonate/bicarbonate) was the running buffer to transport the microdialysis sample through the capillary when detecting glutamate. Detection of GABA from the samples required a different running buffer consisting of 23 mM borate with 120 mM sodium dodecyl sulfate and 0.5% methanol. The samples or standards were sucked into the anodic end by applying a negative pressure (19 psi or 1.34 kg/cm for 0.7 s) at the cathodic end of the capillary. Electrophoretic separation was achieved by applying a high voltage between the anode and the cathode for 12 min, 22 kV for glutamate and 26 kV for GABA.
Histology
After completing the experiment, the animals were euthanized with sodium pentobarbital and decapitated and the brains were dissected out, placed in 10% formalin for 24 h and then transferred to 15% sucrose with 10% formalin for 24 hrs. The brains were then frozen, sectioned (50 µm) on a freezing microtome and stained with Cresyl violet for verification of the probe placement and extensions of the lesion. Only animals with probe placements verified through cerebellar cortex and lobule HVI (simplex, and interpositus nucleus) region were used for data analysis, also animals that presented high level of damage or hemorrhage due to the microdialysis probe insertion were discarded from the study.
Statistical analyses
Neurochemical analyses were performed using area under the curve (AUC) of neurotransmitter concentration as the dependent measure. Data from experiments were subjected to a two-way analysis of variance of the area under the curve (AUC) for the neurotransmitter, followed by subsequent post hoc tests. For behavioral experiments %CR was the dependent measure and data were subjected to three-way mixed model analyses of variance ([Drug (2): × [Paradigm (2): (Conditioning, Pseudoconditioning)] × [Session (6)), followed by post hoc tests (Dunnett’s). Supernova was the statistical software utilized in these analyses. A p value of <0.05 was considered to be statistically significant. . For comparison of mcirodialysis data between young and aged rats, we applied random effects models (Littel, Milliken, Stroup, Wolfinger, and Schabenberger, 2006; Singer and Willet, 2003) Specifically, we applied separate random effects models over several segments of the pre- and post-stimulus period, consisting of models for the time segments 10 to 30, 40 to 60, 70 to 90, and 100 to 120. For each analysis, time was the within subjects factors, and there were 3 between subjects factors, day (day 1, 3, 5), group (pseudo-training vs. training), and age (young vs. old). The random effects analytic method provides the same basic information as traditional repeated measures analysis in terms of longitudinal changes over time.
Results
STUDY 1 - MICRODIALYSIS
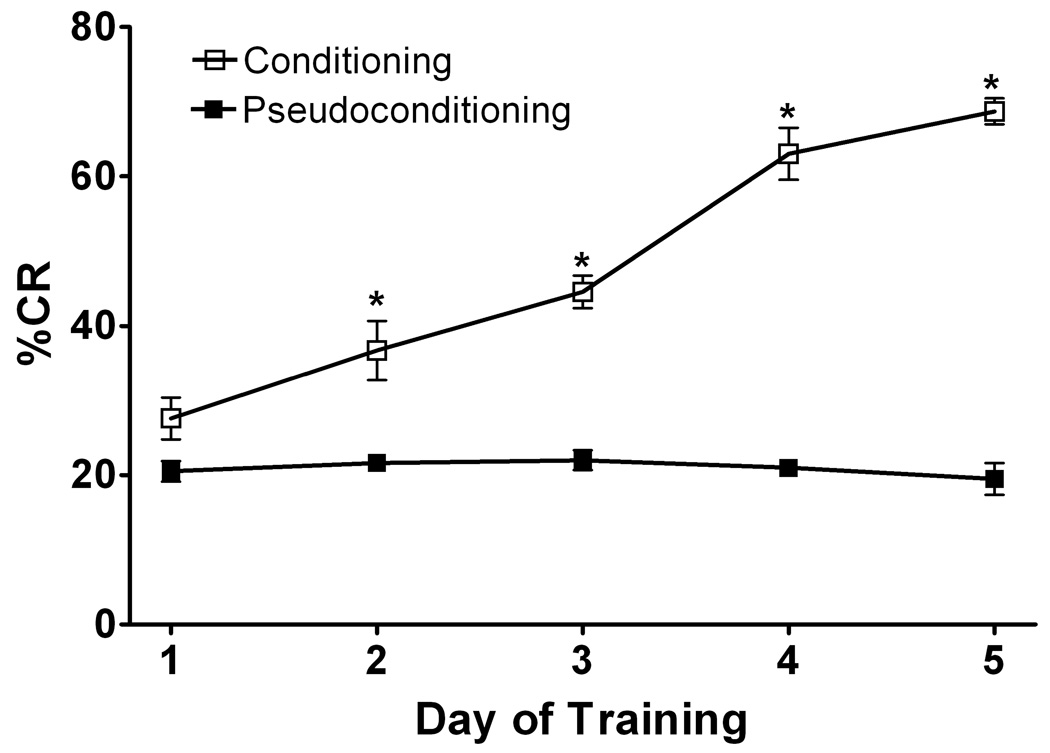
The behavioral data (% conditioned responses) obtained from animals with implanted microdialysis probes was not significantly different from the group without probe implantation (Figure 1). Thus, implantation of the guide cannula and insertion of the microdialysis probe did not cause significant disruption of the cerebellar circuitry involved in the acquisition of the eyeblink conditioning. Rats learned the task over days and were significantly different from pseudoconditioning performance on days 2 – 5 (Figure 1). We examined the extracellular levels of NE, GLU and GABA neurotransmitters on all five days of training. Because microdialysis could only be performed on one day in a given rat, different rats were used for microdialysis results for each day of training. All rats were run on the behavior for 5 days.
Figure 1. Conditioning vs Pseudo-conditioning: Eyelid Conditioning.
Eyelid conditioning was performed over 5 days. The Y-axis shows the percentage of conditioned response (% CR’s), the x-axis represents daily training sessions of 50 trials. Rats in the conditioning group learned this task over days (represented by higher % CR) whereas rats who under went pseudoconditioning did not learn the task. Open boxes represent conditioning rats with microdialysis probes implanted (N=12), black boxes represent pseudoconditioning rats with microdialysis probes implanted (N=12). Closed triangles represent conditioniong rats with no surgery for microdialysis probe implantation (N=6). The conditioning group learned significantly more than pseudoconditioning rats (F 175.41 [1, 40] p<0.001) and there was no significant difference between rats with or without canula implantation.
Norepinephrine
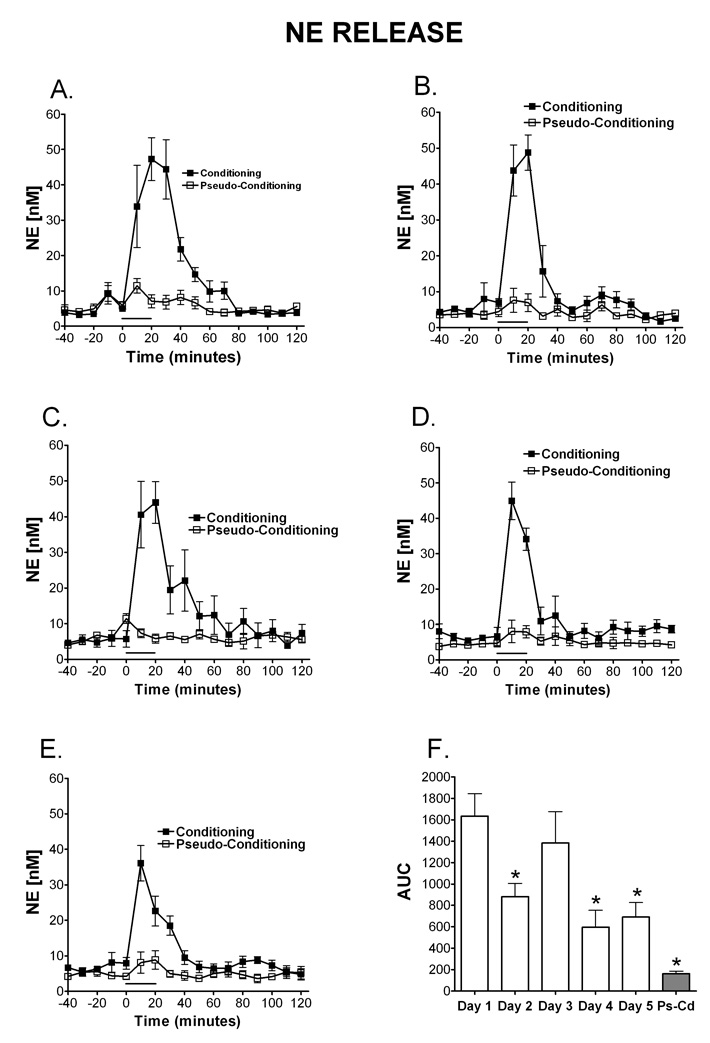
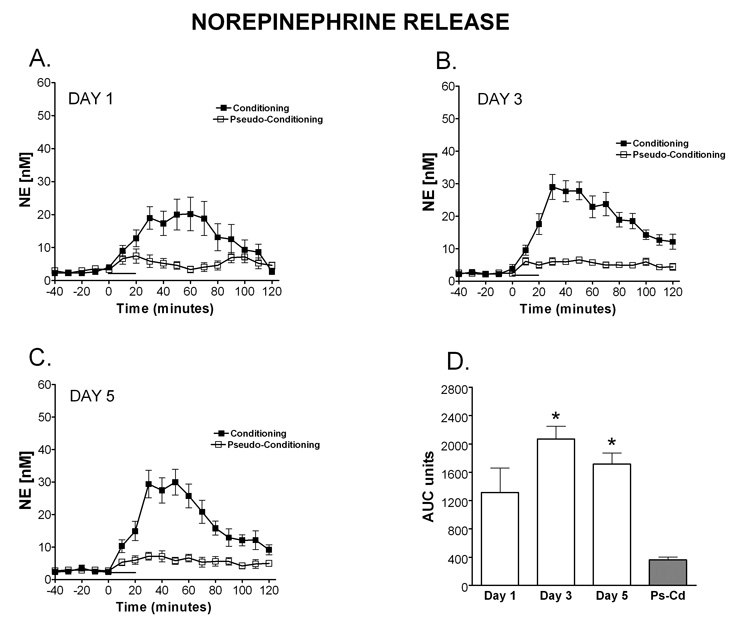
NE release is observed in the microdialysate on all 5 days of training, however the temporal pattern and magnitude of release changes over days of training (Figure 2A–E). On day one of training there is an increase in NE detected in the microdialysate that peaks at the end of the behavioral training and remains significantly above baseline for 50 minutes after the training session ended. A 2 (paradigm: conditioning, pseudoconditioning) × 5 (day: 1–5) between subjects ANOVA revealed a significant main effect of paradigm, [F (1, 27) = 84.20, MSe = 91975.60], with conditioning resulting in higher AUC of NE than pseudoconditioning (Fig 2F). This indicates that the NE release is specific for the learning condition and is not the result of sensory stimulation alone. There was also a significant main effect of day, [F (4, 27) = 4.30], with AUC generally decreasing over days. A Fisher’s LSD revealed that day 1 resulted in significantly higher AUC than days 2, 4 and 5. As shown in Figure 2F, no significant interaction between day and paradigm was present [F (4, 26) = 2.20]. A Fisher’s LSD shows that NE release was significantly greater in the conditioning group on days 1–5 compared to the pseudoconditioning group. These findings can be observed in Figures 2A–E which illustrate the time window of NE release once the training starts. On all days a clear increase in NE levels are observed with the onset of training in the conditioning group. Interestingly, over days NE levels return to baseline levels faster, indicating a larger peak amount of release with a longer duration are observed on the beginning days of training. In contrast, the pseudoconditioning group did not show this pattern of transient NE release which indicates that the NE release observed (conditioning group) is directly related to the learning process in the eyeblink task.
Figure 2. Temporal release of norepinephrine during eyelid conditioning.
Microdialysis was performed in the cerebellar cortex on rats during training in the delay eyelid conditioning task. The time window of NE release once eyelid conditioning starts can be observed on day 1 (A; N=5 Condition, 4 Pseudocondition), day 2 (B; N=5 condition, 4 pseudocondition), day 3 (C; N= 4 condition, 3 pseudocondition), day 4 (D; N=4 condition, 3 pseudocondition) and day 5 (E; N= 4 condition; 4 pseudocondition). On day one of training (A) there was an increase in NE that peaked at the end of the behavioral training and remains significantly above baseline for 70 minutes after the training session. On all days (A–E) a clear increase in NE levels was observed with the onset of training in the conditioning group. Data are expressed as NE [nM] (y-axis) over time in 10 minute dialysate samples (x-axis). Black squares represent conditioning, open squares represent pseudoconditioning. F) Area under the curve (AUC units) representation of NE release for each day of training during Eyelid conditioning. Note that the pseudoconditioning group (Ps-Cd) did not show this pattern of transient NE release (indicates that the NE release observed (conditioning group) is directly related to the learning process in the eyeblink task.) The solid bar underneath the curves indicates the time when the rats were receiving training trials.
GABA
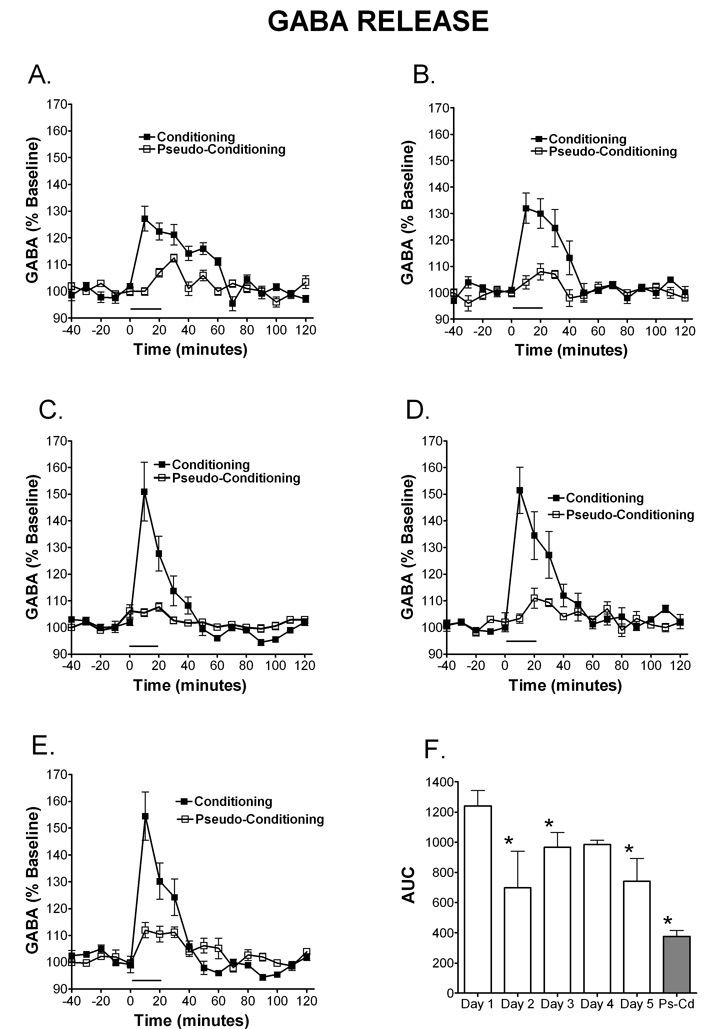
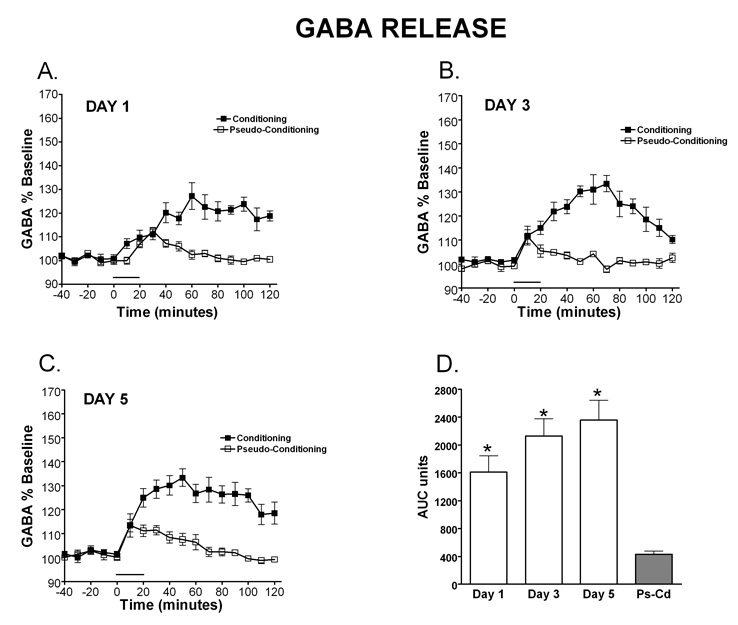
GABA release was also examined over days of training and it can be observed in Figure 3 that the amplitude of GABA release increased over days of training and the time course shortened. Data are expressed as percent of baseline and mean basal levels were 0.12 µM (± 0.1). A (paradigm: conditioning, pseudoconditioning) × 5 (day: 1–5) between subjects ANOVA revealed a significant main effect of paradigm, [F (1, 27) = 92.70, MSe = 33192.80], where conditioning resulted in significantly more GABA release (expressed as AUC units) than the pseudoconditioning (see figure 3F). There was also a significant interaction between paradigm and day, [F (4, 27) = 4.70], which revealed that GABA release was significantly greater in the conditioning group on day 1 compared to days 2, 3 and 5 (p<0.05), one reason for this difference is observed when looking at the time course of the GABA release over days of training. The maximum amplitude of release is lower on Day 1, yet the release is spread over time, thus leading to an overall larger amount of GABA measured over baseline. Figures 3A–E illustrates the time window of GABA release once the training starts. The amount of release increases over time and the time frame of the response sharpens so that GABA release is closely timed with the performance of CR’s by day 3 and sharpens further up to day 5. As observed with NE, the conditioning group resulted in significantly higher AUC on all days except day 5, when compared to the pseudoconditioning group (p<0.05), suggesting that the GABA release during the eyeblink conditioning is associated with the learning of the conditioned response and does not happen just as response to the sensory input carried by the CS and/or US.
Figure 3. Temporal release of GABA during eyelid conditioning.
Microdialysis was performed through the cerebellar cortex and interpositus nuclei on rats during training in the delay eyelid conditioning task. The time window of GABA release once eyelid conditioning starts can be observed on day 1 (A; N=5 condition, 4 pseudocondition), day 2 (B; N=5 condition, 4 pseudocondition), day 3 (C; N= 4 condition, 3 pseudocondition), day 4 (D; N=4 condition, 3 pseudocondition) and day 5 (E; N= 4 condition; 4 pseudocondition).. GABA release was significantly greater in the conditioning group on day 1 compared to days 2, 3 and 5 (p<0.05). Peak magnitude of release increases over time and the time frame of the response sharpens so that GABA release is sharply timed with the performance of CR’s by day 3 and sharpens further up to day 5. Data are expressed as percent of baseline (% baseline) (y-axis). Black squares represent conditioning, open squares represent pseudoconditioning. (F) Area under the curve representation of GABA release for each day of training during eyeblink conditioning. As was observed with NE, the conditioning group resulted in significantly higher AUC for GABA on all days except day 5, when compared to the pseudoconditioning group (Ps-Cd) (p<0.05), suggesting that the GABA release is associated with learning of the conditioned response. The solid bar underneath the curves indicates the time when the rats were receiving training trials.
Glutamate
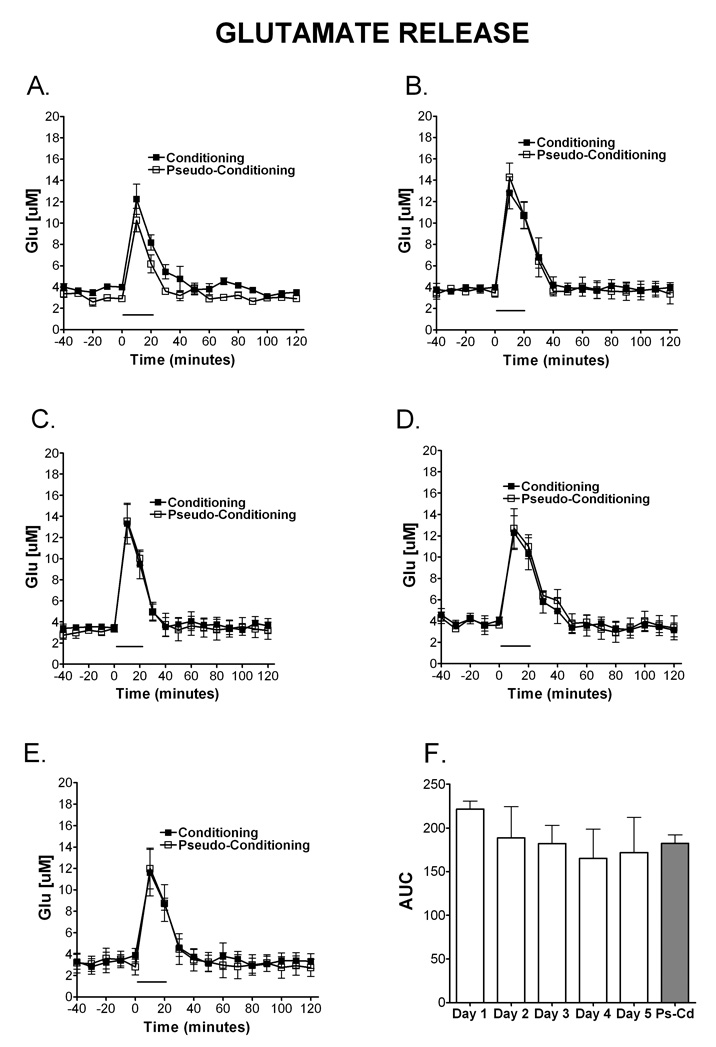
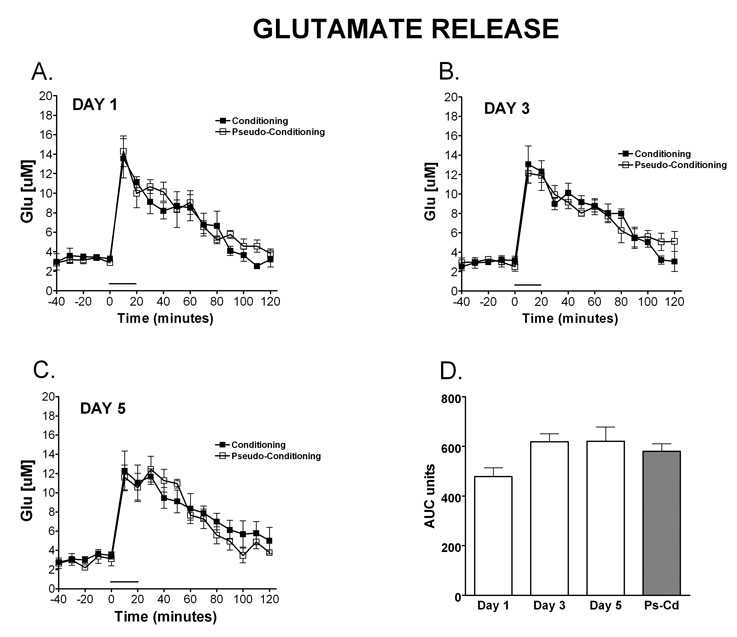
As illustrated in Figure 4F, a 2 (paradigm: conditioning, pseudoconditioning) × 5 (day: 1–5) between subjects ANOVA of the AUC of Glu did not detect main effects of day, [F (4,31) = 0.40, MSe = 2854.80] or paradigm, [F(1,31) = 0.0036], or any interaction between paradigm and day, [F(4,31) = 1.50]. Interestingly, figures 4A–E shows that comparable levels of glutamate are released during training on the eyeblink task for both the conditioning and the pseudoconditioning groups. Contrary to what is observed with NE and GABA, the finding shows that GLU release above the basal levels is not a learning dependent process.
Figure 4. Temporal release of glutamate during eyelid conditioning.
Microdialysis was performed in the cerebellar cortex on rats during training in the delay eyelid conditioning task. The release of Glu over time during eyelid conditioning is shown on day 1 (A; N=4 condition, 4 pseudocondition), day 2 (B; N=4 condition, 4 pseudocondition), day 3 (C; N= 4 condition, 3 pseudocondition), day 4 (D; N=4 condition, 3 pseudocondition) and day 5 (E; N= 4 condition; 4 pseudocondition). Comparable levels of glutamate were released during training on the eyeblink task for both the conditioning and the pseudoconditioning groups. Data are expressed as Glu [µM] (y-axis). Black squares represent conditioning, open squares represent pseudoconditioning. (F) Area under the curve representation of Glu release for each day of training during eyelid conditioning and pseudoconditioning (Ps-Cd, average of days). There were no differences in AUC of Glu. The solid bar underneath the curves indicates the time when the rats were receiving training trials.
STUDY 2 - PHARMACOLOGY
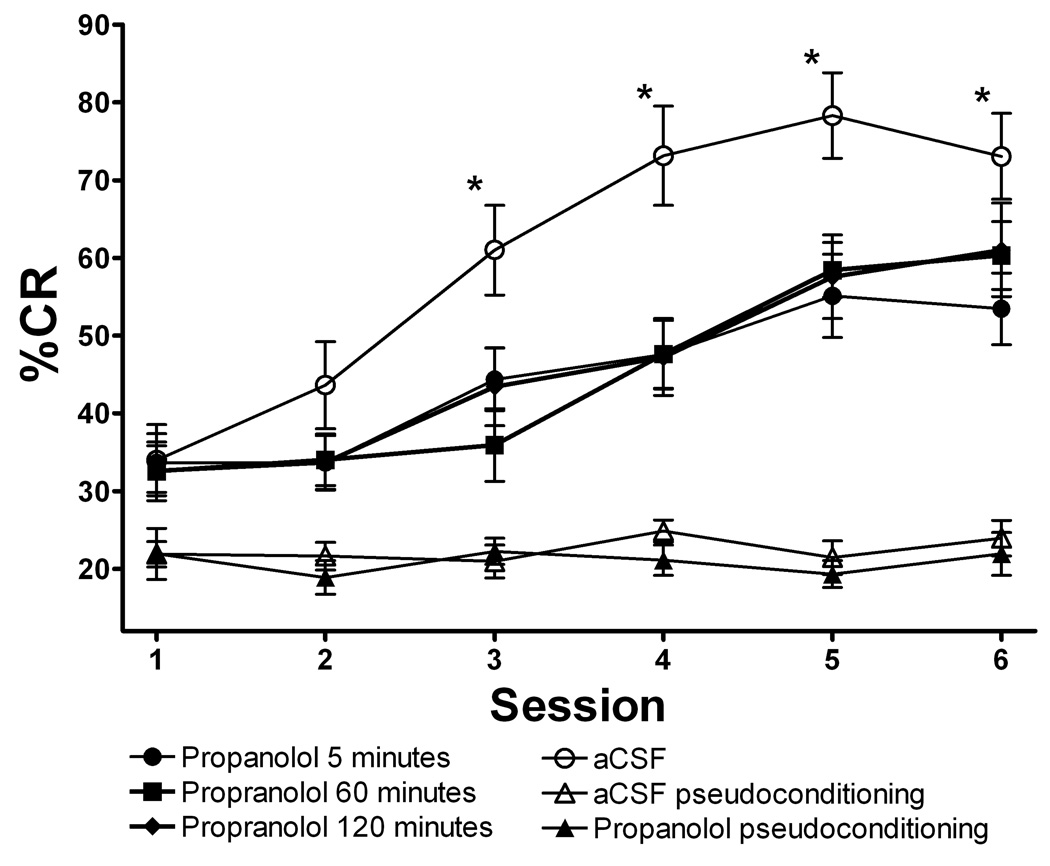
In order to further elucidate the effects of the observed elevation of NE levels in the cerebellum post-training we infused propranolol (1 µl, 100µm) into the cerebellar cortex at various times post-training. Propranolol when administered 5, 60 or 120 minutes following training significantly disrupts learning of CR’s. Figure 5 illustrates the attenuation of learning (shown as % CR) when propranolol is administered 5 minutes post training (significant drug × session interaction [F(5,80) = 2.72, p<0.05]) The effect of propranolol is observed at 5 minutes post training with rats learning significantly less than aCSF on sessions 3, 4, 5 and 6. A similar pattern is observed in rats treated with propranolol at 60 and 120 minutes post training.
Figure 5.
Propranolol administered 5, 60 or 120 minutes following training significantly disrupts learning of CR’s. The X-axis shows training sessions over time, with session 1 being Day 1 AM, 2 Day 1 PM, 3 Day 2 AM, 4 Day 2 PM, 5 Day 3 AM, and 6 Day 3 PM. Learning was attenuated (shown as % CR) when propanolol was administered 5 (N= 6), 60 (N=6), or 120 (N=6) minutes post training, compared to aCSF (N=14) on sessions 3, 4, 5 and 6 (indicated by *). Pseudoconditioning did not differ over sessions or as a result of drug administration (N= 6 psuedocondition control, 6 pseudocondtion propranolol at 5 mintues post training). These data show robust learning in aCSF treated rats and partial learning in propranolol treated rats when administered 5, 60 or 120 minutes post training.
The amplitude of the conditioned response and the unconditioned response were examined on all trials. Analyses of amplitude of both CR and UR for drug treatments 5, 60 and 120 minutes post training failed to detect any significant difference between aCSF and propranolol (Figure 6). The timing of the CR was also not affected by drug treatment. Neither CR onset or CR peak amplitude times were significantly different in any of the drug treatment groups (Figure 7).
Figure 6.
Amplitude of the conditioned response (A,B, C,) or unconditioned response (D, E, F) were analyzed for all drug groups compared with aCSF. There were not differences between control and drug administration groups in either the 5 minute post-training administration (A, D) the 60 minute (B, E) or the 120 minute post-training groups (C, F).
Figure 7.
Timing of the conditioned responses was measured for all groups. The time to onset of the CR (A, B, C) and the time of peak amplitude of the CR (D,E,F) are illustrated for both the aCSF and proranolol treatement groups. No significant differences were observed between groups for either measure.
STUDY 3 – AGING
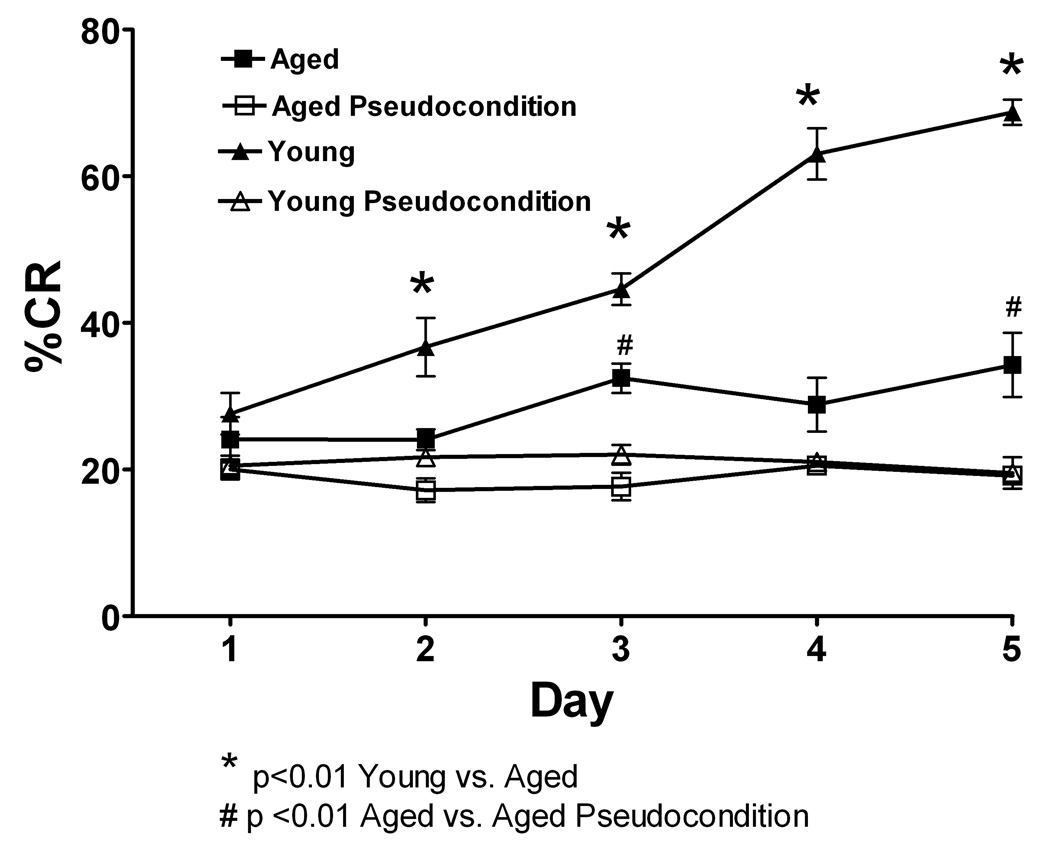
Microdialysis was performed in the cerebellar cortex on aged rats during training in the delay eyelid conditioning task on days 1, 3 and 5. Aged rats are significantly impaired on the acquisition of CR’s in this task when compared with young rats (Figure 8). The aged rats do show acquisition of CR’s over time as they are significantly different from pseudoconditioned aged-matched controls on days 3 and 5 of training (F = 46.3 (1, 40); N= 12 young condition, 12 young pseudocondtion, 12 aged condition, 12 aged pseudocondition). The data for young rats shown here is from the same animals reported in figure 1 and are reproduced here for comparison purposes. Neurotransmitter release was examined on days 1, 3 and 5 of training. However the microdialysis performed and plotted for neurotransmitter release on each day was done with independent groups of rats.
Figure 8.
Eyelid conditioning was performed over 5 days. The Y-axis shows the percentage of conditioned response (% CR’s), the x-axis represents 5 daily training sessions of 50 trials. Black triangles represent young conditioning, open triangles are young pseudoconditioning, black squares represent aging conditioning, open squares are aging pseudoconditioning. Both aging and young rats performed significantly better than their age-matched pseudoconditioning groups. For the conditioning groups, young rats learned the task faster and to a higher performance level than aged rats (p<0.01). * indicates difference between young and aged, # indicates difference between aged conditioning and aged pseudoconditioning.
NE
NE release was observed in the microdialysate on all days of training that were examined. The temporal pattern and magnitude of release changed over days of training (Figure 9). The NE measured increased beginning with the onset of training, however, the peak levels were not observed until 30 minutes, which is just after the training is finished. The NE levels remained at peak levels for at least 40 minutes and then slowly returned to baseline. As compared to the pseudo-training group, the trained group showed increases in NE concentration after the time 0 period. However, the nature of these gains, as well as subsequent declines in NE concentration differed for young as compared to older animals. The results for each of the 5 random effects models are shown in Table 1. As a guide to this table, intercept refers to the average value at the beginning of the time segment. The individual effects of day, group, and age reflect differences as a function of each of these factors at the beginning of the time interval. Finally, the interactions with time reflect the influence of the between subjects variables on changes over the individual time segment.
Figure 9.
Microdialysis was performed in the cerebellar cortex of aged rats during training in the delay eyelid conditioning task. The time window of NE release once eyelid conditioning starts can be observed on day 1 (A; N= 6 condition, 5 pseudocondition), day 3 (B; N= 6 condition, 5 pseudocondition) and day 5 (C; N= 6 condition, 5 pseudocondition). Data are expressed as NE [nM] (y-axis) over time in 10 minute dialysate samples (x-axis). Black squares represent conditioning, open squares represent pseudoconditioning. D) Area under the curve (AUC units) representation of NE release for each day of training during eyelid conditioning.
Table 1.
Model for NE
| Parameters | Time | |||
|---|---|---|---|---|
| 10–30 | 40–60 | 70–90 | 100–120 | |
| Intercept (Value at time start) | 18.78*** | 3.59 | −.24 | 2.28 |
| Day | −.27 | .05 | .25 | .25 |
| Group (pseudo vs training) | 13.70*** | 15.61*** | 10.73*** | 4.16*** |
| Age (Young vs. Old) | −17.93*** | 4.15* | 6.98*** | 3.97*** |
| Time | −.03 | −.15 | −.04 | .11 |
| Day*Time | −.21** | .04 | .04 | −.01 |
| Group*Time | .56* | −.45*** | −.20* | −.19* |
| Age*Time | .10 | .04 | .12 | −.33** |
| Day*Age*Time | .29*** | −.04 | −.06 | .04 |
| Group*Age*Time | −.32 | .49** | −.10 | .15 |
For the first segment measured which encompasses the time of training and one extra microdialysis sample (10 – 30 minutes), the average concentration for young rats was 18.78 nM, the training group was 13.70 nM higher than the pseudo-training group, and the older rats were almost 18 nM (Est. = 17.93 nM) units lower than the younger rats. For the changes in concentration over the 10 to 30 minutes period, there was a significant effect of day, with a lesser increase seen over subsequent days, and a statistically significant group X time interaction with larger gains for the trained group. Finally, the day by time by age interaction for the 10 to 30 minutes period, indicated that increases in NE levels in microdialysate for the older rats were less than those seen for the younger rats. For the segment between 40 and 60 minutes, the training group was almost 16 (Est. = 15.61) nM higher and the old was over 4 (Est. = 4.15) nM higher. This reflects a switch from the previous time segment where the young rats were higher than the older rats and also denotes the fact that the NE concentrations return to baseline much faster among the younger rats. Continuing with the 40–60 minutes time segment, the effect of time interaction with group revealed the training group lower levels of NE. Furthermore, the three way interaction between time, group, and age indicated that the older animals did not return to baseline as was seen for the young rats. For the final two time segments, the training group and the older rats had higher concentrations at the beginning of the time segments. In addition, the group by time interaction for both of these time segments indicated that the training groups had significantly greater declines in concentrations, as compared to the pseudo-conditioning group. Finally, the age by time interaction for the 100–120 minutes time segment reflected the fact that the older trained rats still exhibited declines at this follow-up period, whereas the pseudo-conditioning and younger conditioned rats change trajectories were essentially flat.
GABA
GABA release was also examined over days of training and it can be observed in Figure 10 that the amplitude of GABA release increased over days of training and the GABA levels remained elevated above baseline. Across the three days, the trained groups (young and aged) exhibited gains following the time 0 point, whereas the GABA concentrations for the pseudo-conditioning group were essentially flat. However, there were differences between the two age groups for the training condition, with younger rats exhibiting an earlier peak in concentration followed by rapid declines, which were more pronounced in days 3 and 5. By contrast, the older trained rats exhibited later peaks and did not return to pseudo-conditioning baseline concentrations.
Figure 10.
Microdialysis was performed in the cerebellar cortex on aged rats during training in the delay eyelid conditioning task. The time window of GABA release once eyelid conditioning starts can be observed on day 1 (A; N= 5 condition, 4 pseudocondition), day 3 (B; N= 5 condition, 4 pseudocondition) and day 5 (C; N= 5 condition, 5 pseudocondition). GABA release was significantly greater in the conditioning group on day 1 compared to day 5 (p<0.05). The pattern of release in aged rats changes across days so that by day 5 GABA remains elevated 2 hours after training. Data are expressed as percent of baseline (% baseline) (y-axis). Black squares represent conditioning, open squares represent pseudoconditioning. (D) Area under the curve representation of GABA release for each day of training during eyeblink conditioning
The results of each of the five random effects models are shown in Table 2. For the 10 to 30 minutes time period, the effect of day indicated that concentrations were significantly higher on subsequent days of testing. The effects of group and age revealed that at the start of the time period, the trained group and the younger rats were statistically higher than their counterparts. The three-way interaction between age, group, and time can be seen by comparing figure 3 and figure 10 whereby the young trained rats exhibit much greater gains over this time period, as compared to the conditionined old rats or both groups of pseudo-conditioned rats. Similar effects were seen during the 40 to 60 minutes time period. The main difference is that the effect of age is now reversed, with the old rats averaging almost 13 (Est. = 12.84) units higher than the younger rats. This difference reflects the fact that the concentrations for the younger rats return to low levels relatively rapidly, whereas the GABA concentrations for the old rats are maintained. For the final two time segments (70–90 and 100–120 minutes), the older conditioned rats still had higher GABA concentrations than the younger conditioned rats, who were at the level of the pseudo conditioned rats.
Table 2.
Model for GABA
| Parameters | Time | |||
|---|---|---|---|---|
| 10–30 | 40–60 | 70–90 | 100–120 | |
| Intercept (Value at time start) | 102.47*** | 98.86*** | 93.72*** | 94.53*** |
| Day | 3.50*** | .47 | .38 | −.09 |
| Group (pseudo vs training) | 19.66*** | 12.84*** | 12.47*** | 10.18*** |
| Age (Young vs. Old) | −13.54*** | 8.91*** | 13.45*** | 11.76*** |
| Time | .47* | .29 | .20 | .13 |
| Day*Time | −.20*** | −.09* | −.03 | .05 |
| Group*Time | −.74*** | −.54*** | −.34** | −.29** |
| Age*Time | −.05 | −.45* | −.37* | −.14 |
| Day*Age*Time | .17* | .08 | .05 | −.07 |
| Group*Age*Time | .67** | 1.00*** | .37 | .09 |
GLU
As can be seen in figure 11, there is no difference between the measured glutamate levels in microdialysate in the conditioning or pseudoconditioning paradigms. Neither the young or old rats showed statistically significant differences between the conditioned and pseudo-conditioned groups across the three days of measurement. The results of the give random effects models are shown in Table 3. There are several trends that are apparent in the table. First, the statistically significant effects for age were positive for the three final time periods, indicating that the older rats had higher concentrations. Two statistically significant interactions with age and time indicated that the two age groups exhibited different change functions across the 10–30 and 70–90 minutes time periods. The lack of statistically significant group effects, or relevant interactions across the final four time periods indicating that training did not impact concentrations of GLU.
Figure 11.
Microdialysis was performed in the cerebellar cortex on aged rats during training in the delay eyelid conditioning task. The release of Glu over time during eyelid conditioning is shown on day 1 (A; N= 4 condition, 4 pseudocondition), day 3 (B; N= 4 condition, 4 pseudocondition) and day 5 (C; N= 4 condition, 4 pseudocondition). Comparable levels of glutamate are released during training on the eyeblink task for both the conditioning and the pseudoconditioning groups. Data are expressed as Glu [µ M] (y-axis). Black squares represent conditioning, open squares represent pseudoconditioning. (D) Area under the curve representation of Glu release for each day of training during eyelid conditioning and pseudoconditioning (Ps-Cd, average of days). There were no differences in AUC of Glu.
Table 3.
Model for GLU
| Parameters | Time | |||
|---|---|---|---|---|
| 10–30 | 40–60 | 70–90 | 100–120 | |
| Intercept (Value at time start) | 12.17*** | 3.43*** | 3.09*** | 2.84*** |
| Day | −.13 | .15 | .01 | .12 |
| Group (pseudo vs training) | .65 | −.26 | .82* | .05 |
| Age (Young vs. Old) | .56 | 5.96*** | 3.88*** | 1.44*** |
| Time | −.41*** | −.02 | .00 | .01 |
| Day*Time | .01 | .00 | .00 | −.01 |
| Group*Time | .00 | .03 | −.02 | .03 |
| Age*Time | .22** | −.01 | −.10* | −.05 |
| Day*Age*Time | .02 | −.01 | .01 | .02 |
| Group*Age*Time | −.07 | .00 | .00 | −.07 |
Discussion
The overall results show presynaptic activity of the noradrenergic, GABAergic and glutamatergic neurotransmitters in the cerebellar cortex and interpositus nuclei as the active area of the microdialysis probe extended through both regions. Increases in the extracellular levels for the neurotransmitters NE, GABA and GLU occur during the acquisition of the CR in the delay eyeblink-conditioning paradigm. The literature shows that these neurotransmitters participate in different ways and are essential for memory formation, consolidation and extinction (Attwell, Cooke, and Yeo, 2002a; Farley and Alkon, 1985b; Yeo 2004a). To date there is no evidence directly showing presynaptic release in vivo while the animals perform the learning task. In this study, the extracellular levels of neurotransmitters reported correspond to those captured by the active zone of the microdialysis probe (3.0 mm) from the lobus simplex and interpositus nucleus. We show the magnitude and temporal pattern of release which occurs during training on the delay eyeblink conditioning task. This pattern changes differently for NE and GABA with the magnitude of release decreasing over days of training for NE but increasing for GABA. The temporal pattern for both NE and GABA extends past the training in the early days of training and becomes more closely associated with training as the rat learns the conditioned response. We further demonstrate that the NE overflow observed post training is important for consolidation of the learned response as blockade of the post-synaptic β-adrenergic receptor signal transduction with either propranolol reduces the amount of learned responses over days of training. The critical time period for this effect matches that observed for NE overflow.
Norepinephrine
The data demonstrate that extracellular levels of NE are increased in the cerebellum during training sessions of eyeblink conditioning from day 1 through day 5. On day 1, extracellular levels of NE remains increased over baseline for more than 60 minutes, whereas, as training progresses over days, the peak becomes smaller and NE remains elevated for a shorter time period so that by day 4 the NE signal returns to baseline within 30 minutes. In contrast, when pseudo-conditioning was examined there is no significant change in NE release during or after training on any day of training. This clearly indicates that the increase in NE which was observed during and after CS-US paired training is specifically linked to the combination of both the CS and US.
NE induces synaptic plasticity in Purkinje neurons by selectively improving the signal to noise ratio of evoked versus spontaneous activity, enhancing the sensitivity of cerebellar neurons to both excitatory and inhibitory afferent inputs (Freedman et al, 1976; Moises, Woodward, Hoffer, and Freedman, 1979). The importance of NE during acquisition of motor learning tasks is supported by reports that 6-OHDA induced lesions of the LC disrupt cerebellar motor learning on a runway task (Bickford et al, 1992; Watson et al, 1983) and classical eyeblink conditioning (Winsky et al, 1992). Furthermore, blockade of postsynaptic β-adrenergic receptors with systemic administration of propranolol disrupts acquisition of the delay eyeblink conditioning in both the rabbit (Gould 1998) and rat (Cartford et al, 2002). Previous studies have demonstrated that administering propranolol or Rp-cAMPS directly into the cerebellum prior to training impairs acquisition of CR’s (Cartford et al, 2004b). Interestingly, once the CR has been established by day 5 of training, neither propranolol nor Rp-cAMPS block performance of acquired CRs. Overall, the amount of NE released decreases across days of training consistent with a role of NE early in acquisition and possibly in consolidation (Gilbert, 1974b). This is consistent with work showing the firing of LC neurons demonstrates good discrimination within the first 500 trials of reversal of contingency in a visual discrimination task in monkeys (Kubiak, Rajkowski, Ivanova, and Aston-Jones, 1998) (monkeys are still showing a high number of errors), again suggesting that NE is important during the acquisition phase of learning of this task. The role of NE in consolidation is discussed further at the end of the discussion section.
GABA
As was observed with NE, GABA was released in a learning dependent manner and was specifically associated with paired conditioning and not pseudo-conditioning. As GABA is the predominant neurotransmitter in the cerebellum it was expected that if the cerebellum and interpositus nucleus are involved in the learned response, GABA release would be observed during the time corresponding to the training period. Interestingly, during the first days of training, the increase above baseline in extracellular levels of GABA extends beyond the training period for over 60 minutes. This pattern changes across days of training where the time frame of GABA release shortens and at a time where the behavioral response is nearly maximal the GABA release is now primarily only observed during the behavioral testing.
Much of the work examining GABAergic transmission using either GABA agonists or antagonists has been directed at examining the functional role of the cerebellar cortex and cerebellar nuclei in regard to the memory formation and timing. Discrete infusions of GABA agonists and antagonists into the cerebellar cortex and interpositus nucleus support the hypothesis that both areas are involved in plasticity (Bao et al, 2002; Krupa and Thompson, 1997; Mamounas, Thompson, and Madden, 1987; Schreurs and Alkon, 1993). For example, the expression of CRs is mediated by LTD at the granule to Purkinje synapses and by LTP at the mossy fiber synapses in the cerebellar nuclei (Mauk, Garcia, Medina, and Steele, 1998). GABAergic circuitry in the cerebellar cortex has also been postulated to play a role in post training memory consolidation: these authors demonstrate that delayed infusions of muscimol into the cerebellar cortex at 5 or 45 minutes produced significant impairments of consolidation, suggesting that about 1 hour after training there is a period of cortical activity that is necessary for consolidation of the learned response. This time window is similar to that observed for GABA release (and NE release) observed in this report on the first few days of training. This GABA release likely reflects the activity of the cerebellar circuitry following training. During the later phase of training (days 4 and 5) there is a more discrete release of GABA which shows a higher peak while the timing is confined to the training session. At this time the behavioral response is well trained and the GABA release pattern likely reflects the concomitant activity of the cerebellar circuitry that is associated with the CR. Interestingly, in this report we have measured GABA release from both interpositus and lobus simplex which corresponds to HVI in a rabbit. Unit recordings from these regions do show increased neuronal activity in both regions (Gould et al, 1994). Recent work in the anterior lobe has suggested that inhibition of Purkinje cell activity is the predominant learned unit activity (Green and Steinmetz, 2005) and it will be of significant interest to repeat these studies with microdialysis probes that are more discrete so that we can distinguish interpositus from cortex, and to examine differences between lobus simplex and anterior lobe for the different neurotransmitters.
Glutamate
GLU release measured in this report likely reflects the inputs to the cerebellar cortex and interpositus seems to be activated in both paired and unpaired conditions which may explains why we observe and increase above the basal levels of GLU in both conditions. This also agrees with the hypothesis that learning does not occur prior to afferent signals arriving in the cerebellum, as the glutamate input does not show learning related activity. This does not suggest that GLU is unimportant postsynaptically within the cerebellar cortex or interpositus nucleus, this simply reflects the presynaptic signal entering the cerebellum as the training signal. Glutamate has been shown to have significant post-synaptic effects during training. For example, infusion of the NMDA antagonist AP5 disrupts eyeblink conditioning (Chen and Steinmetz, 2000a). As mentioned above, our results reflect the pre-synaptic events of the glutamatergic system from afferents to the cerebellar cortex and support the idea that learning of the conditioned response does not take place before the signals reach the cerebellum, as there is no change in afferent activity across days of training. It is interesting that GLU release happens in both, paired and unpaired trials of the conditioning sessions and the time period for the release is concomitant with the time period in which the sensory input to the cerebellum is happening. A different scenario is observed for the NE and GABA as described above. Changes in the dynamics of NE release over time suggest that NE plays a major role in the first days of training. The change in timing of the GABA response correlates with the learned response in the rat and possibly reflects the activity of Purkinje neurons and interpositus neurons that show entrained firing that develops over days of training.
NE and consolidation
To further examine the role of NE in consolidation we administered propranolol into the cerebellar cortex at either 5, 60 or 120 minutes post-training. The local administration of propranolol post training diminished the acquisition of CR’s when administered up to 120 minutes post-training. It is important to note that when propranolol was administered post-training that the rats still learned, albeit not to the same extent as controls, indicating that even when the β-noradrenergic signaling is blocked there are other neurotransmitters involved in the formation of memory. It seems that the role of NE is to modulate this effect, consistent with it’s role to increase the signal to noise ratio of other neurotranmsitters (Freedman et al, 1976). It is possible that the inhibition of the beta-noradrenergic receptor makes it difficult to enhance the response of the cerebellar neurons to GABA and possibly GLU during post-training activation of the cerebellar circuitry necessary for consolidation of the learned response. In other words, the lack of the noradrenergic signal (elicited by propranolol administration) impairs the improvement of the signal to noise ratio of learning or consolidation related neuronal firing versus background spontaneous neuronal activity which may explain why the rats would require more training sessions in order to reach a significant level of conditioned responses. One caveat to take into consideration with this data however is that for the second session every day the propranolol was administered 4 –6 hours prior to acquisition, thus could have lingering effects on the 2nd session of the day. This is unlikely as we saw no effects of propranolol on parameters of the CR or UR such as amplitude or timing. Furthermore, if this were to be playing a role then the 120 minute group would show more residual effects of the propranolol than the 5 minute group and this was not observed.
Another explanation for the inhibition of CR’s after blocking the beta-adrenergic receptor at different time points post-training could be associated with the activity that NE exerts by activating the beta-adrenergic receptors which leads to the activation of cAMP/PKA signaling cascade, this was demonstrated previously using the PKA inhibitor Rp-cAMPS which also disrupts the consolidation of the CR’s (Cartford et al, 2004b), although other signaling pathways can also lead to activation of PKA, thus it is possible that the results with PKA reflect activation of one of these alternate pathways in addition to activation via the β-adrenergic receptor. Outside of the cerebellum, cAMP, PKA and phophorylated CREB (pCREB) have been implicated in the establishment of synaptic changes necessary for both short-term and long-term memory formation (Baldwin, Sadeghian, Holahan, and Kelley, 2002; Muller, 2000; Shobe, 2002; Taylor, Birnbaum, Ubriani, and Arnsten, 1999; Vianna et al, 2000) and studies in long term potentiation (LTP) and long term depression (LTD) support these behavioral findings (Huang and Kandel, 1996; Huang et al, 1994; Nayak, Zastrow, Lockteig, Zahniser, and Browning, 1998; Rotenberg, Abel, Hawkins, Kandel, and Muller, 2000). In eyelid conditioning in particular Chen and Steinmetz (Chen and Steinmetz, 2000b) have shown that localized blocking of a range of kinase activity disrupts acquisition but not retention of conditioning in rabbits.
Activation of the cAMP/PKA signaling pathway has been shown to enhance intracellular calcium and promote the release of vesicles containing GABA (Saitow, Suzuki, and Konishi, 2005). Furthermore, increases in intracellular calcium in GABAergic synapses are also modulated by AMPA receptors (Rusakov, Saitow, Lehre, and Konishi, 2005; Satake, Saitow, Rusakov, and Konishi, 2004). Interestingly, Luft and colleagues demonstrated that motor skill learning depends on de novo synthesis of proteins in motor cortex after training. Based on the authors work they hypothesize that consolidation (of motor learning) requires modifications in neuronal circuitry or plastic changes in neuronal structure and established that the disruption of protein synthesis in inter trials can diminish consolidation (Luft and Buitrago, 2005; Luft, Buitrago, Ringer, Dichgans, and Schulz, 2004).
It has been shown that the blockade of non-NMDA receptors by CNQX infusion into the cerebellar cortex is also capable of disrupting learning (Attwell, Rahman, and Yeo, 2001). In addition, the activation of GABA-A receptors in the cerebellar nuclei using muscimol can disrupt the acquisition of CR’s (Hardiman, Ramnani, and Yeo, 1996; Krupa, Thompson, and Thompson, 1993; Ramnani and Yeo, 1996; Yeo, Lobo, and Baum, 1997). Cooke and colleagues published an experiment where they demonstrated that cortical infusions of the GABA-A receptor agonist muscimol delayed by 5 or 45 minutes after a conditioning session disrupted learning, suggesting a role of the cerebellar cortex in consolidation (Cooke, Attwell, and Yeo, 2004). Further support for the role of GABAergic activation post-training is observed in our microdialysis data demonstrating the GABA release is observed for up to 60 minutes post training as well (discussed further above). Together these findings suggest that in addition to various neurotransmitters playing a role in the acquisition of CR’s there are critical temporal effects which have been elucidated for GABA and NE which are critical for the consolidation of CR’s.
Aging effect on neurochemical profile of EBC
The results show that release of the neurotransmitters NE, GABA and GLU occurs during the acquisition of the CR in the delay eyelid conditioning paradigm in aged rats. Furthermore, the data show that aged rats have an alteration in the temporal pattern and magnitude of release of the neurotransmitters NE, GABA and Glu associated to the learning of the CR of eyeblink conditioning when compared with young rats. This deficit appears to be directly associated with the impairments on the acquisition of CR’s observed in aged rats. The literature shows that these neurotransmitters participate in different ways and are essential for memory formation, consolidation and extinction (Attwell, Cooke, and Yeo, 2002b; De Zeeuw and Yeo, 2005a; Farley and Alkon, 1985a; Weis, Klaver, Reul, Elger, and Fernandez, 2004; Yeo, 2004b). .
We have demonstrated in young rats that extracellular levels of NE are increased in the cerebellum during training sessions of eyeblink conditioning from day 1 through day 5. Both propranolol and Rp-cAMPS (activate cAMP-dependent PKA) directly administered into the cerebellum prior to learning impair acquisition of CRs (Cartford, Samec, Fister, and Bickford, 2004a). In young rats the amount of NE released decreases across days of training consistent with a role of NE early in acquisition and possibly in consolidation (Gilbert, 1974a). However, in aged rats there was a very different temporal pattern of release during each day of training and across days of training. Across days of training the amount of release as measured by the AUC increased from day 1 to days 3 and 5; this is associated and consistent with an improvement in learned responses not being observed until much later in the training process. The peak amplitude of release in aged rats is significantly less than that observed in young rats. For example on day1 the peak magnitude of release for young rats is approximately 50 nM and is observed 10–20 minutes after the initiation of training. For aged rats the peak magnitude of release observed on day 1 is approximately 20 nM and is observed at that level for an extended period of time between 30 and 60 minutes post initiation of training, this peak release lasts well into the post training period. In young rats, the NE levels also are observed after the training session on days 1–3, and this is thought to reflect an important aspect of NE function related to consolidation. If NE transmission is blocked post-training with propranolol administered into the cerebellar cortex then this disrupts learning. However the extent of temporal pattern change and the fact that NE levels do not even peak until after training occurs may have a significant impact on information processing within the cerebellum and be one of the reasons that learning of the CR’s is not observed in the aged rats. The NE is simply not present at adequate levels and appropriate time during the training sessions. One thing to note, however, is that the total amount of NE released when examined as AUC, thus independent of timing, is similar for both young and aged rats. The lower amplitude and prolonged time frame of release is also observed in aged rats when NE clearance is studied in the cerebellum of aged rats (Lin, Bickford, Palmer, Cline, and Gerhardt, 1997) suggesting that one potential mechanism underlying these changes is a reduction in clearance of NE via reduced reuptake. However, this may not adequately explain the long term time course change on the order of hours, as these changes in re-uptake would most likely only have effects on extra cellular levels of NE on the order of seconds or minutes. A prolonged increase in plasma catecholamines has also been observed in response to novelty stress in aged rats (Mabry, Gold, and McCarty, 1995) and the relationship between these two changes in time course of catecholamine responses should be explored further.
As was observed with NE, GABA was released in a learning dependent manner and was specifically associated with paired conditioning and not pseudo-conditioning in the aged rats. GABA release in aged rats was also significantly delayed in peak amplitude and duration when compared to the young rats (see data from companion paper), thus the age related changes in the temporal pattern of neurotransmitter release are not selective for NE. This could be an indication of over activity of the incoming signaling carried out by the climbing and mossy fibers. As there was also a change in the temporal pattern of glutamate release, this does suggest that the afferents to the cerebellum are continuing to show activity, even after the training session. This continued activation of afferent input is not observed in young rats. Thus, this underscores that changes in the associative learning circuit outside of the cerebellum are also contributing to the age-related decline in learning of CR’s.
The data show an alteration in the pattern of release for NE, GABA and glutamate in aged rats, strongly suggesting that the deficit observed in the acquisition of the CR’s in aged rats is related to a loss in the synchronization of neurochemical patterns during the acquisition of CR’s. It should also be considered that the changes in the neurochemical pattern observed in the aged animals could be a consequence of processes underlying aging, such as oxidative stress and inflammation for which the radical theory of aging has predicted (Harman, 1956). A decline in the capacity of normal antioxidant defense mechanism has been postulated as a causative factor in aging related decline in the normal activity of different physiological systems (Ames, Shigenaga, and Hagen, 1993b). Damage to proteins, DNA and membranes has also been reported in association with aging (Ames, Shigenaga, and Hagen, 1993a; Bickford, 1993a; Davies and Goldberg, 1987; Gutteridge and Stocks, 1976). Chronic inflammation leads to an over activation of microglia and high levels of pro-inflammatory cytokines and has been shown to deplete learning in the eyeblink conditioning task (Cartford, Gemma, and Bickford, 2002a). The present report agrees with previous data which has demonstrated that deficits in noradrenergic signaling seen in aging are directly associated to the decline in learning capabilities (Bickford, Shukitt-Hale, and Joseph, 1999). More experiments are required to address the different aspects associated with cognitive deficits observed during aging. We have shown with microdialysis that extracellular levels of NE increase at the beginning of training and remain elevated above basal levels for up to 80 minutes after the training session has finished. If we consider the release of NE directly into the cerebellar cortex during the critical time for consolidation, it is reasonable to suggest that NE might also have a critical role providing a source of energy when it is required for encoding memory in the cerebellum.
In conclusion, these studies monitored the dynamics of noradrenergic, GABAergic and glutamatergic release as a consequence of the delay classical eyeblink conditioning in the cerebellum in young and aged rats. These data show that NE and GABA release vary dynamically over time and are specific to the paired training while GLU release is unaltered over days of training and is also observed with unpaired training, suggesting that the information entering the cerebellum is not trained. The timing and amplitude of the NE response is consistent with previous studies demonstrating a role for NE to facilitate acquisition of learned responses in this and other cerebellar dependent learning tasks. The timing of the GABA and NE responses in young rats during the first days of training is consistent with a period of about 1 – 2 hours post training during which the cerebellar circuitry is active which may reflect a period of consolidation. The fact that NE is also elevated after training is consistent with a possible role for NE in the consolidation process. The data showing that blockade of β-adrenergic receptors following learning is consistent with the role of this elevated NE overflow with consolidation of the association between the CS and US. These results clearly indicate a concomitant synchronization among noradrenergic, GABAergic and glutamatergic neurotransmitter systems during delay classical conditioning. It is likely that shifting in the timing or sequences of events within this neurochemical orchestrations as observed in the aged rats might be responsible for declinations in the acquisition rate or event expression of the CR’s of the EBC during aging and different pathological conditions.
Acknowledgements
This research was supported by NIH grants MH070430, AG04418 and the VAMRS.
Reference List
- Aksenov D, Serdyukova N, Irwin K, Bracha V. GABA neurotransmission in the cerebellar interposed nuclei: involvement in classically conditioned eyeblinks and neuronal activity. J.Neurophysiol. 2004;91:719–727. doi: 10.1152/jn.00859.2003. [DOI] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc.Natl.Acad.Sci.U.S.A. 1993b;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc.Natl.Acad.Sci.U.S.A. 1993a;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardenghi P, Barros D, Izquierdo LA, Bevilaqua L, Schroder N, Quevedo J, Rodrigues C, Madruga M, Medina JH, Izquierdo I. Late and prolonged post-training memory modulation in entorhinal and parietal cortex by drugs acting on the cAMP/protein kinase A signalling pathway. Behav.Pharmacol. 1997;8:745–751. doi: 10.1097/00008877-199712000-00010. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P. Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience. 1997;80:697–715. doi: 10.1016/s0306-4522(97)00060-2. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002b;34:1011–1020. doi: 10.1016/s0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Cooke SF, Yeo CH. Cerebellar function in consolidation of a motor memory. Neuron. 2002a;34:1011–1020. doi: 10.1016/s0896-6273(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Ivarsson M, Millar L, Yeo CH. Cerebellar mechanisms in eyeblink conditioning. Annals of the New York Academy of Sciences. 2002;978:79–92. doi: 10.1111/j.1749-6632.2002.tb07557.x. [DOI] [PubMed] [Google Scholar]
- Attwell PJ, Rahman S, Yeo CH. Acquisition of eyeblink conditioning is critically dependent on normal function in cerebellar cortical lobule HVI. J.Neurosci. 2001;21:5715–5722. doi: 10.1523/JNEUROSCI.21-15-05715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Holahan MR, Kelley AE. Appetitive instrumental learning is impaired by inhibition of cAMP- dependent protein kinase within the nucleus accumbens. Neurobiol.Learn.Mem. 2002;77:44–62. doi: 10.1006/nlme.2000.4002. [DOI] [PubMed] [Google Scholar]
- Bao S, Chen L, Kim JJ, Thompson RF. Cerebellar cortical inhibition and classical eyeblink conditioning. Proc.Natl.Acad.Sci.U.S.A. 2002;99:1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier NE, Moore JW. Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response. Experimental Brain Research. 1986;63:341–350. doi: 10.1007/BF00236851. [DOI] [PubMed] [Google Scholar]
- Bickford P. Motor learning deficits in aged rats are correlated with loss of cerebellar noradrenergic function. Brain Research. 1993b;620:133–138. doi: 10.1016/0006-8993(93)90279-v. [DOI] [PubMed] [Google Scholar]
- Bickford P. Motor learning deficits in aged rats are correlated with loss of cerebellar noradrenergic function. Brain Res. 1993a;620:133–138. doi: 10.1016/0006-8993(93)90279-v. [DOI] [PubMed] [Google Scholar]
- Bickford P. Aging and motor learning: a possible role for norepinephrine in cerebellar plasticity. Reviews in the Neurosciences. 1995a;6:35–46. doi: 10.1515/revneuro.1995.6.1.35. [DOI] [PubMed] [Google Scholar]
- Bickford P, Heron C, Young DA, Gerhardt GA, de la Garza R. Impaired acquisition of novel locomotor tasks in aged and norepinephrine-depleted F344 rats. Neurobiology of Aging. 1992;13:475–481. doi: 10.1016/0197-4580(92)90075-9. [DOI] [PubMed] [Google Scholar]
- Bickford PC. A possible role for norepinephrine in cerebellar plasticity. Reviews in Neuroscience. 1995b;6:35–46. doi: 10.1515/revneuro.1995.6.1.35. [DOI] [PubMed] [Google Scholar]
- Bickford PC, Hoffer BJ, Freedman R. Interaction of norepinephrine with Purkinje cell responses to cerebellar afferent inputs in aged rats. Neurobiology of Aging. 1985;6:89–94. doi: 10.1016/0197-4580(85)90023-5. [DOI] [PubMed] [Google Scholar]
- Bickford PC, Shukitt-Hale B, Joseph J. Effects of aging on cerebellar noradrenergic function and motor learning: nutritional interventions. Mech.Ageing Dev. 1999;111:141–154. doi: 10.1016/s0047-6374(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Bowenkamp KE, Lapchak PA, Hoffer BJ, Miller PJ, Bickford PC. Intracerebroventricular glial cell line-derived neurotrophic factor improves motor function and supports nigrostriatal dopamine neurons in bilaterally 6-hydroxydopamine lesioned rats. Experimental Neurology. 1997;145:104–117. doi: 10.1006/exnr.1997.6436. [DOI] [PubMed] [Google Scholar]
- Cartford MC, Allgeier CA, Bickford PC. The effects of beta-noradrenergic receptor blockade on acquisition of eyeblink conditioning in 3-month-old F344 rats. Neurobiol.Learn.Mem. 2002;78:246–257. doi: 10.1006/nlme.2002.4063. [DOI] [PubMed] [Google Scholar]
- Cartford MC, Gemma C, Bickford PC. Eighteen-month-old Fischer 344 rats fed a spinach-enriched diet show improved delay classical eyeblink conditioning and reduced expression of tumor necrosis factor alpha (TNFalpha) and TNFbeta in the cerebellum. J.Neurosci. 2002a;22:5813–5816. doi: 10.1523/JNEUROSCI.22-14-05813.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartford MC, Gemma C, Bickford PC. Eighteen-month-old Fischer 344 rats fed a spinach-enriched diet show improved delay classical eyeblink conditioning and reduced expression of tumor necrosis factor alpha (TNFalpha) and TNFbeta in the cerebellum. J.Neurosci. 2002b;22:5813–5816. doi: 10.1523/JNEUROSCI.22-14-05813.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartford MC, Samec A, Fister M, Bickford PC. Cerebellar norepinephrine modulates learning of delay classical eyeblink conditioning: evidence for post-synaptic signaling via PKA. Learn.Mem. 2004b;11:732–737. doi: 10.1101/lm.83104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartford MC, Samec A, Fister M, Bickford PC. Cerebellar norepinephrine modulates learning of delay classical eyeblink conditioning: evidence for post-synaptic signaling via PKA. Learn.Mem. 2004a;11:732–737. doi: 10.1101/lm.83104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Steinmetz JE. Intra-cerebellar infusion of NMDA receptor antagonist AP5 disrupts classical eyeblink conditioning in rabbits. Brain Research. 2000a;887:144–156. doi: 10.1016/s0006-8993(00)03005-5. [DOI] [PubMed] [Google Scholar]
- Chen G, Steinmetz JE. Microinfusion of protein kinase inhibitor H7 into the cerebellum impairs the acquisition but not the retention of classical eyeblink conditioning in rabbits. Brain Research. 2000b;856:193–201. doi: 10.1016/s0006-8993(99)02429-4. [DOI] [PubMed] [Google Scholar]
- Cheun JE, Yeh HH. Modulation of GABAA receptor-activated current by norepinephrine in cerebellar Purkinje cells. Neuroscience. 1992;51:951–960. doi: 10.1016/0306-4522(92)90532-7. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Attwell PJ, Yeo CH. Temporal properties of cerebellar-dependent memory consolidation. Journal of Neuroscience. 2004;24:2934–2941. doi: 10.1523/JNEUROSCI.5505-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KJ, Goldberg AL. Oxygen radicals stimulate intracellular proteolysis and lipid peroxidation by independent mechanisms in erythrocytes. J.Biol.Chem. 1987;262:8220–8226. [PubMed] [Google Scholar]
- De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr.Opin.Neurobiol. 2005b;15:667–674. doi: 10.1016/j.conb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr.Opin.Neurobiol. 2005a;15:667–674. doi: 10.1016/j.conb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Farley J, Alkon DL. Cellular mechanisms of learning, memory, and information storage. Annu.Rev.Psychol. 1985b;36:419–494. doi: 10.1146/annurev.ps.36.020185.002223. [DOI] [PubMed] [Google Scholar]
- Farley J, Alkon DL. Cellular mechanisms of learning, memory, and information storage. Annu.Rev.Psychol. 1985a;36:419–494. doi: 10.1146/annurev.ps.36.020185.002223. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hoffer BJ, Puro D, Woodward DJ. Noradrenaline modulation of the responses of the cerebellar Purkinje cell to afferent synaptic activity. British Journal of Pharmacology. 1976;57:603–605. doi: 10.1111/j.1476-5381.1976.tb10391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Hoffer BJ, Woodward DJ, Puro D. Interaction of norepinephrine with cerebellar activity evoked by mossy and climbing fibers. Experimental Neurology. 1977;55:269–288. doi: 10.1016/0014-4886(77)90175-3. [DOI] [PubMed] [Google Scholar]
- Gilbert P. How the cerebellum could memorise movements. Nature. 1975;254:688–689. doi: 10.1038/254688a0. [DOI] [PubMed] [Google Scholar]
- Gilbert PF. A theory of memory that explains the function and structure of the cerebellum. Brain Res. 1974b;70:1–18. doi: 10.1016/0006-8993(74)90208-x. [DOI] [PubMed] [Google Scholar]
- Gilbert PF. A theory of memory that explains the function and structure of the cerebellum. Brain Res. 1974a;70:1–18. doi: 10.1016/0006-8993(74)90208-x. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Beta-adrenergic involvement in acquisition vs. extinction of a classically conditioned eye blink response in rabbits. Brain Research. 1998;780:174–177. [PubMed] [Google Scholar]
- Gould TJ, Steinmetz JE. Multiple-unit activity from rabbit cerebellar cortex and interpositus nucleus during classical discrimination/reversal eyelid conditioning. Brain Research. 1994;652:98–106. doi: 10.1016/0006-8993(94)90322-0. [DOI] [PubMed] [Google Scholar]
- Green JT, Steinmetz JE. Purkinje cell activity in the cerebellar anterior lobe after rabbit eyeblink conditioning. Learn.Mem. 2005;12:260–269. doi: 10.1101/lm.89505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge JM, Stocks J. Peroxidation of cell lipids. Med.Lab Sci. 1976;33:281–285. [PubMed] [Google Scholar]
- Hall ME, Hoffer BJ, Gerhardt GA. Rapid and sensitive determination of catecholamines in small tissue samples by high performance liquid chromatography coupled with dual-electrode coulometric electrochemical detection. LC.GC. 1986;7:258–265. [Google Scholar]
- Hardiman MJ, Ramnani N, Yeo CH. Reversible inactivations of the cerebellum with muscimol prevent the acquisition and extinction of conditioned nictitating membrane responses in the rabbit. Exp.Brain Res. 1996;110:235–247. doi: 10.1007/BF00228555. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J.Geront. 1956;11:289–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Heron C, Gould TJ, Bickford P. Acquisition of a runway motor learning task is impaired by a beta adrenergic antagonist in f344 rats. Behavioural Brain Research. 1996;78:235–241. doi: 10.1016/0166-4328(95)00252-9. [DOI] [PubMed] [Google Scholar]
- Hoffer BJ, Freedman R, Woodward DJ, Puro D, Moises H. A functional role for adrenergic input to the cerebellar cortex: interaction of norepinephrine with mossy and climbing fiber excitation and GABA-mediated inhibition. In: Garattini S, et al., editors. Interactions.between.putative.neurotransmitters.in.the.brain. NewYork: Raven.Press; 1978. pp. 231–243. [Google Scholar]
- Huang YY, Kandel ER. Modulation of both the early and the late phase of mossy fiber LTP by the activation of beta-adrenergic receptors. Neuron. 1996;16:611–617. doi: 10.1016/s0896-6273(00)80080-x. [DOI] [PubMed] [Google Scholar]
- Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis dependent late-phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson JK, Thompson RF. Localization of a memory trace in the mammalian brain. Science. 1993;260:989–991. doi: 10.1126/science.8493536. [DOI] [PubMed] [Google Scholar]
- Krupa DJ, Thompson RF. Reversible inactivation of the cerebellar interpositus nucleus completely prevents acquisition of the classically conditioned eye-blink response. Learn.Mem. 1997;3:545–556. doi: 10.1101/lm.3.6.545. [DOI] [PubMed] [Google Scholar]
- Kubiak P, Rajkowski J, Ivanova S, Aston-Jones G. Rapid acquisition of discriminative responding in monkey locus coeruleus neurons. Adv.Pharmacol. 1998;42:956–960. doi: 10.1016/s1054-3589(08)60906-0. [DOI] [PubMed] [Google Scholar]
- Lin AM, Bickford PC, Palmer MR, Cline EJ, Gerhardt GA. Effects of ethanol and nomifensine on NE clearance in the cerebellum of young and aged Fischer 344 rats. Brain Research. 1997;756:287–292. doi: 10.1016/s0006-8993(97)00229-1. [DOI] [PubMed] [Google Scholar]
- Lin AMY, Freund RK, Palmer MR. Sensitization of GABA-induced depressions of cerebellar Purkinje neurons to the potentiative effects of ethanol by B-adrenergic mechanisms in rat brain. Journal of Pharmacology and Experimental Therapeutics. 1993 [PubMed] [Google Scholar]
- Littel RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2006 2nd. [Google Scholar]
- Luft AR, Buitrago MM. Stages of motor skill learning. Mol.Neurobiol. 2005;32:205–216. doi: 10.1385/MN:32:3:205. [DOI] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM, Ringer T, Dichgans J, Schulz JB. Motor skill learning depends on protein synthesis in motor cortex after training. J.Neurosci. 2004;24:6515–6520. doi: 10.1523/JNEUROSCI.1034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabry TR, Gold PE, McCarty R. Age-related changes in plasma catecholamine and glucose responses of F-344 rats to a single footshock as used in inhibitory avoidance training. Neurobiology of Learning & Memory. 1995;64:146–155. doi: 10.1006/nlme.1995.1054. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Thompson RF, Madden J. Cerebellar GABAergic processes: evidence for critical involvement in a form of simple associative learning in the rabbit. Proc.Natl.Acad.Sci.U.S.A. 1987;84:2101–2105. doi: 10.1073/pnas.84.7.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauk MD, Garcia KS, Medina JF, Steele PM. Does cerebellar LTD mediate motor learning? Toward a resolution without a smoking gun. Neuron. 1998;20:359–362. doi: 10.1016/s0896-6273(00)80978-2. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Locus coeruleus lesions and resistance to extinction of a classically conditioned response: involvement of the neocortex and hippocampus. Brain Res. 1982;245:239–249. doi: 10.1016/0006-8993(82)90806-x. [DOI] [PubMed] [Google Scholar]
- McElligott JG, Freedman W. Vestibulo-ocular reflex adaptation in cats before and after depletion of norepinephrine. Exp.Brain Res. 1988;69:509–521. doi: 10.1007/BF00247305. [DOI] [PubMed] [Google Scholar]
- Moises HC, Woodward DJ, Hoffer BJ, Freedman R. Interactions of norepinephrine with Purkinje cell responses to putative amino acid neurotransmitters applied by microiontophoresis. Exp.Neurol. 1979;64:493–515. doi: 10.1016/0014-4886(79)90227-9. [DOI] [PubMed] [Google Scholar]
- Muller U. Prolonged activation of cAMP-dependent protein kinase during conditioning induces long-term memory in honeybees. Neuron. 2000;27:159–168. doi: 10.1016/s0896-6273(00)00017-9. [DOI] [PubMed] [Google Scholar]
- Nayak A, Zastrow DJ, Lockteig R, Zahniser NR, Browning MD. Maintenance of the late-phase of LTP is accompanied by PKA-dependent increase in AMPA receptor synthesis. Nature. 1998;394:680–683. doi: 10.1038/29305. [DOI] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Lee KH, Gibbs E, Dudman JT, Santoro B, Yin D, Thompson RF, Siegelbaum SA, Kandel ER, Morozov A. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 2003;115:551–564. doi: 10.1016/s0092-8674(03)00884-5. [DOI] [PubMed] [Google Scholar]
- Parfitt KD, Hoffer BJ, Bickford-Wimer PC. Potentiation of gamma-aminobutyric acid-mediated inhibition by isoproterenol in the cerebellar cortex: receptor specificity. Neuropharmacology. 1990;29:909–916. doi: 10.1016/0028-3908(90)90141-d. [DOI] [PubMed] [Google Scholar]
- Perrett SP, Ruiz BP, Mauk MD. Cerebellar cortex lesions disrupt learning-dependent timing of conditioned eye blink responses. Journal of Neuroscience. 1993;13:1708–1718. doi: 10.1523/JNEUROSCI.13-04-01708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano O, van Neerven J, Collewijn H, van der Steen J. Changes in VOR adaptation after local injection of beta-noradrenergic agents in the flocculus of rabbits. Acta Otolaryngol. 1991;111:176–181. doi: 10.3109/00016489109137371. [DOI] [PubMed] [Google Scholar]
- Rada P, Mendialdua A, Hernandez L, Hoebel BG. Extracellular glutamate increases in the lateral hypothalamus during meal initiation, and GABA peaks during satiation: microdialysis measurements every 30 s. Behav.Neurosci. 2003;117:222–227. doi: 10.1037/0735-7044.117.2.222. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Yeo CH. Reversible inactivations of the cerebellum prevent the extinction of conditioned nictitating membrane responses in rabbits. J.Physiol. 1996;495(Pt 1):159–168. doi: 10.1113/jphysiol.1996.sp021581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg A, Abel T, Hawkins RD, Kandel ER, Muller RU. Parallel instabilities of long-term potentiation, place cells, and learning caused by decreased protein kinase A activity. Journal of Neuroscience. 2000;20:8096–8102. doi: 10.1523/JNEUROSCI.20-21-08096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Saitow F, Lehre KP, Konishi S. Modulation of presynaptic Ca2+ entry by AMPA receptors at individual GABAergic synapses in the cerebellum. J.Neurosci. 2005;25:4930–4940. doi: 10.1523/JNEUROSCI.0338-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitow F, Suzuki H, Konishi S. beta-Adrenoceptor-mediated long-term up-regulation of the release machinery at rat cerebellar GABAergic synapses. J.Physiol. 2005;565:487–502. doi: 10.1113/jphysiol.2005.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake S, Saitow F, Rusakov D, Konishi S. AMPA receptor-mediated presynaptic inhibition at cerebellar GABAergic synapses: a characterization of molecular mechanisms. Eur.J.Neurosci. 2004;19:2464–2474. doi: 10.1111/j.0953-816X.2004.03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs BG, Alkon DL. Rabbit cerebellar slice analysis of long-term depression and its role in classical conditioning. Brain Res. 1993;631:235–240. doi: 10.1016/0006-8993(93)91540-9. [DOI] [PubMed] [Google Scholar]
- Shobe J. The Role of PKA, CaMKII, and PKC in Avoidance Conditioning: Permissive or Instructive? Neurobiol.Learn.Mem. 2002;77:291–312. doi: 10.1006/nlme.2001.4022. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willet JB. Applied longitudinal data analysis: Modeling change and event occurence. 2003 [Google Scholar]
- Taylor JR, Birnbaum S, Ubriani R, Arnsten AF. Activation of cAMP-dependent protein kinase A in prefrontal cortex impairs working memory performance. Journal of Neuroscience. 1999;19:RC23. doi: 10.1523/JNEUROSCI.19-18-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna MR, Izquierdo LA, Barros DM, Ardenghi P, Pereira P, Rodrigues C, Moletta B, Medina JH, Izquierdo I. Differential role of hippocampal cAMP-dependent protein kinase in short- and long-term memory. Neurochemistry Research. 2000;25:621–626. doi: 10.1023/a:1007502918282. [DOI] [PubMed] [Google Scholar]
- Vianna MR, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc.Natl.Acad.Sci.U.S.A. 2001;98:12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M, McElligott JG. 6-OHDA induced effects upon the acquisition and performance of specific locomotor tasks in rats. Pharmacology and Biochemistry of Behavior. 1983;18:927–934. doi: 10.1016/s0091-3057(83)80016-1. [DOI] [PubMed] [Google Scholar]
- Watson M, McElligott JG. Cerebellar norepinephrine depletion and impaired acquisition of specific locomotor tasks in rats. Brain Research. 1984b;296:129–138. doi: 10.1016/0006-8993(84)90518-3. [DOI] [PubMed] [Google Scholar]
- Watson M, McElligott JG. Cerebellar norepinephrine depletion and impaired acquisition of specific locomotor tasks in rats. Brain Res. 1984a;296:129–138. doi: 10.1016/0006-8993(84)90518-3. [DOI] [PubMed] [Google Scholar]
- Weis S, Klaver P, Reul J, Elger CE, Fernandez G. Temporal and cerebellar brain regions that support both declarative memory formation and retrieval. Cereb.Cortex. 2004;14:256–267. doi: 10.1093/cercor/bhg125. [DOI] [PubMed] [Google Scholar]
- Weiss C, Thompson RF. Delayed acquisition of eyeblink conditioning in aged F1 hybrid (Fischer-344 X Brown Norway) rats. Neurobiology.of.Aging. 1992;13:319–323. doi: 10.1016/0197-4580(92)90045-y. [DOI] [PubMed] [Google Scholar]
- Winsky L, Harvey JA. 6-Hydroxydopamine induced impairment of Pavlovian conditioning in the rabbit. Neurochemistry Research. 1992;17:415–422. doi: 10.1007/BF00969886. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Thompson RF. Classical conditioning of the eyelid response in rabbits as a model system for the study of brain mechanisms of learning and memory in aging. Experimental Aging Research. 1985;11:109–122. doi: 10.1080/03610738508259290. [DOI] [PubMed] [Google Scholar]
- Yeh HH, Woodward DJ. Beta-1 adrenergic receptors mediate noradrenergic facilitation of Purkinje cell responses to gamma-amminobutyric acid in cerebellum of rat. Neuropharmacology. 1983;22:629–639. doi: 10.1016/0028-3908(83)90155-7. [DOI] [PubMed] [Google Scholar]
- Yeo CH. Memory and the cerebellum. Curr.Neurol.Neurosci.Rep. 2004a;4:87–89. doi: 10.1007/s11910-004-0018-4. [DOI] [PubMed] [Google Scholar]
- Yeo CH. Memory and the cerebellum. Curr.Neurol.Neurosci.Rep. 2004b;4:87–89. doi: 10.1007/s11910-004-0018-4. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Experimental Brain Research. 1985;60:99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Lobo DH, Baum A. Acquisition of a new-latency conditioned nictitating membrane response--major, but not complete, dependence on the ipsilateral cerebellum. Learn.Mem. 1997;3:557–577. doi: 10.1101/lm.3.6.557. [DOI] [PubMed] [Google Scholar]