Abstract
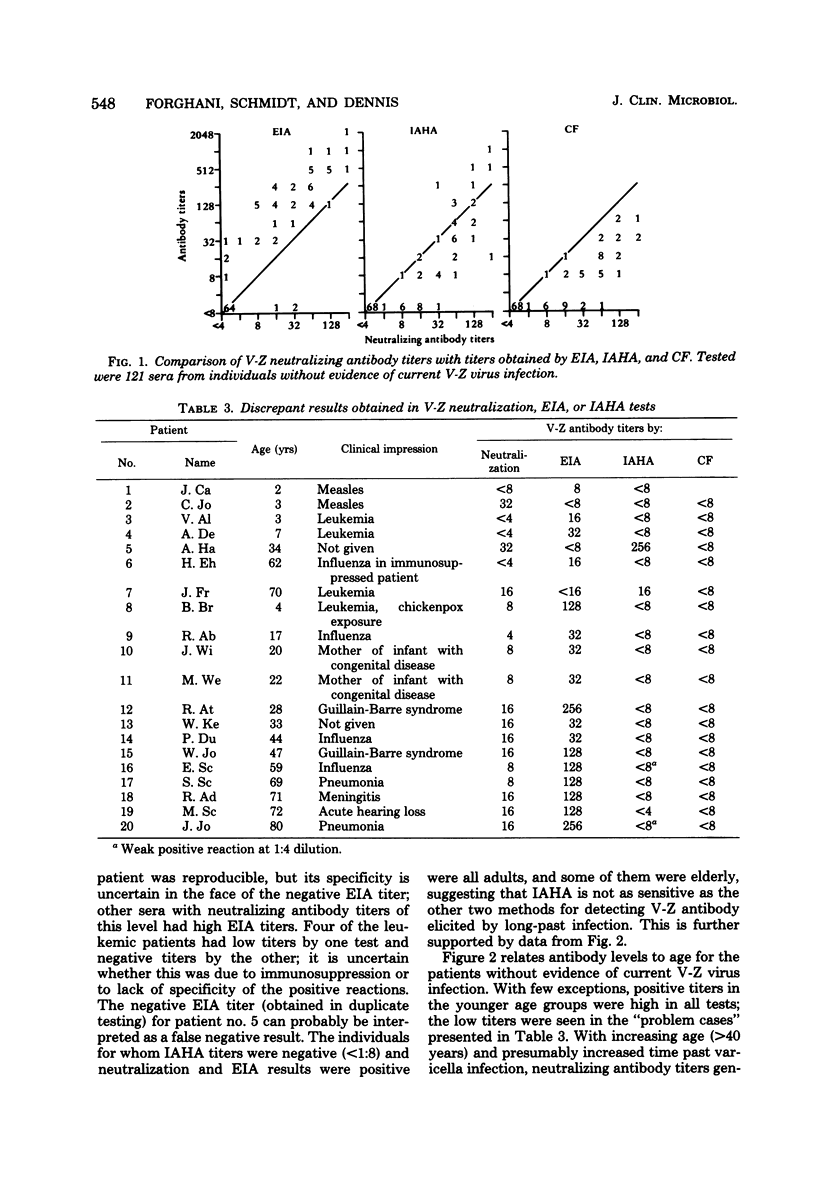
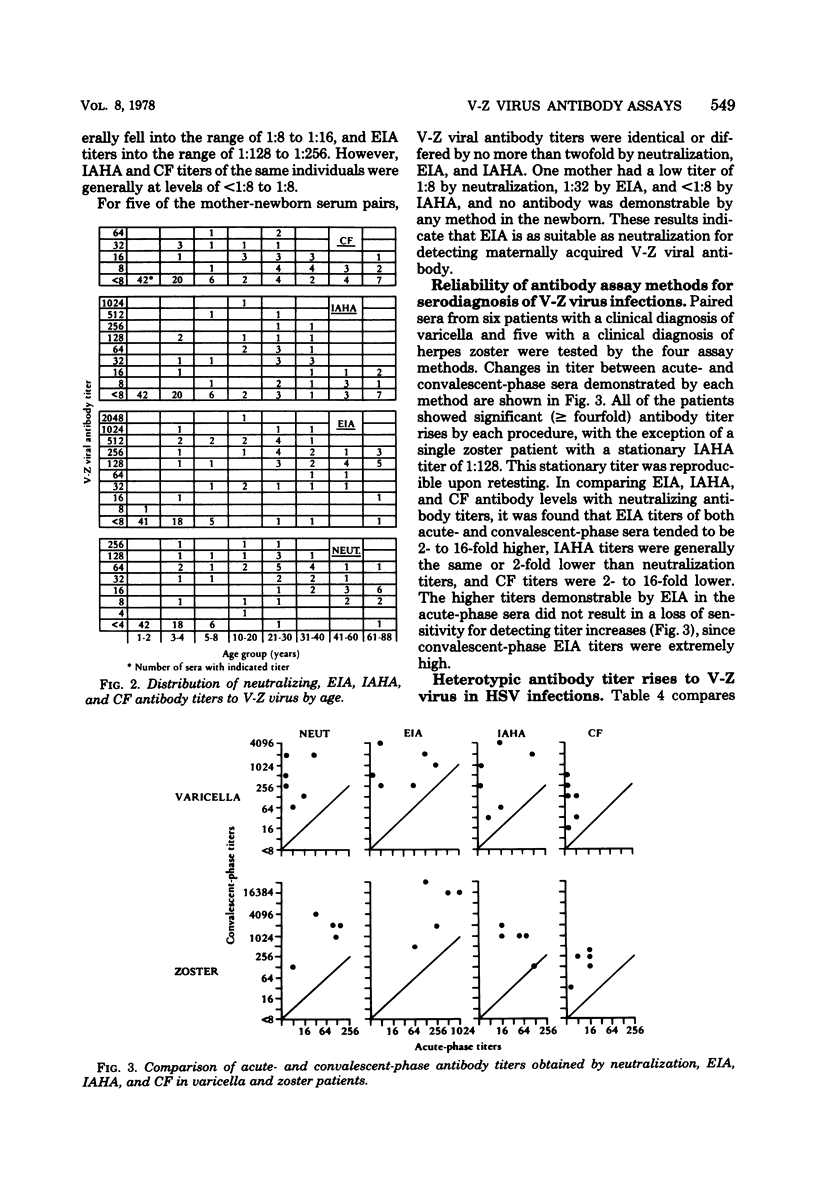
An enzyme immunossay (EIA) was adapted for detection of antibody to varicella-zoster virus, and its sensitivity and specificity were compared with those of neutralization, immune adherence hemagglutination (IAHA), and complement fixation tests. Test sera showed little nonspecific reactivity in the EIA system, and valid results could usually be obtained at serum dilutions as low as 1:8. Demonstration of the presence or absence of varicella-zoster viral antibody by EIA showed 94% correlation with results obtained in neutralization tests, but EIA titers were 2- to 16-fold higher than neutralizing antibody titers. Results by IAHA showed 87% correlation with those obtained by neutralization. No false positive IAHA results were seen, but a number of false negative IAHA results were seen at the 1:8 serum dilution, particularly in older individuals. With increasing age (>40 years), and presumably increased time from varicella infection, neutralizing antibody levels generally declined to 1:8 or 1:16, EIA levels fell to 1:128 or 1:256, and IAHA and complement fixation antibody titers were usually <1:8 or 1:8. EIA and IAHA were as reliable as the neutralization and complement fixation tests for serodiagnosis of varicella and zoster infections. All tests demonstrated heterotypic varicella-zoster antibody titer rises in selected patients with initial herpes simplex virus infections, but fewer heterotypic responses were seen by EIA than by the other methods. EIA offers a rapid, sensitive, and specific method for varicella-zoster antibody assay that is applicable to use in a clinical setting.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Castellano G. A., Hazzard G. T., Madden D. L., Sever J. L. Comparison of the enzyme-linked immunosorbent assay and the indirect hemagglutination test for detection of antibody to cytomegalovirus. J Infect Dis. 1977 Oct;136 (Suppl):S337–S340. doi: 10.1093/infdis/136.supplement_2.s337. [DOI] [PubMed] [Google Scholar]
- Engvall E., Jonsson K., Perlmann P. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim Biophys Acta. 1971 Dec 28;251(3):427–434. doi: 10.1016/0005-2795(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Lennette E. H. Radioimmunoassay of measles virus antigen and antibody in SSPE brain tissue. Proc Soc Exp Biol Med. 1978 Feb;157(2):268–272. doi: 10.3181/00379727-157-40035. [DOI] [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Lennette E. H. Solid phase radioimmunoassay for typing herpes simplex viral antibodies in human sera. J Clin Microbiol. 1975 Nov;2(5):410–418. doi: 10.1128/jcm.2.5.410-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon A. A., Kalter Z. G., Steinberg S., Kuhns W. J. Detection of antibody to Varicella-Zoster virus by immune adherence hemagglutination. Proc Soc Exp Biol Med. 1976 Apr;151(4):762–765. doi: 10.3181/00379727-151-39302. [DOI] [PubMed] [Google Scholar]
- Gillani A., Spence L. Immune adherence hemagglutination test applied to the study of herpes simplex and varicella-zoster virus infections. J Clin Microbiol. 1978 Feb;7(2):114–117. doi: 10.1128/jcm.7.2.114-117.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S. C., Docherty J. J. Detection of antibodies specific for herpes simplex virus in human sera by the enzyme-linked immunosorbent assay. J Infect Dis. 1977 Oct;136 (Suppl):S286–S293. doi: 10.1093/infdis/136.supplement_2.s286. [DOI] [PubMed] [Google Scholar]
- Gravell M., Dorsett P. H., Gutenson O., Ley A. C. Detection of antibody to rubella virus by enzyme-linked immunosorbent assay. J Infect Dis. 1977 Oct;136 (Suppl):S300–S303. doi: 10.1093/infdis/136.supplement_2.s300. [DOI] [PubMed] [Google Scholar]
- KAPSENBERG J. G. POSSIBLE ANTIGENIC RELATIONSHIP BETWEEN VARICELLA ZOSTER VIRUS AND HERPES SIMPLEX VIRUS. Arch Gesamte Virusforsch. 1964 Sep 9;15:67–73. doi: 10.1007/BF01241422. [DOI] [PubMed] [Google Scholar]
- Kalter Z. G., Steinberg S., Gershon A. A. Immune adherence hemagglutination: further observations on demonstration of antibody to varicella-zoster virus. J Infect Dis. 1977 Jun;135(6):1010–1013. doi: 10.1093/infdis/135.6.1010. [DOI] [PubMed] [Google Scholar]
- Leinikki P. O., Passila S. Quantitative, semiautomated, enzyme-linked immunosorbent assay for viral antibodies. J Infect Dis. 1977 Oct;136 (Suppl):S294–S299. doi: 10.1093/infdis/136.supplement_2.s294. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Forghani B., Lennette E. H. Type specificity of complement-requiring and immunoglobulin M neutralizing antibody in initial herpes simplex virus infections of humans. Infect Immun. 1975 Oct;12(4):728–732. doi: 10.1128/iai.12.4.728-732.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Improved yields of cell-free varicella-zoster virus. Infect Immun. 1976 Sep;14(3):709–715. doi: 10.1128/iai.14.3.709-715.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H., Magoffin R. L. Immunological relationship between herpes simplex and varicella-zoster viruses demonstrated by complement-fixation, neutralization and fluorescent antibody tests. J Gen Virol. 1969 Apr;4(3):321–328. doi: 10.1099/0022-1317-4-3-321. [DOI] [PubMed] [Google Scholar]
- Schmidt N. J., Lennette E. H. Neutralizing antibody responses to varicella-zoster virus. Infect Immun. 1975 Sep;12(3):606–613. doi: 10.1128/iai.12.3.606-613.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedmyr A. Varicella virus in HeLa cells. Arch Gesamte Virusforsch. 1965;17(3):495–503. doi: 10.1007/BF01241206. [DOI] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E., Bartlett A. Enzyme immunoassays in diagnostic medicine. Theory and practice. Bull World Health Organ. 1976;53(1):55–65. [PMC free article] [PubMed] [Google Scholar]
- Wallen W. C., Mattson J. M., Levine P. H. Detection of soluble antigen of Epstein-Barr virus by the enzyme-linked immunosorbent assay. J Infect Dis. 1977 Oct;136 (Suppl):S324–S328. doi: 10.1093/infdis/136.supplement_2.s324. [DOI] [PubMed] [Google Scholar]
- Williams V., Gershon A., Brunell P. A. Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J Infect Dis. 1974 Dec;130(6):669–672. doi: 10.1093/infdis/130.6.669. [DOI] [PubMed] [Google Scholar]
- Wong C. L., Castriciano S., Chernesky M. A., Rawls W. E. Quantitation of antibodies to varicella-zoster virus by immune adherence hemagglutination. J Clin Microbiol. 1978 Jan;7(1):6–11. doi: 10.1128/jcm.7.1.6-11.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaia J. A., Oxman M. N. Antibody to varicella-zoster virus-induced membrane antigen: immunofluorescence assay using monodisperse glutaraldehyde-fixed target cells. J Infect Dis. 1977 Oct;136(4):519–530. doi: 10.1093/infdis/136.4.519. [DOI] [PubMed] [Google Scholar]


