Summary
In rod photoreceptors, arrestin localizes to the outer segment (OS) in the light and to the inner segment (IS) in the dark. Here, we demonstrate that redistribution of arrestin between these compartments can proceed in ATP-depleted photoreceptors. Translocation of transducin from the IS to the OS also does not require energy, but depletion of ATP or GTP inhibits its reverse movement. A sustained presence of activated rhodopsin is required for sequestering arrestin in the OS, and the rate of arrestin relocalization to the OS is determined by the amount and the phosphorylation status of photolyzed rhodopsin. Interaction of arrestin with microtubules is increased in the dark. Mutations that enhance arrestin-microtubule binding attenuate arrestin translocation to the OS. These results indicate that the distribution of arrestin in rods is controlled by its dynamic interactions with rhodopsin in the OS and microtubules in the IS and that its movement occurs by simple diffusion.
Introduction
In vertebrate rod photoreceptors, light-activated rhodopsin (Rh*) sets off the phototransduction cascade via the visual G protein transducin. At the same time, rhodopsin kinase initiates the shut-off process through rapid phosphorylation of Rh*. Visual arrestin binds phosphorhodopsin (P-Rh*) with high affinity, preventing its further interaction with transducin. P-Rh* subsequently decays to phosphoopsin, loses all-trans-retinal, is reconstituted with 11-cis-retinal produced by the retinal pigment epithelium, and is dephosphorylated. Ultimately, these processes regenerate the inactive state of Rh that does not bind transducin or arrestin with appreciable affinity.
Prolonged illumination causes massive translocation of arrestin to the outer segment (OS), whereas in the dark arrestin relocalizes to the inner segment (IS) and other proximal compartments of the cell. Transducin moves in the opposite direction. The movement of both proteins is believed to play a role in light and dark adaptation (Arshavsky, 2003; Broekhuyse et al., 1985; Philp et al., 1987; Sokolov et al., 2002; Whelan and McGinnis, 1988). Although the translocation of arrestin and transducin was described 2 decades ago, the underlying mechanism has not been investigated until recently. It was shown that in mice with impaired synthesis of 11-cis-retinal (RPE65−/−), light does not induce arrestin movement to the OS, indicating that active rhodopsin is required for this process (Mendez et al., 2003). The importance of rhodopsin phosphorylation in arrestin movement was addressed using two mouse models deficient in this process: mice expressing a mutant form of rhodopsin in which all the phosphorylation sites at the C terminus were mutated to Ala (completely substituted mutant [CSM]) and rhodopsin kinase knockout (RK−/−) mice (Chen et al., 1999; Mendez et al., 2000). The results of these studies clearly show that rhodopsin phosphorylation is not required for arrestin localization to the OS under constant light exposure. Arrestin and transducin move independently: arrestin translocation is normal in transducin knockout mice, ruling out a role for transducin-mediated signaling in arrestin movement (Mendez et al., 2003), and the movement of trans-ducin is unchanged in arrestin knockout mice (Zhang et al., 2003).
Despite these advances, two basic questions regarding the mechanism of light-dependent translocation of arrestin and transducin in vertebrate photoreceptors remain unanswered. First, it is unclear whether these proteins simply diffuse to their respective destinations or are actively transported there. Second, regardless of the “mode of transportation,” it is not clear how arrestin and transducin are retained in the appropriate compartment.
Here, we investigated these transport mechanisms by manipulating live photoreceptors ex vivo using mouse eyecups. We demonstrate that translocation of both arrestin and transducin is energy independent and occurs by diffusion. Our data indicate that arrestin is drawn to the OS and is sequestered there via direct binding to activated rhodopsin. The release from rhodopsin in the dark leads to the return of arrestin to the IS, where it is retained by its association with the abundant network of microtubules (Eckmiller, 2000). According to our model, the functional state of rhodopsin is the sole factor determining arrestin localization in vertebrate rod photoreceptors.
Results
Does Light-Dependent Translocation of Arrestin and Transducin in Photoreceptors Require Energy?
We found that in mouse eyecup preparations both arrestin and transducin can translocate between the OS and the IS of rod photoreceptors in a manner similar to their light-dependent movement in intact eyes (Figure 1 and Figure 2). We refer to “IS” localization throughout this report for convenience. As can be seen in the figures in this report, when arrestin or transducin are not in the OS, they are distributed from the ellipsoid to the synaptic terminal. The natural pattern of localization of arrestin and transducin allowed us to use eyecups (Figure S1 in the Supplemental Data available with this article online) as an ex vivo photoreceptor cell model amenable to biochemical manipulations.
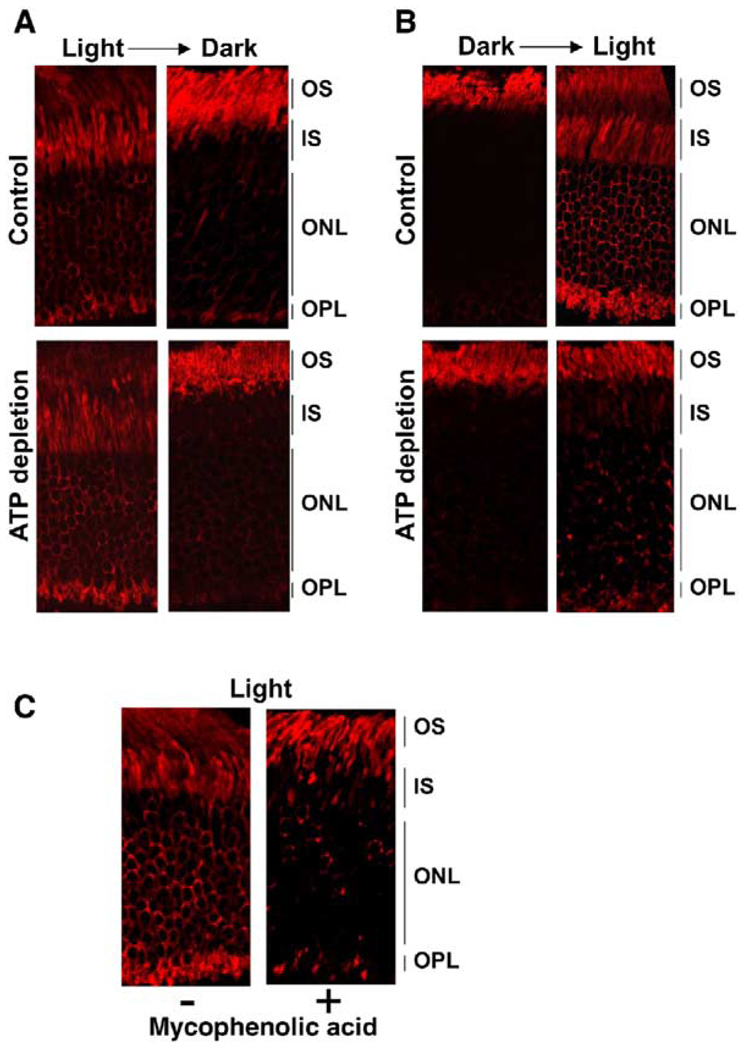
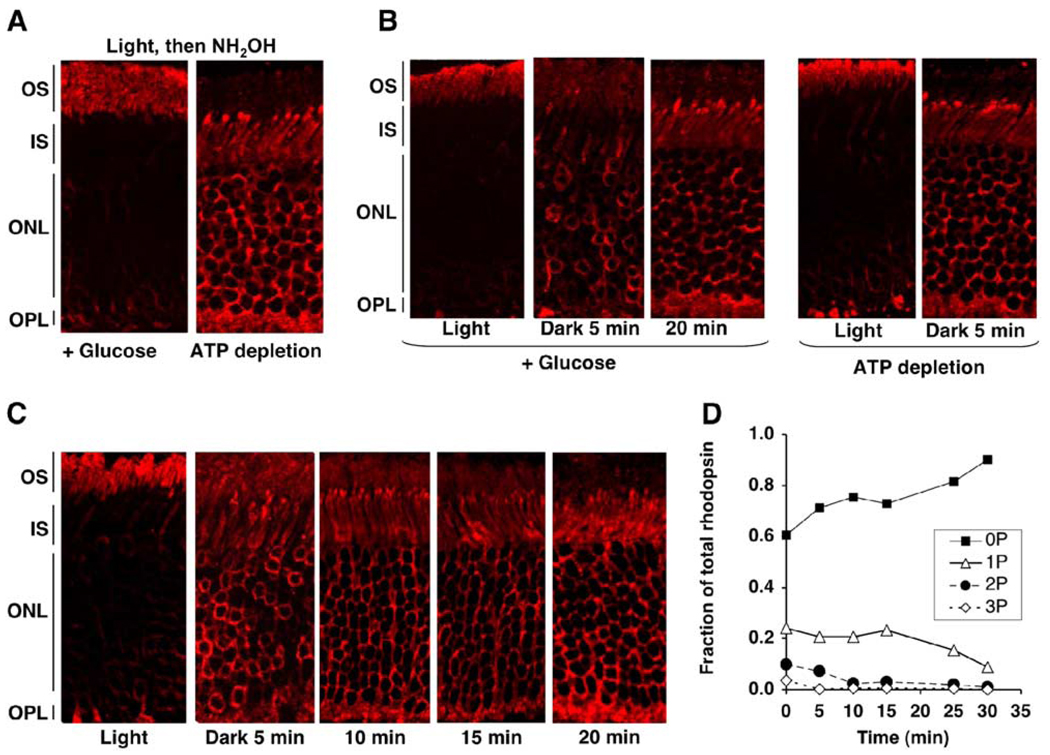
Figure 1. Normal Light-Dependent Movement of Arrestin in ATP-Depleted Retinas.
Mouse eyecups were incubated in DMEM (control) or glucose-free DMEM supplemented with 2 mM deoxyglucose and 10 mM KCN (ATP depletion). (A) Top panels (control): three mouse eyecups were incubated in the dark in DMEM at room temperature; one of the three eyecups (left panel) was fixed after 1 hr of dark adaptation. The two remaining eyecups were exposed to light (600 lux) for 1 hr, at which time one of these eyecups (center) was fixed. The third eyecup was returned to the dark for 1 hr (right) and fixed. The eye-cups were embedded and sectioned, and arrestin was visualized with anti-arrestin antibody, as described in the Experimental Procedures (see Supplemental Data for more details on methods). Lower panels (ATP depletion): three eyecups were incubated in glucose-free DMEM supplemented with 2 mM deoxyglucose and 10 mM KCN (ATP depletion) for 1 hr in the dark and then subjected to the same illumination protocol as in the control: one eyecup was fixed after 1 hr of dark adaptation, the second was fixed following illumination for 1 hr, and the third was fixed after the subsequent return to the dark. Typical images from five independent experiments are presented. (B) After the 1 hr incubation in the dark, control or ATP-depleted eyecups were homogenized in 10% tricholoroacetic acid, and their ATP content was analyzed using a luciferase assay. Bar graphs representing luminescence intensity (means ± SD) from three independent experiments are shown. (C) The ATP-depleted and control eyecups were illuminated and homogenized in urea. Rhodopsin phosphorylation was measured by mass spectrometry as described in the Experimental Procedures and, in more detail, in the Supplemental Data. The distribution of unphosphorylated (black bar) and differentially phosphorylated forms of rhodopsin as a fraction of total rhodopsin (means ± SD) in two independent experiments is shown.
Figure 2. Effects of ATP and GTP Depletion on Light-Dependent Translocation of Transducin.
(A) One pair of eyecups was prepared in the light and incubated in DMEM (control), and another pair was incubated in glucose-free DMEM supplemented with 2 mM deoxyglucose (ATP depletion) and KCN for 1 hr at room temperature. One eyecup from each pair was left in the light, and the other was transferred to dark. After incubation for an additional 1 hr, the eyecups were fixed and sectioned, and the localization of α subunit of transducin was determined using anti-Gαt antibody. (B) Four eyecups were prepared under dim red light from dark-adapted (12 hr) mice. Two eyecups were ATP depleted, and another two were incubated with DMEM (control). After 1 hr in the dark, one eyecup from the ATP-depleted pair and one from the control were fixed. The remaining two eyecups were illuminated at bright room light (1000 lux) for an additional 1 hr prior to fixing. (C) Two eyecups were prepared under dim red light from dark-adapted mice and incubated in DMEM in the presence or absence of 2 mM mycophenolic acid for 4 hr in the dark. Both eye-cups were then transferred to bright room light for 1 hr and analyzed for Gαt localization. Typical images from three (A and B) or two (C) independent experiments are presented.
To determine whether arrestin and/or transducin translocation requires energy, we induced ATP depletion of photoreceptor cells. The eyecups were incubated in a glucose-free medium supplemented with deoxyglucose and KCN, and arrestin movement and transducin movement were compared in control (kept in glucose-rich medium) and energy-starved eyecups.
We found that arrestin movement in the ATP-depleted eyecups proceeds normally in both directions (Figure 1A). To ascertain that ATP depletion indeed occurs under our experimental conditions, we directly measured the ATP content and found that our procedure reduced the ATP level by two orders of magnitude, from 5–10 µg ATP to less than 100 ng per eyecup (Figure 1B). Furthermore, in sharp contrast to the control, in the energy-starved eyecups light did not induce phosphate incorporation into rhodopsin, as measured by mass spectrometry (Kennedy et al., 2001; Nair et al., 2004) (Figure 1C), confirming that the photoreceptor cells were severely ATP depleted.
Similar to arrestin movement, transducin translocation from the IS to the OS in the dark occurred in the ATP-depleted photoreceptors (Figure 2A). However, the light-induced return of transducin from the OS to the IS was inhibited by energy starvation (Figure 2B). Since ATP is required for the synthesis of GTP, we reasoned that transducin cannot translocate to the IS due to the lack of GTP, which is necessary for its release from light-activated rhodopsin. To test this idea, we selectively depleted GTP by treating the eyecups with mycophenolic acid, a membrane-permeant specific inhibitor of inosine monophosphate dehydrogenase, the key enzyme involved in GMP synthesis in photoreceptors (Bowne et al., 2002). We found that, in the GTP-depleted cells, transducin translocation from the OS to the IS was significantly impaired (Figure 2C). Thus, the effect of ATP depletion on transducin movement to the IS in the light is more likely due to the absence of GTP than lack of ATP.
Our finding that ATP is not required for arrestin and transducin translocation suggests that these processes do not involve energy-dependent transport or active gating mechanisms, i.e., they occur by passive diffusion.
The Rate of Protein Diffusion in Photoreceptors
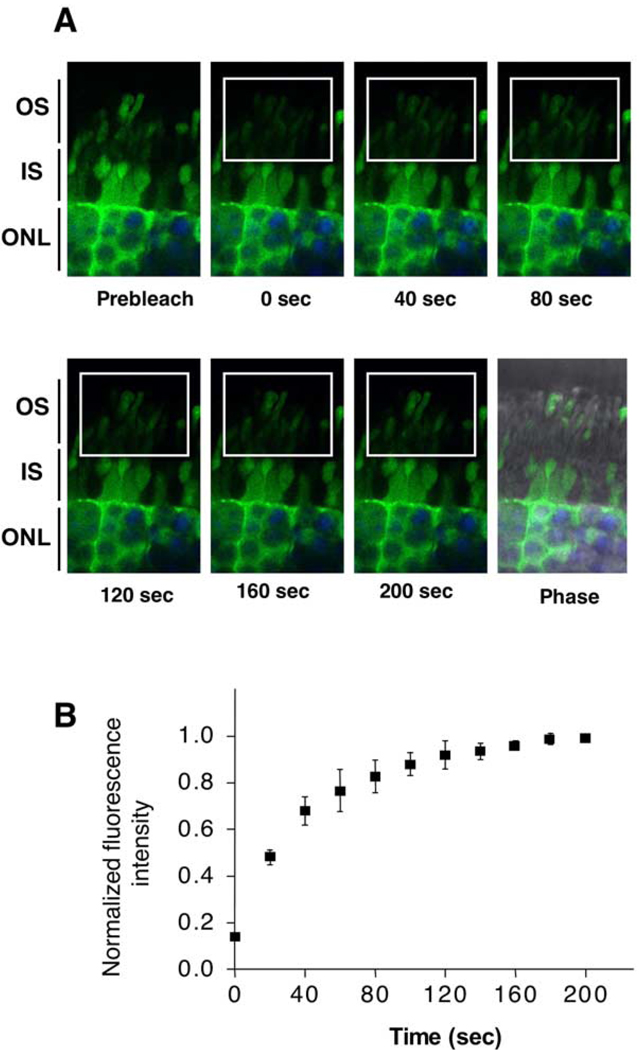
Soluble proteins diffuse rapidly across the cytoplasm of most cells. However, the photoreceptor OS is separated from the IS by a relatively narrow connecting cilium, which could potentially impede diffusion. To estimate the rate of protein diffusion between the IS and OS, we measured fluorescence recovery after photo-bleaching (FRAP) in live mouse photoreceptors using green fluorescent protein (GFP) as a model soluble protein. Live retinas from transgenic mice expressing GFP (Hadjantonakis et al., 1998) were sectioned and visualized using a confocal microscope. An area of a selected visual field containing the OS was bleached, and the recovery of fluorescence was recorded in a series of time-lapse images (Figure 3A). Our results clearly show that the recovery of green fluorescence in the bleached rod OS is rapid, with a half-time of less than 2 min (Figure 3B). The rate of fluorescence recovery in cone outer segments was similar (data not shown). Thus, diffusion of soluble proteins in photoreceptors occurs as rapidly as in other cell types and hence can clearly support light-dependent movement of either arrestin or transducin.
Figure 3. The Rate of Protein Diffusion in Live Photoreceptors.
Eyecups were prepared from GFP-expressing transgenic mice. Retinas were removed and rapidly embedded in low-melt agarose at 25°C–30°C and sectioned on a vibratome. The slices were then immediately analyzed on a confocal microscope. Hoechst nucleic acid dye was added to the media during the eyecup preparation to visualize photoreceptor nuclei. (A) Time-lapse images of GFP fluorescence (green) acquired before (Prebleach) and after photobleaching. The bleached area is denoted by the rectangle. Photoreceptor nuclei stained with Hoechst are shown in blue. (B) The ratio of fluorescence signal in the bleached area to the fluorescence outside the bleached box was determined for each of the recorded images and plotted as a function of time. Means ± SD from four independent experiments measuring GFP fluorescence are shown
However, diffusion per se can only equalize the concentration of a soluble protein in the OS and the inner compartments. This is at odds with the fact that both arrestin and transducin are concentrated in the IS or the OS depending on the light conditions. Thus, free diffusion of soluble arrestin must be restricted, conceivably by its interaction with binding partners, which are specifically localized in the OS or the IS and act as dynamic “sinks,” sequestering arrestin in the OS in the light and in the IS in the dark. Therefore we focused on identifying these sinks for arrestin.
Arrestin Movement to the OS Requires the Sustained Presence of Rh*
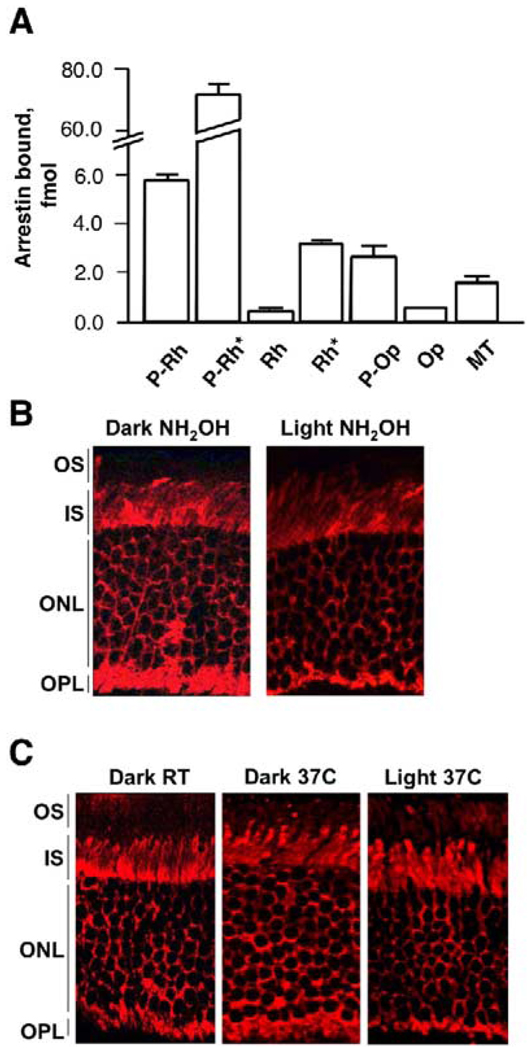
Rhodopsin, which is abundant in the OS and binds arrestin in a light-dependent fashion, appears to be the most likely candidate for this role. This model predicts that a significant reduction in the amount of light-activated rhodopsin in the OS should prevent arrestin translocation. To test this model, we treated eyecups with hydroxylamine, a reagent that causes irreversible decay of Metarhodopsin II by removing all-trans-retinal (Hofmann et al., 1983; Hofmann et al., 1992), or high temperature (37°C), which dramatically accelerates Rh* decay to opsin (Ebrey, 1968; Hagins, 1956; Hurley et al., 1977). We found that illumination of dark-adapted eyecups in the presence of hydroxylamine at room temperature (Figure 4B) or illumination at 37°C (Figure 4C) failed to induce arrestin movement to the OS, in sharp contrast to control eyecups (Figure 1). Spectroscopic measurements (Figure S2) showed that after 15 min at 37°C Rh* almost completely decayed to opsin, whereas at room temperature even after 1 hr at least 50% of the pigment remained in the eyecups, suggesting that the striking effect of temperature on arrestin behavior is likely due to the accelerated decay of Rh* at 37°C. The inhibitory effect of 37°C on arrestin translocation was much more pronounced in the eyecups prepared from older animals (>20 weeks) than in those from young animals (4–6 weeks). In older animals, the retina is more readily detached from the RPE during the eyecup preparation, which apparently exacerbates the diminished ability of the eyecup to regenerate the pigment. The inhibition of arrestin translocation to the OS by rapid inactivation of rhodopsin shows that the sustained presence of either Rh* or P-Rh* in the OS is required for this process.
Figure 4. Movement of Arrestin to the OS Requires Continuous Presence of Active Rhodopsin.
(A) Interaction of arrestin with different forms of rhodopsin and microtubules (MT). The binding assay was performed in vitro using radiolabeled arrestin and ROS membranes containing 0.6 µg of the indicated forms of rhodopsin (dark Rh, Rh*, dark P-Rh, P-Rh*), opsin (Op, P-Op), or MT. Means ± SD from three independent binding experiments performed in duplicate are shown.
(B) Eyecups were treated with 1 mM hydroxylamine in DMEM for 1 hr at room temperature in the dark and then either kept in the dark or exposed to light for an additional 30 min. The eyecups were then fixed, and arrestin was visualized with anti-arrestin antibody.
(C) Dark-adapted eyecups were incubated in DMEM at room temperature, then transferred to 37°C in the dark for 15 min, followed by illumination with 600 lux for 1 hr at 37°C. The eyecups were fixed, and localization of arrestin was determined. Typical images from six experiments are presented.
The Rate of Arrestin Movement to the OS Depends on the Amount of Activated Rhodopsin and Its Phosphorylation
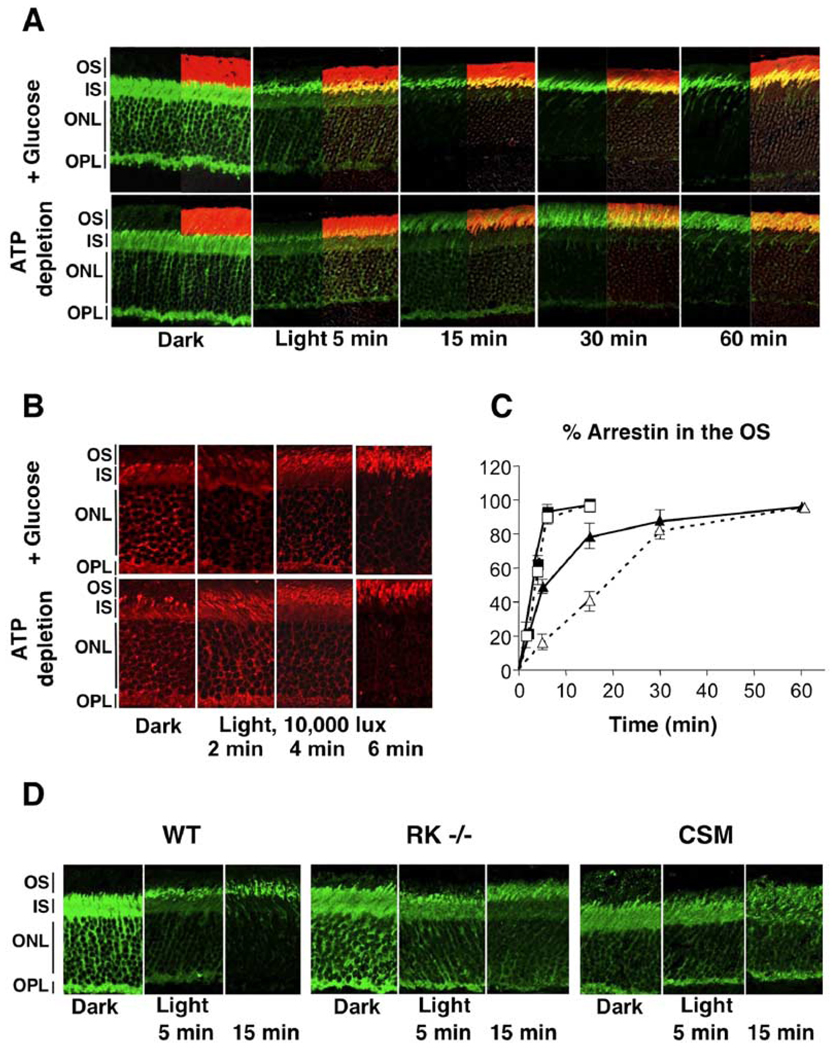
We next investigated the kinetics of arrestin translocation to the OS under two different light intensities in control and ATP-depleted eyecups (Figures 5A–5C). Under 400 lux illumination, which led to accumulation of about 50% of rhodopsin in its bleached state within 1 hr, the rate of arrestin translocation is slower in ATP-depleted eyecups compared to the control. The rate of translocation was dramatically higher in eyecups illuminated by saturating light (10,000 lux, bleaching over 90% rhodopsin in 10 s) as compared to ambient light (Figure 5B and 5C). After the brief saturating illumination, movement of arrestin was complete within 5 min for both control and ATP-depleted eyecups, whereas the half-time of arrestin translocation at 400 lux was 10 and 20 min, respectively. To investigate if the attenuating effect of ATP depletion was specifically due to the lack of rhodopsin phosphorylation rather than another process, we assessed the rate of arrestin translocation in photoreceptors where rhodopsin phosphorylation was precluded either by inactivating rhodopsin kinase gene (RK−/−) or by using mice that express only a mutant form of rhodopsin that cannot be phosphorylated (CSM) (Mendez et al., 2000). We found that in both these animal models, the rate of redistribution of arrestin to the OS is significantly slower than that in the wild-type animals (Figure 5D), which resembles the effect of ATP depletion on the rate of arrestin translocation (Figure 5A).
Figure 5. Effect of Rhodopsin Phosphorylation on the Rate of Arrestin Redistribution from the IS to the OS.
(A) Eyecups from wild-type mice were prepared in the dark and either were kept in DMEM (+ Glucose) or were ATP depleted. Eyecups were then exposed to 400 lux light at room temperature for the indicated times prior to fixing. Localization of arrestin (green) and rhodopsin (red) was determined using immunofluorescence microscopy.
(B) Eyecups from wild-type mice were prepared in the dark and either ATP depleted or kept in DMEM (+ Glucose). They were then illuminated for 10 s with 10,000 lux light at room temperature and fixed at the indicated times, and localization of arrestin (red) was determined.
(C) Graph summarizing the data represented in (A) and (B). The y axis shows the percentage of total arrestin translocated to the OS at the indicated times. Arrestin localization at 10,000 lux is represented by solid squares (with ATP) or by open squares (without ATP) (n = 3). Arrestin localization at 400 lux is depicted by closed triangles (+ATP) and open triangles (−ATP). Means ± SD from three independent experiments measuring fluorescence intensity are shown.
(D) Eyecups were prepared in the dark from wild-type mice (WT), rhodopsin kinase knockout mice (RK−/−), and transgenic mice expressing the completely substituted mutant (CSM) of rhodopsin, in which all the phosphorylation sites are substituted by alanines. Following the light exposure (400 lux) for the indicated times, the eyecups were fixed and stained with anti-arrestin (green) antibodies as in (A).
Together, these results indicate that both the affinity of activated rhodopsin for arrestin and the amount of rhodopsin that can bind arrestin regulate the extent and the rate of arrestin movement to the OS.
Return of Arrestin to the IS in the Dark
If light-activated rhodopsin is the sole factor responsible for drawing arrestin to the OS and retaining it there, inactivation of rhodopsin should result in arrestin translocation from the OS to the IS. The process of rhodopsin inactivation involves decay (dissociation of all-trans-retinal), regeneration (reconstitution with 11-cis-retinal), and dephosphorylation. The actual sequence of these events and their relative importance for arrestin release from rhodopsin are not known. Consistent with the fact that the majority of arrestin does not localize to the OS in the dark, arrestin does not bind in vitro to Rh and opsin, forms that are predominantly found in the dark (Figure 4A). Thus, elimination of activated forms of rhodopsin should facilitate arrestin return to the IS.
To test this prediction, we illuminated the eyecups (600 lux, 1 hr) to induce arrestin movement to the OS and then treated the eyecups with hydroxylamine. Arrestin remained in the OS of hydroxylamine-treated photoreceptors, demonstrating that the elimination of Rh* and P-Rh* is insufficient to trigger arrestin return to the IS (Figure 6A, left panel). Since arrestin has a measurable affinity for phosphorylated opsin (P-Op) and dark P-Rh (Figure 4A), the interaction of arrestin with P-Op may explain its retention in the OS. To test this idea, the eyecups were ATP depleted prior to light exposure and arrestin translocation to the OS and then treated with hydroxylamine. In the absence of ATP, arrestin readily returned to the IS even in the light (Figure 6A, right panel). Thus, unphosphorylated opsin cannot retain arrestin in the OS.
Figure 6. Movement of Arrestin from the OS to the IS Requires Dissociation of the Rhodopsin-Arrestin Complex.
(A) Eyecups were prepared from dark-adapted mice in the dark and either kept in DMEM (+ Glucose) or ATP depleted. They were then exposed to light at room temperature for 1 hr, treated with hydroxylamine for 15 min, and fixed. Localization of arrestin was determined by immunofluorescence microscopy.
(B) Eyecups were prepared in the dark and either ATP depleted for 1 hr in the dark or kept in DMEM. They were then illuminated for 1 hr with ambient light at room temperature to cause movement of arrestin to the OS and then transferred to the dark. At the indicated times, eyecups were fixed, and localization of arrestin was determined.
(C) Kinetics of arrestin translocation to the IS in vivo. Mice were exposed to light (600 lux) for 1 hr and then returned to the dark. At the indicated times, animals were sacrificed. Enucleated eyes were placed in fixing solution, and cornea and lens were rapidly removed to allow instantaneous fixing.
(D) Kinetics of rhodopsin dephosphorylation in vivo. The contralateral eyes from the animals used in (B) were homogenized in 0.5 ml of 7 M urea, and the phosphorylation status of rhodopsin in the eyecups was determined by mass spectrometry.
To test if the absence of rhodopsin phosphorylation accelerates the departure of arrestin from the OS, we compared the rate of arrestin movement from the OS to the IS in the dark in control and ATP-depleted eyecups (Figure 6B). We found that arrestin returns to the IS substantially faster in the energy-starved eyecups, suggesting that rhodopsin dephosphorylation is required for arrestin translocation to the IS.
If rhodopsin dephosphorylation is a limiting factor for arrestin release from the OS, the return of arrestin to the IS should not occur faster than rhodopsin dephosphorylation. To test this idea, we compared the rates of rhodopsin dephosphorylation and arrestin movement from the OS to the IS in the dark in vivo. Mice were light adapted for 1 hr and then returned to the dark for different periods of time; one eye from each animal was used to determine the localization of arrestin by immunofluorescence (Figure 6C), the other eye was used to measure rhodopsin phosphorylation by mass spectrometry (Figure 6D). Double- and triple-phosphorylated forms of rhodopsin disappeared within 10 min, which closely correlated with the rate of arrestin return to the IS. Interestingly, monophosphorylated rhodopsin was detected even after the departure of the bulk of arrestin, in agreement with previous findings that rhodopsin monophosphorylation is insufficient for high-affinity arrestin binding in vitro (Gurevich and Benovic, 1993) and in vivo (Mendez et al., 2000).
Collectively, our data demonstrate that the sustained presence of substantial amounts of light-activated rhodopsin, phosphorylated or not, in the OS is necessary and sufficient to induce arrestin relocalization into this compartment. The rate of its movement to the OS is increased with the amount of activated rhodopsin and binding affinity (e.g., P-Rh* versus Rh*) of rhodopsin for arrestin. The rate of arrestin return to the IS is determined by the rate of its release from the complex with rhodopsin.
Arrestin Interactions with Microtubules
Since the binding of arrestin to light-activated rhodopsin concentrates it in the OS, it stands to reason that its accumulation in the IS in the dark requires an alternative binding partner in this compartment. This interaction partner must be sufficiently abundant in the IS, considering the high expression of arrestin in rods (Hamm and Bownds, 1986). We have recently demonstrated that visual arrestin directly interacts with micro-tubules (Nair et al., 2004). Microtubules are strikingly abundant in the IS (Eckmiller, 2000), with a substantially lower presence in the OS, where the majority of polymerized tubulin is found in the axoneme (Eckmiller, 2000; McGinnis et al., 2002). Interestingly, it was previously observed that a fraction of arrestin is retained in the axoneme of dark-adapted Xenopus rods (McGinnis et al., 2002).
To investigate whether arrestin binding to microtubules plays a role in its light-dependent translocation, we examined the association of arrestin with cytoskeletal fractions in light- and dark-adapted mouse retinas (Figure 7). OS fractions were isolated from mice that were kept in the dark for varying time intervals as well as light-adapted animals. The isolated OS were then extracted with 1.0% Triton X-100 (these conditions solubilize >95% of rhodopsin) and fractionated to obtain the detergent-insoluble cytoskeletal fraction (Nair et al., 2004). We found that virtually all arrestin in the OS was detergent soluble in the light, whereas within 15 min in the dark, a significant proportion of arrestin became associated with the cytoskeleton (Figure 7A). This fraction of arrestin remained bound to the cytoskeleton for up to 12 hr (data not shown) until light exposure of the dark-adapted mice triggered its return to the detergentsoluble fraction.
Figure 7. Light-Dependent Interaction of Arrestin with the Cytoskeleton.
(A) Mice were exposed to light (600 lux) for 1 hr and then transferred to the dark. The animals were sacrificed, the eyes were removed, and retinas were isolated. Outer segments were prepared and fractionated to obtain detergent-soluble and -insoluble (cytoskeleton) fractions as described in the Experimental Procedures. These fractions were analyzed by Western blot using anti-arrestin (Arr) and anti-tubulin (Tb) antibodies.
(B) Mice were either dark adapted for 12 hr or light adapted for 1 hr at 600 lux. Following the brief vortexing required for the separation of OS (as in [A]), the remaining retina was collected and solubilized with 1% Triton X-100. The microtubule-rich detergent-insoluble cytoskeletal fractions from the dark- and light-adapted retinas were analyzed for the presence of tubulin and arrestin. The bar graph represents the mean ± SD of the Western blot signal for arrestin from three independent preparations.
(C) The cytoskeletal preparation from dark-adapted ROS was incubated with bovine ROS membrane vesicles containing regenerated phosphorhodopsin for 15 min at room temperature in the dark or light. The samples were then loaded on a 50% glycerol gradient and centrifuged to separate the membranes from the cytoskeletal fraction, which was subsequently analyzed for the presence of arrestin and tubulin by Western blot. Typical blots from three experiments are presented.
In parallel, we investigated the interaction of arrestin with the non-OS cytoskeleton (Figure 7B). Following the detachment of OS by vortexing of the retina, the remaining tissue was homogenized and solubilized in 1% Triton X-100, and the cytoskeleton was pelleted by centrifugation. The amount of arrestin in this fraction was dramatically reduced in the light-adapted retinas, whereas the amount of tubulin remained the same. This result suggests that in the dark a fraction of arrestin in the IS and cell bodies is bound to microtubules and that it dissociates from the cytoskeleton upon illumination.
Since the amount of cytoskeleton-associated arrestin is dramatically reduced by light, we reasoned that the removal of arrestin from the cytoskeleton is induced by the emergence of a more “competitive” binding partner, light-activated rhodopsin. To test this idea, we added phosphorhodopsin-containing membranes, in light or dark, to the isolated microtubule-enriched cytoskeletal preparation containing bound arrestin. Figure 7C shows that the addition of light-activated (but not dark) phosphorhodopsin removes arrestin from the cytoskeleton. The dissociation occurred within seconds (Figure S3), demonstrating that the interaction of arrestin with microtubules is dynamic and indicating that arrestin can readily leave its binding sites on the cytoskeleton and move to the OS as soon as activated rhodopsin becomes available.
These results further demonstrate that redistribution of arrestin from microtubules to activated rhodopsin can occur simply by the law of mass action and does not require energy.
Arrestin Mutants with Increased Affinity for Microtubules Demonstrate Impaired IS to OS Translocation In Vivo
To test the idea that dynamic interactions with microtubules regulate arrestin movement in vivo, we used transgenic mice expressing two arrestin mutants with enhanced affinity for microtubules. We constructed mouse arrestin (1–377) that does not have the regulatory C-tail (ΔC) (Nair et al., 2004) and arrestin (L374A, V375A,F376A) (3A), in which three bulky hydrophobic residues anchoring the C tail to the body of the molecule are replaced with alanines (Gurevich, 1998; Hirsch et al., 1999). Mice expressing the ΔC and 3A mutants in photoreceptors were bred into arrestin knockout background (Xu et al., 1997). The transgenic line with the highest level of ΔC expressed it only at the level of 10% ± 2% of the arrestin in wild-type mice. Therefore, for our comparative studies we used arrestin+/− mice (expression 46% ± 5%) and the line expressing the 3A mutant at 44% ± 8% of the wild-type level.
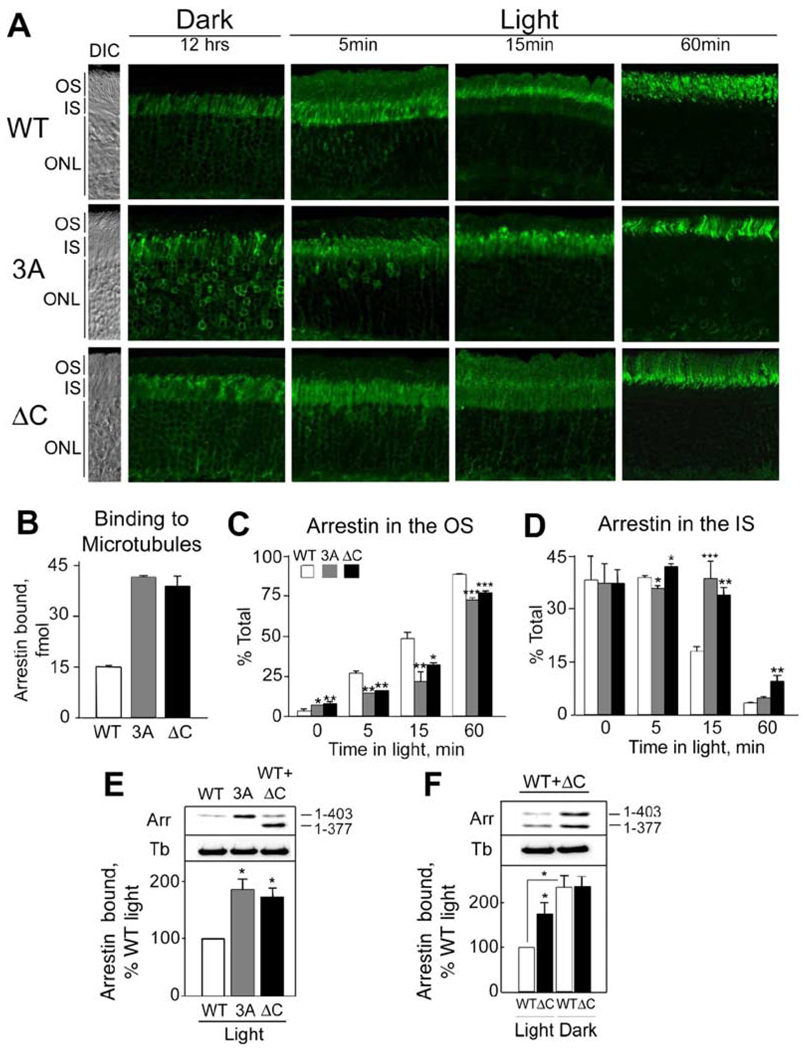
In the direct binding assay, 3A and ΔC mutants retained the normal ability to bind P-Rh* and bound to microtubules substantially better than wild-type arrestin (Figure 8B). In vivo in fully dark-adapted photoreceptors, the majority of wild-type arrestin was localized in the IS and in the cell bodies, with only 3.1% ±1.1% of the immunofluorescence present in the OS (Figures 8A, 8C, and 8D; see also Table S1). The proportion of both mutants in the OS of the dark-adapted retinas was 2-to 2.5-fold higher. After 5 min in the light (2700 lux), 27% of wild-type arrestin has moved to the OS, in contrast to about half of this amount of the mutant arrestins. At 15 min, 48% of wild-type arrestin was already present in the OS, whereas only 21% of 3A and 32% of the ΔC mutant had moved to the OS. Accordingly, the departure of both mutants from the IS was delayed (Table S1). As expected, in fully light-adapted retinas (60 min) most of the wild-type arrestin (89%) was found in the OS, with the ΔC and 3A mutants reaching a some-what lower level (77% and 73%, respectively; Table S1). At the same time, a significantly higher proportion of the 3A mutant (23%) was still found in the cell bodies and perinuclear areas compared to wild-type arrestin (8%). In agreement with this distribution, we found that the amount of both mutants bound to the non-OS cytoskeletal fraction in vivo in fully light-adapted animals is substantially greater than that of wild-type arrestin (Figure 8E).
Figure 8. Light-Dependent Translocation of Wild-Type and Mutant Arrestins In Vivo.
(A) Animals were dark-adapted overnight and then exposed to 2700 lux light for the indicated periods of time. DIC images were acquired in parallel (panels on the left) and used to identify OS, IS, and outer nuclear layer (ONL).
(B) Microtubules (20 µg) were incubated with 200 fmol of the indicated radiolabeled arrestin at 30°C for 20 min. Bound arrestin was separated from free arrestin as described in the Experimental Procedures (see Supplemental Data for more details). Means ± SD from three independent direct binding experiments performed in duplicate are shown.
(C) Percentage of wild-type or mutant arrestin localized in the OS. Average percentage (±SD) of wild-type (WT) and mutant (3A or ΔC) arrestin detected in the OS and the IS at indicated time points is shown. Statistical significance of the differences is indicated above the corresponding bars: *p < 0.05, **p < 0.01, ***p < 0.001, as compared to WT.
(D) Localization of the arrestins in the IS.
(E) The detergent-insoluble non-ROS cytoskeleton fractions were obtained from the light-adapted retinas of arrestin+/− mice (WT), transgenic mice expressing 3A on the background of arrestin knockout (3A), and mice expressing the truncated (ΔC) arrestin mutant on the arrestin+/−background. The non-ROS cytoskeletal fractions obtained as in Figure 6B were compared with respect to the presence of arrestin. The bar graph represents quantification (means ± SD) of arrestin Western blot signal from three independent preparations.
(F) The effect of light on association of wild-type (WT) and truncated (ΔC) arrestins with the non-ROS cytoskeleton was investigated in mice expressing the ΔC mutant on the arrestin+/−background. Typical blots and means ± SD from three experiments are presented.
The size difference between wild-type arrestin and the truncated mutant allowed us to compare their microtubule association in the same animals in vivo. To this end, we bred the ΔC transgene into arrestin+/− background and compared the amounts of the two arrestin species in the non-OS cytoskeletal fractions prepared from light- and dark-adapted animals (Figure 8F). Light reduced the amount of microtubule-associated wild-type arrestin to a much greater extent than the amount of ΔC mutant. Even though the expression level of full-length arrestin is five times greater than that of the truncated species, the amounts of the two proteins associated with microtubules in the dark are essentially the same, clearly demonstrating enhanced affinity of the ΔC mutant for microtubules in the living animal. Interestingly, the ΔC mutant was markedly concentrated in the proximal part of the fully light adapted OS, in contrast to both wild-type and the 3A mutant, which are distributed fairly evenly along the long axis of the OS. This peculiar distribution lends further support to the idea that light-activated rhodopsin recruits arrestin to the OS and holds it there. The expression level of the ΔC mutant is significantly lower than that of wild-type, and so en route from the IS it should be exhausted by rhodopsin in the proximal part of the OS, leaving relatively little available for the distal part.
Discussion
We have investigated the mechanism of light-dependent redistribution of arrestin and transducin in vertebrate photoreceptors. We found that this movement does not require ATP, likely occurring by passive diffusion. Our data indicate that arrestin localization is determined by its interaction with activated rhodopsin in the OS in the light and microtubules in the inner compartments of the cell in the dark.
Arrestin and Transducin Translocation Do Not Require Energy
Movement of arrestin in both directions, from the IS to the OS upon illumination and the return to the IS from the OS in the dark, occurs in severely ATP-depleted eyecups (Figure 1). The extent of ATP depletion was confirmed by direct measurements of ATP level (Figure 1B) as well as the lack of light-dependent rhodopsin phosphorylation in the photoreceptors (Figure 1C). In contrast to arrestin, light-induced transducin movement to the IS requires ATP, apparently because ATP is needed for GTP synthesis (Figure 2). The return of transducin to the OS in the dark is not affected by ATP depletion. Based on this combined evidence, we rule out active transport and active gating as mechanisms for arrestin movement.
It has been reported (Marszalek et al., 2000) that, in kinesin II knockout animals, rhodopsin remains in the IS, implicating kinesin-mediated transport in the process of rhodopsin delivery to the OS. In these mice, the movement of arrestin was impaired as well, so that a significant pool was retained in the IS in the light. Superficially, this observation appears to be at odds with our conclusions. However, the incomplete translocation of arrestin in the kinesin knockout mice can be explained by trapping of arrestin by rhodopsin, which is accumulated in the IS of these animals.
Diffusion is the only mechanism consistent with our findings, but is it fast enough? To answer this question, we used FRAP to measure the rate of diffusion of a model soluble protein, GFP (Figure 3). Our results indicate that the diffusion through the narrow connecting cilium is fast enough to account for the observed rates of arrestin and transducin movement.
However, diffusion per se can only equalize protein concentration across the cell. Thus, preferential localization of arrestin in the OS in the light or the IS in the dark requires binding partners acting as sinks for soluble arrestin. Central to this model is the idea that binding of arrestin to at least one target must be regulated by light. Our studies show that light-activated rhodopsin recruits arrestin to the OS.
Rhodopsin Activation Is Necessary and Sufficient for Arrestin Translocation to the OS
In fully dark-adapted photoreceptors, virtually all rhodopsin exists in its inactive unphosphorylated form, dark Rh. Light converts rhodopsin into Rh*. This functional form activates transducin and is also specifically recognized by rhodopsin kinase that can incorporate up to seven phosphates into its C terminus, generating active phosphorhodopsin, P-Rh*. Rh* and P-Rh* then decay to opsin and phosphoopsin (P-Op), respectively. Phosphoopsin is dephosphorylated, and opsins are regenerated with 11-cis-retinal to inactive Rh ready for the next round of activation. Arrestin preferentially binds P-Rh* and, with much lower affinity, to Rh*, phosphorylated dark rhodopsin (P-Rh), and P-Op (Figure 4A). There are only two functional forms of rhodopsin that arrestin virtually does not bind: dark Rh and opsin (Figure 4A; Gurevich, 1998; Gurevich and Benovic, 1993; Hirsch et al., 1999).
Our findings (Figure 1 and Figure 4) are in full agreement with earlier reports (Mendez et al., 2003; Zhang et al., 2003) that rhodopsin activation is necessary to induce the translocation of arrestin to the OS. Here, we show that generation of Rh* is also sufficient for arrestin movement. According to our model, arrestin moves by passive diffusion along the concentration gradient created by the direct binding of arrestin to Rh*, which acts as a dynamic sink removing free arrestin from the cytoplasm and sequestering it at the disc membranes. Thus, rapid inactivation of Rh* should prevent arrestin translocation to the OS. This prediction is clearly supported by the experimental evidence, as accelerated inactivation of rhodopsin by hydroxylamine or elevated temperature (Figure 4) prevented light-induced translocation of arrestin, consistent with previous observations (Mangini et al., 1994). The requirement for the sustained presence of Rh* supports the idea that rhodopsin “attracts” arrestin to the OS by directly binding to it, rather than indirectly by setting off a hypothetical transducin-independent signaling pathway (Mendez et al., 2003; Zhang et al., 2003).
Our model is further supported by the finding that, at a moderate light level, the rate of arrestin movement to the OS is reduced in ATP-depleted photoreceptors or in mice with impaired rhodopsin phosphorylation (Figure 5), likely because the affinity of arrestin for Rh* is lower than that to P-Rh*. Furthermore, we found that a saturating flash of light, which instantly converts over 90% of rhodopsin to Rh*, greatly accelerates arrestin redistribution in both control and ATP-depleted cells (Figure 5B). Although the affinity of arrestin for Rh* is much lower than that for P-Rh*, both proteins are present in the OS at extremely high (millimolar) concentrations (Hamm and Bownds, 1986), which favors the formation of the Rh*-arrestin complex. An increase in either the affinity (P-Rh* versus Rh*) or the concentration of the bleached pigment can facilitate arrestin translocation to the OS, indicating that arrestin moves to the OS simply in accordance with the law of mass action. However, a more detailed kinetic analysis, involving precise measurements of arrestin and active rhodopsin concentrations, and a real-time study of fluorescently tagged arrestin, will be required before any additional mechanisms can be excluded. Our finding that arrestin can move to the OS within minutes after a saturating flash shows that the rate of diffusion of arrestin is similar to that of GFP. Thus, at subsaturating light levels the rate of arrestin relocalization to the OS is limited solely by the availability of the sink, which is the combined pool of Rh*, P-Rh*, and P-Op.
Dissociation of Arrestin from Rhodopsin Determines the Rate of Its Return to the IS
If binding to rhodopsin is sufficient to cause the translocation of arrestin to the OS, is the dissociation of the complex sufficient for its return to the IS? We found that, if rhodopsin was inactivated by hydroxylamine treatment after arrestin had already moved to the OS in light, the return of arrestin to the IS was inhibited (Figure 6A). In the absence of retinal, only P-Op has sufficient affinity for arrestin to retain it in the OS (Figure 4A). Indeed, when the hydroxylamine treatment was performed on ATP-depleted eyecups, arrestin readily returned to the IS. Importantly, this return occurred even in the light. This confirmed the notion that the presence of either the activated (e.g., Rh*) or the phosphorylated (e.g, P-Op) form of rhodopsin is necessary and sufficient to keep arrestin in the OS, i.e., rhodopsin is the sink for arrestin in the OS. We also found that the rate of return of arrestin to the IS in the dark closely correlated with rhodopsin dephosphorylation, particularly with the rate of disappearance of multiphosphorylated forms (Figure 6B and 6C). Moreover, in eyecups where phosphorylated rhodopsin was absent due to ATP depletion, arrestin returned to the IS much faster than in control eyecups, further supporting the idea that the release of arrestin from the complex limits the rate of its redistribution.
Potential Role of Microtubules as the Sink for Arrestin in the IS
While arrestin release from rhodopsin allows it to move freely, it does not explain why in the dark arrestin accumulates in the IS, rather than evenly distributing throughout the cytoplasm of the photoreceptor cell. Recently, in a series of elegant experiments in transgenic frog photoreceptors, it has been demonstrated that, in sharp contrast to the evenly distributed GFP, arrestin-GFP fusion protein accumulates in the IS in the dark and in the OS upon illumination (Peet et al., 2004). It is reasonable to hypothesize that the interaction with an alternative binding partner concentrates arrestin in the IS in the dark, just as the rhodopsin interaction confines it in the OS in the light.
Several studies have implicated the cytoskeleton in the regulation of arrestin function. In amphibian rods, a fraction of arrestin is retained in the dark-adapted OS, where it colocalizes with the axoneme (McGinnis et al., 2002), and in mouse rods arrestin was detected near microtubules by electron microscopy (Nir and Ransom, 1993). We have recently described direct binding of visual arrestin to microtubules (Nair et al., 2004). Experiments with purified proteins show that microtubules and rhodopsin compete for arrestin binding, suggesting that the interactions of arrestin with rhodopsin and microtubules are mutually exclusive (Figure 7C; Nair et al., 2004). Mouse arrestin partitions to the microtubulerich cytoskeletal fraction in the dark in vivo, whereas the exposure of animals to light reverses this association (Figure 7A and 7B). Taken together, these results support the hypothesis that microtubules can serve as a “default” arrestin binding partner, where it is sequestered in the dark and from which it is quickly released when a binding partner with higher affinity, such as Rh*, is generated by illumination. The remarkable abundance of microtubules in the IS (Eckmiller, 2000) can explain how the relatively low-affinity interaction concentrates arrestin in this compartment in the dark. This model is supported by our data that the increase in affinity of arrestin to microtubules by 3A and ΔC mutations results in a substantial delay in its light-induced translocation to the OS (Figure 8). While our biochemical experiments and analysis of the localization of arrestin mutants in the 3A and ΔC transgenic mice support the hypothesis that in the dark microtubules in the IS can serve as the sink for free arrestin, the existence of additional or alternative mechanisms cannot be ruled out.
Of Mice and Flies
Two recent elegant studies of the translocation of visual arrestin in Drosophila (Lee and Montell, 2004; Lee et al., 2003) demonstrated that, in the invertebrate eye, arrestin interacts with membrane phosphoinositides (PI), and this interaction is required for its light-induced translocation to the rhabdomere. Apparently, arrestin “piggybacks” on the PI-rich vesicles that move with the help of the eye-enriched myosin III NINAC. The authors proposed an intriguing hypothesis that a similar mechanism may operate in the vertebrate photoreceptors. Indeed, bovine visual arrestin binds inositol phosphates IP4 and IP6 (Palczewski et al., 1991a). In fact, mammalian visual and nonvisual arrestins bind various polyanions, such as multiphosphorylated peptides (Puig et al., 1995), heparin (Gurevich et al., 1994; Palczewski et al., 1991b), and IP6 (Gaidarov et al., 1999; Gurevich et al., 1994). However, this low-affinity binding is mediated by the site in the arrestin N domain that binds receptor-attached phosphates (reviewed in Gurevich and Gurevich, 2004), in contrast to the high-affinity PI binding site localized in the C domain of nonvisual arrestins (Gaidarov et al., 1999). It was directly demonstrated that, in sharp contrast to Drosophila visual arrestin and both mammalian nonvisual arrestins, mammalian visual arrestin does not have a high-affinity PI interaction site (Gaidarov et al., 1999). It is also noteworthy that in dark-adapted fly photoreceptors arrestin is evenly distributed in the cytoplasm (Lee and Montell, 2004; Lee et al., 2003), whereas in the vertebrate system it is concentrated in the IS.
Our data demonstrate that ATP is not required for the translocation of vertebrate visual arrestin, ruling out the participation of active transport. Thus, the difference in the mechanism of arrestin translocation in vertebrate and invertebrate photoreceptors joins the list of other important differences between the two systems that already includes the whole signaling cascade downstream of rhodopsin and the effect of light on the polarization of the photoreceptor membrane.
Model of Arrestin Translocation
Collectively, our data suggest the following mechanism of arrestin translocation (Figure S4). In the dark, rhodopsin exists as inactive Rh, and arrestin is concentrated in the inner compartments, where microtubules are abundant. Soluble arrestin is at a dynamic equilibrium with the cytoskeleton bound form; its concentration is uniform throughout the cytoplasm and relatively low. Upon illumination, generation of Rh* promptly depletes soluble arrestin in the OS cytoplasm, so that free arrestin diffuses along the concentration gradient to the OS. Since the binding affinity of arrestin for microtubules is low, the drop of the concentration of free arrestin in the IS results in its quick release from microtubules, which keep supplying free arrestin for this process as long as it is depleted due to its binding to rhodopsin. When illumination is terminated, regeneration and dephosphorylation of phosphoopsin creates Rh and opsin, neither of which bind arrestin, so that it can reassociate with microtubules, concentrating primarily in the IS. As the concentration of microtubules throughout photoreceptor cells remains unchanged regardless of illumination, the functional state of rhodopsin is the sole determinant of arrestin localization. Furthermore, since both the dissociation of arrestin from the cytoskeleton and its diffusion are rapid, not only the extent, but also the speed, of arrestin redistribution to the OS is dictated by the amount of emerging active rhodopsin. It is tempting to speculate that the similarly energy-independent movement of transducin (Figure 2) may also be governed by its dynamic interactions with binding partners in the OS and IS that have yet to be identified.
It has been proposed that light-dependent redistribution of both transducin and arrestin contributes to light adaptation. It has recently been demonstrated that light sensitivity of rods changes about 10-fold under illumination conditions that induce the translocation of 90% of transducin from the OS (Sokolov et al., 2002). However, along with the movement of transducin, light also causes the translocation of arrestin. Therefore, to determine the extent of the sensitivity change that can be ascribed to the translocation of each of these proteins, one needs an animal model in which one protein moves normally and the other does not. Physiological studies of animal models expressing arrestin or transducin that do not move will ultimately yield definitive answers about the contribution of each of these proteins’ movements to light adaptation.
Relocalization of signaling molecules is one of the mechanisms that neurons use to adjust their function to changing circumstances. Our findings suggest that in many cases these proteins move by passive diffusion, the direction of which is governed by differential availability of their interaction partners that act as sinks. This strategy may help to reduce otherwise crippling demands on energy production in the cell.
Experimental Procedures
Animals and Tissue Preparation
Animal research was conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee. Animals were dark adapted in complete darkness and exposed to light of the indicated intensity. The mice were anesthetized with isoflurane and sacrificed by cervical dislocation, and the eyes were enucleated. The eyecups were prepared by removing the cornea and lens under a dissection microscope (see Supplemental Data and Figure S1 for details) and maintained in culture in DMEM supplemented with or depleted of certain ingredients as required by a particular experiment.
Transgenic Mice
Mutagenesis by PCR was used to introduce Glu378Stop for ΔC and Leu374Ala, Val375Ala, Phe376Ala triple mutation for 3A into the mouse arrestin coding sequence (GenBank M24086), which was subcloned between the opsin promoter and the MP1 polyadenylation sequence. Transgenic mice were generated by standard procedures at the University of Southern California according to approved NIH and USC guidelines. Transgene-positive mice were bred into arrestin knockout background (Xu et al., 1997). After two rounds of breeding, transgene- and arrestin knockout-positive mice that had no wild-type arrestin alleles were identified by PCR.
Rhodopsin kinase knockout and mice expressing the CSM mutant of rhodopsin were described previously (Mendez et al., 2003).
The transgenic mice expressing GFP [TgN(GFPU)5Nagy], generated originally in the laboratory of Andras Nagy (Hadjantonakis et al., 1998), were obtained on a C57BL/6 genetic background from the Jackson Laboratory. In this strain, the expression of GFP is controlled by a hybrid CAG promoter, which drives high-level mosaic expression in all tissues including photoreceptors.
Immunofluorescence
Eyecups were fixed in 4% paraformaldehyde for 1 hr at room temperature and embedded in 3.5% melted agarose. The blocks of hardened agarose containing the eyecups were cut in a vibratome to obtain 100 µm sections. The sections were permeabilized and blocked by incubation in a 100 µl drop of PBS containing 1% BSA and 0.1% Triton X-100 for 1 hr in a humidified box at room temperature. The sections were then incubated with anti-arrestin or anti-transducin antibody diluted 1:500 in the same buffer for 1 hr, washed with PBS, and then incubated with secondary Cy3-conjugated affinity-purified donkey anti-rabbit IgG (Jackson Immunoresearch Laboratories, Inc.) for 1 hr followed by three washes with PBS. The preparations were then mounted on a coverslip using Antifade reagent (Molecular Probes) and visualized in a fluorescence microscope. Alternatively, eyecups were fixed and stained using frozen sections as described earlier (Mendez et al., 2003) and viewed using a Zeiss LSM 510 laser scanning confocal microscope. Quantification of the relative amount of arrestin in the OS and IS was determined using MetaMorph software.
ATP Depletion of Eyecups
Eyecups were incubated in DMEM free of glucose and sodium pyruvate and supplemented with 2 mM deoxyglucose and 10 mM KCN (pH 7.5) for 1 hr in the dark at room temperature. For direct measurements of ATP content, the eyecups were homogenized in 10% tricloroacetic acid, and the level of ATP in the extract was measured using Sigma Luciferase ATP Determination Kit according to the manufacturer’s instructions.
Analysis of Rhodopsin Phosphorylation by Mass Spectrometry
Mouse eyecups or eyes were homogenized in 7 M urea and centrifuged, and the pellet was washed and then incubated with endoproteinase aspN (Roche, Indianapolis, IN). The lysate was centrifuged and fractionated by capillary HPLC. The eluted samples were then analyzed on a Finnegan LCQ deca ion trap mass spectrometer in positive ion mode. To obtain the relative amounts of the various phosphorylated species in a given sample, we integrated the area under the peaks, as described in detail in the Supplemental Data and in Kennedy et al. (2001) and Lee et al. (2002).
FRAP
Eyes from GFP-expressing transgenic mice were placed in DMEM containing 1 mM Hoechst nuclei-staining dye, and the eyecups were prepared. The eyecups were quickly embedded in 1.5% lowmelt agarose dissolved in DMEM, sectioned on a vibratome to produce 150 micron sections, and immediately imaged on a Zeiss LSM 510 laser scanning confocal microscope. For best results, imaging had to be completed in less than 2 hr after the death of the animal. Once an appropriate area of the photoreceptor cell was selected and subjected to photobleaching, time-lapse images of GFP (433 nm excitation, and 488 nm emission) and Hoechst (390 excitation, 420 emission) were recorded. The data were analyzed using Zeiss software.
In Vitro Direct Arrestin Binding Assays
Arrestins were labeled by in vitro translation, and urea-treated rod OS membranes were prepared, phosphorylated, and regenerated with 11-cis-retinal as described (Gurevich and Benovic, 1993). In vitro-translated arrestins were incubated with the various functional forms of rhodopsin either in the dark or in room light, then cooled on ice and loaded onto 2 ml of Sepharose 2B columns equilibrated with 10 mM Tris-HCl (pH 7.4), 100 mM NaCl. Bound arrestin eluted with the membranes in the void volume. Arrestin binding to microtubules in vitro was performed using purified (>99%) tubulin (Cytoskeleton, Inc.). Briefly, tubulin was polymerized in the presence of GTP and taxol, separated from unpolymerized tubulin by centrifugation, and mixed with in vitro-translated arrestins. Microtubules with bound arrestin were pelleted by centrifugation and dissolved in 1% SDS and 50 mM NaOH, and arrestin was quantified by liquid scintillation counting.
Association of Arrestin with Cytoskeleton In Vivo
The retinas were removed from the eyes, placed in 0.1 ml of 8% Optiprep (Sigma), vortexed at a medium speed for 1 min, and then centrifuged at 12,000 rpm for 1 min. The supernatant containing ROS was collected, and the pellet was resuspended and centrifuged three more times. The combined supernatants were centrifuged at 12,000 rpm for 5 min to obtain the ROS-enriched pellet, which was then solubilized in 10 mM Tris, 125 mM NaCl, 1% Triton X-100. The ROS-depleted pellet was resuspended in 10 mM HEPES, 5 mM MgCl2, 100 mM KCl, 1 mM EGTA supplemented with protease inhibitors as well as DNase and RNase to reduce viscosity, and solubilized with 1% Triton X-100. The lysed ROS or non-ROS fractions were incubated for 10 min at room temperature and then centrifuged at 12,000 rpm for 15 min. The supernatants were loaded onto a 200 µl cushion of 50% glycerol and centrifuged at 100,000 rpm for 20 min. The supernatants and the pellets representing the cytoskeletal fraction were collected for further experimentation or Western blot analysis.
Supplementary Material
Acknowledgments
We are grateful to Drs. Cheryl M. Craft, Jeffrey L. Benovic, Rosalie K. Crouch, Narsing A. Rao, and Clay Smith for mouse visual arrestin clone, rhodopsin kinase, 11-cis-retinal, and anti-arrestin antibodies, respectively. We thank Tatiana Vishnivetskaya and Olga Nichols for technical assistance. Image collection and analysis were performed in part through the use of the VUMC Cell Imaging Core Resource (supported by NIH grants EY08126, CA68485, DK20593, DK58404, and HD15052). We also thank Qiang Wang, Heather Ezelle, and Galina Dvoryanchikova (UMSM Departments of Pharmacology, Immunology, and Ophthalmology, respectively) for help with animals and the confocal microscopy facility of UMSM for assistance with image acquisition. This work was supported by NIH rhodopgrants GM 060019 and NEI 012982 (V.Z.S.), EY11500 and GM 63097 Na(V.V.G.), EY 06641 (J.B.H.), NS45117 and MH62651 (E.V.G.), and EY012155 and EY012703 (J.C.), and an American Heart Association (Florida Affiliate) postdoctoral fellowship (K.S.N.). S.M.H. is a recipirhoent of predoctoral NIH Training Grant GM07628.
Footnotes
Supplemental Data
The Supplemental Data, which include Supplemental Results, Discussion, Experimental Procedures, and figures and one supplemental table, can be found with this article online at http://www.neuron.org/cgi/content/full/46/4/555/DC1/.
References
- Arshavsky VY. Protein translocation in photoreceptor light adaptation: a common theme in vertebrate and invertebrate vision. Sci. STKE. 2003;2003:PE43. doi: 10.1126/stke.2003.204.pe43. [DOI] [PubMed] [Google Scholar]
- Bowne SJ, Sullivan LS, Blanton SH, Cepko CL, Blackshaw S, Birch DG, Hughbanks-Wheaton D, Heckenlively JR, Daiger SP. Mutations in the inosine monophosphate dehydrogenase 1 gene (IMPDH1) cause the RP10 form of autosomal dominant retinitis pigmentosa. Hum. Mol. Genet. 2002;11:559–568. doi: 10.1093/hmg/11.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekhuyse RM, Tolhuizen EF, Janssen AP, Winkens HJ. Light induced shift and binding of S-antigen in retinal rods. Curr. Eye Res. 1985;4:613–618. doi: 10.3109/02713688508999993. [DOI] [PubMed] [Google Scholar]
- Chen CK, Burns ME, Spencer M, Niemi GA, Chen J, Hurley JB, Baylor DA, Simon MI. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc. Natl. Acad. Sci. USA. 1999;96:3718–3722. doi: 10.1073/pnas.96.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrey TG. The thermal decay of the intermediates of rhodopsin in situ. Vision Res. 1968;8:965–982. doi: 10.1016/0042-6989(68)90071-0. [DOI] [PubMed] [Google Scholar]
- Eckmiller MS. Microtubules in a rod-specific cytoskeleton associated with outer segment incisures. Vis. Neurosci. 2000;17:711–722. doi: 10.1017/s0952523800175054. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Krupnick JG, Falck JR, Benovic JL, Keen JH. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J. 1999;18:871–881. doi: 10.1093/emboj/18.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV. The selectivity of visual arrestin for light-activated phosphorhodopsin is controlled by multiple nonredundant mechanisms. J. Biol. Chem. 1998;273:15501–15506. doi: 10.1074/jbc.273.25.15501. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Benovic JL. Visual arrestin interaction with rhodopsin Sequential multisite binding ensures strict selectivity toward light-activated phosphorylated rhodopsin. J. Biol. Chem. 1993;268:11628–11638. [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The molecular acrobatics of arrestin activation. Trends Pharmacol. Sci. 2004;25:105–111. doi: 10.1016/j.tips.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Chen CY, Kim CM, Benovic JL. Visual arrestin binding to rhodopsin Intramolecular interaction between the basic N terminus and acidic C terminus of arrestin may regulate binding selectivity. J. Biol. Chem. 1994;269:8721–8727. [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech. Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Hagins WA. Flash photolysis of rhodopsin in the retina. Nature. 1956;177:989–990. doi: 10.1038/177989b0. [DOI] [PubMed] [Google Scholar]
- Hamm HE, Bownds MD. Protein complement of rod outer segments of frog retina. Biochemistry. 1986;25:4512–4523. doi: 10.1021/bi00364a010. [DOI] [PubMed] [Google Scholar]
- Hirsch JA, Schubert C, Gurevich VV, Sigler PB. The 2.8 A crystal structure of visual arrestin: a model for arrestin’s regulation. Cell. 1999;97:257–269. doi: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- Hofmann KP, Emeis D, Schnetkamp PP. Interplay between hydroxylamine, metarhodopsin II and GTP-binding protein in bovine photoreceptor membranes. Biochim. Biophys. Acta. 1983;725:60–70. doi: 10.1016/0005-2728(83)90224-4. [DOI] [PubMed] [Google Scholar]
- Hofmann KP, Pulvermuller A, Buczylko J, Van Hooser P, Palczewski K. The role of arrestin and retinoids in the regeneration pathway of rhodopsin. J. Biol. Chem. 1992;267:15701–15706. [PubMed] [Google Scholar]
- Hurley JB, Ebrey TG, Honig B, Ottolenghi M. Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts. Nature. 1977;270:540–542. doi: 10.1038/270540a0. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Lee KA, Niemi GA, Craven KB, Garwin GG, Saari JC, Hurley JB. Multiple phosphorylation of rhodopsin and the in vivo chemistry underlying rod photoreceptor dark adaptation. Neuron. 2001;31:87–101. doi: 10.1016/s0896-6273(01)00340-3. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Montell C. Light-dependent translocation of visual arrestin regulated by the NINAC myosin III. Neuron. 2004;43:95–103. doi: 10.1016/j.neuron.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Lee KA, Craven KB, Niemi GA, Hurley JB. Mass spectrometric analysis of the kinetics of in vivo rhodopsin phosphorylation. Protein Sci. 2002;11:862–874. doi: 10.1110/ps.3870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Xu H, Kang LW, Amzel LM, Montell C. Light adaptation through phosphoinositide-regulated translocation of Drosophila visual arrestin. Neuron. 2003;39:121–132. doi: 10.1016/s0896-6273(03)00390-8. [DOI] [PubMed] [Google Scholar]
- Mangini NJ, Garner GL, Okajima TI, Donoso LA, Pepperberg DR. Effect of hydroxylamine on the subcellular distribution of arrestin (S-antigen) in rod photoreceptors. Vis. Neurosci. 1994;11:561–568. doi: 10.1017/s0952523800002467. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- McGinnis JF, Matsumoto B, Whelan JP, Cao W. Cytoskeleton participation in subcellular trafficking of signal transduction proteins in rod photoreceptor cells. J. Neurosci. Res. 2002;67:290–297. doi: 10.1002/jnr.10120. [DOI] [PubMed] [Google Scholar]
- Mendez A, Burns ME, Roca A, Lem J, Wu LW, Simon MI, Baylor DA, Chen J. Rapid and reproducible deactivation of rhodopsin requires multiple phosphorylation sites. Neuron. 2000;28:153–164. doi: 10.1016/s0896-6273(00)00093-3. [DOI] [PubMed] [Google Scholar]
- Mendez A, Lem J, Simon M, Chen J. Light-dependent translocation of arrestin in the absence of rhodopsin phosphorylation and transducin signaling. J. Neurosci. 2003;23:3124–3129. doi: 10.1523/JNEUROSCI.23-08-03124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Hanson SM, Kennedy MJ, Hurley JB, Gurevich VV, Slepak VZ. Direct binding of visual arrestin to microtubules determines the differential subcellular localization of its splice variants in rod photoreceptors. J. Biol. Chem. 2004;279:41240–41248. doi: 10.1074/jbc.M406768200. [DOI] [PubMed] [Google Scholar]
- Nir I, Ransom N. Ultrastructural analysis of arrestin distribution in mouse photoreceptors during dark/light cycle. Exp. Eye Res. 1993;57:307–318. doi: 10.1006/exer.1993.1129. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Pulvermuller A, Buczylko J, Gutmann C, Hofmann KP. Binding of inositol phosphates to arrestin. FEBS Lett. 1991a;295:195–199. doi: 10.1016/0014-5793(91)81416-6. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Pulvermuller A, Buczylko J, Hofmann KP. Phosphorylated rhodopsin and heparin induce similar conformational changes in arrestin. J. Biol. Chem. 1991b;266:18649–18654. [PubMed] [Google Scholar]
- Peet JA, Bragin A, Calvert PD, Nikonov SS, Mani S, Zhao X, Besharse JC, Pierce EA, Knox BE, Pugh EN., Jr Quantification of the cytoplasmic spaces of living cells with EGFP reveals arrestin-EGFP to be in disequilibrium in dark adapted rod photoreceptors. J. Cell Sci. 2004;117:3049–3059. doi: 10.1242/jcs.01167. [DOI] [PubMed] [Google Scholar]
- Philp NJ, Chang W, Long K. Light-stimulated protein movement in rod photoreceptor cells of the rat retina. FEBS Lett. 1987;225:127–132. doi: 10.1016/0014-5793(87)81144-4. [DOI] [PubMed] [Google Scholar]
- Puig J, Arendt A, Tomson FL, Abdulaeva G, Miller R, Hargrave PA, McDowell JH. Synthetic phosphopeptide from rhodopsin sequence induces retinal arrestin binding to photoactivated unphosphorylated rhodopsin. FEBS Lett. 1995;362:185–188. doi: 10.1016/0014-5793(95)00225-x. [DOI] [PubMed] [Google Scholar]
- Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Jr, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Whelan JP, McGinnis JF. Light-dependent subcellular movement of photoreceptor proteins. J. Neurosci. Res. 1988;20:263–270. doi: 10.1002/jnr.490200216. [DOI] [PubMed] [Google Scholar]
- Xu J, Dodd RL, Makino CL, Simon MI, Baylor DA, Chen J. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 1997;389:505–509. doi: 10.1038/39068. [DOI] [PubMed] [Google Scholar]
- Zhang H, Huang W, Zhu X, Craft CM, Baehr W, Chen CK. Light-dependent redistribution of visual arrestins and transducin subunits in mice with defective phototransduction. Mol. Vis. 2003;9:231–237. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.