Abstract
The dawning of this millennium broke new ground in life science and technology, presented us genomic and proteomic revolution, nanotechnology innovation, and high performance liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS) used for separating and identifying new chemical entities at pico-, or even femto-concentrations. Applications of these high technologies to the traditional Chinese medicine (TCM) opened a new chapter in the ancient medicine, and prompted us to re-evaluate the thousand-year-old phytomedicine–ginseng from current perspectives. We, therefore, collected the latest information (mostly within 10 years) on ginseng, and condensed the information into two parts of this review serial. The present part covers etymology of ginseng, its pharmacognosy (natural origin, physical appearance, chemical properties, and specie identification), its cultivation and processing-related metabolic changes in active ingredients, standardized analytical methods used for quality control of various ginseng products, modern analytical methods used to identify and classify more than 100 chemical entities (many were recently unfolded) derived from ginseng species and their metabolites. The global markets and production of ginseng and relevant government regulations are herein updated to exchange information and understandings about current people’s uses and cultivation of ginseng. The second part of the review serial will classify all these 100 chemical entities separated from various ginseng species into different groups based on their structural similarities, and summarize bioactivities of these entities. The second part of the review serial will also focus on recent findings of ginseng pharmacology and its clinical trials for various diseases, and brief side effects of ginseng.
Keywords: Ginseng, ginsenosides, ginseng market, pharmacognosy, phytomedicine, traditional chinese medicine
INTRODUCTION
About 25 years ago when we just started our careers as life scientists focused on, in part, traditional Chinese medicine, we did an astonishing experiment that still remains vivid in our mind today and has drawn our great interests, since then, to the thousand years old of herbal medicine— Ginseng. In that experiment, outbred male CD mice were randomly divided into two groups. Each mouse was clasped with a two-gram metal weight on their tails. One group of the mice was orally administered concentrated extract of red ginseng, and another was given only water as the control. One hour later after the administration, all mice were placed in a large sink filled with water to swim and the swim times were counted. The untreated control mice only swam for an average time of 20 min, whereas, the ginseng-treated mice swam for more than one hour. One of the 20 treated mice even swam for more than two hours! We witnessed the most noticeable feature of ginseng in increasing resistance to physical, chemical and biological stress and boosting general vitality. We have firmly believed the anti-fatigue and adaptogenic effects of ginseng since then, and paid our great interests to all ginseng-related research and medicinal applications.
Nowadays, herbal medicine has received much attention and is recommended as a natural alternative to maintain one’s health. At the beginning of this millennium, we have seen explosive revolutions in life science, including genomics and proteomics, nanotechnology and its applications in the traditional Chinese medicine (TCM) [1], and high performance liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS) used for separating and identifying new chemical entities [2]. These revolutions have been enriching our knowledge of ginseng, identifying new chemical entities from various ginseng species, and improving our understanding of this millennium herbal medicine. The pharmacopoeias of several countries, including China, Japan, Germany, Austria, United Kingdom, and France, have collected information about ginseng characterization.
The empirical knowledge of ginseng and its adaptogenic effects were passed on by oral tradition and literal records in ancient times when technology-based objective analyses, diagnoses, and treatments were almost impossible. As a result, today, the facts and fictions about ginseng’s medical effectiveness are mixed in the literature, and need to be critically analyzed, re-evaluated and verified. It has been difficult to validate some of the medicinal benefits of ginseng because the old results and records of ginseng were not obtained with modern analytical tools. In addition, different studies using a wide variety of ginseng species that were harvested from different places and processed, preserved, and extracted differently could produce contradictory results and records. Indeed, harvest time, places and process procedures could cause differences in quality of ginseng and the resultant study reports. Now it is a perfect time for us to re-evaluate ginseng from current perspectives, and exchange ideas and information in this regard. This all-inclusive paper was designed for this purpose to review information collected within the recent 10 years, narrate the history of folk uses of ginseng and its etymology, summarize current findings of ginseng related to its pharmacognosy, phytochemical analysis and methods, pharmacology, and new clinical applications. More importantly, We will critically analyze the relevant information and data from multidisciplinary approaches, and list all chemical entities found from ginseng species.
ETYMOLOGY
The English word ginseng derives from the Chinese term rénshēn (simplified: 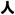
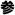 ; traditional:
; traditional: 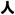
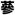 ), literally “man root” (referring to the root’s characteristic forked shape, resembling the human body and the legs of a man). The difference between rénshēn and “ginseng” is explained by the fact that the English pronunciation derives from a Japanese reading of these Chinese characters. However, the current Japanese word for these characters
), literally “man root” (referring to the root’s characteristic forked shape, resembling the human body and the legs of a man). The difference between rénshēn and “ginseng” is explained by the fact that the English pronunciation derives from a Japanese reading of these Chinese characters. However, the current Japanese word for these characters 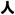
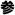 (ninjin) means carrot, and ginseng is referred to in Japanese as
(ninjin) means carrot, and ginseng is referred to in Japanese as 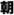
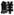
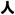
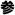 (chosen ninjin), adopting the name of the last dynasty of Korea
(chosen ninjin), adopting the name of the last dynasty of Korea 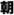
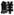 (Choson). The Korean name is
(Choson). The Korean name is 

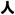
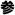 (goryo insam).
(goryo insam).
Ginseng refers to the root of several species in the plant genus Panax (C.A. MEYER Araliaceae). The botanical name “Panax” was given by the Russian botanist Carl Anton von Meyer (1795–1855) in 1843 in the Bulletin of Physics and Mathematics published by the Academy of Petersburg Institute [3]. In Greek, “pan” means all and “axos” means cure. As a whole, Panax indicates “all-heal”. Panax was applied to this genus because Carl Linnaeus (1707–1778, Swedish, who introduced the consistent use of binomial names for both plants and animals and validly published over 9,000 plant names) was aware of its wide use in Chinese medicine.
Among them, Panax ginseng (Panax ginseng C.A. Meyer) is the most widely used ginseng and is indigenous to the Far East countries, most notably China and Korea [4]. Panax ginseng was first reported cultivated around 11 BC and has a medical history of more than two thousand years [5].
PHARMACOGNOSY
Ginseng grows in the Northern Hemisphere, typically in cooler climates. They are slow-growing perennial plants with fleshy roots [6, 7]. The plant grows 6 to 18 inches tall with 1 to 3 umbels of 15 to 30 flowers and greenish-yellow corollas. The fruit is a pea-sized, globular to reniform, scarlet, smooth and glossy drupe, which contains 2 seeds [8]. The plant usually bears three leaves, each with three to five leaflets 2 to 5 inches long. In China, Korea, Japan and Russia, the cultivated ginseng roots are harvested when the plant is 3–6-year old. The treasured aromatic root resembles a small parsnip that forks as it matures. It should be noted that ginseng is not derived from the rhizomes (the thick stem that lies flat along the ground with roots and leaves growing from it).
The wild American ginseng (Panax quinquefolium, Araliaceae family) is a plant familiar to many people in the Southern Appalachian region. For several generations, “digging sand” has been an enjoyable and profitable activity for many mountain people. American ginseng is native to many states, east of the Mississippi River, and can also be found in the mountainous regions of the Southern states where a cool temperate climate exists. Wisconsin is the largest producer of Panax quinquefolium. Ginseng also grows naturally in the Eastern provinces of Canada, mainly in provinces of British Columbia and Ontario. Ginseng is a tender perennial. The first frosts of fall kill the leafy top, but a new top grows up the following spring, from an underground bud on the perennial root. It takes seven or eight years for the wild American ginseng plants to grow to maturity in a natural woodland habitat.
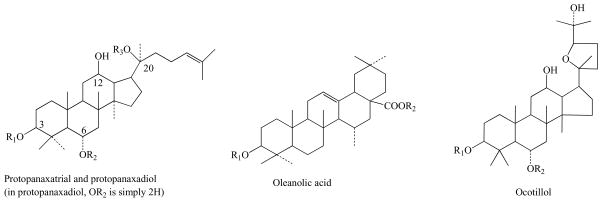
Ginseng is characterized by the presence of ginsenosides. Ginsenosides are triterpenes saponins considered to be the main bioactive principles of ginseng (Fig. 1). Ginsenosides have been shown to interact with numerous membrane proteins such as ion channels, transporters and receptors, resulting in a broad range of physiological activities [9, 10]. The ginsenoside content and its metabolites are varying depending on the Panax species, the plant age, the part of the plant, the preservation method, the season of harvest, and the extraction method [11, 12]. The ginsenoside content variability can also be in part ascribed to natural variations such as kind of soil, weather conditions, geographical location and different production procedures. Table 1 lists various concentrations of ginsenoside and its metabolites that develop in complexity with age of ginseng [13].
Fig. 1.
Main structures of ginsenosides, including protopanaxadiol, protopanaxatriol, oleanolic acid and ocotillol.
Table 1.
Changes of Content of Ginsenosides (Saponins) in Ginseng with its Cultivation Years [13]. Thin Layer Chromatography and Colorimetric Method were Used for Quantitative Comparison of Saponin Contents of Ginseng Harvested in Different Years After Cultivation
| Years | Total saponins (%) | Rb (%) | Rg (%) | Ro (%) |
|---|---|---|---|---|
| 2 | 1.97 | 0.88 | 0.54 | 0.13 |
| 3 | 2.20 | 1.03 | 0.62 | 0.17 |
| 4 | 4.75 | 2.27 | 1.10 | 0.40 |
| 5 | 4.60 | 2.08 | 1.19 | 0.21 |
| 6 | 3.84 | 1.94 | 0.81 | 0.29 |
| 9 | 3.81 | 2.32 | 0.46 | 0.40 |
PROCESSING, CULTIVATION AND CLASSIFICATION
There are two different ways of processing ginseng after harvest: air drying and steaming the roots; the former results in white ginseng and the latter produces red ginseng. Interestingly, after these two different processes, the roots differ in their content of saponins [4] and this may be the reason for the variable actions of different ginseng products.
The so-called white ginseng is peeled and then air dried to reduce the water content to 12% or less. White ginseng may contain less of the therapeutic constituents. It is thought by some that enzymes contained in the root break down these constituents in the process of drying. Drying in the sun bleaches the root to a yellowish-white color. The so-called red ginseng (simplified Chinese: 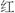
 ; traditional Chinese:
; traditional Chinese: 
 ), is Panax ginseng that is not peeled but steamed, which gives the roots a glossy reddish-brown coloring. Steaming the root is thought to change the biochemical composition of ginseng and prevent the breakdown of the active ingredients. The roots are then sun-dried. Red ginseng is frequently marinated in an herbal brew which results in the root becoming extremely brittle. This version of ginseng is traditionally associated with stimulating sexual function and increasing energy [4]. Red ginseng is always produced from cultivated roots, usually from either China or South Korea.
), is Panax ginseng that is not peeled but steamed, which gives the roots a glossy reddish-brown coloring. Steaming the root is thought to change the biochemical composition of ginseng and prevent the breakdown of the active ingredients. The roots are then sun-dried. Red ginseng is frequently marinated in an herbal brew which results in the root becoming extremely brittle. This version of ginseng is traditionally associated with stimulating sexual function and increasing energy [4]. Red ginseng is always produced from cultivated roots, usually from either China or South Korea.
Ginseng cultivated in Korea is classified into three types, depending on how it is processed: fresh ginseng (less than 4 years old), white ginseng (4–6 years old and dried after peeling), and red ginseng (harvested when 6 years old, steamed and dried) [14]. Red ginseng is not skinned before it is steamed or otherwise heated and subsequently dried. In the course of the steaming process, ginseng starch is gelatinized, causing an increase in saponin content. Traditionally red ginseng has been used to restore and enhance normal well-being, and is often referred to as an adaptogenic.
Wild ginseng is one that has not been planted and cultivated domestically, rather it is that which grows naturally and is harvested from wherever it is found to be growing. It is traditionally considered to be superior to field farmed ginseng, and has been shown to contain higher levels of ginsenoside. Wild ginseng is relatively rare and even increasingly endangered, due in large part to high demand for the product in recent years, which has led to the wild plants being sought out and harvested faster than new ones can grow to reach maturity. Wild ginseng can be either Asian or American and can be processed to be red ginseng.
There are differences in visual appearance between wild and cultivated ginseng roots. The wild roots are dark tan in color, gnarled in appearance and show many concentric growth rings. They are often forked. Some of them resemble the body of a man. Wild roots are generally small in size and light in weight. One distinctive characteristic of a wild root is a long neck. The cultivated roots are cream colored, smooth and fat and exhibit few concentric growth rings. Cultivated roots are often large and heavy. They are most often shaped like a carrot. Ginseng grown from cultivated seed will typically have a short neck.
There are wood grown American ginseng programs in Wisconsin, Maine, Tennessee, Virginia and North Carolina [15]. The wood grown plants have comparable value to wild grown ginseng of similar age. The wood planting of ginseng restores natural habitats and removes pressure from any remaining wild ginseng.
With extinction of wild American ginseng in many regions due to wild harvest and the threat from the widespread fungus disease, a system called wild simulated ginseng production is emerging in the United States [16]. Using this production system, landowners may establish naturalized populations of wild American ginseng on the forest floor in their privately-owned woodlands. If managed correctly, these natural stands of ginseng will be perpetual. A natural stand of undisturbed wild ginseng renews itself by self-seeding. Careful harvest of mature plants can take place, in wild simulated ginseng patches, without taking the site out of production [15]. Young seedling ginseng plants will just grow up to take their place.
The most commonly used Panax species are listed below in the order of the size of the consumer market although Panax quinquefolius occupies its global market almost equivalent to Panax ginseng [4]:
Panax ginseng: Korean or Asian ginseng; found mostly in northern China, Korea, and eastern Russia.
Panax quinquefolius: American ginseng; found in southern Canada and in the United States. It is now cultivated in the Canadian provinces of Ontario and British Columbia and the American state of Wisconsin. P. quinquefolius is now also grown in northern China.
Panax notoginseng: found in China, it is also named Tienchi or Sanchi (San qi in Chinese pronunciation). It is now cultivated on a large scale in Yunan province of China [17, 18]. It is a hemostatic ingredient in the traditional Chinese medicine, Yunnan Bai Yao. It may not be adaptogenic.
Panax japonicus: Japanese ginseng.
Panax vietnamensis: discovered in Vietnam, the southernmost ginseng found.
Panax pseudoginseng: grown in Nepal and eastern Himalayas.
The following plants are referred to as ginsengs, or ginseng alternatives, but they may not be adaptogenic:
Gynostemma pentaphyllum: Southern ginseng, aka Jiaogulan.
Eleutherococcus senticosus: Siberian ginseng. It was found in eastern Siberian as an adaptogen [19] with a name of Siberian ginseng as a marketing ploy; it has a woody root instead of a fleshy root; it contains eleutherosides rather than ginsenosides, hence, it is not regarded as ginseng.
Pseudostellaria heterophylla: Prince ginseng.
Withania somnifera: Indian ginseng, aka Ashwagandha.
Pfaffia paniculata: Brazilian ginseng.
Lepidium meyenii: Peruvian ginseng, aka Maca.
Oplopanax horridus: Alaskan ginseng.
Angelica sinensis: Female ginseng, aka Dong Quai.
These plants are sometimes referred to as ginsengs, but they are either from a different family or genus. Only Jiaogulan (a traditional Chinese herbal medicine) actually contains compounds closely related to ginsenosides, although ginsenosides alone are not believed to determine the effectiveness of whole ginseng. Since each of these plants has different uses, one should research their properties before use [5].
GINSENG PRODUCTION, PRODUCTS AND GLOBAL MARKETS
Approximately two million pounds of ginseng were grown in intensive cultivation under artificial shade in Wisconsin in 1994 [20]. Ginseng cultivation has been practiced there since 1900. In 1994, production in Ontario, Canada, exceeded one and one-half million pounds. In 1994, production of ginseng in British Columbia, Canada, exceeded one-half million pounds. In 1994, artificial shade grown roots were selling for $30 to $40 per pound. They sold for $18 to $30 per pound in 1995, $10 to $22 per pound in 1996, and only $6 to $18 per pound in 1997 [21]. Current prices are below or close to the costs of production, thus causing a shrinkage of the artificial shade cultivated ginseng industry in North America.
Most of the ginseng, grown or gathered from the wild in the United States, is exported to Asian countries for sale. Hong Kong has traditionally absorbed the bulk of North American ginseng, accounting for a consistent 80 percent of all purchases of unprocessed root [16]. Ginseng growers and gatherers in the United States and Canada produce about four million pounds of dried roots for export to Asia each year. Ginseng was the second-highest selling herbal supplement in the United States in 2000, with gross retail sales of $62 million [22]. Europe is a market area that is quite used to medicinal and other herbs. It has been subjected only to Asian ginseng until recently when a small amount of American ginseng was shipped there. One of the restrictions to this marketplace is that they do not want or won’t allow any soil on the roots. This is the opposite to Asian buyers who want the root a bit dirty. Some adjustments will have to be made in ginseng handling procedures in order to market ginseng there.
One of the primary reasons for declining prices of cultivated American ginseng is increased production in China. Hundreds of acres of American ginseng are being grown under artificial shade in Liaoning, Jilin and Heilongjiang Provinces. Chinese ginseng experts have been buying American ginseng seed from Canada for the past 20 years. The Chinese have become very skillful at growing excellent quality cultivated American ginseng roots. China now is self-sufficient in cultivating American ginseng [20].
It was reported that yield per acre of cultivated ginseng could be as high as 2,500 pounds of dried root. Establishment costs for one acre of ginseng beds, under wood lath shade or under polypropylene shade cloth, varies from $20,000 to $40,000 depending upon the current prices of materials needed.
The root of ginseng contains active chemical components called ginsenosides (or panaxosides) that are thought to be responsible for the herb’s medicinal properties [23]. Ginseng products are sold in tablets (regular and chewable), capsules, liquid extracts, tinctures, carbonated drinks, graded root (bulk or gift packs), powdered root (bulk or packaged), sliced root, chips, soft gels, and teas as well as creams or other preparations for external use. These products are mainly produced using Panax ginseng and Panax quinquefolius.
Biological effects of ginseng products depend on many factors [24]: degree of chemical variation; content of individual compounds which vary significantly; dosage form (i.e., capsule, tablet, liquid extract, etc.); amount of finished product ingested; and the effective dosage range (i.e., the dosage range in which ginseng is likely to have a biological effect on the body).
GOVERNMENTAL REGULATIONS ON GINSENG PRODUCTS
In China, as of this writing, there were 911 ginseng-related products registered to the Chinese Food and Drug Administration (SFDA, www.sfda.gov.cn) as a kind of Chinese medicinal formula that contains different amounts of ginseng and its ingredients. These products are formulated as extract, compounded extract, or tincture of ginseng root, capsules containing extract of ginseng root or leaves, and ginsenosides. In addition, there were 46 types of food products containing ginseng and its extracts under the SFDA inspection. Ginseng species were well described in the Chinese Pharmacopoeia when the second edition of the Pharmacopoeia was published in 1963, and the information is periodically updated to the current seventh edition [25].
Wild American ginseng is regarded as an endangered species plant. Therefore, there are regulations on its harvest, sale and shipping. To keep track of the movement of wild American ginseng a form has to be filled out and stamped by a Canadian Agriculture and Agri-food officer before a shipment can be released for export. This is an ‘Application for Permit to Export Endangered Species’ form, and it is available from www.cites.org. In USA, the Center for Food Safety and Applied Nutrition of the U.S. Food and Drug Administration (FDA) monitors ginseng quality and safety. According to federal regulations, products making claims fall into two classes. Class I refers to manipulated products (e.g., standardized ginseng extract) or products containing added ingredients (e.g., softgels containing standardized ginseng extract). These products must contain at least 100% of the claim throughout the shelf life of the product. Thus, an Asian ginseng product standardized to 4% ginsenosides must contain at least 4% ginsenosides throughout the shelf life of the product. Class II refers to naturally occurring, non-manipulated ingredients (e.g., Asian ginseng root powder). These products must contain at least 80% of the claimed amount of the specified ingredient over the shelf life of the product. [Code of Federal Regulations 21 CFR101]. If an Asian ginseng root powder product claims 4% ginsenosides, that product must contain at least 80% of 4% (i.e., 3.2%) for the shelf life of the product.
The FDA has special labeling requirements for dietary supplements and treats them as foods, not drugs, to ensure quality and safety. These dietary supplements include vitamins, minerals, herbs or other botanicals, amino acids, enzymes, and/or other ingredients intended to supplement the diet. The Code of Federal Regulations, 21CFR310.528, states that drug products containing active ingredients offered over-the-counter for use as an aphrodisiac are any product that bears labeling claims that it will arouse or increase sexual desire, or that it will improve sexual performance, is an aphrodisiac drug product. Anise, cantharides, donqual, estrogens, fennel, ginseng, golden seal, gotu kola, Korean ginseng, lico-rice, mandrake, methyltestosterone, minerals, nux vomica, Pega Palo, sar-saparilla, strychnine, testosterone, vi-tamins, yohimbine, yohimbine hydrochloride, and yohimbinum have been presented as ingredients in such drug products (www.fda.gov/cder/otcmonographs).
Section 10806(b) (1) of the Farm Security and Rural Investment Act of 2002 (the Farm Bill) (Pub.L.107–171), signed into law on May 13, 2002, by President Bush, states that the term “ginseng” may only be considered to be a common or usual name (or part thereof) for any herb or herbal ingredient derived from a plant classified within the genus Panax, and only labeling or advertising for herbs or herbal ingredients classified within that genus may include the term “ginseng.” (www.fda.gov/ora/fiars). Section 10806(b)(2) amended section 403 of the Federal Food, Drug, and Cosmetic Act (the Act) (21 U.S.C. 343) by adding the provision that a food or dietary supplement is misbranded “if it purports to be or is represented as ginseng, unless it is an herb or herbal ingredient derived from a plant classified within the genus Panax.”
This amendment to the Act means that no food, food ingredient, dietary ingredient or dietary supplement may be identified as “ginseng” unless it is a botanical within the genus Panax. However, many dietary supplements and their ingredients that are or that contain the botanical Eleutherococcus senticosus are often identified using the term “Siberian ginseng.” Because plants within the genus Eleutherococcus are not the same genus as Panax, these products cannot use the term “ginseng” in their name (Table 2). Accordingly, the dietary ingredient Eleutherococcus senticosus or a dietary supplement containing it must identify the ingredient using the Latin binomial Eleutherococcus senticosus or the standardized common name “eleuthero.” The term “Siberian ginseng” cannot be used.
Table 2.
Distinguishing Ginseng from Eleutherococcus (or Siberian Ginseng; www.fda.gov/ora/fiars)
| Latin Binomial | Standardized Common Name |
|---|---|
| Eleutherococcus senticosus | Eleuthero (it is not ginseng) |
| Panax ginseng | Asian ginseng |
| Panax pseudoginseng var. japonica | Japanese ginseng |
| Panax pseudoginsneg var. notoginseng | Tienchi ginseng |
| Panax quinquefolis | American ginseng |
GENERAL PHYTOCHEMICAL CLASSIFICATION OF GINSENG AND GINSENOSIDES
Careful and logical classification of active principals of ginseng was not pursued timely in the past probably because it is boring and time-consuming work that needs due diligence to gather all scattered species of information together and catch up with the constant changes and additions of newly-found chemical entities from ginseng species into the ginseng’s phytochemical data base. To provide the world a comprehensive list of all chemical entities separated from ginseng, we searched for the information as much as we can, and found that there are more than 100 chemical entities from ginseng species reported. The detailed chemical structures and related bioactivities of these 100 chemical entities will be reported in the next part of this review serial. We herein offered our classification of ginseng’s phytochemistry based on the best of our knowledge and the up-to-date information obtained.
In general, active or inactive chemical entities obtained from ginseng species can be classified into five categories ranked below in the order of their bioactivities: saponins (Fig. 1), polysaccharides, polyynes, flavonoids, and volatile oils.
-
Saponins (or sapogenin glycosides). Saponin is a type of glycoside that widely distributes in plants. Each saponin consists of a sugar and a sapogenin, the latter constitutes the aglucon moiety. The sapogenin may be a steroid or a triterpene. The sugar moiety may be a glucose, maltose, fructose, galactose, pentose, or methylpentose. Ginseng’s saponins are generally called ginsenosides (Rx), which are considered as the main active principals of ginseng and often used as a marker for the quality control of ginseng drugs and commercial products. The basic structure of ginsenosides is similar. They consist of a gonane steroid nucleus with 17 carbon atoms arranged in four rings. The characteristic biological responses for each ginsenoside are attributed to the differences in the type, position, and number of sugar moieties attached by the glycosidic bond at C-3, C-6, and C-20 [5, 13, 20]. Ginsenosides are amphipathic in nature. The hydroxyl (OH) group of ginsenosides allows both interactions between the polar head of the membrane phospholipids and the beta-OH group of cholesterol, while the hydrophobic steroid backbone can interact with the hydrophobic side chains of fatty acids and cholesterol. Indeed, these physiochemical interactions are greatly determined by the numbers and sites of polar hydroxyl groups on each ginsenoside. Up to now more than 100 ginsenosides have been isolated from Panax species and most of them exhibit four types of aglycone moieties:
protopanaxadiol (or aglycone (20S)-protopanaxadiol): dammarane-type ginsenosides including ginsenosides Ra1, Ra2, Ra3, Rb1, Rb2, Rb3, notoginsenoside R4, Rs1, Rs2, Rs3, Rs4, and malonylginsenoside Rb1, Rb2, Rc and Rd. The metabolism pathway of the protopanaxadiol has been extensively investigated resulting in identification and characterization of several active metabolites. Table 3 lists these metabolites.
protopanaxatriol (or aglycone (20S)-protopanaxatriol): also dammarane-type ginsenosides including ginsenosides Re, Rf, Rg1, and notoginsenoside R1. The main structural difference between protopanaxatriol and protopanaxadiol is that the latter holds only H element at C6.
oleanolic acid (or aglycone oleanolic acid): including ginsenosides Ro that is an oleanane triterpenoid, chikuset-susasaponin-V Rb1, Rb2, Rc, Rd, Re, and Rg1.
ocotillol-type.
Polysaccharides: these are water-soluble and include panaxane A to U. The acidic polysaccharides (MW 10,000–150,000 Dalton) have immunomodulating and antiproliferative effects. They contain various sugar moieties, uronic acid, and less than 5% protein by weight. Recent studies have identified an acidic polysaccharide, referred to as ‘Ginsan’, with noted immunostimulatory activity [26, 27].
Polyynes: the polyynes are a group of organic compounds with alternating single and triple bonds. The term polyyne simply implies the presence of several alkynes. In ginseng, these include falacrinol (panaxynol), falcarintriol (panaxytriol), acetic acid or linolenic acid.
Flavonoids.
Volatile oils: Qiu et al. [28] recently used comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry or flame ionization detector to characterize and quantify the chemical composition of volatile oil in the redixes of Panax ginseng harvested from Changbai Mountain in Jilin province, China. These ginseng samples were 3, 5 and 8 years old, respectively. They tentatively identified total 36 terpenoids in the ginseng volatile oil based on the mass library search and retention index of each oil entity. The study found that the following components of the volatile oil significantly increased with the age of the ginseng: α-cadinol, α-bisabolol, thujopsene, and n-hexadecanoic acid.
Table 3.
Structures and Bioactivity of New Compounds from Protopanaxadiols
| No. | Name | R1 | R3 | Main activity | References |
|---|---|---|---|---|---|
| 1 | Compound K (IH-901) | H | -Glc | Apoptosis, anti-proliferation | [40–44] |
| 2 | Compound Y (IH-902) | H | -Glc-Ara(p) | Antigenotoxic | [41] |
| 3 | Compound Mc (IH-903) | H | -Glc-Ara(f) | Antigenotoxic antitumor | [41, 45, 46] |
| 4 | Compound Mx | H | -Glc-Xyl | Antitumor | [46, 47] |
| 5 | PPD | H | H | Apoptosis, anti-proliferation | [48, 49] |
| 6 | A | =O | Glc | No | [43] |
A = 12β-hydroxydammar-3-one-20 (S)-O-β-D-glucopyranoside.
Four malonyl derivatives of ginsenosides Rb1, Rb2, Rc and Rd were well described by Fuzzati [29]. The malonyl derivatives and ginsenoside Ro (an oleanane-type triterpenoid) are also called “acidic” ginsenosides while the other are usually named “neutral” ginsenosides. Due to the fact that ginseng is a very popular phytomedicine used all around the world, a huge quantity of work has been carried out during the last 30 years in order to develop analytical methods for the identification, quantification and quality control of ginsenosides in raw plant materials, extracts and marketed products. One of the main goals of these researches was to differentiate the ginsenosides pattern between the different Panax species in order to avoid adulteration or misidentification. Moreover, studies of changes in ginsenosides composition due to different traditional processing of Panax ginseng roots such as white and red ginseng have been undertaken.
PHYTOCHEMICAL ANALYSIS OF GINSENG
Total content in ginsenosides and Rb1/Rg1 ratio are used for the standardization of ginseng products. In particular, ratios differ among species: Rb1/Rg1 values usually between 1 and 3 are characteristic of Panax ginseng (Asia ginseng), while Rb1/Rg1 values around 10 or greater are indicative of Panax quinquefolius (American ginseng). Also, the presence or absence of the marker compounds is used for species differentiation [29]. The absence of ginsenoside Rf is used to identify Panax quinquefolius and to exclude adulteration. Reviewing the huge amount of literature produced about the analytical methods for ginsenosides, one can notice different quantitative results depending on the employed methodology. Total ginsenosides content in Panax ginseng varied from 0.2 to 2% for main roots and from 4 to 9% for root hair. These results confirm what our Professor of pharmacognosy taught us 25 years ago that “it is smart to buy ginseng root hair at low price because per kg of root hair contains higher amount of active ginsenosides than per kg of main root does”. Panax quinquefolius roots have been showed to possess a total ginsenosides content ranging from 4 up to 10%. Although the main source of ginsenosides from ginseng is the root, both the leaf and berry parts of ginseng also contain significant quantities of ginsenosides [30].
In order to evaluate the quality of the products on the US market, the American Botanical Council started the Ginseng Evaluation Program (GEP) in 1993 [24, 31]. The GEP is an analytical evaluation that measures product content and consistency, and compares these to the label claims. The GEP developed and validated testing methodologies for consumer products, and analyzed more than 500 Asian, American, and Siberian ginseng products using HPLC to profile and assay ginsenosides and/or eleutherosides (for the full text of the methodologies used by the GEP, please refer to www.herbalgram.org). The identities of the reference ginsenosides and eleutherosides were determined by spectroscopic means (nuclear magnetic resonance, mass spectroscopy, LC/MS), and their purity was established by HPLC analysis [31]. Of the numerous ginsenosides that have been identified from ginseng, Rb1, Rb2, Rc, Rd, Re, and Rg1 have been chosen for reference standards for ginseng products [32]. However, the GEP evaluated quality of ginseng products by testing for the presence and amount of seven major ginsenosides Rb1, Rb2, Rc, Rd, Re, Rf and Rg1 [31] (Table 4). Each product was evaluated for lot-to-lot consistency as demonstrated by the following three factors: 1. percent of lots with an Rb1/Rg1 ginsenoside value within an acceptable range for Asian ginseng; 2. percent of lots that met claim for total ginsenoside content; and 3. percent relative standard deviation (%RSD) of the lot-to-lot total ginsenoside content.
Table 4.
Structures of 7 Ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1) Commonly Used for Quality Control of Ginseng Products
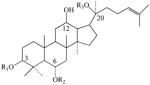 R1 R1
|
OR2 | R3 | |
|---|---|---|---|
| Rb1 | Glucose-2→ 1-glucose | 2H | Glucose-6→1-glucose |
| Rb2 | Glucose-2→1-glucose | 2H | Glucose-6→1-arabnose(pyr) |
| Rc | Glucose-2→1-glucose | 2H | Glucose-6→1-arabnose(fur) |
| Rd | Glucose-2→1-glucose | 2H | Glucose |
| Re | H | O-glucose-2→1-rhamnose | Glucose |
| Rf | H | O-glucose-2→1-glucose | H |
| Rg1 | H | O-glucose | Glucose |
The GEP found substantial variation in the use of the term “standardized”. For instance, some products employed the term “standardized” with no claim about the content in ginsenosides of the extract. Other products were standardized to contain a certain percentage of ginsenosides, usually 4–7%. The results of GEP showed that the majority of standardized products analyzed met the minimal standards of quality control. However, the GEP recommended that a more complete labeling should be introduced in order to clarify the ginsenosides dose that could be expected per unit. Furthermore, an acceptable range of ginsenosides content should be established by the industry, Pharmacopoeia, and government regulatory agencies. To alleviate the difficulty in quantitatively assessing the active ingredients of ginseng, recently researchers have employed the use of standardized extracts of both Panax ginseng (G115, marketed as Ginsana) and Panax quinquefolium (CNT-2000 from Chai-Na-Ta Corp., Langley, B.C., Canada). These changes hold the potential to enable improvements for assessing both quality control of ginseng products and efficacy of proposed activity from ginseng [30].
Recently, U.S. Pharmacopoeia published monographs for Panax quinquefolius and Panax ginseng roots and extract [33]. The total content in ginsenosides, calculated as the sum of ginsenosides Rb1, Rb2, Rc, Re, Rg1 and Rd and determined by HPLC-UV, is not less than 4% for P. quinquefolius roots and 10% for the extracts. Panax ginseng roots were defined to contain not less than 0.2% Rg1 and 0.1% Rb1 using the same method as Panax quinquefolius. A different HPLC-UV method is employed for the analyses of P. ginseng extracts which contains not less than 3.0% of ginsenosides Rb1, Rb2, Rc, Re, Rg1 and Rd. European Pharmacopoeia published a monograph on Panax ginseng roots in which the content of ginsenosides Rb1 and Rg1, determined by HPLC-UV, is not less than 0.4% [34]. Concerning the literature data, besides the natural variation due to the heterogeneity of the plant material, the main reason of this variability of ginsenosides content is attributable to the choice of the compounds to be quantified in ginseng. Some authors quantified neutral ginsenosides Rb1, Rb2, Rc, Re, Rg1 and Rd stating that they make up 90% of total saponin content [35]. However, other authors showed that the content of acidic saponins malonyl-ginsenoside Rb1, Rb2, Rc, and Rd represent between 35 and 60% of the total content of ginsenosides in both Panax ginseng and Panax quinquefolius [36]. Since malonyl-ginsenosides are likely to release ginsenosides upon consumption of ginseng products, the content of sole neutral saponins may not reflect the potency of the product. Furthermore, the relative contents of acidic and neutral ginsenosides could be used for the determination of the age and the processing of ginseng samples (Table 1).
ANALYTICAL METHODS
Among all the classical techniques usually employed for phytochemical analyses, high-performance liquid chromatography (HPLC) has been the method of choice for the analysis of ginsenosides in the last 20 years [29], and even in the today’s world [37]. HPLC-UV is the commonly used method for the quantification of ginsenosides in plant material, extracts and marketed products. The choice of this technique is mainly due to the large availability of HPLC-UV instrumentation in analytical laboratories. However, because of the weak UV absorption of ginsenosides, detection is performed at 200–205 nm producing chromatograms with high level of baseline noise and, consequently, poor sensitivity. Evaporative light scattering detector was proven to be a valuable alternative detection method for the HPLC analysis of ginsenosides which produces stable base line chromatograms and allows to extend the choice of solvent systems for enhanced chromatographic separation [38]. A limitation of both UV and Evaporative light scattering detectors is the lack of information on the identity of chromatographic peaks that is usually obtained by injection of standard compounds.
The use of LC/MS/MS allows the on-line identification of ginsenosides producing important structural information such as molecular weight, sugar unit sequence and aglycone moiety [2]. Hence, ginseng drugs derived from different Panax species were differentiated on the basis of their ginsenosides distribution by application of LC/MS/MS methodology. Furthermore, the LC/MS/MS technique demonstrated to be a highly sensitive and specific analytical method for the quantification of ginsenosides. However, even if this methodology is still too expensive to be used in routine analyses it remains an essential tools for pharmacokinetics and metabolism studies [2]. Major drawbacks of the use of HPLC for routine analyses of ginsenosides are that it is time consuming with long sample preparation and analysis times (usually more than 60 min), due to the presence of the high number of constituents to be separated.
Ultra-performance LC-quadrupole TOF MS combined with multivariate statistical analysis was recently developed to characterize principal components from five Panax species [39]. A total of 25 saponins were identified in each complex sample obtained from the powder of the five Panax species after methanol and water extraction. The method takes less than 20 min to separate and identify the 25 saponins. Near infrared spectroscopy seems to be the technique of the future for the routine analyses of ginsenosides. Near infrared spectroscopy was applied with success in the determination of ginsenosides in plant material showing a precision and accuracy comparable with HPLC. This technique is rapid, does not need extensive sample preparation and is simple to use in routine operations. However, the instrument has to be calibrated on several samples with known ginsenosides concentrations obtained with a suitable reference method. Concerning the quantitative data, a great variability in the content of ginsenosides is found in literature. This variability can be in part ascribed to natural variations such as kind of soil, weather conditions, geographical location and different production procedures. However, these sources of variability should be minimized with the introduction of good agricultural practices and good manufacturing practices. Furthermore, the divergence in the reported levels of ginsenosides among investigators can also be attributed to different sample handling, such as extraction, different testing methodologies and interpretation. In particular, many researchers do not take into account malonyl-ginsenosides which were demonstrated to represent up to 60% of the total content in ginsenosides. Since ginseng is one of the most sold oriental herbal medicines in the world, it is important that the authorities of countries adopt common analytical methodologies in order to establish precise quality standards and have a control on the marketed products. A first step has been taken by both USP [33] and European Pharmacopoeia [34], which has recently introduced monographs for Panax ginseng and Panax quinquefolius [33] plant material and extract. However, harmonization of specifications and analytical methodologies has still to be reached and would be highly appreciated.
CONCLUSIONS
Recent revolutions in life sciences urged us to re-evaluate the millennium old herb medicine – ginseng from multiple perspectives, and to critically analyze the existing data in order to separate ‘signals’ form ‘noises’. Ginseng refers to the root of several species in the plant genus Panax. Although specie differences in the genus can cause variation in pharmacological effects of ginseng, there are other factors that can also change the quality of ginseng’s products and result in deviation in its pharmacological effects. These factors include growing conditions, e.g., soil conditions, sunlight, rainfall, temperature, etc.; time of harvest, i.e., age and life cycle stage of ginseng at harvest, time of year when it is harvest; method of drying; storage conditions and duration of storage; and processing procedures. For quality control purpose, numerous ginsenosides have been chosen as reference standards to measure active ingredient content and product quality consistency in ginseng products. These ginsenosides include RB1, Rb2, Rc, Rd, Re, and Rf. Recently, Rg1, G115 (Ginsana) and CNT-2000 were also added to the list. Global governmental regulations on ginseng’s products and their import and export (in particular, the wild ginseng) are improved and enforced to confront the challenges from the rapid growth of the market. Updating pharmacopeias of countries with new information on ginseng and its products is necessary. Recent introduction of various analytical methods with high sensitivity and specificity, especially the LC/MS/MS, into ginseng field not only facilitates its analytical procedures, but also reveals new molecule entities in ginseng. These newly discovered molecules and their relevant pharmacological effects will be updated in the second part of the review serial. Only comprehensive integration of data and information into systems that organically interact and inter-depend on each other ensures a correct understanding of the old phytomedicine.
ABBREVIATIONS
- GEP
Ginseng Evaluation Program
- HPLC
high performance liquid chromatography
- LC/MS/MS
high performance liquid chromatography coupled with tandem mass spectrometry
- TCM
Traditional Chinese Medicine
References
- 1.Jia L. Global governmental investment in nanotechnologies. Curr NanoSci. 2005;1:263–266. doi: 10.2174/157341305774642957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu XD, Jia L. The conduct of drug metabolism studies considered good practice (I): analytical systems and in vivo studies. Curr Drug Metab. 2007;8:815–821. doi: 10.2174/138920007782798153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Court WE. Ginseng: The Genus Panax. Harwood Academic Publishers; Newark, New Jersey: 2000. [Google Scholar]
- 4.Yun TK. Brief introduction of Panax ginseng C.A. Meyer. J Korean Med Sci. 2001;16:53–55. doi: 10.3346/jkms.2001.16.S.S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coates P, Blackman M, Crag G. Encyclopedia of Dietary Supplements. Marcel Dekker; New York, NY: 2005. Ginseng, Asian (Panax ginseng) pp. 265–277. [Google Scholar]
- 6.Blumenthal M, Goldberg A, Brinckman J. Herbal medicine: expanded commission monographs. Lippincott Williams & Wilkins; Newton, MA: 2000. Ginseng root; pp. 170–177. [Google Scholar]
- 7.WHO. WHO Monographs on Selected Medicinal Plants. Geneva: World Health Organization; 1999. Radix Ginseng; pp. 168–182. [Google Scholar]
- 8.Fleming T. Physicians’ desk references for herbal medicine. 2. Medical Economics Co; Montvale, NJ: 2000. pp. 346–351. [Google Scholar]
- 9.Blumenthal M. Asian ginseng: potential therapeutic uses. Adv Nurse Practice. 2001;9:26–28. [PubMed] [Google Scholar]
- 10.Tyler VE. The honest herbal-A sensible guide to the use of herbs and related remedies. 3. New York: Haworth Press; 1993. [Google Scholar]
- 11.Liberti LE, Der Mardersian DA. Evaluation of commercial ginseng products. J Pharm Sci. 1978;10:1487–1489. doi: 10.1002/jps.2600671050. [DOI] [PubMed] [Google Scholar]
- 12.Philipson JD, Anderson LA. Ginseng quality safety and efficacy. Pharm J. 1984;232:161–165. [Google Scholar]
- 13.Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27–38. doi: 10.1016/0378-8741(92)90057-x. [DOI] [PubMed] [Google Scholar]
- 14.Jang DJ, Lee MS, Shin BC, Lee YC, Ernst E. Red ginseng for treating erectile dysfunction: a systematic review. Br J Clin Pharmacol. 2008;66:444–450. doi: 10.1111/j.1365-2125.2008.03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persons S. American ginseng: green gold. Bright Mountain Books Inc; Asheville, NC., USA: 1994. [Google Scholar]
- 16.Bozak G, Bailey WG. Ginseng production in north America. International Conference of Ginseng and Allied Plants; Harbin, China: 1995. [Google Scholar]
- 17.Zhao Y, Wang W, Han L, Rayburn ER, Hill DL, Wang H, Zhang R. Isolation, structural determination, and evaluation of the biological activity of 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol [20(S)-25-OCH3-PPD], a novel natural product from Panax notoginseng. Med Chem. 2007;3:51–60. doi: 10.2174/157340607779317508. [DOI] [PubMed] [Google Scholar]
- 18.Jiang B, Wang C, Han Y, Hu X, Zheng L, Zhao Y. Isolation and identification of minor bioactive saponins from the leaves of Panax notoginseng. Zhong Yao Cai. 2004;27:489–91. [PubMed] [Google Scholar]
- 19.Davydov M, Krikorian AD. Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. (Araliaceae) as an adaptogen: a closer look. J Ethnopharmacol. 2000;72:345–393. doi: 10.1016/s0378-8741(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 20.Hankins A. The Business of Herbs. Vol. 25 Jemez Springs; New Mexico: 1997. The Chinese Ginseng Industry. [Google Scholar]
- 21.Hankins A. Producing and marketing wild simulated ginseng in forest and agroforestry systems. 2000 http://www.ext.vt.edu/pubs/forestry/354-312/354-312.pdf.
- 22.Blumenthal M. Herb sales down 15% in mainstream market. Herbalgram. 2001;51:69. [Google Scholar]
- 23.Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 24.Hall T, Lu ZZ, Yat PN, Fitzloff JF, Arnason JT, Awang DVC, Fong HHS, Blumenthal M. Evaluation of consistency of standardized Asian ginseng products in the ginseng evaluation program. HerbalGram. 2001;52:31–45. [Google Scholar]
- 25.Pan European Federation of TCM Societies. The development of Chinese Pharmacopoeia. 2007 http://www.pefots.com.
- 26.Kim KH, Lee YS, Jung IS, Park SY, Chung HY, Lee IR, Yun YS. Acidic polysaccharide from Panax ginseng, ginsan, induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Planta Medica. 1998;64:110–5. doi: 10.1055/s-2006-957385. [DOI] [PubMed] [Google Scholar]
- 27.Shim JY, Han Y, Ahn JY, Yun YS, Song JY. Chemoprotective and adjuvant effects of immunomodulator ginsan in cyclophosphamide-treated normal and tumor bearing mice. International Journal of Immunopathology Pharmacology. 2007;20:487–497. doi: 10.1177/039463200702000307. [DOI] [PubMed] [Google Scholar]
- 28.Qiu Y, Lu X, Pang T, Ma CF, Li X, Xu GW. Determination of radix ginseng volatile oils at different ages by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. J Sep Sci. 2008;31:3451–3457. doi: 10.1002/jssc.200800253. [DOI] [PubMed] [Google Scholar]
- 29.Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B. 2004;812:119–133. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 30.Kitts DD, Hu C. Efficacy and safety of ginseng. Pub Health Nutr. 2000;3(4A):473–485. doi: 10.1017/s1368980000000550. [DOI] [PubMed] [Google Scholar]
- 31.Hall T, Lu ZZ, Yat PN, Fitzloff JF, Arnason JT, Awang DVC, Fong HHS, Blumenthal M. An introduction to the ginseng evaluation program. HerbalGram. 2001;52:27–30. [Google Scholar]
- 32.Ma YC, Zhu J, Luo L. A comparative evaluation of ginsenosides in commercial ginseng products and tissue culture samples using HPLC. J Herb Spices Med Plants. 1995;3:41–50. [Google Scholar]
- 33.USP 27 NF 22, United States Pharmacopeial Convention Inc., Rockville, MD, 2004, p. 2005
- 34.European Pharmacopoeia. 4. Council of Europe; Strasbourg: 2002. p. 1244. [Google Scholar]
- 35.Li W, Fitzloff JF. HPLC determination of ginsenosides content in ginseng dietary supplements using ultraviolet detection. J Liq Chromatogr Related Tech. 2002;25:2485–2495. [Google Scholar]
- 36.Court WA, Hendel JG, Elmi J. Reversed-phase high-performance liquid chromatography determination of ginsenosides of Panax quinquefolium. J Chromatogr A. 1996;755:11–17. [Google Scholar]
- 37.Zhou W, Li J, Li X, Yan Q, Zhou P. Development and validation of a reversed-phase HPLC method for quantitative determination of ginsenosides Rb1, Rd, F2, and compound K during the process of biotransformation of ginsenoside Rb1. J Sep Sci. 2008;31:921–925. doi: 10.1002/jssc.200700406. [DOI] [PubMed] [Google Scholar]
- 38.Kwon SW, Han SB, Park H, Kim JM, Park MK, Park JH. Liquid chromatographic determination of less polar ginsenosides in processed ginseng. J Chromatogr A. 2001;921:335–339. doi: 10.1016/s0021-9673(01)00869-x. [DOI] [PubMed] [Google Scholar]
- 39.Xie G, Plumb R, Su M, Xu Z, Zhao AH, Qiu MF, Long X, Liu Z, Jia W. Ultra-performance LC/TOF MS analysis of medicinal Panax herbs for metabolomic research. J Sep Sci. 2008;31:1015–1026. doi: 10.1002/jssc.200700650. [DOI] [PubMed] [Google Scholar]
- 40.Akao T, Kanaoka M, Kobashi K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration: measurement of compound K by enzyme immunoassay. Biol Pharm Bull. 1998;21:245–249. doi: 10.1248/bpb.21.245. [DOI] [PubMed] [Google Scholar]
- 41.Lee BH, Lee SJ, Hui JH. In vitro antigenotoxic activity of novel ginseng saponin metabolites formed by intestinal bacteria. Planta Medica. 1998;64:500–503. doi: 10.1055/s-2006-957501. [DOI] [PubMed] [Google Scholar]
- 42.Shin JE, Park EK. Cytotoxicity of compound K (IH-901) and ginsenoside Rh2, main biotransformants of ginseng saponins by bifidobacteria, against some tumor cells. J Ginseng Res. 2003;27:129–134. [Google Scholar]
- 43.Chen GT, Yang M, Song Y, Lu ZQ, Zhang JQ, Huang HL, Wu LJ, Guo DA. Microbial transformation of ginsenoside Rb1 by Acremonium strictum. Appl Microbiol Biotech. 2008;77:1345–1350. doi: 10.1007/s00253-007-1258-4. [DOI] [PubMed] [Google Scholar]
- 44.Lee JY, Shin JW, Chun KS. Antitumor promotional effects of a novel intestinal bacterial metabolite (IH-901) derived from the protopanaxadiol type ginsenosides in mouse skin. Carcinogenesis. 2005;26:359–367. doi: 10.1093/carcin/bgh313. [DOI] [PubMed] [Google Scholar]
- 45.Bea EA, Park SY, Kim DH. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. 2000;23:1481–1485. doi: 10.1248/bpb.23.1481. [DOI] [PubMed] [Google Scholar]
- 46.Han Y, Sun B, Hu X, Zhang H, Jiang B, Spranger MI, Zhao Y. Transformation of bioactive compounds by Fusarium sacchari fungus isolated from the soil-cultivated ginseng. J Agri Food Chem. 2007;55:9373–9. doi: 10.1021/jf070354a. [DOI] [PubMed] [Google Scholar]
- 47.He K, Liu Y, Yang Y, Li P, Yang L. A dammarane glycoside derived from ginsenoside Rb3. Chem Pharm Bull. 2005;53:177–9. doi: 10.1248/cpb.53.177. [DOI] [PubMed] [Google Scholar]
- 48.Zhang XR, Zhang D, Xu JH, Gu JK, Zhao YQ. Determination of 25-OH-PPD in rat plasma by high-performance liquid chromatography-mass spectrometry and its application in rat pharmacokinetic studies. J Chromatogr B. 2007;858:65–70. doi: 10.1016/j.jchromb.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 49.Popovich DG, Kitts DD. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch Biochem Biophys. 2000;406:1–8. doi: 10.1016/s0003-9861(02)00398-3. [DOI] [PubMed] [Google Scholar]