Abstract
The newly described Streptococcus pneumoniae serotype 6C accounted for 2.3% (16/709) of meningitis cases and 3.2% (3/95) of nasopharyngeal isolates from healthy individuals in Brazil. The strains were multidrug resistant (18.8%) and genetically diverse. Despite low serotype 6C prevalence, continuous surveillance is necessary to guide vaccine strategies.
Keywords: Streptococcus pneumoniae, serotype 6C, epidemiology, meningitis, carriage
Streptococcus pneumoniae is a significant cause of morbidity and mortality especially among children < 2 years age and the elderly (WHO, 2007). The antiphagocytic polysaccharide capsule is the major virulence determinant of S. pneumoniae (Kadioglu et al., 2008). Of the 91 known capsular serotypes (Park et al., 2007b), approximately 20 are associated with > 80% of invasive pneumococcal disease (IPD) (Hausdorff et al., 2000).
The serotypes 6C and 6A biosynthetic loci are identical except for the presence of different wciN genes that encode distinct glycosyl transferases (Park et al., 2007a). The two serotypes are not resolved by classical quelling serotyping (Park et al., 2007a). Classically serotyped 6A pneumococci (CS6As) are associated with nasopharyngeal (NP) carriage and IPD in all ages (du Plessis et al., 2008; Granat et al., 2007; Reis et al., 2008).
The currently available 7-valent pneumococcal conjugate vaccine (PCV7) has been highly effective against the seven serotypes that were predominant in children prior to its implementation in the United States (CDC, 2008; WHO, 2007). PCV7 contains serotype 6B and cross-protects against 6A, however recent surveillance data indicates that PCV7 is ineffective against serotype 6C (Carvalho et al., 2009; Park et al., 2008).
We show here the prevalence of serotype 6C within a well-defined collection of CS6As collected in Brazil from meningitis cases and NP carriage.
Antimicrobial susceptibility testing employed broth microdilution. Minimum inhibitory concentrations (MICs) of 10 key antibiotics were determined using year 2007 Clinical and Laboratory Standards Institute guidelines (CLSI, 2007). Intermediate penicillin resistance (MICs of 0.12-1.0 μg/ml) or full penicillin resistance (MICs ≥ 2.0 μg/ml) was considered penicillin non-susceptible.
CS6As were subtyped using a triplex PCR reaction that detects cpsA (conserved capsular biosynthetic locus; 160 bp), serogroup 6 (250 bp) and the 6C-specific gene wciN6C [727 bp] (da Gloria Carvalho et al., 2009). DNA extraction and PCR were performed as described (http://www.cdc.gov/ncidod/biotech/strep/pcr.htm). Pulsed field gel electrophoresis (PFGE) of chromosomal SmaI (Sigma, S. Louis, MO) digests was performed as described (McEllistrem et al., 2000; Tenover et al., 1995). Multilocus sequence typing (MLST) was performed (Enright and Spratt, 1998) on eight isolates representing the two PFGE clusters and three PFGE outliers.
CS6As consisting of 47 (6.6%) CSF isolates from a collection of 709 pneumococci identified in metropolitan Salvador, Brazil during 12 years (1996-2007) of bacterial meningitis surveillance, and 9 NP isolates (Reis et al., 2008), were tested by triplex PCR to resolve serotypes 6C and serotype 6A. Of 56 CS6As tested, 16/47 (34%) from meningitis patients were PCR-positive for wciN6C, indicating that 4.4% (31/709) and 2.3% (16/709) of meningitis cases were caused by serotypes 6A and 6C, respectively. In addition, 6A and 6C represented 6.4% and 3.2% (3/95) of carriage isolates, respectively. Low prevalences of serotype 6C disease and colonization has also been observed in South Africa (du Plessis et al., 2008) and Portugal (Nunes et al., 2009).
There were no significant differences between serotypes 6A and 6C with respect to ages and genders of meningitis patients. Serotype 6C meningitis cases were associated with a significantly lower case fatality rate than serotype 6A cases (0% [0 of 15 cases] vs. 28%[8 of 29 cases], respectively, P = 0.02) (Table 1). These findings are in contrast to observations in South Africa (du Plessis et al., 2008) where significantly different case fatality rates between serotypes 6A and 6C were not observed.
Table 1.
Characteristics of meningitis cases caused by S. pneumoniae serotypes 6A and 6C in Salvador, Brazil, 1996-2007
| Characteristic | No. of isolates (%) |
P-value | |
|---|---|---|---|
| 6A (n = 31) | 6C (n = 16)1 | ||
| Age | |||
| < 5 years | 15/31 (48.4) | 4/15 (26.7) | |
| ≥ 5 years | 16/31 (51.6) | 11/15 (73.3) | |
| Male gender | 17/31 (54.8) | 11/15 (73.3) | |
| Case fatality rate (no. of deaths/no. of cases) | 8/292 (27.6) | 0/15 (0.0) | 0.02 |
| Penicillin non-susceptible | 3/31 (9.7) | 1/16 (6.3) | |
| Sxt3 non-susceptible | 17/31 (54.8) | 12/16 (75.0) | |
For 1 patient the epidemiological and clinical data were not available (i.e., age, sex, outcome).
Two patients were transferred to another hospital.
Sxt = trimethoprim /sulfamethoxazole.
Table 2 lists the antibiotic susceptibility of all serotype 6C isolates identified. Among meningitis case CS6As, 71% (22 of 31) of 6A and 81.3% (13 of 16) of 6C were non-susceptible to at least one antibiotic. Similar proportions of serotype 6A and 6C meningitis isolates were non-susceptible to penicillin or trimethoprim/sulfamethoxazole (Sxt). Among 6C isolates, 6.3% (1 of 16) and 75% (12 of 16) were non-susceptible to penicillin and Sxt, respectively, while 10% of 6A isolates were penicillin-nonsusceptible and 55% were Sxt-nonsusceptible. The high frequency of Sxt resistance among meningitis CS6As reflects widespread use of this drug in Brazil (Reis et al., 2008). Multidrug resistance was found among 19.4% (6 of 31) and 18.8% (3 of 16) of serotype 6A and 6C meningitis isolates, respectively. Of CS6As obtained during a NP carriage study of a healthy population in Salvador (Reis et al., 2008), 2/3 (67%) 6C isolates and 1/6 6A (16.7%) isolates were multi-drug resistant.
Table 2.
Antimicrobial Susceptibilities of Streptococcus pneumoniae serotype 6C isolates in metropolitan Salvador, Brazil from 1996-20071
| Antimicrobial agents | MIC (μg/ml)2 |
No. (%) of isolates3 |
||||
|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | S | I | R | |
| Cefotaxime | 0.016 - 64 | 0.016 | 0.031 | 19 (100) | — | — |
| Chloramphenicol | 0.016 - 64 | 2.0 | 4.0 | 17 (89.5) | NA | 2 (10.5) |
| Clindamycin | 0.016 - 64 | 0.031 | 0.062 | 19 (100) | — | — |
| Erythromycin | 0.016 - 64 | 0.062 | 0.25 | 18 (94.7) | — | 1 (5.3) |
| Ofloxacin | 0.016 - 64 | 1.0 | 2.0 | 19 (100) | — | — |
| Penicillin | 0.016 - 64 | 0.031 | 0.062 | 18 (94.7) | 1 (5.3) | — |
| Rifampicin | 0.016 - 64 | 0.031 | 0.062 | 19 (100) | — | — |
| Tetracycline | 0.016 - 64 | 0.5 | 8.0 | 15 (78.9) | — | 4 (21.1) |
| Trimethoprim / Sulfamethoxaxole | 0.0625/1.1875 – 32/608 | 1.0 | 2.0 | 6 (31.6) | 11 (57.9) | 2 (10.5) |
| Vancomycin | 0.016 - 64 | 0.5 | 0.5 | 19 (100) | NA | — |
A total of 19 isolates were tested (16 isolates from meningitis patients and 3 isolates from NP carriages).
MICs were determined by the broth microdilution method (CLSI, 2007). MIC50 and MIC90 concentrations at which the growth of 50 and 90%, respectively, of the isolates is inhibited.
S, susceptible; I, intermediate; R, resistant; NA, not applicable; —, no isolates were identified. The breakpoints used to define susceptibility categories were those recommended by the Clinical Laboratory Standards Institute (CLSI, 2007).
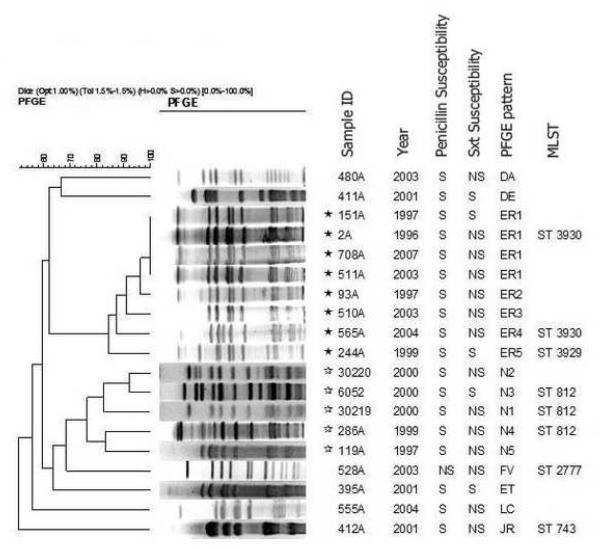
PFGE of serotype 6C chromosomal digests revealed two clusters and 6 outlier patterns (Figure 1). Cluster I (black stars) represented eight meningitis isolates whereas cluster II (white stars) represented two meningitis isolates and three NP carriage isolates. PFGE Cluster I isolates were associated with two newly-identified STs, ST3929 and ST3930, which differed by only two loci. ST3930 differed by only 1 or 2 loci from 5 CS6As (NP and lower respiratory tract isolates) recovered in Finland, the United States, and Poland (www.mlst.net). PFGE Cluster II isolates were associated with ST812, which is a single locus variant (SLV) of ST753 from a CS6A meningitis isolate recovered in Brazil and a double locus variant of ST2789 from a type 6C carriage isolate recovered in Portugal (Nunes et al, 2009). The PFGE outlier correlated with ST2777, which was also from a type 6C CSF isolate recovered in Brazil (www.mlst.net). It is interesting that ST2777 is a SLV of ST338 from the clone Colombia23F-26 that is associated with antibiotic non-susceptible 23F and 23A isolates (Pai et al., 2005). The final PFGE outlier (ST743) was previously associated with serotype 34 meningitis and NP isolates (www.mlst.net). Overall, these data are consistent with other studies indicating both a high degree of genetic diversity within serotype 6C and its long-term existence within the species (Jacobs et al., 2009; Nunes et al., 2009; Park et al., 2007a). In our study, the first 6C isolate identified was isolated in March, 1996 (strain no. 2A). Vaccine pressure could potentially select for the emergence of pre-existing 6C clones and 6C variants that arise through serotype switching.
Fig. 1.
PFGE analysis showing serotype 6C isolates recovered from meningitis cases and carriage: Cluster I (black stars) comprised of eight meningitis isolates (nos. 151A, 2A, 708A, 511A, 93A, 510A, 565A and 244A), belonging to ST 3930; Cluster II (white stars), comprised of two meningitis isolates (nos. 286A and 199A) and three 6C NP carriage isolates (nos. 30220, 6052, 30219), belonging to ST 812. The other six isolates are genetic outliers as judged by PFGE-relationships (Sxt, trimethoprim / sulfametoxazole; S, susceptible; NS, non-susceptible).
In Brazil, PCV7 will probably be implemented within the next several years in young children (Brasil, 2008). Although PCV7 does not protect against 6C disease (da Gloria Carvalho et al., 2009; Park et al., 2008), we found that the prevalence of serotype 6C among meningitis isolates is low (2.3%). Nonetheless, serotype replacement in disease incidence is a concern, where non-PCV7 serotypes such as type 6C could possibly emerge as important pathogens due to removal of vaccine serotype strain competitors from the NP reservoir (Hicks et al., 2007; Moore et al., 2008). A slight increase of 6C IPD has been documented in the post-PCV7 era among adults in the United States, where 6C has become the prevalent serogroup 6 serotype (da Gloria Carvalho et al., 2009; Moore et al., 2008; Park et al., 2008). We emphasize that these data showing a predominance of 6C from the United States primarily indicate high efficacy of PCV7 against serotypes 6A and 6B, rather than 6C emergence. Continuous pneumococcal serotype surveillance is necessary to evaluate the impact and suitability of current conjugate vaccines in developing countries such as Brazil.
Acknowledgements
We thank Dr Marise D. Asensi (Instituto Oswaldo Cruz, FIOCRUZ, Rio de Janeiro, Brazil for her help in the PFGE performance. We are grateful for the global pneumococcal MLST database (Imperial College London, funded by the Wellcome Trust). This study was supported by grants from the Brazilian National Research Council (CNPq - 491345/2005-4 and 478685/2007-6), Research Support Foundation for the State of Bahia (FAPESB - 1431040054051), and National Institute of Health, USA (D43 TW00919 and R01 TW007303).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Centers for Disease Control and Prevention (CDC) Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction--eight states, 1998-2005. MMWR Morb Mortal Wkly Rep. 2008;57:144–148. [PubMed] [Google Scholar]
- Clinical Laboratory Standards Institute (CLSI) Seventeenth Informational Supplement. Approved Standard M100-S17. Wayne, PA: 2007. Performance Standards for Antimicrobial Susceptibility Testing. [Google Scholar]
- da Carvalho MG, Pimenta FC, Gertz RE, Jr., Joshi HH, Trujillo AA, Keys LE, Findley J, Moura IS, Park IH, Hollingshead SK, Pilishvili T, Whitney CG, Nahm MH, Beall BW. PCR-Based Quantitation and Clonal Diversity of the Current Prevalent Invasive Serogroup 6 Pneumococcal Serotype, 6C, in the United States in 1999 and 2006 to 2007. J Clin Microbiol. 2009;47:554–559. doi: 10.1128/JCM.01919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Plessis M, von Gottberg A, Madhi SA, Hattingh O, de Gouveia L, Klugman KP. Serotype 6C is associated with penicillin-susceptible meningeal infections in human immunodeficiency virus (HIV)-infected adults among invasive pneumococcal isolates previously identified as serotype 6A in South Africa. Int J Antimicrob Agents. 2008;32(Suppl 1):S66–70. doi: 10.1016/j.ijantimicag.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144(Pt 11):3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- Brasil. Ministério da Saúde. Secretaria Executiva . Mais saúde: direito de todos: 2008-2011. Editora do Ministério da Saúde; Brasília: 2008. [Google Scholar]
- Granat SM, Mia Z, Ollgren J, Herva E, Das M, Piirainen L, Auranen K, Makela PH. Longitudinal study on pneumococcal carriage during the first year of life in Bangladesh. Pediatr Infect Dis J. 2007;26:319–324. doi: 10.1097/01.inf.0000257425.24492.11. [DOI] [PubMed] [Google Scholar]
- Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis. 2000;30:100–121. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, Jackson D, Thomas A, Beall B, Lynfield R, Reingold A, Farley MM, Whitney CG. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196:1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- Jacobs MR, Bajaksouzian S, Bonomo RA, Good CE, Windau AR, Hujer AM, Massire C, Melton R, Blyn LB, Ecker DJ, Sampath R. Occurrence, distribution, and origins of Streptococcus pneumoniae Serotype 6C, a recently recognized serotype. J Clin Microbiol. 2009;47:64–72. doi: 10.1128/JCM.01524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6:288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- McEllistrem MC, Stout JE, Harrison LH. Simplified protocol for pulsed-field gel electrophoresis analysis of Streptococcus pneumoniae. J Clin Microbiol. 2000;38:351–353. doi: 10.1128/jcm.38.1.351-353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MR, Gertz RE, Jr., Woodbury RL, Barkocy-Gallagher GA, Schaffner W, Lexau C, Gershman K, Reingold A, Farley M, Harrison LH, Hadler JL, Bennett NM, Thomas AR, McGee L, Pilishvili T, Brueggemann AB, Whitney CG, Jorgensen JH, Beall B. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197:1016–1027. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- Nunes S, Valente C, Sa-Leao R, de Lencastre H. Temporal trends and molecular epidemiology of recently described serotype 6C of Streptococcus pneumoniae. J Clin Microbiol. 2009;47:472–474. doi: 10.1128/JCM.01984-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai R, Gertz RE, Whitney CG, Beall B. Clonal association between Streptococcus pneumoniae serotype 23A, circulating within the United States, and an internationally dispersed clone of serotype 23F. J Clin Microbiol. 2005;43:5440–5444. doi: 10.1128/JCM.43.11.5440-5444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Moore MR, Treanor JJ, Pelton SI, Pilishvili T, Beall B, Shelly MA, Mahon BE, Nahm MH. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J Infect Dis. 2008;198:1818–1822. doi: 10.1086/593339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Park S, Hollingshead SK, Nahm MH. Genetic basis for the new pneumococcal serotype, 6C. Infect Immun. 2007a;75:4482–4489. doi: 10.1128/IAI.00510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007b;45:1225–1233. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis JN, Palma T, Ribeiro GS, Pinheiro RM, Ribeiro CT, Cordeiro SM, da Silva Filho HP, Moschioni M, Thompson TA, Spratt B, Riley LW, Barocchi MA, Reis MG, Ko AI. Transmission of Streptococcus pneumoniae in an urban slum community. J Infect. 2008;57:204–213. doi: 10.1016/j.jinf.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Pneumococcal conjugate vaccine for childhood immunization--WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104. [PubMed] [Google Scholar]