Abstract
Trifluoperazine (TFP), a phenothiazine, is a commonly used antipsychotic drug whose therapeutic effects are attributed to its central anti-adrenergic and antidopaminergic actions. However, TFP is also a calmodulin (CaM) antagonist and alters the Ca binding properties of calsequestrin (CSQ). The CaM and CSQ proteins are known modulators of sarcoplasmic reticulum (SR) Ca release in ventricular myocytes. We explored TFP actions on cardiac SR Ca release in cells and single type-2 ryanodine receptor (RyR2) channel activity in bilayers. In intact and permeabilized ventricular myocytes, TFP produced an initial activation of RyR2-mediated SR Ca release and over time depleted SR Ca content. At the single channel level, TFP or nortryptiline (NRT; a tricyclic antidepressant also known to modify CSQ Ca binding) increased the open probability (Po) of CSQ-free channels with an EC50 of 5.2 μM or 8.9 μM (respectively). This Po increase was due to elevated open event frequency at low drug concentrations while longer mean open events sustained Po at higher drug concentrations. Activation of RyR2 by TFP occurred in the presence or absence of CaM. TFP may also inhibit SR Ca uptake as well as increase RyR2 opening. Our results suggest TFP and NRT can alter RyR2 function by interacting with the channel protein directly, independent of its actions on CSQ or CaM. This direct action may contribute to the clinical adverse cardiac side effects associated with these drugs.
Introduction
Trifluoperazine (TFP) is clinically used to treat psychotic disorders, agitation, and dementia. It is associated with a broad range of adverse side effects on cardiac performance including QT prolongation, tachycardia, and arrhythmia [4, 17, 19]. Additionally, TFP is a well-known calmodulin (CaM) antagonist [4] that when bound to CaM blocks its ability to interact with its target enzymes [5, 36]. TFP is also known to bind to calsequestrin (CSQ) altering its conformation and consequently its Ca-binding capacity [24].
Type-2 ryanodine receptor (RyR2) mediated Ca release from the sarcoplasmic reticulum (SR) is key to cardiac muscle function. CaM and CSQ both are known to modulate the RyR2-mediated Ca release. The action of CaM on RyR2 may be indirect via CaM-dependent protein kinase (CaMKII) or direct by CaM binding to the RyR2 protein itself [3, 12, 21, 38]. The action of CSQ on cardiac cellular Ca handling is also multifaceted. CSQ is a lowaffinity high-capacity intra-SR Ca buffer and is one element of an intra-SR RyR2 regulatory complex [1, 3, 8, 11, 13, 15, 26, 29, 33]. Alteration of the CSQ-RyR2 interaction can lead to disorders of cardiac Ca regulation and arrhythmia [15, 20, 26]. Indeed, TFP disruption (as well as other compounds like nortryptiline) of the CSQRyR2 interaction could explain some of this drug's cardiotoxic side effects [24].
Here, we show that TFP activates single RyR2 channels in a dose-dependent, but CaM- and CSQ-independent manner. This correlates well with the action of TFP on spontaneous RyR2-mediated Ca sparks in ventricular myocytes. We therefore propose that TFP likely affects SR Ca handling in ventricular myocytes through multiple mechanisms including a direct stimulation of the RyR2 channel. This direct action could play a substantial role in the clinical adverse cardiac side effects associated with this drug.
Materials and methods
Intracellular Ca measurements
Cardiac ventricular myocytes were enzymatically isolated from adult cats and rabbits using methods described previously [27, 30] and approved by the Institutional Animal Care and Use Committee. Intracellular Ca concentration ([Ca]i) was measured in intact and permeabilized ventricular myocytes with a fluorescence laser scanning confocal microscope (Radiance 2000 MP, Bio-Rad, UK) equipped with a ×40 oil-immersion objective (N.A.=1.3). The Ca indicator Fluo-4 was excited by 488 nm light from an argon ion laser and emitted fluorescence was measured at >515 nm. Spontaneous SR Ca release events (sparks) were studied in saponin-permeabilized ventricular myocytes as described previously [39]. After permeabilization, cells were placed in a solution composed of (mM): K aspartate 100; KCl 15; KH2PO4 5; MgATP 5; EGTA 0.35; CaCl2 0.12; MgCl2 0.75; phosphocreatine 10; HEPES 10; Fluo-4 pentapotassium salt 0.03; creatine phosphokinase 5 U/ml; dextran (MW, 40,000) 8%, and pH 7.2. Free Ca concentration of this solution was adjusted to 150 nM (calculated using WinMAXC 2.05, Stanford University, CA). All experiments were performed at room temperature. Images were acquired in linescan mode (3 ms per line; pixel size 0.12 μm). Ca sparks were detected and analyzed using the SparkMaster program [25] with the threshold criteria set at 3.8. Analysis included spark frequency (sparks×s−1×(100 μm)−1), amplitude (ΔF/F0), full duration at half-maximal amplitude (FDHM; ms) and full width at half-maximal amplitude (FWHM; μm). The F0 was considered to be the initial fluorescence recorded under steady-state conditions and ΔF=F−F0.
Intact ventricular myocytes were loaded with the Ca indicator Fluo-4 by 20 min incubation in Tyrode solution containing 20 μM Fluo-4/AM (Fluo-4 acetoxymethyl ester) at room temperature. Tyrode solution was composed in mM: NaCl 140; KCl 4; CaCl2 2, MgCl2 1; glucose 10; HEPES 10; pH 7.4 adjusted with NaOH. 15 min were allowed for de-esterification of the dye. Whole-cell Ca transients were obtained by averaging the entire cellular fluorescence signal from the line scanned and presented as background-subtracted normalized fluorescence (F/F0). Ca transients were evoked by electrical field stimulation with suprathreshold voltage pulses applied through a pair of extracellular platinum electrodes at a frequency of 0.5 Hz.
For both intact and permeabilized cells, SR Ca load was determined from the peak amplitude of the cytosolic free Ca transient induced by the rapid application of 20 mM caffeine. This concentration of caffeine fully activates RyR2s [24] and leads to the synchronized release of total Ca stored in the SR [3].
Single RyR2 channel recording
Heavy SR microsomes were prepared from rat ventricle using methods described previously [6]. Planar lipid bilayers were formed from a 5:4:1 mixture (50 mg/ml in decane) of bovine brain phosphatidylethanolamine, phosphatidylserine, and phosphatidylcholine. Bilayers were formed across a 100-μm diameter hole in a 20-μm thick Teflon partition that separated two 1 ml compartments. One of these compartments (cis) was virtually grounded and filled with a HEPES-Tris solution (250 mM HEPES, 120 mM Tris, pH 7.4). The other chamber was filled with HEPES-Ca solution (250 mM HEPES, 53 mM Ca(OH)2, pH 7.4). Then, 500 mM CsCl and 5–15 μg heavy SR microsomes were added to the cis chamber. Immediately upon observation of ion channel activity, the solutions in both compartments were exchanged at a rate of 4 ml/min (for 5 min) to establish the desired test conditions. Unless otherwise specified, the holding potential was held constant at 0 mV and recordings were made at room temperature (20–22°C). Single RyR2 channel recordings sampled at 100 μs/pt and filtered at 1 kHz (8-pole Bessel). Single channel analysis was done using pCLAMP9 software (Axon Instruments/Molecular Devices). Open times, closed times, event frequency and open probability were defined using the half-amplitude threshold method. Channel incorporation always resulted in the cytosolic side of the RyR2 channel facing the cis solution/chamber [10, 35].
After incorporation of a single RyR2 channel into the bilayer, the luminal side of the channel was subjected (unless specified differently) to a 15 min 10–53 mM Ca solution prewash to promote spontaneous dissociation of CSQ (if present) from the channel as previously described [26]. Note that the cytosolic side of the RyR2 channel was not subjected to this high salt wash and thus cytosolic protein–protein interactions were not affected by this prewash.
A mixture of BAPTA and dibromo-BAPTA was used to buffer free Ca concentration in the cis compartment to 1 μM. The required buffer solution was devised using WinMAXC 2.05 (see above) and subsequently verified by Ca electrode.
Chemicals and drugs
Fluo-4 was purchased from Molecular Probes/Invitrogen (Carlsbad, CA). BAPTA (1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid), 5,5′-Dibromo-BAPTA (1,2-Bis (2-amino-5-bromophenoxy)ethane-N,N,N′,N′-tetraacetic acid) were obtained from Fluka (Milwaukee, WI). CaCl2 standard for calibration was purchased from World Precision Instruments Inc (Sarasota, FL). Phospholipids were obtained from Avanti Polar Lipids (Alabaster, Alabama). High-purity ryanodine was obtained from Calbiochem (San Diego, CA). Caffeine, amitriptyline, nortriptyline and TFP were purchased from Sigma-Aldrich (St Louis, MO). Park et al. [24] demonstrated convincingly that TFP is membrane permeable. This is consistent with the reported partition coefficients among the phenothiazine derivatives. For our studies, the TFP was dissolved in water. The wild-type cardiac CSQ protein was kindly provided to our lab by Drs. Pompeo Volpe and Alessandra Nori (University of Padova, Italy). All other chemicals were either from Fluka or Sigma, and were reagent grade.
Statistics
Some results are presented as mean±SEM of n measurements (or channels). Statistical comparisons (unpaired) between means were performed using a Student's t test with significance defined at the p<0.05 level.
Results
TFP action on single RyR2 channels
Single RyR2 channel function was measured in planar lipid bilayer studies. Sample single channel recordings in four different experimental conditions are shown in Fig. 1. The all-points histograms (right) represents 5 min of recording in each condition. With no TFP present (control), the open probability was 0.15. Addition of TFP (5, 20, 75 μM) to the cytosolic side of the channel substantially increased Po but did not change the unit Ca current carried by the channel. When 75 μM TFP was present, occasional sojourns to a subconductance state (which was ∼34% of the full open level) were observed. The presence of this subconductance state at high TFP concentrations did not substantially influence the overall Po determinations.
Figure 1.
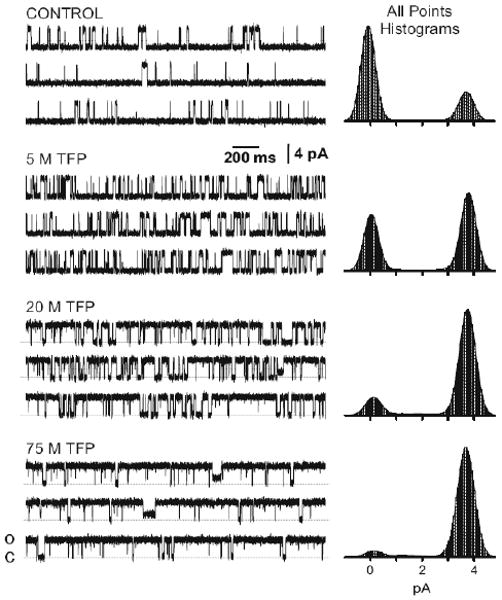
TFP Action on Single RyR2 Channels. Sample single channel recordings before (control) and after application of three different TFP concentrations. Open events are shown as upward deflections. The holding potential was 0 mV and the cytosolic free Ca is 1 μM. All recordings shown were made on the same RyR2 channel. All-points histograms generated from 5 min of recording in each condition.
The Po vs. TFP dose relationship determined from ten different RyR2 channels is presented in Fig. 2a. A curve fit to the means suggests a TFP EC50 of 5.2 μM with a Hill coefficient of 1.8. Fig. 2b shows how open ovent frequency (filled triangles) and mean open event duration (open triangles) changed as a function of TFP concentration. Open event frequency increased between 1 and 10 μM TFP and then decreased at higher TFP levels. Mean open event duration became longer with increasing [TFP]. Thus, the TFP-dependent Po change is due primarily to increased event frequency at low TFP doses (<10 μM) and by sustained long open events at higher TFP doses. As described earlier, the RyR2 channel is regulated by both CaM and CSQ [9–12, 28] and TFP alters these proteins [24, 37]. The possibility that TFP action on RyR2 function was due to TFP disruption of CaM-RyR2 or CSQ RyR2 regulation is addressed in Fig. 3. First, CaM and CSQ modulate RyR2 from opposite sides of the channel (cytosol and luminal, respectively). Figure 3a shows sample recordings in control conditions and after 100 μM TFP was added either to the cytosolic or luminal side of the channel. The increase of Po evoked by TFP addition was identical irrespective of whether the drug was added to the cytosolic or the luminal side of the chamber. Note that these studies do not differentiate whether the drug was acting on one or both sides of the channel. The lack of sidedness simply indicates that TFP acts from both sides of the membrane or that the membrane is not a substantial barrier for TFP to reach its site of action.
Figure 2.
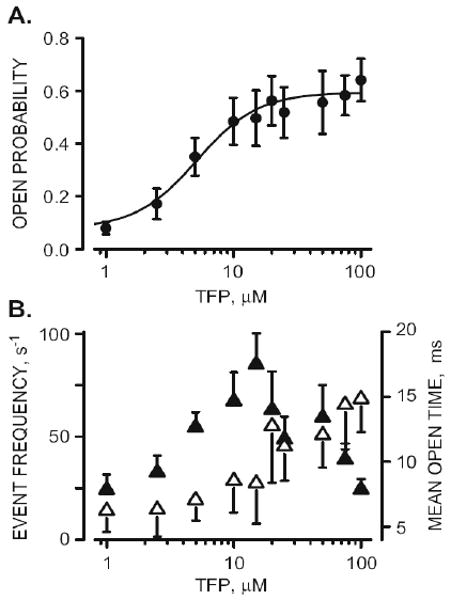
Single RyR2 TFP activation. A. TFP dose–response (Po vs. TFP dose) curve representing results collected from ten different RyR2 channels. Points represent means (±SEM). The curve (fit to means) is a Hill relationship with an EC50 of 5.2±1.9 and 1.8 Hill coefficient. B. Open event frequency filled triangles) and mean open time (open triangles) as a function of TFP concentration. Data represent results collected from ten different RyR2 channels
Figure 3.
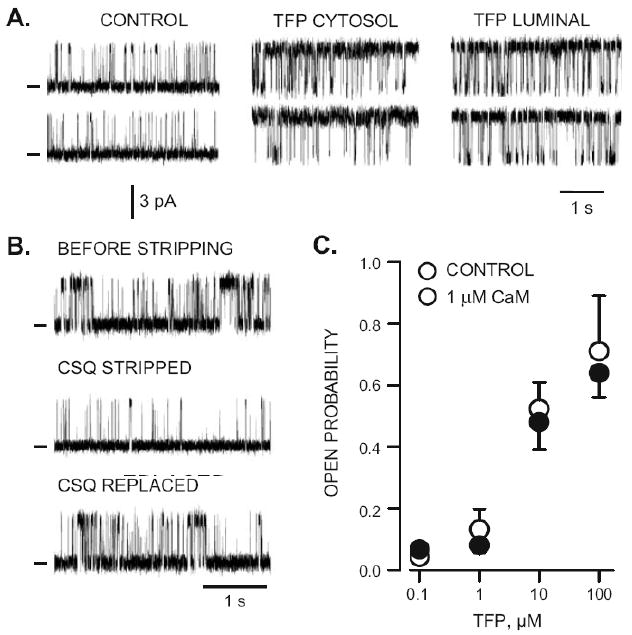
CSQ and CaM dependence of TFP action. A. Sample single RyR2 channel recordings before (control) and after 100 μM TFP was added to either the cytosolic or luminal side of the channel. Open events are upward deflections. Holding potential was 0 mV and cytosolic free Ca was 1 μM. All recordings were made from the same channel. B. Sample single RyR2 channel recordings before, after endogenous CSQ was stripped from the channel and after subsequent addition of luminal CSQ (5 μg/ml). These recordings were made from the same channel. Open events are upward. C. TFP dose–response in the absence (filled circles) and presence of 1 μM cytosolic CaM (open circles). The points here represent means (±SEM) of determination on six to ten different single RyR2 channels
All the single RyR2 channels in our studies were prestripped of any CSQ that may have been associated with them. This was done by pre-treating channels with high luminal Ca levels (>10 mM for 15 min) which is known to promote dissociation of the CSQ from the RyR2 channel complex [2, 14, 26]. The effectiveness of the stripping process is demonstrated in Fig. 3b, where sample recordings in three experimental conditions are shown (before stripping, CSQ stripped, and CSQ replaced). All these recordings were made in the presence of 1 μM cytosolic Ca and 1 mM luminal Ca (some luminal Cswas also present to provide charge carrier). The CSQ replacement was done by adding 5 μg/ml of the CSQ protein to the luminal chamber [26]. Note that the Po in the CSQ stripped condition (Fig. 3b, middle trace) is similar to that of the CSQ stripped control RyR2 recordings shown in Figs. 1 and 3a. Since all TFP challenged single channels in this study were CSQ stripped, the TFP actions reported here cannot be due to TFP disruption of the CSQ-RyR2 regulatory process.
The rate of CaM-RyR dissociation [3, 34] suggests that little, if any, CaM is likely to be associated with the channel by the time its activity is recorded in our bilayer studies. This is consistent with the observation that addition of 1 μM CaM to the cytosolic side of the RyR2 changed Po in our control conditions (0.067±0.026, n=5 vs. 0.042±0.025, n=4). Although this was not a statistically significant difference, the change was of the anticipated magnitude and in the expected direction [31]. This small inhibitory action of CaM on RyR2 Po is not illustrated here. Instead, Figure 3c compares Po in the absence (filled circles) and presence of 1 μM cytosolic CaM (open circles) at four different TFP concentrations. The action of TFP on RyR2 Po did not depend on whether CaM was present. This suggests the TFP actions reported here are not likely due to TFP disruption of a CaM-RyR2 regulatory process.
Park et al. [24] have shown that several tricyclic antidepressant drugs alter CSQ Ca binding capacity (as TFP does). Recently, we have shown that the tricyclic antidepressant amitriptyline (AMT) activates single RyR2 channels similar to the action of TFP shown here [40]. The action of TFP, AMT, NRT (nortriptyline, an AMT metabolite) and caffeine are compared in Fig. 4b. But first, Figure 4a illustrates the actions of NRT. Like TFP, NRT clearly activated CSQ-free RyR2 channels. The NRT dose– response relationship reveals an EC50 of 8.9 μM with a Hill coefficient of 2.13. Open event frequency increased between 1–15 μM NRT and then decreased at higher NRT levels, whereas mean open time remained relatively constant over the NRT concentration range tested. Figure 4b shows that caffeine (open circles) only begins to substantially activate the channel at concentrations greater than 100 μM (open circles). The caffeine EC50 measured here was 1.1 mM with a Hill coefficient of 0.99. The maximal Po reached was near 1.0. Figure 4b also includes TFP, AMT, and NRT dose–response results. All three of these psychoactive drugs activated the channel to different extents at concentrations less than 10 μM. The maximal Po's evoked by AMT, TFP, NRT, and were ∼0.7, ∼0.6, and ∼0.3, respectively.
Figure 4.
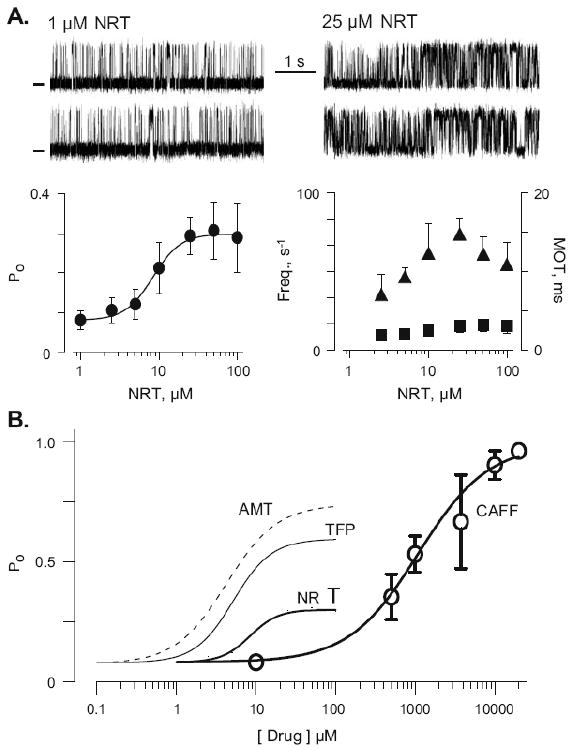
Comparison of TFP, NRT, AMT and caffeine actions on single RyR2 channels. A. Top panels show sample single RyR2 channel recordings in 1 and 25 μM NRT (drug applied to cytosolic chamber). Open events are upward deflections. Holding potential was 0 mV and cytosolic free Ca was 1 μM. Recordings were all made from the same channel. Bottom panels show summary NRT dose response data from six to eight different channels as well as open frequency (filled triangles) and mean open time (filled squares) results. B. Comparison of caffeine (CAFF), AMT, TFP, and NRT dose response data. Dotted line represents AMT results from our lab presented elsewhere [40]
TFP action on Ca signaling in ventricular myocytes
Next, we studied how TFP affects elementary Ca release events (Ca sparks) in saponin-permeabilized ventricular myocytes. Figure 5a shows representative confocal linescan images (space vs. time) and corresponding subcellular ΔF/F0 profiles recorded from the marked points. Sparks were monitored before (control), during the first and third minute (1 and 3 min) following application of 50 μM TFP. TFP initially increased spark frequency by 57% followed by suppression of spark activity by 50% compared to control. Average Ca spark frequencies in control conditions were 9.5±1.0 and 14.9±1.1 and 4.7±0.6 sparks×s−1 × (100 μm)−1 (n=9) during the first and third minute after TFP application, respectively. TFP initially increased spark width and duration by 13% and 23%, respectively. During the later phase (measured after 3 min of TFP application), spark amplitude and width were significantly decreased by 25% and 21%, respectively, but not spark duration. Ca spark properties in permeabilized cells in these three experimental conditions are provided in Table 1. This TFP action was at least partially reversible after the drug was washed out. We tested whether the observed transient stimulation of Ca spark activity was associated with changes in SR Ca load. Figure 5b shows representative linescan images and corresponding ΔF/F0 profiles of Ca transients evoked by 20 mM caffeine before (control) and after 3 min of a 50-μM TFP application. Control peak amplitude of caffeine-evoked Ca transients (ΔF/F0) was 2.8±0.3 (n=4) and after 3 min of TFP application was 1.6± 0.2 (n=4). Thus, TFP application decreased SR Ca load by 43%. A similar action of TFP on [Ca]i handling was observed in intact ventricular myocytes. We found that TFP (100 μM) evoked SR Ca release and this was followed by a depletion of SR Ca load (Fig. 5c). The amplitude of this TFP-induced Ca release in intact cells was 18±6% (n=4) of the control caffeine-induced Ca transient. After the initial release evoked by TFP, SR Ca load in the intact cells was decreased to 56±8% (n=4) of control. We also found that TFP significantly inhibited action-potential-evoked Ca transient amplitude by 60– 70%. Additionally, we observed that the decay kinetics of Ca transients were slower by ∼50% in the presence of TFP (Fig. 5c), suggesting that [Ca]i removal by either the SR Ca pump (SERCA) and/or Na–Ca exchange (NCX) might be affected by TFP. The [Ca]i decline of the caffeine-induced Ca transient is due primarily to NCX because SERCA removal is short circuited by the open RyR2 channels. The action-potential-induced transient [Ca]I decline is the result of the combined activities of SERCA and NCX [3]. We did not observe any substantial TFP action on the kinetics of the caffeine-induced [Ca]i transient (Fig. 5c). This implies that the slower decline of the action-potential-induced Ca transient is likely the result of SERCA inhibition by TFP. TFP inhibition of SERCA is consistent with early reports of TFP actions on the SR Ca pump [16].
Figure 5.
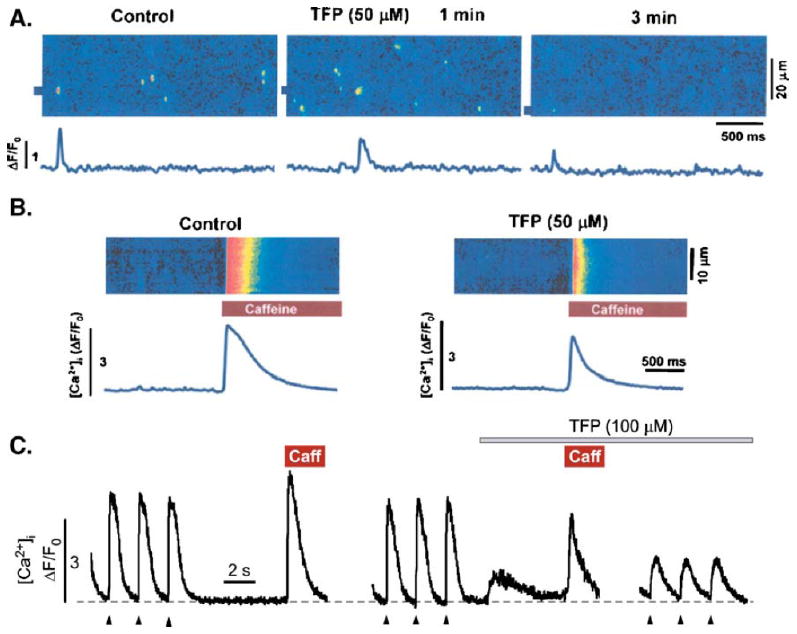
TFP Action on SR Ca release and load in permeabilized and intact ventricular myocytes. a Linescan images of permeabilized ventricular myocytes and corresponding ΔF/F0 profiles before and within 1 and 3 min of 50μM TFP exposure. The ΔF/F0 profiles represent the marked locations in the linescan images. b Global SR Ca release transients (linescan and ΔF/F0 rofiles) evoked by 20 mM caffeine before (control) and after 3 min of 50 μM TFP exposure. The reduced peak Ca release in the presence of TFP indicates a reduction in SR Ca load. c Whole-cell Ca transients (ΔF/F0 profiles) evoked by electrical field stimulation (arrow heads; 0.5 Hz), caffeine (10 mM), and TFP (100 μM) recorded from intact rabbit ventricular myocytes. SR Ca load (caffeine-induced Ca transient) was measured before and after TFP application
These data are in agreement with the idea that stimulation of RyR2 by TFP causes a transient stimulation of SR Ca release in both permeabilized and intact cells. The subsequent decrease in RyR2 activity is likely a consequence of SR Ca content depletion due to higher Ca leak from the SR and reduced SR Ca uptake. This TFP action is very similar to the action of AMT on SR Ca handling in ventricular myocytes [40].
Discussion
The phenothiazine TFP and tricyclic antidepressants NRT are commonly prescribed antipsychotic drug that have adverse, and potentially lethal cardiac side effects [4, 17, 19]. These drugs are known to alter CSQ Ca binding [24] and CSQ is known to be an important regulator of single RyR2 channel function [14, 26]. The same is true for CaM [5, 31, 36, 37]. These facts piqued our interest in defining how TFP and NRT may alter single RyR2 channel function and/or RyR2-mediated Ca release in cells.
The therapeutically effective TFP and NRT plasma concentrations are thought to be in the 10−7 to 10−6 M range [4, 32]. Toxic levels of these drugs have been reported to be in the 1 to 10 μM range [17–19, 28]. In our hands, we found no detectable (statistically significant) action of TFP or NRT on single RyR2 function or RyR2- mediated Ca release when these drugs were applied at their therapeutic concentrations (<1 μM). This suggests that alteration of RyR2-mediated Ca signaling is likely not involved in their normal therapeutic actions. However, our results show that the TFP and NRT substantially activate single RyR2 channels with EC50's of 5.2 and 8.9 μM, respectively. Thus, doses greater than 1 μM may contribute to the adverse cardiac side effects of these drugs by acting on single RyR2 channel function.
These drugs (>1 μM) activated RyR2 channels independently of CSQ and CaM. This suggests that the drugs may do this by binding directly on the RyR2 protein. However, Park et al. (2005) reported KDS of TFP and NRT action on CSQ Ca binding of 14 and 11 μM, respectively. Others have reported that TFP inhibits the cardiac hERG K channel with an IC50 between 9 and 21 μM [7]. Additionally, tricyclic antidepressants are known to block Nacurrents with an IC50 of about 20 μM [22, 23]. Thus, TFP and NRT action on single RyR2 function appears to be just one of several possibly cardiotoxic actions of these drugs. The TFP action on RyR2 channels reported here occurred at smaller TFP concentrations than those described above. This could imply that the TFP action on single RyR2 channels is of greater significance than these other reported actions. However, this may be an overly simplistic interpretation. For example, Nau et al. (2000) reported substantial block of cardiac Nacurrents during repetitive depolarization pulses (∼55% block at 5 Hz) in the presence of just 1 μM AMT. Thus, although the relative steady-state affinities are suggestive, they may not accurately reflect how these drugs act in the dynamic environment of the cell.
In ventricular myocytes, we show that TFP transiently increased Ca spark frequency and reduced SR Ca content within a few minutes. Increased spark frequency is thought to reduce SR Ca load and this will in turn eventually down regulate spark frequency [9]. In addition, inhibition of SERCA by TFP would also contribute to depletion SR Ca load. The transient increase in spark frequency we observed here may be a consequence of a TFP-evoked increase in single RyR2 opening frequency. The increased opening frequency could be due to a direct TFP action of the RyR2 channel as we observed in our bilayer studies. TFP actions on CaM and/or CSQ may also indirectly contribute. We cannot conclude unequivocally whether the effect of TFP on Ca sparks is direct or indirect (or a combination of both). Since the cytosolic environment is controlled in permeabilized cells by the perfusion solution (which in our experiments did not contain CaM), it can be argued that endogenous CaM was largely washed out in those studies. Therefore, CaM is unlikely to play a role in the TFP effect on sparks in our studies. The does not apply to CSQ. Since TFP permeates cell membranes, it may indeed be interacting with CSQ inside the SR of both intact and permeabilized cells in our studies. Although we cannot make any definitive statements about the relative contributions of direct and indirect (via CSQ) TFP actions at this point, we believe that both are likely playing a part here. Also, it is important to note that the experimental conditions were not identical in our cellular and bilayer studies (due to the usual technical considerations) and this should always be considered when interpreting results like these.
This action of TFP on RyR2-mediated Ca signaling in myocytes and single RyR2 channel function was reminiscent of those of caffeine. One clear difference is that the caffeine EC50 of single RyR2 activation was 1.1 mM while the TFP EC50 was 5.2 μM. Another is that high doses of caffeine-activated single RyR2 channels to Po levels approaching 1 while high doses of TFP activated channels to a Po level of ∼0.6. High NRT doses activated channels to a lesser extent (Po ∼0.3). This implies that TFP and NRT may not be good substitutes for caffeine for rapidly assessing SR Ca load (where maximal RyR2 opening is required). However, the lower EC50's and effectiveness of TFP and NRT may make them interesting pharmacological probes in SR Ca release studies were less robust RyR2 activation is desirable.
Acknowledgments
This work was supported by National Institutes of Health Grants HL80101 & HL62231 (to LAB), HL57832 & AR54098 (to MF), and an American Heart Association Grant AHA0530309Z (to AVZ). We are very thankful to Drs. Pompeo Volpe and Alessandra Nori for kindly providing the CSQ protein. We would also like to thank Dr. Josefina Ramos-Franco and Ms. Alma Nani for their expert assistance.
References
- 1.Arai M, Otsu K, MacLennan DH, Alpert NR, Periasamy M. Effect of thyroid hormone on the expression of mRNA encoding sarcoplasmic reticulum proteins. Circ Res. 1991;69(2):266–276. doi: 10.1161/01.res.69.2.266. [DOI] [PubMed] [Google Scholar]
- 2.Beard NA, Casarotto MG, Wei L, Varsanyi M, Laver DR, Dulhunty AF. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca and phosphorylation. Biophys J. 2005;88(5):3444–3454. doi: 10.1529/biophysj.104.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bers DM. Excitation–contraction coupling and cardiac contractile force. Kluwer; Norwell, MA USA: 2001. [Google Scholar]
- 4.Buckley NA, Sanders P. Cardiovascular adverse effects of antipsychotic drugs. Drug Saf. 2000;23(3):215–228. doi: 10.2165/00002018-200023030-00004. [DOI] [PubMed] [Google Scholar]
- 5.Cachia PJ, Van Eyk J, Ingraham RH, McCubbin WD, Kay CM, Hodges RS. Calmodulin and troponin C: a comparative study of the interaction of mastoparan and troponin I inhibitory peptide [104–115] Biochemistry. 1986;25(12):3553–3562. doi: 10.1021/bi00360a013. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain BK, Volpe P, Fleischer S. Calcium-induced calcium release from purified cardiac sarcoplasmic reticulum vesicles. General characteristics. J Biol Chem. 1984;259(12):7540–7546. [PubMed] [Google Scholar]
- 7.Choi SY, Koh YS, Jo SH. Inhibition of human ether-a-gogo-related gene Kchannel and IKr of guinea pig cardiomyocytes by antipsychotic drug trifluoperazine. J Pharmacol Exp Ther. 2005;313(2):888–895. doi: 10.1124/jpet.104.080853. [DOI] [PubMed] [Google Scholar]
- 8.di Barletta MR, Viatchenko-Karpinski S, Nori A, Memmi M, Terentyev D, Turcato F, Valle G, Rizzi N, Napolitano C, Gyorke S, Volpe P, Priori SG. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2006;114(10):1012–1019. doi: 10.1161/CIRCULATIONAHA.106.623793. [DOI] [PubMed] [Google Scholar]
- 9.Eisner DA, Trafford AW, Diaz ME, Overend CL, O'Neill SC. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc Res. 1998;38(3):589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- 10.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82(4):893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 11.Fliegel L, Ohnishi M, Carpenter MR, Khanna VK, Reithmeier RA, MacLennan DH. Amino acid sequence of rabbit fasttwitch skeletal muscle calsequestrin deduced from cDNA and peptide sequencing. Proc Natl Acad Sci U S A. 1987;84(5):1167–1171. doi: 10.1073/pnas.84.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fruen BR, Bardy JM, Byrem TM, Strasburg GM, Louis CF. Differential Ca() sensitivity of skeletal and cardiac muscle ryanodine receptors in the presence of calmodulin. Am J Physiol Cell Physiol. 2000;279(3):C724–C733. doi: 10.1152/ajpcell.2000.279.3.C724. [DOI] [PubMed] [Google Scholar]
- 13.Fujii J, Willard HF, MacLennan DH. Characterization and localization to human chromosome 1 of human fast-twitch skeletal muscle calsequestrin gene. Somat Cell Mol Genet. 1990;16(2):185–189. doi: 10.1007/BF01233048. [DOI] [PubMed] [Google Scholar]
- 14.Gyorke I, Hester N, Jones LR, Gyorke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86(4):2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77(2):245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 16.Ho MM, Scales DJ, Inesi G. The effect of trifluoroperazine on the sarcoplasmic reticulum membrane. Biochim Biophys Acta. 1983;730(1):64–70. doi: 10.1016/0005-2736(83)90317-6. [DOI] [PubMed] [Google Scholar]
- 17.Hull BE, Lockwood TD. Toxic cardiomyopathy: the effect of antipsychotic–antidepressant drugs and calcium on myocardial protein degradation and structural integrity. Toxicol Appl Pharmacol. 1986;86(2):308–324. doi: 10.1016/0041-008x(86)90061-x. [DOI] [PubMed] [Google Scholar]
- 18.Jusic N, Lader M. Post-mortem antipsychotic drug concentrations and unexplained deaths. Br J Psychiatry. 1994;165(6):787–791. doi: 10.1192/bjp.165.6.787. [DOI] [PubMed] [Google Scholar]
- 19.Kelly HG, Fay JE, Laverty SG. Thioridazine hydrochloride (Mellaril): its effect on the electrocardiogram and a report of two fatalities with electrocardiographic abnormalities. Can Med Assoc J. 1963;89:546–554. [PMC free article] [PubMed] [Google Scholar]
- 20.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, Zhang W, Roden DM, Jones LR, Franzini-Armstrong C, Pfeifer K. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116(9):2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meissner G, Henderson JS. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca and is modulated by Mg, adenine nucleotide, and calmodulin. J Biol Chem. 1987;262(7):3065–3073. [PubMed] [Google Scholar]
- 22.Nau C, Seaver M, Wang SY, Wang GK. Block of human heart hH1 sodium channels by amitriptyline. J Pharmacol Exp Ther. 2000;292(3):1015–1023. [PubMed] [Google Scholar]
- 23.Pancrazio JJ, Kamatchi GL, Roscoe AK, Lynch C., 3rd Inhibition of neuronal Nachannels by antidepressant drugs. J Pharmacol Exp Ther. 1998;284(1):208–214. [PubMed] [Google Scholar]
- 24.Park IY, Kim EJ, Park H, Fields K, Dunker AK, Kang C. Interaction between cardiac calsequestrin and drugs with known cardiotoxicity. Mol Pharmacol. 2005;67(1):97–104. doi: 10.1124/mol.104.005744. [DOI] [PubMed] [Google Scholar]
- 25.Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol. 2007;293(3):C1073–C1081. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 26.Qin J, Valle G, Nani A, Nori A, Rizzi N, Priori SG, Volpe P, Fill M. Luminal Ca regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J Gen Physiol. 2008;131(4):325–334. doi: 10.1085/jgp.200709907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubenstein DS, Lipsius SL. Premature beats elicit a phase reversal of mechanoelectrical alternans in cat ventricular myocytes. A possible mechanism for reentrant arrhythmias. Circulation. 1995;91(1):201–214. doi: 10.1161/01.cir.91.1.201. [DOI] [PubMed] [Google Scholar]
- 28.Rudorfer MV, Robins E. Fatal nortriptyline overdose, plasma levels and in vivo methylation of tricyclic antidepressants. Am J Psychiatry. 1981;138(7):982–983. doi: 10.1176/ajp.138.7.982. [DOI] [PubMed] [Google Scholar]
- 29.Scott BT, Simmerman HK, Collins JH, Nadal-Ginard B, Jones LR. Complete amino acid sequence of canine cardiac calsequestrin deduced by cDNA cloning. J Biol Chem. 1988;263(18):8958–8964. [PubMed] [Google Scholar]
- 30.Shannon TR, Guo T, Bers DM. Ca scraps: local depletions of free [Ca] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca reserve. Circ Res. 2003;93(1):40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- 31.Smith JS, Rousseau E, Meissner G. Calmodulin modulation of single sarcoplasmic reticulum Ca-release channels from cardiac and skeletal muscle. Circ Res. 1989;64(2):352–359. doi: 10.1161/01.res.64.2.352. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen B, Kragh-Sorensen P, Larsen NE, Hvidberg EF. The practical significance of nortriptyline plasma control. A prospective evaluation under routine conditions in endogenous depression. Psychopharmacology (Berl) 1978;59(1):35–39. doi: 10.1007/BF00428027. [DOI] [PubMed] [Google Scholar]
- 33.Treves S, Vilsen B, Chiozzi P, Andersen JP, Zorzato F. Molecular cloning, functional expression and tissue distribution of the cDNA encoding frog skeletal muscle calsequestrin. Biochem J. 1992;283(Pt 3):767–772. doi: 10.1042/bj2830767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tripathy A, Xu L, Mann G, Meissner G. Calmodulin activation and inhibition of skeletal muscle Ca release channel (ryanodine receptor) Biophys J. 1995;69(1):106–119. doi: 10.1016/S0006-3495(95)79880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tu Q, Velez P, Cortes-Gutierrez M, Fill M. Surface charge potentiates conduction through the cardiac ryanodine receptor channel. J Gen Physiol. 1994;103(5):853–867. doi: 10.1085/jgp.103.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vandonselaar M, Hickie RA, Quail JW, Delbaere LT. Trifluoperazine-induced conformational change in Ca()-calmodulin. Nat Struct Biol. 1994;1(11):795–801. doi: 10.1038/nsb1194-795. [DOI] [PubMed] [Google Scholar]
- 37.Weiss B. Techniques for measuring the interaction of drugs with calmodulin. Methods Enzymol. 1983;102:171–184. doi: 10.1016/s0076-6879(83)02018-2. [DOI] [PubMed] [Google Scholar]
- 38.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266(17):11144–11152. [PubMed] [Google Scholar]
- 39.Zima AV, Picht E, Bers DM, Blatter LA. Partial inhibition of sarcoplasmic reticulum Ca release evokes long-lasting Ca release events in ventricular myocytes: role of luminal Ca in termination of ca release. Biophys J. 2008a;94(5):1867–1879. doi: 10.1529/biophysj.107.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zima AV, Qin J, Fill M, Blatter LA. Tricyclic antidepressant amitriptyline alters sarcoplasmic reticulum calcium handling in ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008b;295(5):H2008–H2116. doi: 10.1152/ajpheart.00523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


