Abstract
Transglutaminases (TGs) are Ca2+-dependent enzymes that catalyze a variety of modifications of glutaminyl (Q) residues. In the brain, these modifications include the covalent attachment of a number of amine-bearing compounds, including lysyl (K) residues and polyamines, which serve to either regulate enzyme activity or attach the TG substrates to biological matrices. Aberrant TG activity is thought to contribute to Alzheimer disease, Parkinson disease, Huntington disease, and supranuclear palsy. Strategies designed to interfere with TG activity have some benefit in animal models of Huntington and Parkinson diseases. The following review summarizes the involvement of TGs in neurodegenerative diseases and discusses the possible use of selective inhibitors as therapeutic agents in these diseases.
Keywords: cystamine, neurodegeneration, polyamines, transglutaminase, γ-glutamylpolyamines, γ-glutamyl-ε-lysine
Cerebral transglutaminases
Eight active transglutaminases (TGs) (TGs 1–7 and factor XIIIa) are expressed in mammals, of which TGs 1–3 (Kim et al. 1999)1 and 6 (Hadjivassilou et al. 2008) are present in human brain. The major reaction thus far attributed to the cerebral TGs is transamidation. In this reaction the carboxamide moiety of a Q residue [-C(O)NH2] is converted to a substituted carboxamide [-C(O)NHR] by nucleophilic attack of an amine [RNH2] such as various mono-, di-, and polyamines or the ε amino group of a K residue (Lorand and Graham 2003). Of the possible transamidation linkages, the γ-glutamyl-ε-lysine [Nε-(γ-l-glutamyl)-l-lysine] (GGEL) isopeptide linkage formed between Q and K resides, is the most commonly studied. GGEL bonds occur both within and between polypeptide chains, and thereby contribute to the formation of stable soluble and insoluble polymers. TGs also cross-link proteins via bis-γ-glutamylpolyamine bridges between Q residues (Piacentini et al. 1988). These linkages are formed by two successive transamidations: the first utilizes a free polyamine to generate a γ-glutamylpolyamine residue, which becomes the amine-bearing substrate for a second transamidation. bis-γ-Glutamylpolyamine cross-links are formed at least as frequently as those involving GGEL (Piacentini et al. 1988). Under physiological conditions, the majority of cross-linking bonds are generated outside of cells where the concentrations of Ca2+ are sufficiently high enough to stimulate the catalysis of these bonds by TGs. TG 2 and 3 have three Ca2+ binding sites and the current indications are that the catalysis of GGEL bonds requires the occupation of all three sites (Datta et al. 2006).
Intracellular Ca2+ concentrations rarely match the extracellular concentrations. Moreover, GTP acts in cells as an endogenous inhibitor of TGs (Bergamini et al. 1987). Nevertheless, intracellular TG-catalyzed reaction products can be detected in normal cells, especially those products related to polyamination (Piacentini et al. 1988). The consequences of polyamination in the brain, however, are poorly understood as only a limited number of polyaminated proteins have been identified (Tucholski et al. 1999). Of these, phospholipase A2 (PLA2) is especially interesting since the polyamination of this enzyme may contribute to the inflammation associated with neurodegeneration. PLA2 produces two groups of pro-inflammatory mediators: leukotrienes and prostaglandins. Polyamination of PLA2 results in a 3-fold increase in activity (Cordella-Miele et al. 1993) that may persist for the life of the protein given the inability of most peptidases to hydrolyze γ-glutamylamine (GGEL, γ-glutamylpolyamine and bis-γ-glutamylpolyamine) linkages (Fink and Folk 1981). Thus, polyamination may represent a unique post-translational modification of enzymes that permanently affects activity. This situation contrasts with other types of covalent post-translational modifications, such as phosphorylation that typically are transient.
Increased TG(s) in neurodegenerative diseases
Transglutaminase activity is widespread in brain (Kim et al. 1999) and is present in primary cultures of neurons and astrocytes (Perry et al. 1995; Caccamo et al. 2004). TG 2 is associated with the extracellular matrix, cell membranes and cytosol of neurons, and TG activity has been identified in synaptosomes (Pastuszko et al. 1986), mitochondria (Krasnikov et al. 2005), and nuclei (Lesort et al. 1998). The activity, expression and amounts of individual TG enzymes are increased in a variety of neurodegenerative diseases. TG activity is significantly elevated in the affected cerebral regions in Alzheimer disease (AD) (Johnson et al. 1997; Kim et al. 1999), Huntington disease (HD) (Karpuj et al. 1999; Lesort et al. 1999), and supranuclear palsy (Zemaitaitis et al. 2003). These increases in activity are accompanied by gains in the amount of TG 1 and TG 2 proteins in AD brain (Kim et al. 1999; Bonelli et al. 2002), and also of TG 2 protein in the brains of HD (Lesort et al. 1999) and supranuclear palsy (Zemaitaitis et al. 2003) patients. Increased TG 2 protein is also found in the CSF of AD (Bonelli et al. 2002) and Parkinson disease (PD) (Vermes et al. 2004) patients.
Not only are the amounts of TG increased in AD, HD and supranuclear palsy, but the conditions favoring the activation of these enzymes are also enhanced in these diseases. These conditions include elevations in intracellular Ca2+ due to glutamate-mediated excitotoxity (Caccamo et al. 2004) and other perturbations in Ca2+ homeostasis (Mattson 2007) as well as decreases in GTP concentrations following from losses in energy production (Lin and Beal 2006).
The number of TG 2 transcripts is also increased in HD (Lesort et al. 1999) and supranuclear palsy (Zemaitaitis et al. 2003), and a shortened alternate transcript of TG 2 encoding a form of enzyme missing the GTP binding domain is expressed in AD brain (Festoff et al. 2002). A number of mechanisms may account for the increased transcription and translation of TGs in neurodegenerative disorders. The TG 1 promoter has Ca2+ (Kawabe et al. 1998), retinoid (Polakowska et al. 1999), cAMP, Sp1, and AP1 responsive elements (Medvedev et al. 1999), while the TG 2 promoter contains elements that respond to retinoids (Nagy et al. 1996; Yan et al. 1996), interleukin 6, transforming growth factor β1 (Ritter and Davies 1998), and tumor necrosis factor-α (Kuncio et al. 1998). The inflammatory mediators are likely to act via the NF-κB (Nuclear factor κB) binding region in the TG 2 promoter (Kuncio et al. 1998; Kim et al. 2008). NF-κB translocation and DNA binding are stimulated by tumor necrosis factor-α and glutamate, both of which have been shown to increase TG 2 expression in microglia and astrocytes (Campisi et al. 2004; Park et al. 2004). As noted earlier, inflammation accompanies neurodegeneration and TGs may contribute to this response via the sustained activation of polyaminated PLA2. The possibility that TGs may contribute to their continued activation highlights the necessity of limiting the activity of these enzymes in neurodegenerative disorders.
Increased TG-catalyzed products in neurodegenerative diseases
Increased TG activity in neurodegenerative disorders is accompanied by an increase in TG-catalyzed products. Selkoe et al. (1982a,b) demonstrated that cerebral TGs catalyze the in vitro polymerization of cytoskeletal elements, and hypothesized that TGs might facilitate paired helical formation in AD tangles. TG 2 and GGEL cross-links were subsequently shown to co-localize with the tangles (Miller and Anderton 1986; Johnson et al. 1997) and TG2 was shown to co-localize with plaques (Zhang et al. 1998) in AD brain. Components of the plaques or tangles, including β-amyloid (Aβ) (Ikura et al. 1993; Dudek and Johnson 1994; Ho et al. 1994; Rasmussen et al. 1994), the Dutch mutation of Aβ (Q22 → E22) (Dudek and Johnson 1994), tau (Miller and Anderton 1986; Dudek and Johnson 1993; Miller and Johnson 1995; Appelt and Balin 1997; Murthy et al. 1998; Tucholski et al. 1999), and the non-Aβ component derived from α-synuclein (Jensen et al. 1995) are TG substrates. The in vitro products of the reaction of these substrates with TG bear a striking resemblance to the insoluble polymers found in AD brain (Jensen et al. 1995; Appelt and Balin 1997; Hartley et al. 2008).
Huntington disease is caused by a CAG expansion in the huntingtin (htt) gene that encodes a length of contiguous Q residues [polyglutamine (Qn)] in the N-terminus of the expressed protein. Green (1993) hypothesized that the expanded Qn region would favor the formation of TG-catalyzed GGEL linkages and lead to the formation of htt-containing aggregates. In support of this hypothesis, expanded Qn domains are excellent TG substrates (Kahlem et al. 1996; Cooper et al. 1997a; Gentile et al. 1998; Lesort et al. 1999; Zainelli et al. 2005) and mutant htt is present in HD aggregates (DiFiglia et al. 1995) as are GGEL crosslinks (Zainelli et al. 2003).
The increased cerebral aggregation seen in HD, PD, and supranuclear palsy is also associated with a comparable increase in GGEL immunoreactivity within the polymers (Zemaitaitis et al. 2000; Zainelli et al. 2003; Andringa et al. 2004). Although some concerns have been raised about the specificity of GGEL antibodies in immunoblots (Johnson and LeShoure 2004), the increase in protein-associated GGEL in AD brain has been unequivocally confirmed using mass spectrometric techniques (Kim et al. 1999; Nemes et al. 2004).
As noted earlier, the isopeptide bonds in γ-glutamylamine linkages are resistant to proteolysis (Fink and Folk 1981). Moreover, the ability to metabolize free γ-glutamylamines in brain is limited. Consequently, γ-glutamylamines are excised intact during proteolysis and are present in brain and CSF (Jeitner et al. 2001, 2008; Dedeoglu et al. 2002). A several-fold increase in free GGEL has been measured in the brains of HD patients (Dedeoglu et al. 2002), and the amount of GGEL in the CSF of patients with AD, PD (Sárvari et al. 2002), or HD (Jeitner et al. 2001, 2008; Dedeoglu et al. 2002) is also increased relative to control CSF. The increase in CSF GGEL reported in HD is also matched by comparable increases in the amounts of CSF γ-glutamylspermidine, γ-glutamylputrescine and bis-γ-glutamylputrescine (Jeitner et al. 2008). CSF contains higher (μM) quantities of γ-glutamylspermidine than GGEL (< μM) in accord with the suggestion noted above that TGs predominantly catalyze polyamination over Q → K cross-linking within the brain.
Possible mechanisms for TG-mediated neurotoxicity
Although the formation of insoluble protein aggregates has been proposed to account for the toxic actions of TGs, the role of such aggregates in the etiology of diseases such as HD is controversial (Kuemmerle et al. 1999; Sieradzan and Mann 2001). Indeed, mice that lacked TG 2 and over-express mutant htt had 30% more brain aggregates and still lived longer than their TG 2- and mutant htt-expressing littermates (Mastroberardino et al. 2002). The increased aggregation is unlikely to have been due to compensatory cross-linking by TG 1 and 3, since the TG 2-deficient mice also exhibited a 10-fold decrease in the number of GGEL linkages. It was subsequently shown that GGEL cross-links serve to produce soluble Qn aggregates, whereas polyaminated or unmodified Qn domains spontaneously aggregate to form insoluble polymers (Lai et al. 2004; Konno et al. 2005). These observations suggest that TG 2-catalyzed GGEL bond formation generates soluble aggregates that may be neurotoxic.
Another possibility is that rather than being toxic per se, the soluble and insoluble polymers cause neuronal death by sequestering critical proteins within the aggregates. These proteins could include, for example, glyceraldehyde 3-phosphate dehydrogenase, α-ketoglutarate dehydrogenase, and histones (Cooper et al. 1997b, 2000; Gentile et al. 1998) in HD, and ubiquitin, HSP27, parkin, and α-synuclein in AD (Nemes et al. 2004). It has also been suggested that congestion of proteasomes may contribute to CAG-expansion and other neurodegenerative diseases (Cooper et al. 2002; Wang et al. 2008). In support of this hypothesis, components of the ubiquitin proteasome system are found in HD aggregates (Bennett et al. 2007).
The above observations suggest the following model for the contribution of TGs to neurodegenerative diseases. Early in these diseases, TGs predominantly catalyze polyamination reactions. As these diseases progress, TGs begin to form more GGEL cross-links, which stabilize soluble toxic protein aggregates, eventually leading to removal of key proteins and to a fatal congestion of proteasomes.
TGs as potential therapeutic targets in neurodegenerative diseases
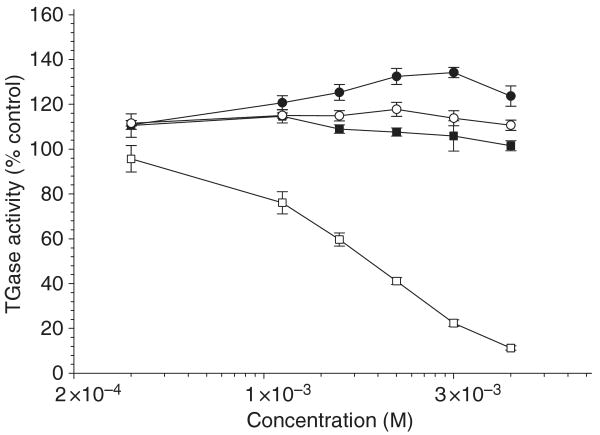
Several authors have raised the possibility that TG inhibitors may be of therapeutic benefit in neurodegenerative diseases (e.g. Cooper et al. 2002; Gentile and Cooper 2004), and one such in vitro inhibitor – cystamine – is beneficial in murine models of HD and PD (e.g. Dedeoglu et al. 2002; Van Raamsdonk et al. 2005; Stack et al. 2008). We have shown that cysteamine, the reduced form of cystamine, is a competitive inhibitor/alternative substrate of TG 2 (Jeitner et al. 2005). Cysteamine attenuates polyamination by acting as an alternative TG 2 substrate, presumably forming Nβ-(γ-l-glutamyl)-cysteamine linkages (Jeitner et al. 2005). In addition to cysteamine, cystamine is metabolized to hypotaurine and taurine, and cystamine treatment in mice leads to increased brain cysteine levels (Fox et al. 2004; Pinto et al. 2005). We tested the ability of hypotaurine, taurine, cysteine, and cysteamine to inhibit TG 2. Of the tested compounds, only cysteamine was able to inhibit TG 2-catalyzed polyamination (Fig. 1). As neither cystamine nor cysteamine can be detected (detection limit ≤ 20 μM) in the brains of cystamine-treated YAC128 (HD) mice (Pinto et al. 2005), the conversion of cystamine to cysteamine, and then to Nβ-(γ-l-glutamyl)-cysteamine, is likely to be rapid.
Fig. 1.
Effect of cysteamine, cysteine, hypotaurine, and taurine on TG activity. TG activity in the presence of cysteamine (□), cysteine (■) hypotaurine (○), or taurine (●) was determined by measuring the incorporation of radiolabeled putrescine into N,N-dimethylcasein as described by Jeitner et al. (2005) using tritiated putrescine and his-tagged guinea pig TG 2 (Gillet et al. 2004). The data are depicted as percent of the control (21 910 ± 1731 dpm per tube) and represent the mean ± SEM of four separate experiments. The data with cysteamine at concentrations ≥ 2.5 mM and taurine at 5 and 10 mM were significantly different from that of the control (p < 0.05, paired t-test).
Prolonged cystamine treatment results in decreased TG activity (Dedeoglu et al. 2002; Van Raamsdonk et al. 2005), even though cysteamine is not an irreversible TG inhibitor (Jeitner et al. 2005). Thus, another mechanism must account for the diminished TG activity. We hypothesize that cystamine-derived cysteamine inhibits the binding of transcription factors to TG promoters and thereby limits the transcription of TGs. In support of this hypothesis, cysteamine attenuates the DNA binding of AP1 and NF-κB (Goldstone et al. 1995), and binding sites for these factors are present in the TG 1 and TG 2 promoters (Kuncio et al. 1998; Medvedev et al. 1999; Kim et al. 2008).
As indicated above, cystamine has multiple biological actions in the brain, including raising the levels of cysteine (Fox et al. 2004; Pinto et al. 2005). Cystamine also causes elevation of brain-derived neurotropic factor (Borrell-Pages et al. 2006) and possibly attenuates apoptosis through inhibition of caspase 3 activity (Lesort et al. 2003). Recently, we discovered another potentially beneficial property of cystamine. Cystamine, at concentrations as low as 15 μM, significantly attenuated dopamine-induced macroautophagy in SH SY5Y cells, whereas cysteamine had no effect (Fig. 2). Dopamine reduced the viability of SH SY5Y cells by 48 ± 3% (mean ± SEM, n = 11) after 24 h, while the combination of dopamine and cystamine (15 μM) only reduced viability by 24 ± 2% (p < 0.05, paired t-test). In these experiments, the cells were pre-treated with cystamine for 2 h, and then treated for a further 24 h (i.e. 26 h). Importantly, the treatment of cells with cystamine for only 4 h prior to the application of dopamine was as effective as the 26-h cystamine treatment (Fig. 2). This observation suggests that cystamine primes the cells against the induction of macroautophagy.
Fig. 2.
Effect of cyst(e)amine on dopamine-induced macroautophagy. SH SY5Y cells at 70% confluence in a 75 cm2 flask were detached with 0.05% trypsin : verscene (1 : 1), then collected into 50 mL 10% fetal bovine serum, 90% Dulbecco's modified eagle medium prior to seeding onto 24 multi-well plates at 1 mL per well. Twenty-four hours later the cells were treated with either cystamine (■) or cysteamine (□) for 2 h. The cells were then incubated for an additional 24 h together with 10−4 M dopamine to induce macroautophagy as described by Gomez-Santos et al. (2003). The data from these studies are depicted as the mean ± SEM of four separate experiments. The cells were also treated with cystamine for 4 h then washed three times with Hank's balanced salt solution (HBBS) at 37°C, followed by 24 h incubation with 100 μM dopamine (gray open circles). The data from these studies represent the mean of two individual experiments that did not vary by more than 5% of the mean. At the end of the incubations, the cells were washed twice with HBSS at 37°C then incubated with 250 μL of 1 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide in HBSS for 30 min at 37°C, for the determination of viability. The resulting formazan precipitates were then solubilized with 200 μL DMSO. The viability of cells treated with cystamine at 15 μM and 100 μM dopamine (■) was significantly different than that of cells treated with dopamine alone (p < 0.05, paired t-test.
Given the multiplicity of biological activities attributed to cyst(e)amine, it is difficult to assign the therapeutic benefit of this agent to the inhibition of TGs per se. Moreover, excess cysteamine has been reported to be harmful in at least one setting. Thus, Frankel and Schipper (1999) noted that cysteamine induces the appearance of iron-rich (peroxidase-positive) cytoplasmic inclusions in cultured rat astroglia, which are identical to glial inclusions that progressively accumulate in substantia nigra and other subcortical brain regions with advancing age.
The positive results obtained with a mouse model of HD in which the animals lacked TG 2 are a more compelling argument for the involvement of TGs in neurodegeneration (Mastroberardino et al. 2002) than the results obtained with cystamine. In this regard, several groups are actively synthesizing more selective TG inhibitors than cystamine as possible therapeutic agents. These inhibitors include dihydroisoxazole derivatives, peptide-bound 1,2,4-thiadiazoles, peptides containing diazo-5-oxo-l-norleucine in place of glutamine, α,β-unsaturated amides and epoxides (Pardin et al. 2008a,b). Pardin et al. have recently focused their attention on trans-cinnamoyl benzotriazole amides and 3-(substituted cinnamoyl)pyridines [azachalcones], which are potent reversible TG inhibitors (Pardin et al. 2008a,b), and have discovered a triazole compound that inhibits guinea pig TG 2 with a Ki value of ∼170 nM (Pardin et al. 2008b). Stein and colleagues have discovered another series of reversible TG inhibitors that are thieno[2,3-d]pyrimidin-4-one acylhyd-razide derivatives (Duval et al. 2005; Case and Stein 2007).
Finally, Sohn et al. (2003) have developed a novel strategy that blocks the polyamination of PLA2. This group noted that uteroglobin and lipocortin-1 contain common sequences that antagonize the interaction of TG 2 with PLA2. Synthesized variants of these sequences prevented both the polyamination of PLA2 and experimentally-induced allergic conjunctivitis in experimental animals. These results suggest that rather than inhibiting TG 2 directly, some benefit may be derived from targeting the interaction of TGs and specific pathogenic TG substrates. Polyaminated PLA2 may be one such target in neurodegenerative disorders.
Conclusion and future prospects
Although TGs do not cause neurodegenerative diseases directly, the current evidence suggests that this family of enzymes contributes to the neuropathology once the disease process has begun. It is anticipated that potent TG inhibitors will soon be evaluated for their therapeutic potential in cellular and animal models of HD and other neurodegenerative diseases. Care will be required to ensure that these inhibitors are sufficiently selective so as not to affect crucial TG reactions critical to normal metabolic processes or to inhibit blood clot formation.
Acknowledgments
Part of the authors' work cited herein was supported by the National Institutes of Health grant PO1 AG14930.
Abbreviations
- AD
Alzheimer disease
- Aβ
β-amyloid
- GGEL
γ-glutamyl-ε-lysine [Nε-(γ-l-glutamyl)-l-lysine]
- HD
Huntington disease
- htt
huntingtin
- PD
Parkinson disease
- PLA2
phospholipase A2
- Qn
polyglutamine
- TG
transglutaminase
Footnotes
Given the need for brevity, the number of citations has been restricted/limited.
Conflicts of interest: All authors declare no conflicts of interests.
References
- Andringa G, Lam KY, Chegary M, Wang X, Chase TN, Bennett MC. Tissue transglutaminase catalyzes the formation of α-synuclein crosslinks in Parkinson's disease. FASEB J. 2004;18:932–934. doi: 10.1096/fj.03-0829fje. [DOI] [PubMed] [Google Scholar]
- Appelt DM, Balin BJ. The association of tissue transglutaminase with human recombinant tau results in the formation of insoluble filamentous structures. Brain Res. 1997;745:21–31. doi: 10.1016/s0006-8993(96)01121-3. [DOI] [PubMed] [Google Scholar]
- Bennett EJ, Shaler TA, Woodman B, Ryu KY, Zaitseva TS, Becker CH, Bates GP, Schulman H, Kopito RR. Global changes to the ubiquitin system in Huntington's disease. Nature. 2007;448:704–708. doi: 10.1038/nature06022. [DOI] [PubMed] [Google Scholar]
- Bergamini CM, Signorini M, Poltronieri L. Inhibition of erythrocyte transglutaminase by GTP. Biochim Biophys Acta. 1987;916:149–151. doi: 10.1016/0167-4838(87)90222-6. [DOI] [PubMed] [Google Scholar]
- Bonelli RM, Aschoff A, Niederwieser G, Heuberger C, Jirikowski G. Cerebrospinal fluid tissue transglutaminase as a biochemical marker for Alzheimer's disease. Neurobiol Dis. 2002;11:106–110. doi: 10.1006/nbdi.2002.0535. [DOI] [PubMed] [Google Scholar]
- Borrell-Pages M, Canals JM, Cordelieres FP, et al. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest. 2006;116:1410–1424. doi: 10.1172/JCI27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo D, Campisi A, Curro M, Li Volti G, Vanella A, Ientile R. Excitotoxic and post-ischemic neurodegeneration: involvement of transglutaminases. Amino Acids. 2004;27:373–379. doi: 10.1007/s00726-004-0117-1. [DOI] [PubMed] [Google Scholar]
- Campisi A, Caccamo D, Li Volti G, Curro M, Parisi G, Avola R, Vanella A, Ientile R. Glutamate-evoked redox state alterations are involved in tissue transglutaminase upregulation in primary astrocyte cultures. FEBS Lett. 2004;578:80–84. doi: 10.1016/j.febslet.2004.10.074. [DOI] [PubMed] [Google Scholar]
- Case A, Stein RL. Kinetic analysis of the interaction of tissue transglutaminase with a nonpeptidic slow-binding inhibitor. Biochemistry. 2007;46:1106–1115. doi: 10.1021/bi061787u. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Sheu KF, Burke JR, Onodera O, Strittmatter WJ, Roses AD, Blass JP. Polyglutamine domains are substrates of tissue transglutaminase: does transglutaminase play a role in expanded CAG/poly-Q neurodegenerative diseases? J Neurochem. 1997a;69:431–434. doi: 10.1046/j.1471-4159.1997.69010431.x. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Sheu KR, Burke JR, Onodera O, Strittmatter WJ, Roses AD, Blass JP. Transglutaminase-catalyzed inactivation of glyceraldehyde 3-phosphate dehydrogenase and α-ketoglutarate dehydrogenase complex by polyglutamine domains∼of pathological length. Proc Natl Acad Sci USA. 1997b;94:12604–12609. doi: 10.1073/pnas.94.23.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJL, Wang J, Pasternack R, Fuchsbauer HL, Sheu RK, Blass JP. Lysine-rich histone (H1) is a lysyl substrate of tissue transglutaminase: possible involvement of transglutaminase in the formation of nuclear aggregates in (CAG)n/Qn expansion diseases. Dev Neurosci. 2000;22:404–417. doi: 10.1159/000017470. [DOI] [PubMed] [Google Scholar]
- Cooper AJL, Jeitner TM, Gentile V, Blass JP. Cross linking of polyglutamine domains catalyzed by tissue transglutaminase is greatly favored with pathological-length repeats: does transglutaminase activity play a role in (CAG)n/Qn-expansion diseases? Neurochem Int. 2002;40:53–67. doi: 10.1016/s0197-0186(01)00058-4. [DOI] [PubMed] [Google Scholar]
- Cordella-Miele E, Miele L, Beninati S, Mukherjee AB. Transglutaminase-catalyzed incorporation of polyamines into phospholipase A2. J Biochem. 1993;113:164–173. doi: 10.1093/oxfordjournals.jbchem.a124021. [DOI] [PubMed] [Google Scholar]
- Datta S, Antonyak MA, Cerione RA. Importance of Ca2+-dependent transamidation activity in the protection afforded by tissue transglutaminase against doxorubicin-induced apoptosis. Biochemistry. 2006;45:13163–13174. doi: 10.1021/bi0606795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeoglu A, Kubilus JK, Jeitner TM, et al. Therapeutic effects of cystamine in a murine model of Huntington's disease. J Neurosci. 2002;22:8942–8950. doi: 10.1523/JNEUROSCI.22-20-08942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase K, et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995;14:1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Johnson GVW. Transglutaminase catalyzes the formation of sodium dodecyl sulfate-insoluble, Alz-50-reactive polymers of tau. J Neurochem. 1993;61:1159–1162. doi: 10.1111/j.1471-4159.1993.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Johnson GVW. Transglutaminase facilitates the formation of polymers of the beta-amyloid peptide. Brain Res. 1994;651:129–133. doi: 10.1016/0006-8993(94)90688-2. [DOI] [PubMed] [Google Scholar]
- Duval E, Case A, Stein RL, Cuny GD. Structure-activity relationship study of novel tissue transglutaminase inhibitors. Bioorg Med Chem Lett. 2005;15:1885–1889. doi: 10.1016/j.bmcl.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Festoff BW, SantaCruz K, Arnold PM, Sebastian CT, Davies PJA, Citron BA. Injury-induced “switch” from GTP-regulated to novel GTP-independent isoform of tissue transglutaminase in the rat spinal cord. J Neurochem. 2002;81:708–718. doi: 10.1046/j.1471-4159.2002.00850.x. [DOI] [PubMed] [Google Scholar]
- Fink ML, Folk JE. γ-Glutamylamine cyclotransferase. An enzyme involved in the catabolism of ε-(γ-glutamyl)lysine and other gamma-glutamylamines. Mol Cell Biochem. 1981;38(Spec No):59–67. doi: 10.1007/BF00235688. [DOI] [PubMed] [Google Scholar]
- Fox JH, Barber DS, Singh B, et al. Cystamine increases L-cysteine levels in Huntington's disease transgenic mouse brain and in a PC12 model of polyglutamine aggregation. J Neurochem. 2004;91:413–422. doi: 10.1111/j.1471-4159.2004.02726.x. [DOI] [PubMed] [Google Scholar]
- Frankel D, Schipper HM. Cysteamine pretreatment of the astroglial substratum (mitochondrial iron sequestration) enhances PC12 cell vulnerability to oxidative injury. Exp Neurol. 1999;160:376–385. doi: 10.1006/exnr.1999.7214. [DOI] [PubMed] [Google Scholar]
- Gentile V, Cooper AJL. Transglutaminases - possible drug targets in human diseases. Curr Drug Targets CNS Neurol Disord. 2004;3:99–104. doi: 10.2174/1568007043482552. [DOI] [PubMed] [Google Scholar]
- Gentile V, Sepe C, Calvani M, Melone MA, Cotrufo R, Cooper AJL, Blass JP, Peluso G. Tissue transglutaminase-catalyzed formation of high-molecular-weight aggregates in vitro is favored with long polyglutamine domains: a possible mechanism contributing to CAG-triplet diseases. Arch Biochem Biophys. 1998;352:314–321. doi: 10.1006/abbi.1998.0592. [DOI] [PubMed] [Google Scholar]
- Gillet SM, Chica RA, Keillor JW, Pelletier JN. Expression and rapid purification of highly active hexahistidine-tagged guinea pig liver transglutaminase. Protein Expr Purif. 2004;33:256–264. doi: 10.1016/j.pep.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Goldstone SD, Fragonas JC, Jeitner TM, Hunt NH. Transcription factors as targets for oxidative signalling during lymphocyte activation. Biochim Biophys Acta. 1995;1263:114–122. doi: 10.1016/0167-4781(95)00088-x. [DOI] [PubMed] [Google Scholar]
- Gomez-Santos C, Ferrer I, Santidrian AF, Barrachina M, Gil J, Ambrosio S. Dopamine induces autophagic cell death and alpha-synuclein increase in human neuroblastoma SH-SY5Y cells. J Neurosci Res. 2003;73:341–350. doi: 10.1002/jnr.10663. [DOI] [PubMed] [Google Scholar]
- Green H. Human genetic diseases due to codon reiteration: relationship to an evolutionary mechanism. Cell. 1993;74:955–956. doi: 10.1016/0092-8674(93)90718-6. [DOI] [PubMed] [Google Scholar]
- Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Acshlimann D. Autoantibodies in gluten ataxia recognize a novel neural transglutaminase. Ann Neurol. 2008;64:332–343. doi: 10.1002/ana.21450. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Zhao C, Speier AC, Woodard GA, Li S, Li Z, Walz T. Transglutaminase induces protofibril-like amyloid beta-protein assemblies that are protease-resistant and inhibit long-term potentiation. J Biol Chem. 2008;283:16790–16800. doi: 10.1074/jbc.M802215200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho GJ, Gregory EJ, Smirnova IV, Zoubine MN, Festoff BW. Cross-linking of beta-amyloid protein precursor catalyzed by tissue transglutaminase. FEBS Lett. 1994;349:151–154. doi: 10.1016/0014-5793(94)00663-6. [DOI] [PubMed] [Google Scholar]
- Ikura K, Takahata K, Sasaki R. Cross-linking of a synthetic partial-length (1–28) peptide of the Alzheimer beta/A4 amyloid protein by transglutaminase. FEBS Lett. 1993;326:109–111. doi: 10.1016/0014-5793(93)81772-r. [DOI] [PubMed] [Google Scholar]
- Jeitner TM, Bogdanov MB, Matson WR, et al. Nε-(γ-L-Glutamyl)-L-lysine (GGEL) is increased in cerebrospinal fluid of patients with Huntington's disease. J Neurochem. 2001;79:1109–1112. doi: 10.1046/j.1471-4159.2001.00673.x. [DOI] [PubMed] [Google Scholar]
- Jeitner TM, Delikatny EJ, Ahlqvist J, Capper H, Cooper AJL. Mechanism for the inhibition of transglutaminase 2 by cystamine. Biochem Pharmacol. 2005;69:961–970. doi: 10.1016/j.bcp.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Jeitner TM, Matson WR, Folk JE, Blass JP, Cooper AJL. Increased levels of gamma-glutamylamines in Huntington disease CSF. J Neurochem. 2008;106:37–44. doi: 10.1111/j.1471-4159.2008.05350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PH, Sorensen ES, Petersen TE, Gliemann J, Rasmussen LK. Residues in the synuclein consensus motif of the alpha-synuclein fragment, NAC, participate in transglutaminase-catalysed cross-linking to Alzheimer-disease amyloid β A4 peptide. Biochem J. 1995;310:91–94. doi: 10.1042/bj3100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson GVW, LeShoure R., Jr Immunoblot analysis reveals that isopeptide antibodies do not specifically recognize the ε-(γ-glutamyl)lysine bonds formed by transglutaminase activity. J Neurosci Methods. 2004;134:151–158. doi: 10.1016/j.jneumeth.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Johnson GVW, Cox TM, Lockhart JP, Zinnerman MD, Miller ML, Powers RE. Transglutaminase activity is increased in Alzheimer's disease brain. Brain Res. 1997;751:323–329. doi: 10.1016/s0006-8993(96)01431-x. [DOI] [PubMed] [Google Scholar]
- Kahlem P, Terre C, Green H, Djian P. Peptides containing glutamine repeats as substrates for transglutaminase-catalyzed cross-linking: relevance to diseases of the nervous system. Proc Natl Acad Sci USA. 1996;93:14580–14585. doi: 10.1073/pnas.93.25.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpuj MV, Garren H, Slunt H, Price DL, Gusella J, Becher MW, Steinman L. Transglutaminase aggregates huntingtin into nonamyloidogenic polymers, and its enzymatic activity increases in Huntington's disease brain nuclei. Proc Natl Acad Sci USA. 1999;96:7388–7393. doi: 10.1073/pnas.96.13.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabe S, Ikuta T, Ohba M, Chida K, Ueda E, Yamanishi K, Kuroki T. Cholesterol sulfate activates transcription of transglutaminase 1 gene in normal human keratinocytes. J Invest Dermatol. 1998;111:1098–1102. doi: 10.1046/j.1523-1747.1998.00441.x. [DOI] [PubMed] [Google Scholar]
- Kim SY, Grant P, Lee JH, Pant HC, Steinert PM. Differential expression of multiple transglutaminases in human brain Increased expression and cross-linking by transglutaminases 1 and 2 in Alzheimer's disease. J Biol Chem. 1999;274:30715–30721. doi: 10.1074/jbc.274.43.30715. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park YW, Lee YS, Jeoung D. Hyaluronic acid induces transglutaminase II to enhance cell motility; role of Rac1 and FAK in the induction of transglutaminase II. Biotechnol Lett. 2008;30:31–39. doi: 10.1007/s10529-007-9496-1. [DOI] [PubMed] [Google Scholar]
- Konno T, Morii T, Shimizu H, Oiki S, Ikura K. Paradoxical inhibition of protein aggregation and precipitation by transglutaminase-catalyzed intermolecular cross-linking. J Biol Chem. 2005;280:17520–17525. doi: 10.1074/jbc.M413988200. [DOI] [PubMed] [Google Scholar]
- Krasnikov BF, Kim SY, McConoughey SJ, et al. Transglutaminase activity is present in highly purified nonsynaptosomal mouse brain and liver mitochondria. Biochemistry. 2005;44:7830–7843. doi: 10.1021/bi0500877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuemmerle S, Gutekunst CA, Klein AM, Li XJ, Li SH, Beal MF, Hersch SM, Ferrante RJ. Huntington aggregates may not predict neuronal death in Huntington's disease. Ann Neurol. 1999;46:842–849. [PubMed] [Google Scholar]
- Kuncio GS, Tsyganskaya M, Zhu J, Liu SL, Nagy L, Thomazy V, Davies PJA, Zern MA. TNF-α modulates expression of the tissue transglutaminase gene in liver cells. Am J Physiol. 1998;274:G240–G245. doi: 10.1152/ajpgi.1998.274.2.G240. [DOI] [PubMed] [Google Scholar]
- Lai TS, Tucker T, Burke JR, Strittmatter WJ, Greenberg CS. Effect of tissue transglutaminase on the solubility of proteins containing expanded polyglutamine repeats. J Neurochem. 2004;88:1253–1260. doi: 10.1046/j.1471-4159.2003.02249.x. [DOI] [PubMed] [Google Scholar]
- Lesort M, Attanavanich K, Zhang J, Johnson GVW. Distinct nuclear localization and activity of tissue transglutaminase. J Biol Chem. 1998;273:11991–11994. doi: 10.1074/jbc.273.20.11991. [DOI] [PubMed] [Google Scholar]
- Lesort M, Chun W, Johnson GVW, Ferrante RJ. Tissue transglutaminase is increased in Huntington's disease brain. J Neurochem. 1999;73:2018–2027. [PubMed] [Google Scholar]
- Lesort M, Lee M, Tucholski J, Johnson GVW. Cystamine inhibits caspase activity Implications for the treatment of polyglutamine disorders. J Biol Chem. 2003;278:3825–3830. doi: 10.1074/jbc.M205812200. [DOI] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- Mastroberardino PG, Iannicola C, Nardacci R, et al. ‘Tissue’ transglutaminase ablation reduces neuronal death and prolongs survival in a mouse model of Huntington's disease. Cell Death Differ. 2002;9:873–880. doi: 10.1038/sj.cdd.4401093. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Medvedev A, Saunders NA, Matsuura H, Chistokhina A, Jetten AM. Regulation of the transglutaminase I gene Identification of DNA elements involved in its transcriptional control in tracheobronchial epithelial cells. J Biol Chem. 1999;274:3887–3896. doi: 10.1074/jbc.274.6.3887. [DOI] [PubMed] [Google Scholar]
- Miller CC, Anderton BH. Transglutaminase and the neuronal cytoskeleton in Alzheimer's disease. J Neurochem. 1986;46:1912–1922. doi: 10.1111/j.1471-4159.1986.tb08513.x. [DOI] [PubMed] [Google Scholar]
- Miller ML, Johnson GVW. Transglutaminase cross-linking of the tau protein. J Neurochem. 1995;65:1760–1770. doi: 10.1046/j.1471-4159.1995.65041760.x. [DOI] [PubMed] [Google Scholar]
- Murthy SN, Wilson JH, Lukas TJ, Kuret J, Lorand L. Cross-linking sites of the human tau protein, probed by reactions with human transglutaminase. J Neurochem. 1998;71:2607–2614. doi: 10.1046/j.1471-4159.1998.71062607.x. [DOI] [PubMed] [Google Scholar]
- Nagy L, Saydak M, Shipley N, et al. Identification and characterization of a versatile retinoid response element (retinoic acid receptor response element-retinoid X receptor response element) in the mouse tissue transglutaminase gene promoter. J Biol Chem. 1996;271:4355–4365. doi: 10.1074/jbc.271.8.4355. [DOI] [PubMed] [Google Scholar]
- Nemes Z, Devreese B, Steinert PM, Van Beeumen J, Fesus L. Cross-linking of ubiquitin, HSP27, parkin, and alpha-synuclein by γ-glutamyl-ε-lysine bonds in Alzheimer's neurofibrillary tangles. FASEB J. 2004;18:1135–1137. doi: 10.1096/fj.04-1493fje. [DOI] [PubMed] [Google Scholar]
- Pardin C, Pelletier JN, Lubell WD, Keillor JW. Cinnamoyl inhibitors of tissue transglutaminase. J Org Chem. 2008a;73:5766–5775. doi: 10.1021/jo8004843. [DOI] [PubMed] [Google Scholar]
- Pardin C, Roy I, Lubell WD, Keillor JW. Reversible and Competitive Cinnamoyl Triazole Inhibitors of Tissue Transglutaminase. Chem Biol Drug Des. 2008b;72:189–196. doi: 10.1111/j.1747-0285.2008.00696.x. [DOI] [PubMed] [Google Scholar]
- Park KC, Chung KC, Kim YS, Lee J, Joh TH, Kim SY. Transglutaminase 2 induces nitric oxide synthesis in BV-2 microglia. Biochem Biophys Res Commun. 2004;323:1055–1062. doi: 10.1016/j.bbrc.2004.08.204. [DOI] [PubMed] [Google Scholar]
- Pastuszko A, Wilson DF, Ereciska M. A role for transglutaminase in neurotransmitter release by rat brain synaptosomes. J Neurochem. 1986;46:499–508. doi: 10.1111/j.1471-4159.1986.tb12996.x. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Mahoney SA, Haynes LW. Transglutaminase C in cerebellar granule neurons: regulation and localization of substrate cross-linking. Neuroscience. 1995;65:1063–1076. doi: 10.1016/0306-4522(94)00556-k. [DOI] [PubMed] [Google Scholar]
- Piacentini M, Martinet N, Beninati S, Folk JE. Free and protein-conjugated polyamines in mouse epidermal cells Effect of high calcium and retinoic acid. J Biol Chem. 1988;263:3790–3794. [PubMed] [Google Scholar]
- Pinto JT, Van Raamsdonk JM, Leavitt BR, Hayden MR, Jeitner TM, Thaler HT, Krasnikov BF, Cooper AJL. Treatment of YAC128 mice and their wild-type littermates with cystamine does not lead to its accumulation in plasma or brain: implications for the treatment of Huntington disease. J Neurochem. 2005;94:1087–1101. doi: 10.1111/j.1471-4159.2005.03255.x. [DOI] [PubMed] [Google Scholar]
- Polakowska RR, Graf BA, Falciano V, LaCelle P. Transcription regulatory elements of the first intron control human transglutaminase type I gene expression in epidermal keratinocytes. J Cell Biochem. 1999;73:355–369. [PubMed] [Google Scholar]
- Rasmussen LK, Sorensen ES, Petersen TE, Gliemann J, Jensen PH. Identification of glutamine and lysine residues in Alzheimer amyloid beta A4 peptide responsible for transglutaminase-catalysed homopolymerization and cross-linking to α 2M receptor. FEBS Lett. 1994;338:161–166. doi: 10.1016/0014-5793(94)80356-0. [DOI] [PubMed] [Google Scholar]
- Ritter SJ, Davies PJA. Identification of a transforming growth factor-beta1/bone morphogenetic protein 4 (TGF-beta1/ BMP4) response element within the mouse tissue transglutaminase gene promoter. J Biol Chem. 1998;273:12798–12806. doi: 10.1074/jbc.273.21.12798. [DOI] [PubMed] [Google Scholar]
- Sárvari M, Karpati L, Fésüs L, Deli L, Muszbek L, Nemes Z. Competitive enzyme-linked immunosorbent assay for N ε γ-glutamyl lysine. Anal Biochem. 2002;311:187–190. doi: 10.1016/s0003-2697(02)00422-0. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Abraham C, Ihara Y. Brain transglutaminase: in vitro crosslinking of human neurofilament proteins into insoluble polymers. Proc Natl Acad Sci USA. 1982a;79:6070–6074. doi: 10.1073/pnas.79.19.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ, Ihara Y, Salazar FJ. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982b;215:1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Sieradzan KA, Mann DM. The selective vulnerability of nerve cells in Huntington's disease. Neuropathol Appl Neurobiol. 2001;27:1–21. doi: 10.1046/j.0305-1846.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- Sohn J, Kim TI, Yoon YH, Kim JY, Kim SY. Novel transglutaminase inhibitors reverse the inflammation of allergic conjunctivitis. J Clin Invest. 2003;111:121–128. doi: 10.1172/JCI15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack EC, Ferro JL, Kim J, et al. Therapeutic attenuation of mitochondrial dysfunction and oxidative stress in neurotoxin models of Parkinson's disease. Biochim Biophys Acta. 2008;1782:151–162. doi: 10.1016/j.bbadis.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Tucholski J, Kuret J, Johnson GVW. Tau is modified by tissue transglutaminase in situ: possible functional and metabolic effects of polyamination. J Neurochem. 1999;73:1871–1880. [PubMed] [Google Scholar]
- Van Raamsdonk JM, Pearson J, Bailey CD, Rogers DA, Johnson GVW, Hayden MR, Leavitt BR. Cystamine treatment is neuroprotective in the YAC128 mouse model of Huntington disease. J Neurochem. 2005;95:210–220. doi: 10.1111/j.1471-4159.2005.03357.x. [DOI] [PubMed] [Google Scholar]
- Vermes I, Steur EN, Jirikowski GF, Haanen C. Elevated concentration of cerebrospinal fluid tissue transglutaminase in Parkinson's disease indicating apoptosis. Mov Disord. 2004;19:1252–1254. doi: 10.1002/mds.20197. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang CE, Orr A, Tydlacka S, Li SH, Li XJ. Impaired ubiquitin-proteasome system activity in the synapses of Huntington's disease mice. J Cell Biol. 2008;180:1177–1189. doi: 10.1083/jcb.200709080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan ZH, Noonan S, Nagy L, Davies PJA, Stein JP. Retinoic acid induction of the tissue transglutaminase promoter is mediated by a novel response element. Mol Cell Endocrinol. 1996;120:203–212. doi: 10.1016/0303-7207(96)03826-9. [DOI] [PubMed] [Google Scholar]
- Zainelli GM, Ross CA, Troncoso JC, Muma NA. Transglutaminase cross-links in intranuclear inclusions in Huntington disease. J Neuropathol Exp Neurol. 2003;62:14–24. doi: 10.1093/jnen/62.1.14. [DOI] [PubMed] [Google Scholar]
- Zainelli GM, Dudek NL, Ross CA, Kim SY, Muma NA. Mutant huntingtin protein: a substrate for transglutaminase 1, 2, and 3. J Neuropathol Exp Neurol. 2005;64:58–65. doi: 10.1093/jnen/64.1.58. [DOI] [PubMed] [Google Scholar]
- Zemaitaitis MO, Lee JM, Troncoso JC, Muma NA. Transglutaminase-induced cross-linking of tau proteins in progressive supranuclear palsy. J Neuropathol Exp Neurol. 2000;59:983–989. doi: 10.1093/jnen/59.11.983. [DOI] [PubMed] [Google Scholar]
- Zemaitaitis MO, Kim SY, Halverson RA, Troncoso JC, Lee JM, Muma NA. Transglutaminase activity, protein, and mRNA expression are increased in progressive supranuclear palsy. J Neuropathol Exp Neurol. 2003;62:173–184. doi: 10.1093/jnen/62.2.173. [DOI] [PubMed] [Google Scholar]
- Zhang W, Johnson BR, Suri DE, Martinez J, Bjornsson TD. Immunohistochemical demonstration of tissue transglutaminase in amyloid plaques. Acta Neuropathol. 1998;96:395–400. doi: 10.1007/s004010050910. [DOI] [PubMed] [Google Scholar]