Abstract
Flaxseed is a rich source of lignan and has been shown to reduce androgen levels in men with prostate cancer. Polycystic ovarian syndrome (PCOS), a common endocrine disorder among women in their reproductive years, also is associated with high levels of androgens and is frequently accompanied by hirsutism, amenorrhea and obesity. This clinical case study describes the impact of flaxseed supplementation (30 g/day) on hormonal levels in a 31-year old woman with PCOS. During a four month period, the patient consumed 83% of the flaxseed dose. Heights, weights, and fasting blood samples taken at baseline and 4-month follow-up indicated the following values: BMI (36.0 vs. 35.7m/kg2); insulin (5.1 vs. 7.0 uIU/ml); total serum testosterone (150 ng/dl vs. 45 ng/dl); free serum testosterone (4.7 ng/dl vs. 0.5 ng/dl); and % free testosterone (3.1% vs. 1.1%). The patient also reported a decrease in hirsutism at the completion of the study period. The clinically-significant decrease in androgen levels with a concomitant reduction in hirsutism reported in this case study demonstrates a need for further research of flaxseed supplementation on hormonal levels and clinical symptoms of PCOS.
Keywords: Androgens, Flaxseed, Polycystic Ovarian Syndrome, Diet, Intervention Studies
INTRODUCTION
Polycystic Ovarian Syndrome (PCOS) affects approximately 4 to 8% of women of reproductive age. (Carmina & Azziz, 2006) PCOS is an endocrine disorder defined by four key features: 1) ovulatory and menstrual dysfunction; 2) hyperandrogenemia; 3) clinical features of hyperandrogenism (i.e., hirsutism, acne and androgenic alopecia); and 4) polycystic ovaries (Azziz et al, 2006, Marshall, 2001, Norman 2002)
To date, the study of diet in the treatment of PCOS has focused on weight reduction since roughly half of women with PCOS are obese, and weight loss is helpful in normalizing hormonal levels and clinical symptoms (Gambineri et al, 2002, Marshall, 2001, King, 2006). While other components of the diet have been suggested as potentially beneficial in ameliorating the hormonal disequilibrium associated with PCOS, beyond weight management the current dietary recommendations provided for this disease parallel those established for the general population, i.e., a low fat, moderate protein diet with increased amounts of fruits, vegetables, and whole grains. (Norman et al, 2002)
Over the past few decades, high fiber diets have been shown to influence the hormonal milieu (Adlercreutz et al. 1987, Monroe et al. 2007) and may hold promise in the treatment of PCOS. Previous research suggests that consumption of high lignan foods may cause binding of testosterone in the enterohepatic circulation and subsequent excretion (Adlercreutz et al, 1987). Lignan also has been found to increase levels of sex hormone binding globulin and thus reduce the amount of free circulating testosterone (Adlercreutz et al, 1987, Martin et al., 1996), though such a reduction has not been observed in all studies (Low et al, 2005). Lignan also may hinder the production of 5 α–reductase (the enzyme responsible for converting testosterone into dihydrotestosterone, the more biologically active and potent form)(Evans et al, 1995). Subsequent research by Morton and colleagues (1997) found that high lignan diets may be protective against prostate cancer, another disorder which is linked to high androgen levels.
Flaxseed, a food generally renowned for its omega-3 fatty acid content, also is one of the richest sources of dietary lignan, having levels that are 800-fold over that of most other foods, (Thompson, 1995). Prior studies on the use of flaxseed or isolated lignan suggest that it may decrease androgen levels and normalize lipid levels; however, most of this research has been conducted in male subjects. (Adlercreutz et al, 1987, Demark-Wahnefried et al, 2001, Shultz & Leklam, 1983, Slavin et al, 1997). Currently, there are no published reports on the use of flaxseed in the treatment of PCOS, even though it also is an androgen-related disorder. The following case study provides preliminary evidence that flaxseed supplementation may indeed help regulate androgen levels in women with PCOS.
METHODS
The patient was a 31-year-old college-educated, white female who was newly diagnosed with PCOS. She had yet to begin any sort of pharmacotherapy and expressed an interest in flaxseed supplementation after hearing about an NIH-funded phase II clinical trial that we were conducting among men with prostate cancer (CA85740). The patient was counseled that no data yet existed on flaxseed supplementation and PCOS, however was intrigued by study findings among men with prostate cancer which showed a reduction in serum testosterone. She elected to pursue flaxseed supplementation independently and this report chronicles her experience in the clinical care setting
The flaxseed product that was recommended to this patient was a product that we had used in two previously reported studies and was commercially available (Demark-Wahnefried et al, 2001, Demark-Wahnefried et al, 2003); Alena® now marketed under the name of Mega Omega® (ENRECO, Inc, Newton, WI), is comprised primarily of stabilized ground flaxseed (75 g/100g weight), but also contains emulsifiers (for ease of mixing), as well as oat flour and fructose (to increase palatability). The patient was provided with instruction regarding storage and use of the product and was encouraged to start with a dose of 10 grams for the first 3-days, 20 grams for the next 3-days and onto a dose of 30 grams/day for the duration of the 4-month study period. This stepped dose approach had been used successfully in our previous studies, and was helpful in minimizing the gastrointestinal discomfort associated with this high fiber food. (Demark-Wahnefried et al, 2001, Demark-Wahnefried et al, 2003). Given that flaxseed is usually not consumed alone but is mixed into various foods, we also provided the patient with a list of foods recommended by previous study participants, such as mixing it into applesauce, yogurt, juices, grits, oatmeal or sprinkling it onto cereal. The patient also received log sheets and was asked to record the dose consumed each day, i.e., all of the dose, less than the full dose but at least ¾ of it, less than ¾’s of the full dose but at least ½ of it, less than ½ of the dose but at least ¼ of it or less than ¼ of the dose. The patient also was contacted weekly throughout the 4-month period to assess if there were questions or problems, and to review adherence.
Measures
At baseline, the patient’s height was measured using a fixed stadiometer, and at both baseline and follow-up her weight was assessed on a routinely calibrated platform scale. At both time points, the patient was instructed to report for phlebotomy after an overnight fast of 12-hours. Blood was drawn via venipuncture and collected in serum separator vacutainers. After allowing the blood to clot at room temperature, the tube was centrifuged, and sera was analyzed directly thereafter using immunochemiluminometric methods on a Beckman Coulter Access 2 system with kits for ultrasensitive insulin and testosterone (Beckman Coulter, Inc., Fullerton, CA)(Allauzen et al, 1994; Allauzen et al, 1995, Brutis & Ashwood 1996). Assays were conducted at the Duke University Health System Clinical Laboratory, a College of American Pathologists (CAP) and Clinical Laboratory Improvement Act (CLIA) certified laboratory. To provide high levels of quality assurance test performance was validated prior to patient-sample testing using three levels of assayed commercial control material (BioRad Liquichek Immunoassay Plus) at the beginning of each 8 hour shift (as well as following instrument maintenance or assay calibration). Results were compared to acceptable recovery ranges established by the manufacturer’s assayed values, and overall day-to-day recovery. Cumulative and monthly comparison reports (compared against roughly 40 other CLIA-laboratories) indicated that the Duke laboratory had standard deviation indexes (SDI) which were -.31 for insulin and -.19 for testosterone in the ranges corresponding to the case study’s levels, indicating excellent interlaboratory accuracy. The imprecision associated with the assays themselves were less than 10% for insulin (Krouwer & Rabinowitz, 1984, National Committee of Clinical Laboratory Standards, 1984), and less than 20% for testosterone (Krouwer & Rabinowitz, 1984, National Committee of Clinical Laboratory Standards, 1999). The percentage of free testosterone was derived from assayed androgen levels.
RESULTS
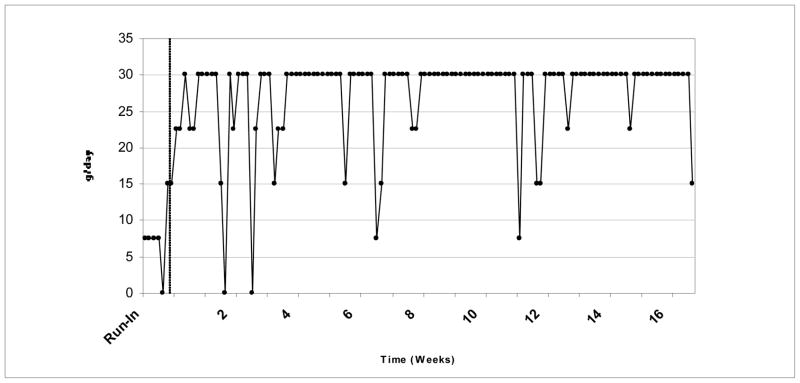
After the initial run-in period, the patient adhered to the daily 30 g. dose of flaxseed 93 days out of a possible 112 days during the observational period. Daily intake over the 4-month period is depicted in Figure 1, with roughly 83% of the recommended dose consumed.
Figure 1.
Daily consumption of flaxseed during 4-month period.
Pre-post levels of physiologic measures are provided in Table 1 along with normal reference ranges. A significant decrease in androgen levels was observed, with a 70% decrease in total serum testosterone, an 89% decrease in free serum testosterone, and a 65% decrease in the % free testosterone observed. The patient also reported a decrease in hirsutism at the conclusion of the 4-month period, though no formal measures were taken, In addition, despite a modest amount of weight loss (0.9 kg) the patient’s insulin levels increased 37%; however, remained well within the reference range.
TABLE 1.
Patient’s physiologic measurements at baseline and 4-month follow-up period.
| Baseline | 4-month follow-up | |
|---|---|---|
| Weight (kg) | 95.4 | 94.5 |
| BMI (kg/m2) | 36 | 35.7 |
| Insulin (uIU/ml)a | 5.1 | 7.0 |
| Total serum testosterone (ng/dL)b | 150 | 45 |
| Free serum testosterone (ng/dL)c | 4.7 | 0.5 |
| % free testosteroned | 3.1 | 1.1 |
The normal reference range for insulin is 1.9–23 uIU/ml
The normal reference range for total serum testosterone for adult females is 12–72 g/dL
The normal reference range for free serum testosterone for adult females is 0.3–1.9 g/dL
The normal reference range for percentage free testosterone for adult females is 0.9–3.8
DISCUSSION
To our knowledge this is the first report of the effect of flaxseed on PCOS. Findings suggest that flaxseed may have a profound impact on testosterone levels, and also may diminish symptoms associated with hyperandrogenism, such as hirsutism. The reductions in androgen levels observed in this case study far surpass those reported with any other dietary intervention conducted to date. For example, Holte and colleagues (1995) reported a decrease of 36% in serum testosterone among (n=13) PCOS patients who participated in a weight reduction intervention and experienced a mean weight loss of 12.4 kg. An effect size of similar magnitude was observed by Kiddy and associates (1992) who reported a 31% decrease in free testosterone levels over a 6–7 month period with a 1000 kcal low fat diet among (n=24) women with PCOS. An earlier study by Pasquali and colleagues (1989) reported comparable results during an 8-month period with a 1000–1500 kcal diet of a 36% decrease in testosterone levels in (n=20) women with PCOS. Further, our results which show significant declines in both free and total testosterone ranging from 70–89% are in clear contrast to those of Huber-Buchholz and associates (1999), who tested a diet and exercise weight management intervention (n=18) and observed a 21% decrease in free testosterone with a concomitant 10% increase in total testosterone. Thus, the data from this case study are intriguing since results suggest that the reductions in androgen levels afforded by flaxseed supplementation are roughly 2–4 fold higher than those reported with other dietary interventions conducted previously.
The reductions in androgen levels observed in this case study of PCOS also exceed those reported with flaxseed supplementations among men with either prostate cancer or abnormal prostatic biopsies. In a pilot study of flaxseed supplementation and concurrent dietary fat restriction among men with prostate cancer (n=25), Demark-Wahnefried and associates (2001) found only a 15% decrease in testosterone levels; however this study was only conducted over a period averaging 34-days. Furthermore, no difference in testosterone levels was observed with flaxseed supplementation and dietary fat restriction in a study of men with abnormal prostatic biopsies (n=15) (Demark-Wahnefried et al, 2003). Thus, the magnitude of effect on androgen levels observed in this case study is fairly impressive, as is the accompanying self-report of diminished hirsutism (a troubling and prevalent symptom in this patient population).
Currently the standard of care treatment for women with PCOS ranges from lifestyle modification to pharmacological interventions. Lifestyle modifications are associated with diet, weight loss, and exercise programs. Pharmacological interventions include; antiandrogens (Spironolactone, Flutamide), insulin lower agents (Metformin and thiazolidinediones), and estrogen-progestin combination (Oral contraceptives). (Dronavalli et al, 2007) While effective, such treatment is associated with substantial cost and may cause various side effects, such as irregular menstruation, gastrointestinal symptoms, weight gain, and increased insulin resistance. In contrast, the costs and side effects associated with flaxseed supplementation are minimal in comparison and primary relate to flaxseed’s laxative effect.
The limitations of this case study are clear, in that the data emanate from a sample of only one and from data collected at only two time points (baseline and follow-up). Furthermore, while we attempted to reduce variability in androgen assessments by performing phlebotomy during identical windows of time during the day, and by the same laboratory, the direct assays used to assess androgen levels, have been criticized for their unreliability, especially within ranges that fall below 300 ng/dl. (Rosner et al, 2007). Finally, clinical symptoms of PCOS were not collected using a standardized protocol and validated instruments. Despite these limitations, the data are still compelling. Such data point to the need for further research in this area, to more thoroughly explore the effects of flaxseed supplementation on hormonal markers and clinical symptoms associated with PCOS.
Acknowledgments
The authors thank ENRECO, Inc., Newton, WI for provision of the flaxseed supplement used in this investigation.
References
- Adlercreutz H, Hockerstedt K, Hockerstedt K, Bannwart C, Bloigu S, Hamalainen E, Fotsis T, Ollus A. Effect of dietary components, including lignans and phytoestrogens, on enterohepatic circulation and liver metabolism of estrogens and on sex hormone binding globulin (SHBG) Journal of Steroid Biochemistry. 1987;27:1135–1144. doi: 10.1016/0022-4731(87)90200-7. [DOI] [PubMed] [Google Scholar]
- Allauzen S, Salhi SL, Piechaczyk M, Bastide M, Pau B, Bounani M. An efficient immunization protocol for production of monoclonal antibodies against human soluble insulin. Immunoloy Letters. 1994;40:1–6. doi: 10.1016/0165-2478(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Allauzen S, Mani JC, Granier C, Pau B, Bounani M. Epitope mapping and binding analysis of insulin monoclonal antibodies using a biosensor approach. Journal of Immunological Methods. 1995;183:27–32. doi: 10.1016/0022-1759(95)00020-b. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Androgen Excess Society. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. Journal of Clinical Endocrinology & Metabolism. 2006;91:4237–45. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- Brutis CA, Ashwood ER, editors. Tietz fundamentals of clinical chemistry. 4. Philadelphia, PA: W.B. Saunders; 1996. pp. 671–672. [Google Scholar]
- Carmina E, Azziz R. Diagnosis, phenotype, and prevalence of polycystic ovary syndrome. Fertility & Sterility 86 Suppl. 2006;1:S7–8. doi: 10.1016/j.fertnstert.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Price DT, Polascik TJ, Robertson CN, Anderson EE, Paulson DF, Walther PJ, Gannon M, Vollmer RT. Pilot study of dietary fat restriction and flaxseed supplementation in men with prostate cancer before surgery: exploring the effects on hormonal levels, prostate-specific antigen, and histopathologic features. Urology. 2001;58:47–52. doi: 10.1016/s0090-4295(01)01014-7. [DOI] [PubMed] [Google Scholar]
- Demark-Wahnefried W, Robertson CN, Walther PJ, Polascik TJ, Paulson DF, Vollmer RT. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate-specific antigen. Urology. 2004;63:900–904. doi: 10.1016/j.urology.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Dronavalli S, Ehrmann DA. Pharmacologic therapy of polycystic ovary syndrome. Clinical Obstetrics and Gynecology. 2007;50:244–254. doi: 10.1097/GRF.0b013e31802f35a0. [DOI] [PubMed] [Google Scholar]
- Evans BA, Griffiths K, Morton MS. Inhibition of 5α-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. Journal of Endocrinology. 1995;147:295–302. doi: 10.1677/joe.0.1470295. [DOI] [PubMed] [Google Scholar]
- Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. International Journal of Obesity and Related Metabolic Disorders. 2002;26:883–896. doi: 10.1038/sj.ijo.0801994. [DOI] [PubMed] [Google Scholar]
- Holte J, Bergh T, Berne C, Wide L, Lithell H. Restored insulin sensitivity but persistently increased early insulin secretion after weight loss in obese women with polycystic ovary syndrome. Journal of Clinical Endocrinology and Metabolism. 1995;80:2586–2593. doi: 10.1210/jcem.80.9.7673399. [DOI] [PubMed] [Google Scholar]
- Huber-Buchholz MM, Carey DGP, Norman RJ. Restoration of reproductive potential by lifestyle modification in obese polycystic ovary syndrome: role of insulin sensitivity and luteinizing hormone. Journal of Clinical Endocrinology & Metabolism. 1999;84:1470–1474. doi: 10.1210/jcem.84.4.5596. [DOI] [PubMed] [Google Scholar]
- Kiddy DS, Hamilton-Fairley D, Bush A, Short F, Anyaoku V, Reed MJ, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clinical Endocrinology. 1992;36:105–111. doi: 10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed] [Google Scholar]
- Low YL, Taylor JI, Grace PB, Dowsett M, Folkerd E, Doody D, et al. Polymorphisms in the CYP19 gene may affect the positive correlations between serum and urine phytoestrogen metabolites and plasma androgen concentrations in men. Journal of Nutrition. 2005;135:2680–2686. doi: 10.1093/jn/135.11.2680. [DOI] [PubMed] [Google Scholar]
- King J. Polycystic Ovary Syndrome. Journal of Midwifery & Women’s Health. 2006;51:415–422. doi: 10.1016/j.jmwh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clinical Chemistry. 1984;30:290–292. [PubMed] [Google Scholar]
- Marshall K. Polycystic ovary syndrome: clinical considerations. Alternative Medicine Review. 2001;6:272–292. [PubMed] [Google Scholar]
- Martin ME, Haourigui M, Pelissero C, Benassayag C, Nunez EA. Interactions between phytoestrogens and human sex steroid binding protein. Life Sciences. 1996;58:429–36. doi: 10.1016/0024-3205(95)02308-9. [DOI] [PubMed] [Google Scholar]
- Monroe KR, Murphy SP, Henderson BE, Kolonel LN, Stanczyk FZ, Adlercreutz H, Pike MC. Dietary Fiber Intake and Endogenous Serum Hormone Levels in Naturally Postmenopausal Mexican American Women: The Multiethnic Cohort Study. Nutrition and Cancer. 2007;58:127–135. doi: 10.1080/01635580701327935. [DOI] [PubMed] [Google Scholar]
- Morton MS, Chan PS, Cheng C, Blacklock N, Matos-Ferreira A, Abranches-Monteiro L. Lignans and isoflavonoids in plasma and prostatic fluid in men: samples from Portugal, Hong Kong, and the United Kingdom. Prostate. 1997;32:122–128. doi: 10.1002/(sici)1097-0045(19970701)32:2<122::aid-pros7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- National Committee for Clinical Laboratory Standards. Tentative guideline – user evaluation of precision performance of clinical chemistry devices, EP5-T 4. 1984;(8) [Google Scholar]
- National Committee for Clinical Laboratory Standards. Approved guideline – evaluation of precision performance of clinical chemistry devices, EP5-A 19. 1999;(2) [Google Scholar]
- Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinology and Metabolism. 2002;13:251–257. doi: 10.1016/s1043-2760(02)00612-4. [DOI] [PubMed] [Google Scholar]
- Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. Journal of Clinical Endocrinology & Metabolism. 2007;92:405–13. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- Shultz TD, Leklem JE. Nutrient intake and hormonal status of premenopausal vegetarian Seventh-day Adventists and premenopausal nonvegetarians. Nutrition and Cancer. 1983;4:247–259. doi: 10.1080/01635588209513765. [DOI] [PubMed] [Google Scholar]
- Slavin J, Jacobs D, Marquart L. Whole-grain consumption and chronic disease: protective mechanisms. Nutrition and Cancer. 1997;27:14–21. doi: 10.1080/01635589709514495. [DOI] [PubMed] [Google Scholar]
- Thompson LU. Flaxseed, lignans, and cancer. In: Cunnane SC, Thompson LU, editors. Flaxseed in human nutrition. Chicago, IL: AOCS Press; 1995. pp. 219–236. [Google Scholar]