Abstract
It is well recognized that the renin-angiotensin system plays an important role in the regulation of arterial pressure and sodium homeostasis. Recent years, many studies have shown that local tissue angiotensin II levels are differentially regulated and cannot be explained on the basis of circulating concentrations. All of the components needed for angiotensin II generation are present within the various compartments in the kidney including the renal interstitium and the tubular network. The cascade of the renin-angiotensin system demonstrates three major possible sites for the pharmacological interruption of the renin-angiotensin system: the interaction of renin with its substrate, angiotensinogen, the angiotensin converting enzyme, and angiotensin II type 1 receptors. This brief article will focus on the role of the intratubular renin-angiotensin system in the pathophysiology of hypertension and the responses to the renin-angiotensin system blockade by renin inhibitors, angiotensin converting enzyme inhibitors and angiotensin II type 1 receptor blockers.
Keywords: Renin-angiotensin system, hypertension, kidney, angiotensin converting enzyme inhibitors, angiotensin II type 1 receptor blockers
INTRODUCTION
In recent years, the focus of interest on the role of the renin-angiotensin (Ang) system (RAS) in the regulation of arterial pressure and in the pathophysiology of hypertension has changed to a major emphasis on the role of the local/tissue RAS in specific tissues [1]. Various studies have demonstrated the importance of the tissue RAS in the brain [2], heart [3], adrenal glands [4], and vasculature [5], as well as in the kidney [6]. Presently, Ang converting enzyme (ACE) inhibitors (ACEI) or Ang II type 1 (AT1) receptor blockers (ARB) are recommended as first-line therapy for hypertensive patients with diabetic nephropathy [7]. The four large trials performed on type 2 diabetes showed that ARBs prevent the development of clinical proteinuria in microalbuminuric patients (IRbesartan in patients with type 2 diabetes and MicroAlbuminuria (IRMA) [8] and MicroAlbuminuria Reduction with VALsartan (MARVAL) studies [9]) and delay the progression of nephropathy towards end-stage renal failure in patients with overt nephropathy (Irbesartan Diabetic Nephropathy Trial (IDNT) [10] and Reduction of Endpoints in Non-insulin dependent diabetes mellitus with the Angiotensin II Antagonist Losartan (RENAAL) studies [11]). In the IDNT study, irbesartan showed the better renoprotective effect compared with amlodipine in patients at a late stage of type 2 diabetic nephropathy, independently from the blood pressure-lowering effects of the drugs [10, 12]. Recently, the Diabetics Exposed to Telmisartan And enalaprIL (DETAIL) trial has directly compared ACEIs and ARBs in patients with type 2 diabetes, and shown the renoprotective effects of these drugs, respectively [13]. ACEIs and ARBs are effective on these patients because of their ability to block the local RAS [14]. Furthermore, Nakao et al. [15] reported that the combination of ACEIs and ARBs was significantly better than each individual drug in renal survival of non-diabetic patients with reduced renal function and moderate daily urine protein excretion. Locally generated Ang II may be involved in the pathogenic mechanisms of chronic renal diseases [16]. The beneficial effects of RAS blockers may be a consequence of the reduction of intraglomerular capillary pressure [17], and also, a result of their antiproteinuric effect [18], antiinflammatory [19], antiproliferative [20], and antifibrotic [21, 22] properties. This brief article will focus on the role of the intratubular RAS in the pathophysiology of hypertension and the responses to RAS blockade.
INTRATUBULAR LOCALIZATION OF THE RENIN-ANGIOTENSIN SYSTEM COMPONENTS
Using in situ hybridization, Ingelfinger et al. [23] demonstrated that the angiotensinogen (AGT) gene was specifically present in the proximal tubules. Terada et al. [24] reported that AGT mRNA was expressed largely in the proximal convoluted tubules and proximal straight tubules, and that there were small amounts in glomeruli and vasa recta as revealed by reverse transcription and polymerase chain reaction. Richoux et al. [25] and Darby et al. [26, 27] showed by immunohistochemistry that renal AGT protein is specifically located in proximal convoluted tubules. Kobori et al. [28] also showed that there was strong positive immunostaining for AGT protein in proximal convoluted tubules and proximal straight tubules, and weak positive staining in glomeruli and vasa recta; however, there was no staining in distal tubules or collecting ducts.
Yanagawa et al. [29] and Moe et al. [30, 31] showed that renin mRNA and renin-like activity could be demonstrated in cultured proximal tubular cells. In addition, low but measurable renin concentrations in proximal tubule fluid have been reported in rats [32]. Interestingly, Prieto-Carrasquero et al. [33] recently reported that renin mRNA and protein are expressed in the principal cells of distal tubules of rats. Moreover, they demonstrated that renin in the distal tubular cells is upregulated by Ang II infusion and this upregulation depends on AT1 receptor activation [34]. They conclude that renin in distal tubular cells and renin in juxtaglomerular cells are separately regulated.
In terms of ACE, abundant expression of ACE mRNA [35] and protein [36] were shown in brush border of proximal tubules of human kidney. ACE has also been measured in proximal and distal tubular fluid but is more abundant in proximal tubule fluid [37].
There are two major types of Ang II receptors, AT1 receptor and type 2 (AT2) receptor, but there is much less AT2 receptors expression in adult kidneys [38, 39]. AT1 receptor mRNA has been localized to proximal convoluted and straight tubules, thick ascending limb of the loop of Henle, cortical and medullary collecting duct cells, glomeruli, arterial vasculature, vasa recta, and juxtaglomerular cells [24]. In rodents, two subtypes of AT1 receptors (AT1A and AT1B) have been demonstrated in the vasculature and glomerulus and in all nephron segments [39]. The AT1A receptor is the predominant subtype in nephron segments, whereas the AT1B receptor is more abundant than AT1A receptor in the glomerulus [40]. Studies using polyclonal and monoclonal antibodies to the AT1 receptors demonstrated that AT1 receptor protein has been localized on vascular smooth muscle cells throughout the vasculature, including the afferent and efferent arterioles and mesangial cells [41]. AT1 receptors are also present on proximal tubule brush border and basolateral membranes, thick ascending limb epithelia, distal tubules, collecting ducts, glomerular podocytes, and macula densa cells [38, 39, 41].
INTRARENAL RENIN-ANGIOTENSIN SYSTEM IN HYPERTENSION
Many studies have demonstrated that Ang II vasoconstricts both pre-glomerular and post-glomerular arterioles. Ang II exerts powerful vascular effects that elicit decreases in renal blood flow and, to a lesser extent, in glomerular filtration rate, therefore, there is usually an increase in filtration fraction [42]. All of the components needed for Ang II generation are present within the various comportments in the kidney including the renal interstitium and the tubular network. Some of the interstitial Ang II is derived from locally formed AGT and may not be dependent on circulating Ang II or AGT. In vivo and in vitro studies have shown that Ang II stimulates intrarenal AGT mRNA localized in proximal tubular cells [28, 43, 44]. Studies in Ang II-infused rats have demonstrated that augmentation of intrarenal Ang II is due, in part, to uptake of circulating Ang II via an AT1 receptor mechanism and also to sustained or enhanced intrarenal production of Ang II [45]. Schunkert et al. [44] showed that plasma Ang II upregulates renal AGT gene expression and downregulates renal renin gene expression. Kobori et al. [46] demonstrated that there were significant increases in intrarenal AGT protein, as well as AGT mRNA level, in response to 2 weeks of Ang II infusion in rats. This augmentation mechanism may be responsible for sustained or enhanced generation of AGT, leading to continued intrarenal production of Ang II under conditions of elevated circulating concentrations. Kobori et al. [47] demonstrated that urinary AGT excretion rates directly relate to kidney Ang II content, but not plasma Ang II content and suggested that urinary excretion rate of AGT provides a specific index of intrarenal AGT production in Ang II-dependent hypertension [48]. This increase is not due to increased proteinuria or the development of hypertension since urinary protein excretion in volume-dependent hypertensive rats was significantly increased more than in Ang II-dependent hypertensive rats; however, urinary AGT excretion was significantly lower in volume-dependent hypertensive rats than in Ang II-dependent hypertensive rats. The increased amounts of intact AGT in urine in Ang II-dependent hypertension suggests augmented AGT levels throughout the nephron. To the extent that renin and ACE are available along the nephron, the AGT provides substrate for continued Ang I generation and Ang II conversion in segments beyond the proximal tubules [49–51]. The sustained increase in intrarenal Ang II in a setting of hypertension can lead to progressive renal injury, proliferation and fibrosis associated with activation of several major cytokines and growth factors [52–55].
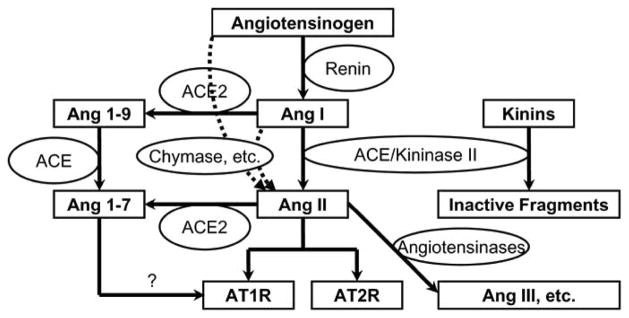
The cascade of the RAS (Fig. 1) demonstrates three major possible sites of the pharmacological interruption of the RAS: the interaction of renin with its substrate, AGT, the ACE, and the AT1 receptors.
Fig. 1.
The cascade of the renin-angiotensin system and its interaction with the kinin system.
RENIN INHIBITORS
AGT is converted into Ang I by renin (Fig. 1). It has often been suggested that the renin step should be one of the most attractive targets for the RAS blockade for two important reasons: 1) the interaction of renin with its substrate, AGT, is a rate-limiting step [56], 2) renin has a species-specificity for its substrate [57].
Fisher and Hollenberg [58] examined renal plasma flow in healthy young men receiving a low sodium intake to activate the RAS. Renin expression in principal cells of collecting ducts is further increased in Ang II-dependent hypertension [33]. Dose-responses were evaluated for different types of blockade of the RAS: ACEIs (captopril, lisinopril, and ramipril) and renin inhibitors (enalkiren and zankiren). To their surprise, the renal vasodilator response to the renin inhibitor, enalkiren, exceeded their expectations. Enalkiren (A-64662, IC50 = 0.8 nM) induced a larger increase in renal plasma flow than captopril. Similar responses were observed with zankiren (A-72517, IC50 = 1 nM). El-Amrani et al. [59] also compared systematically the renal vascular response to a renin inhibitor, remikiren (R042-5892, IC50 = 0.8 nM), an ARB (losartan), and an ACEI (lisinopril) in guinea pigs. The renin inhibitors display species specificity because renin structure varies with species. The guinea pig was selected because remikiren developed for primates, is also effective in the guinea pig. This study also showed that renin inhibitor induced a substantially larger increase in renal plasma flow, glomerular flow rate, diuresis, and natriuresis-as in the humans. The authors suggested that the potentiated response to renin inhibition could reflect greater lipophilicity and tissue penetration, leading to a local, intrarenal action at the site of Ang II production. Fisher et al. [60] suggested that renin inhibition is far more effective than ACE inhibition in blocking Ang II formation in the case of the kidney. Aliskiren (SPP100, IC50 = 0.6 nM) has the potential to become the first orally active renin inhibitor that provides a true alternative to ACEIs and ARBs in therapy for hypertension. Aliskiren acts as a transition state mimetic, inhibiting renin via hydrogen bonding of both the central hydroxy group and amino function to the catalytic Asp32 and Asp215 residues [61, 62]. Aliskiren is one of the most potent renin inhibitors yet identified with high species specificity for primate renin [62]. Nussberger et al. [63] showed that aliskiren dose-dependently decreased plasma Ang II levels in humans following oral administration. Stanton et al. [64] showed that aliskiren inhibits the production of Ang I and II in healthy volunteers and reduces blood pressure. Gradman et al. [65] showed that the administration of aliskiren is as effective as the same amount of irbesartan in lowering blood pressure. Whether or not, renin inhibition by aliskiren results in protection from cardiovascular and renal diseases, similar to that seen for ACEIs and ARBs, needs to be researched.
ANGIOTENSIN CONVERTING ENZYME INHIBITORS
The principal action of ACE inhibition is a disruption of the conversion of Ang I to Ang II and consequently, inhibition of the Ang II effects, such as vasoconstriction, growth promotion and sodium reabsorption (Fig. 1). ACE primarily cleaves a C-terminal dipeptide from substrates and is also known as peptidyl dipeptidase A. Important physiological substrates of ACE are Ang I and bradykinin. Ang I is hydrolyzed by ACE to form the potent vasopressor octa-peptide Ang II. ACE hydrolyzes a wide range of polypeptide substrates, including substance P, luteinizing hormone-releasing hormone, acetyl-Ser-Asp-Lys-Pro, and neurotensin [66, 67]. The specificity of ACE for Ang I is relatively low, and ACE inhibition triggers additional events as a result of protection of other peptides. ACE is also recognized as kininase II and ACEIs block the degradation of bradykinin which may also exert important effects [68].
Kinins act as endogenous vasodilators via stimulation of nitric oxide and release of vasodilatory prostaglandins. In the kidney, kinins contribute to the renal vasodilatory actions of ACEIs, mainly in the medullary circulation [69–73].
A recently described enzyme, termed ACE2, cleaves a single amino acid from Ang I to form Ang 1–9 and from Ang II to form Ang 1–7 [74, 75]. Ang 1–7 exerts significant vasodilator and natriuretic actions that may partially counteract the effects of Ang II [76]. Despite sharing many biochemical properties with ACE, ACE2 is insensitive to classic ACEIs [74, 77]. ACE2 expression is limited mainly to endothelial cells of the arteries, arterioles, and venules in the heart and kidney [74, 75, 77]. ACE2 is also expressed in renal tubular epithelium and vascular smooth muscle cells of the intrarenal arteries and coronary blood vessels [74, 78]. Ang 1–7, an Ang fragment generated from Ang I by action of several endopeptidases, is another peptide degraded by ACE. Although, Ang 1–7 can interact with AT1 receptors, it has been suggested that the peptide is a ligand for novel receptors, which are different from AT1 receptors or AT2 receptors, and that the vasodilatory actions of Ang 1–7 are mediated by prostaglandins and nitric oxide leading to vasodilation, natriuresis and growth inhibition [79, 80].
Multiple lines of evidence have suggested that an alternative pathway to the ACE exists for Ang II generation in the heart, large arteries, and the kidneys [81]. It has been well established that in some patient treated with ACEIs, plasma levels of Ang II return to pre-treatment levels despite effective inhibition of plasma ACE [82, 83]. This phenomenon has been attributed to actions of non-ACE enzymes that may convert Ang I to Ang II. For example, chymase is localized in heart, blood vessels, lungs and kidneys [84]. Takai et al. reported that rat vascular tissues contain ACE as the only Ang II-forming enzyme, while the vascular tissues of human, monkey, dog and hamster contain chymase in addition to ACE as Ang II forming enzymes [85]. Hollenberg et al. [14] also studied the response to Ang I in human and rabbits, and they observed marked species-specificity in vascular Ang II-forming pathways. They reported that although plasma Ang II formation is dependent on ACE, only 30–40% of the conversion of Ang I to Ang II in the human artery depends on this enzyme, whereas the rest depends on chymostatin. They also showed that Ang II formation in rodents appears to be almost entirely dependent on ACE, therefore, ACEIs will suppress Ang II completely in these animals, which contrasts with results in dogs, primates, and humans. Sadjadi et al. [86] showed that chymase activity is upregulated in the ischemic kidney of a two-kidney, one-clip renovascular hypertensive rat model. They also provided additional insight into the role of ACE-independent production of Ang II by the chymase pathway.
Hollenberg et al. also studied healthy, normotensive men under low salt intake [87]. They administrated Ang I at different doses. Then an ACEI, enalapril, was administered. During ACE inhibition, only the highest dose of Ang I raised plasma Ang II levels. Responses of plasma aldosterone concentration and blood pressure were in excellent accord with reduction in Ang II formation. However, the decrease in renal plasma flow was substantially less inhibited than expected, taking in account that ACE is also responsible for bradykinin degradation and thus vasodilator prostaglandins and nitric oxide accumulation. Data from this study showed that ACE inhibition led to non-uniform changes in the response to exogenous Ang I suggesting that intrarenal conversion of Ang I to Ang II also can occur by an ACE-independent pathway. The antihypertensive benefit of combining ACEI therapy with ARB therapy is most likely due to the blockade of ACE-independent pathway of Ang II generation.
ANGIOTENSIN II TYPE 1 RECEPTOR BLOCKERS
Both ACEIs and ARBs target RAS, although their mechanisms of action differ considerably. While ACEIs currently occupy a prominent position in the therapeutic strategies used in hypertension, they are not necessarily the most logical or effective way to suppress the RAS. Blocking Ang II at the receptor levels is an attractive alternative, since ACEIs reduce but do not completely block the production of Ang II.
ARBs are specific non-peptide Ang II receptor antagonists. They work by blocking AT1 receptors at the tissue level. Several factors including the level of Ang II and the number of AT1 receptors available influence the actions of ARB. The number of AT1 receptors on the cell surface determines the magnitude of the blocker’s effect. The powerful actions of intrarenal Ang II acting via stimulation of AT1 receptors on the vascular, glomerular, and tubular structures provide a synchronous cascade of effects contributing to the ability of the kidney to retain over 99% of the filtered sodium. The effects of Ang II not only on proximal nephron reabsorption but also on distal nephron transport function coupled with the associated actions of elevated aldosterone levels markedly increase the sodium-retaining capability of the kidney. The chronic high sodium diet has been reported to stimulate AT1 receptor mRNA expression in renal afferent arterioles [88]. Sodium depletion has been associated with increased number of AT1 receptors and an increased response to ARB. By binding AT1 receptors, an ARB decreases aldosterone, vasopressin, and catecholamine release ACE [89–94]. ARB also causes vascular vasodilation and inhibition of sodium and water reabsorption in the kidney. Collectively, these effects lead to a reduction in blood pressure [95].
Inhibition of AT1 receptors is associated with increases in renin secretion that leads to more Ang II formation. Accumulation of Ang II, as a result of ARB, can theoretically compete with ARB at the receptors and thus diminish therapeutic efficiency of the treatment. However this issue still remains controversial. Kobori et al. [96] have shown that intrarenal Ang II is independently regulated from plasma Ang II in Ang II-infused rat. They showed that an ARB, olmesartan, treatment decreased kidney Ang II levels while plasma Ang II concentration actually increased. In this model, kidney and urinary AGT levels were also inhibited by olmesartan and urinary AGT was closely linked to intrarenal Ang II in Ang II-infused rats.
The increase in Ang II after ARB allows stimulation of AT2 receptors. Activation of AT2 receptors is associated with increased tissue release of nitric oxide, guanylate cyclase, and tissue bradykinin [97]. In contrast to AT1 receptors, AT2 receptors have antigrowth properties and stimulate programmed cell death. Thus, the effects of AT2 receptors stimulation seem to counterbalance the effects of AT1 receptors.
Taking in account that ACEIs do not inhibit completely Ang II formation and that ARBs lead to Ang II accumulation with a possible underlying “escape mechanism,” should ACEIs and ARBs be used together? Ang II, generated by non-ACE mechanisms, could be inhibited at the receptor level by the ARB. While inhibition of the RAS via AT1 receptors could be strengthened, nitric oxide-dependent vasodilator pathways activated by ACEIs would remain intact. On the other hand, AT2 receptor-mediated actions could be activated by RAS shifted to AT2 receptors. Therefore, the addition of ACEIs to ARBs would be beneficial. The Randomized Evaluation of Strategies fOr Left Ventricular Dysfunction (RESOLVD) trial revealed that a combination of an ACEI and an ARB decreased blood pressure and improved the ejection fraction more than treatment with either drug alone in patients with congestive heart failure [98]. The Valsartan in Heart Failure Trial (Val-HeFT) demonstrated that a combination of an ACEI and an ARB reduced hospitalization for heart failure in patients with congestive heart failure by 30%, although no decrease in all-cause mortality was observed [99]. We are also waiting for the reports from ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET) and Telmisartan Randomized AssessmeNt Study in aCEi iNtolerant patients with cardiovascular Disease (TRANSCEND). These trials are expected to provide new insights into the optimal treatment of hypertensive patients.
CONCLUSIONS
ARBs could be superior over ACEIs in terms of renal protection and as an antihypertensive agent. ARBs probably cause better inhibition of the effects of intrarenal RAS via AT1 receptors. However, ACEIs have a potential contribution through the stimulation of alternative vasodilation pathways. The potential beneficial effects of renin inhibitors appear to be not completely explored. Intrarenal RAS response to a combined therapy with ACEIs and ARBs may be beneficial in conditions with activated RAS.
Acknowledgments
Y.S is a recipient of a fellowship from the Kanae Foundation for Life and Socio-medical Science (Tokyo, Japan) and M.C. P-C is a recipient of a fellowship from the American Heart Association, southeast affiliate. Research performed in authors’ laboratories was supported by National Heart, Lung, and Blood Institute Grant (R01HL 026371), by Center of Biomedical Research Excellence Grant from National Center for Research Resources (P20RR017659), and by the millennium Health Excellence Fund from Louisiana Board of Regents. The authors also acknowledge critical reviews and valuable comments of L. Gabriel Navar, Ph.D. (Tulane University).
References
- 1.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation. 1994;89:493–498. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- 2.Bader M, Ganten D. It’s renin in the brain: Transgenic animals elucidate the brain renin angiotensin system. Circ Res. 2002;90:8–10. [PubMed] [Google Scholar]
- 3.Dell’Italia LJ, Meng QC, Balcells E, et al. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest. 1997;100:253–258. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzocchi G, Malendowicz LK, Markowska A, Albertin G, Nussdorfer GG. Role of adrenal renin-angiotensin system in the control of aldosterone secretion in sodium-restricted rats. Am J Physiol Endocrinol Metab. 2000;278:E1027–1030. doi: 10.1152/ajpendo.2000.278.6.E1027. [DOI] [PubMed] [Google Scholar]
- 5.Muller DN, Bohlender J, Hilgers KF, et al. Vascular angiotensin-converting enzyme expression regulates local angiotensin II. Hypertension. 1997;29:98–104. doi: 10.1161/01.hyp.29.1.98. [DOI] [PubMed] [Google Scholar]
- 6.Campbell DJ, Lawrence AC, Towrie A, Kladis A, Valentijn AJ. Differential regulation of angiotensin peptide levels in plasma and kidney of the rat. Hypertension. 1991;18:763–773. doi: 10.1161/01.hyp.18.6.763. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 8.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 9.Viberti G, Wheeldon NM. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: A blood pressure-independent effect. Circulation. 2002;106:672–678. doi: 10.1161/01.cir.0000024416.33113.0a. [DOI] [PubMed] [Google Scholar]
- 10.Lewis EJ, Hunsicker LG, Clarke WRT, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 11.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 12.Rodby RA, Rohde RD, Clarke WR, et al. The irbesartan type II diabetic nephropathy trial: Study design and baseline patient characteristics. For the collaborative study group. Nephrol Dial Transplant. 2000;15:487–497. doi: 10.1093/ndt/15.4.487. [DOI] [PubMed] [Google Scholar]
- 13.Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351:1952–1961. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 14.Hollenberg NK. Renal implications of angiotensin receptor blockers. Am J Hypertens. 2001;14:237S–241S. doi: 10.1016/s0895-7061(01)02133-1. [DOI] [PubMed] [Google Scholar]
- 15.Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): A randomised controlled trial. Lancet. 2003;361:117–124. doi: 10.1016/S0140-6736(03)12229-5. [DOI] [PubMed] [Google Scholar]
- 16.Graciano ML, Cavaglieri Rde C, Delle H, et al. Intrarenal renin-Angiotensin system is upregulated in experimental model of progressive renal disease induced by chronic inhibition of nitric oxide synthesis. J Am Soc Nephrol. 2004;15:1805–1815. doi: 10.1097/01.asn.0000131528.00773.a9. [DOI] [PubMed] [Google Scholar]
- 17.Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985;76:612–619. doi: 10.1172/JCI112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weidmann P, Schneider M, Bohlen L. Therapeutic efficacy of different antihypertensive drugs in human diabetic nephropathy: An updated meta-analysis. Nephrol Dial Transplant. 1995;10 (Suppl 9):39–45. [PubMed] [Google Scholar]
- 19.Wu LL, Cox A, Roe CJ, Dziadek M, Cooper ME, Gilbert RE. Transforming growth factor beta 1 and renal injury following subtotal nephrectomy in the rat: Role of the renin-angiotensin system. Kidney Int. 1997;51:1553–1567. doi: 10.1038/ki.1997.214. [DOI] [PubMed] [Google Scholar]
- 20.Wolf G, Haberstroh U, Neilson EG. Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol. 1992;140:95–107. [PMC free article] [PubMed] [Google Scholar]
- 21.Peters H, Border WA, Noble NA. Targeting TGF-beta overexpression in renal disease: Maximizing the antifibrotic action of angiotensin II blockade. Kidney Int. 1998;54:1570–1580. doi: 10.1046/j.1523-1755.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 22.Oikawa T, Freeman M, Lo W, Vaughan DE, Fogo A. Modulation of plasminogen activator inhibitor-1 in vivo: A new mechanism for the antifibrotic effect of renin-angiotensin inhibition. Kidney Int. 1997;51:164–172. doi: 10.1038/ki.1997.20. [DOI] [PubMed] [Google Scholar]
- 23.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger rna in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mrnas in rat kidney. Kidney Int. 1993;43:1251–1259. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 25.Richoux JP, Cordonnier JL, Bouhnik J, et al. Immunocytochemical localization of angiotensinogen in rat liver and kidney. Cell Tissue Res. 1983;233:439–451. doi: 10.1007/BF00238309. [DOI] [PubMed] [Google Scholar]
- 26.Darby IA, Congiu M, Fernley RT, Sernia C, Coghlan JP. Cellular and ultrastructural location of angiotensinogen in rat and sheep kidney. Kidney Int. 1994;46:1557–1560. doi: 10.1038/ki.1994.445. [DOI] [PubMed] [Google Scholar]
- 27.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 28.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanagawa N, Capparelli AW, Jo OD, Friedal A, Barrett JD, Eggena P. Production of angiotensinogen and renin-like activity by rabbit proximal tubular cells in culture. Kidney Int. 1991;39:938–941. doi: 10.1038/ki.1991.117. [DOI] [PubMed] [Google Scholar]
- 30.Henrich WL, McAllister EA, Eskue A, Miller T, Moe OW. Renin regulation in cultured proximal tubular cells. Hypertension. 1996;27:1337–1340. doi: 10.1161/01.hyp.27.6.1337. [DOI] [PubMed] [Google Scholar]
- 31.Moe OW, Ujiie K, Star RA, et al. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leyssac PP. Changes in single nephron renin release are mediated by tubular fluid flow rate. Kidney Int. 1986;30:332–339. doi: 10.1038/ki.1986.189. [DOI] [PubMed] [Google Scholar]
- 33.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, et al. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutierrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sibony M, Gasc JM, Soubrier F, Alhenc-Gelas F, Corvol P. Gene expression and tissue localization of the two isoforms of angiotensin I converting enzyme. Hypertension. 1993;21:827–835. doi: 10.1161/01.hyp.21.6.827. [DOI] [PubMed] [Google Scholar]
- 36.Schulz WW, Hagler HK, Buja LM, Erdos EG. Ultrastructural localization of angiotensin I-converting enzyme (EC 3.4.15.1) and neutral metalloendopeptidase (EC 3.4.24.11) in the proximal tubule of the human kidney. Lab Invest. 1988;59:789–797. [PubMed] [Google Scholar]
- 37.Casarini DE, Boim MA, Stella RC, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol. 1997;272:F405–409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 38.Wang ZQ, Millatt LJ, Heiderstadt NT, Siragy HM, Johns RA, Carey RM. Differential regulation of renal angiotensin subtype at1a and AT2 receptor protein in rats with angiotensin-dependent hypertension. Hypertension. 1999;33:96–101. doi: 10.1161/01.hyp.33.1.96. [DOI] [PubMed] [Google Scholar]
- 39.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol. 1999;277:F437–446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 40.Bouby N, Hus-Citharel A, Marchetti J, Bankir L, Corvol P, Llorens-Cortes C. Expression of type 1 angiotensin II receptor subtypes and angiotensin II-induced calcium mobilization along the rat nephron. J Am Soc Nephrol. 1997;8:1658–1667. doi: 10.1681/ASN.V8111658. [DOI] [PubMed] [Google Scholar]
- 41.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, el-Dahr SS. Immunohistochemical localization of ang II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol. 1997;273:F170–177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 42.Navar LG, Harrison-Bernard LM, Imig JD, Wang CT, Cervenka L, Mitchell KD. Intrarenal angiotensin II generation and renal effects of AT1 receptor blockade. J Am Soc Nephrol. 1999;10 (Suppl 12):S266–272. [PubMed] [Google Scholar]
- 43.Ingelfinger JR, Jung F, Diamant D, et al. Rat proximal tubule cell line transformed with origin-defective sv40 DNA: Autocrine ang II feedback. Am J Physiol. 1999;276:F218–227. doi: 10.1152/ajprenal.1999.276.2.F218. [DOI] [PubMed] [Google Scholar]
- 44.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol. 1992;263:E863–869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 45.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics. 1999;1:3–9. doi: 10.1152/physiolgenomics.1999.1.1.3. [DOI] [PubMed] [Google Scholar]
- 50.Rohrwasser A, Morgan T, Dillon HF, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 51.Ding Y, Davisson RL, Hardy DO, et al. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem. 1997;272:28142–28148. doi: 10.1074/jbc.272.44.28142. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz-Ortega M, Lorenzo O, Ruperez M, Konig S, Wittig B, Egido J. Angiotensin II activates nuclear transcription factor kappab through AT(1) and AT(2) in vascular smooth muscle cells: Molecular mechanisms. Circ Res. 2000;86:1266–1272. doi: 10.1161/01.res.86.12.1266. [DOI] [PubMed] [Google Scholar]
- 53.Ma LJ, Nakamura S, Whitsitt JS, Marcantoni C, Davidson JM, Fogo AB. Regression of sclerosis in aging by an angiotensin inhibition-induced decrease in pai-1. Kidney Int. 2000;58:2425–2436. doi: 10.1046/j.1523-1755.2000.00426.x. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura S, Nakamura I, Ma L, Vaughan DE, Fogo AB. Plasminogen activator inhibitor-1 expression is regulated by the angiotensin type 1 receptor in vivo. Kidney Int. 2000;58:251–259. doi: 10.1046/j.1523-1755.2000.00160.x. [DOI] [PubMed] [Google Scholar]
- 55.Taal MW, Chertow GM, Rennke HG, et al. Mechanisms underlying renoprotection during renin-angiotensin system blockade. Am J Physiol Renal Physiol. 2001;280:F343–355. doi: 10.1152/ajprenal.2001.280.2.F343. [DOI] [PubMed] [Google Scholar]
- 56.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 57.Hatae T, Takimoto E, Murakami K, Fukamizu A. Comparative studies on species-specific reactivity between renin and angiotensinogen. Mol Cell Biochem. 1994;131:43–47. doi: 10.1007/BF01075723. [DOI] [PubMed] [Google Scholar]
- 58.Fisher ND, Hollenberg NK. Is there a future for renin inhibitors? Expert Opin Investig Drugs. 2001;10:417–426. doi: 10.1517/13543784.10.3.417. [DOI] [PubMed] [Google Scholar]
- 59.el Amrani AI, Menard J, Gonzales MF, Michel JB. Effects of blocking the angiotensin II receptor, converting enzyme, and renin activity on the renal hemodynamics of normotensive guinea pigs. J Cardiovasc Pharmacol. 1993;22:231–239. doi: 10.1097/00005344-199308000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Fisher ND, Hollenberg NK. Renin inhibition: What are the therapeutic opportunities? J Am Soc Nephrol. 2005;16:592–599. doi: 10.1681/ASN.2004100874. [DOI] [PubMed] [Google Scholar]
- 61.Rahuel J, Rasetti V, Maibaum J, et al. Structure-based drug design: The discovery of novel nonpeptide orally active inhibitors of human rennin. Chem Biol. 2000;7:493–504. doi: 10.1016/s1074-5521(00)00134-4. [DOI] [PubMed] [Google Scholar]
- 62.Wood JM, Maibaum J, Rahuel J, et al. Structure-based design of aliskiren, a novel orally effective renin inhibitor. Biochem Biophys Res Commun. 2003;308:698–705. doi: 10.1016/s0006-291x(03)01451-7. [DOI] [PubMed] [Google Scholar]
- 63.Nussberger J, Wuerzner G, Jensen C, Brunner HR. Angiotensin II suppression in humans by the orally active renin inhibitor aliskiren (spp100): Comparison with enalapril. Hypertension. 2002;39:E1–8. doi: 10.1161/hy0102.102293. [DOI] [PubMed] [Google Scholar]
- 64.Stanton A, Jensen C, Nussberger J, O’Brien E. Blood pressure lowering in essential hypertension with an oral renin inhibitor, aliskiren. Hypertension. 2003;42:1137–1143. doi: 10.1161/01.HYP.0000101688.17370.87. [DOI] [PubMed] [Google Scholar]
- 65.Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP. Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation. 2005;111:1012–1018. doi: 10.1161/01.CIR.0000156466.02908.ED. [DOI] [PubMed] [Google Scholar]
- 66.Naqvi N, Liu K, Graham RM, Husain A. Molecular basis of exopeptidase activity in the C-terminal domain of human angiotensin I-converting enzyme: Insights into the origins of its exopeptidase activity. J Biol Chem. 2005;280:6669–6675. doi: 10.1074/jbc.M412638200. [DOI] [PubMed] [Google Scholar]
- 67.Carretero OA. Novel mechanism of action of ACE and its inhibitors. Am J Physiol Heart Circ Physiol. 2005;289:H1796–1797. doi: 10.1152/ajpheart.00781.2005. [DOI] [PubMed] [Google Scholar]
- 68.Bonner G. Kinin-related effects of angiotensin-converting enzyme inhibition. Clin Physiol Biochem. 1990;8 (Suppl 1):6–15. [PubMed] [Google Scholar]
- 69.Kon V, Fogo A, Ichikawa I. Bradykinin causes selective efferent arteriolar dilation during angiotensin I converting enzyme inhibition. Kidney Int. 1993;44:545–550. doi: 10.1038/ki.1993.279. [DOI] [PubMed] [Google Scholar]
- 70.Mattson DL, Roman RJ. Role of kinins and angiotensin II in the renal hemodynamic response to captopril. Am J Physiol. 1991;260:F670–679. doi: 10.1152/ajprenal.1991.260.5.F670. [DOI] [PubMed] [Google Scholar]
- 71.Heller J, Kramer HJ, Horacek V. The effect of kinin and prostaglandin inhibitors on the renal response to angiotensin-converting enzyme inhibition: A micropuncture study in the dog. Pflugers Arch. 1994;427:219–224. doi: 10.1007/BF00374527. [DOI] [PubMed] [Google Scholar]
- 72.Zimmerman BG, Raich PC, Vavrek RJ, Stewart JM. Bradykinin contribution to renal blood flow effect of angiotensin converting enzyme inhibitor in the conscious sodium-restricted dog. Circ Res. 1990;66:234–240. doi: 10.1161/01.res.66.1.234. [DOI] [PubMed] [Google Scholar]
- 73.Omoro SA, Majid DS, El-Dahr SS, Navar LG. Kinin influences on renal regional blood flow responses to angiotensin-converting enzyme inhibition in dogs. Am J Physiol. 1999;276:F271–277. doi: 10.1152/ajprenal.1999.276.2.F271. [DOI] [PubMed] [Google Scholar]
- 74.Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 75.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 76.Ferrario CM. Contribution of angiotensin-(1–7) to cardiovascular physiology and pathology. Curr Hypertens Rep. 2003;5:129–134. doi: 10.1007/s11906-003-0069-y. [DOI] [PubMed] [Google Scholar]
- 77.Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 78.Oudit GY, Crackower MA, Backx PH, Penninger JM. The role of ace2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13:93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 79.Benter IF, Diz DI, Ferrario CM. Cardiovascular actions of angiotensin(1–7) Peptides. 1993;14:679–684. doi: 10.1016/0196-9781(93)90097-z. [DOI] [PubMed] [Google Scholar]
- 80.Osei SY, Ahima RS, Minkes RK, Weaver JP, Khosla MC, Kadowitz PJ. Differential responses to angiotensin-(1–7) in the feline mesenteric and hindquarters vascular beds. Eur J Pharmacol. 1993;234:35–42. doi: 10.1016/0014-2999(93)90703-k. [DOI] [PubMed] [Google Scholar]
- 81.Siragy HM, Bedigian M. Mechanism of action of angiotensin-receptor blocking agents. Curr Hypertens Rep. 1999;1:289–295. doi: 10.1007/s11906-999-0036-3. [DOI] [PubMed] [Google Scholar]
- 82.Juillerat L, Nussberger J, Menard J, et al. Determinants of angiotensin II generation during converting enzyme inhibition. Hypertension. 1990;16:564–572. doi: 10.1161/01.hyp.16.5.564. [DOI] [PubMed] [Google Scholar]
- 83.Rousseau MF, Konstam MA, Benedict CR, et al. Progression of left ventricular dysfunction secondary to coronary artery disease, sustained neurohormonal activation and effects of ibopamine therapy during long-term therapy with angiotensin-converting enzyme inhibitor. Am J Cardiol. 1994;73:488–493. doi: 10.1016/0002-9149(94)90680-7. [DOI] [PubMed] [Google Scholar]
- 84.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–22357. [PubMed] [Google Scholar]
- 85.Takai S, Sakaguchi M, Jin D, Yamada M, Kirimura K, Miyazaki M. Different angiotensin II-forming pathways in human and rat vascular tissues. Clin Chim Acta. 2001;305:191–195. doi: 10.1016/s0009-8981(01)00379-5. [DOI] [PubMed] [Google Scholar]
- 86.Sadjadi J, Kramer GL, Yu CH, Burress Welborn M, 3rd, Chappell MC, Gregory Modrall J. Angiotensin converting enzyme-independent angiotensin ii production by chymase is up-regulated in the ischemic kidney in renovascular hypertension. J Surg Res. 2005;127:65–69. doi: 10.1016/j.jss.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 87.Hollenberg NK, Fisher ND, Price DA, Williams GH. Effect of ACE inhibition on pressor, renal vascular, and adrenal responses to infusion of angiotensin I in normal subjects eating a low-salt diet. Am J Hypertens. 2000;13:498–503. doi: 10.1016/s0895-7061(99)00223-x. [DOI] [PubMed] [Google Scholar]
- 88.Ruan X, Wagner C, Chatziantoniou C, Kurtz A, Arendshorst WJ. Regulation of angiotensin II receptor AT1 subtypes in renal afferent arterioles during chronic changes in sodium diet. J Clin Invest. 1997;99:1072–1081. doi: 10.1172/JCI119235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takishita S, Muratani H, Sesoko S, et al. Short-term effects of angiotensin II blockade on renal blood flow and sympathetic activity in awake rats. Hypertension. 1994;24:445–450. doi: 10.1161/01.hyp.24.4.445. [DOI] [PubMed] [Google Scholar]
- 90.Xu L, Brooks VL. Ang II chronically supports renal and lumbar sympathetic activity in sodium-deprived, conscious rats. Am J Physiol. 1996;271:H2591–2598. doi: 10.1152/ajpheart.1996.271.6.H2591. [DOI] [PubMed] [Google Scholar]
- 91.DiBona GF, Jones SY, Sawin LL. Angiotensin receptor antagonist improves cardiac reflex control of renal sodium handling in heart failure. Am J Physiol. 1998;274:H636–641. doi: 10.1152/ajpheart.1998.274.2.H636. [DOI] [PubMed] [Google Scholar]
- 92.Cervenka L, Wang CT, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of goldblatt hypertensive rats. Hypertension. 1999;33:102–107. doi: 10.1161/01.hyp.33.1.102. [DOI] [PubMed] [Google Scholar]
- 93.Peng Y, Knox FG. Comparison of systemic and direct intrarenal angiotensin II blockade on sodium excretion in rats. Am J Physiol. 1995;269:F40–46. doi: 10.1152/ajprenal.1995.269.1.F40. [DOI] [PubMed] [Google Scholar]
- 94.Munoz-Garcia R, Maeso R, Rodrigo E, et al. Acute renal excretory actions of losartan in spontaneously hypertensive rats: Role of AT2 receptors, prostaglandins, kinins and nitric oxide. J Hypertens. 1995;13:1779–1784. [PubMed] [Google Scholar]
- 95.Navar LG, Harrison-Bernard LM, Imig JD, Cervenka L, Mitchell KD. Renal responses to AT1 receptor blockade. Am J Hypertens. 2000;13:45S–54S. doi: 10.1016/s0895-7061(99)00248-4. [DOI] [PubMed] [Google Scholar]
- 96.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–1132. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–1242. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- 98.McKelvie RS, Yusuf S, Pericak D, et al. and their combination in congestive heart failure: Randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The resolvd pilot study investigators. Circulation. 1999;100:1056–1064. doi: 10.1161/01.cir.100.10.1056. [DOI] [PubMed] [Google Scholar]
- 99.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]