Abstract
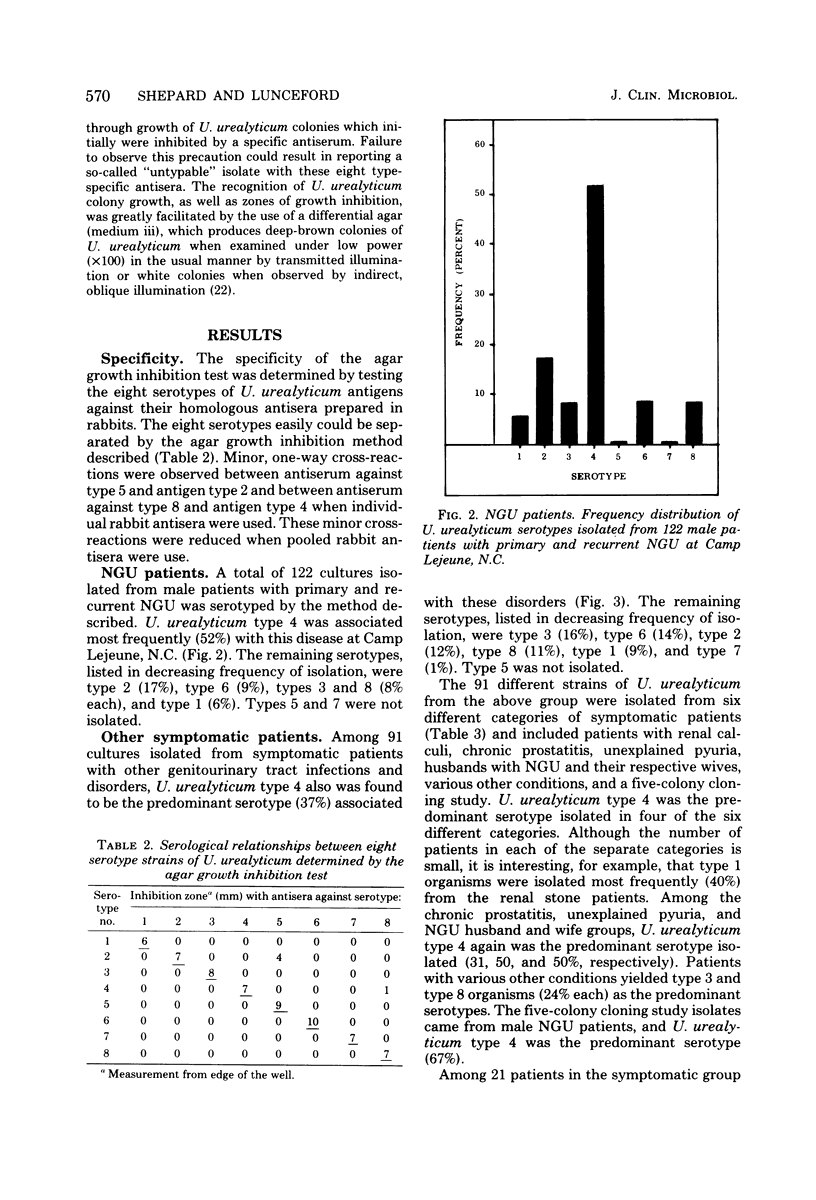
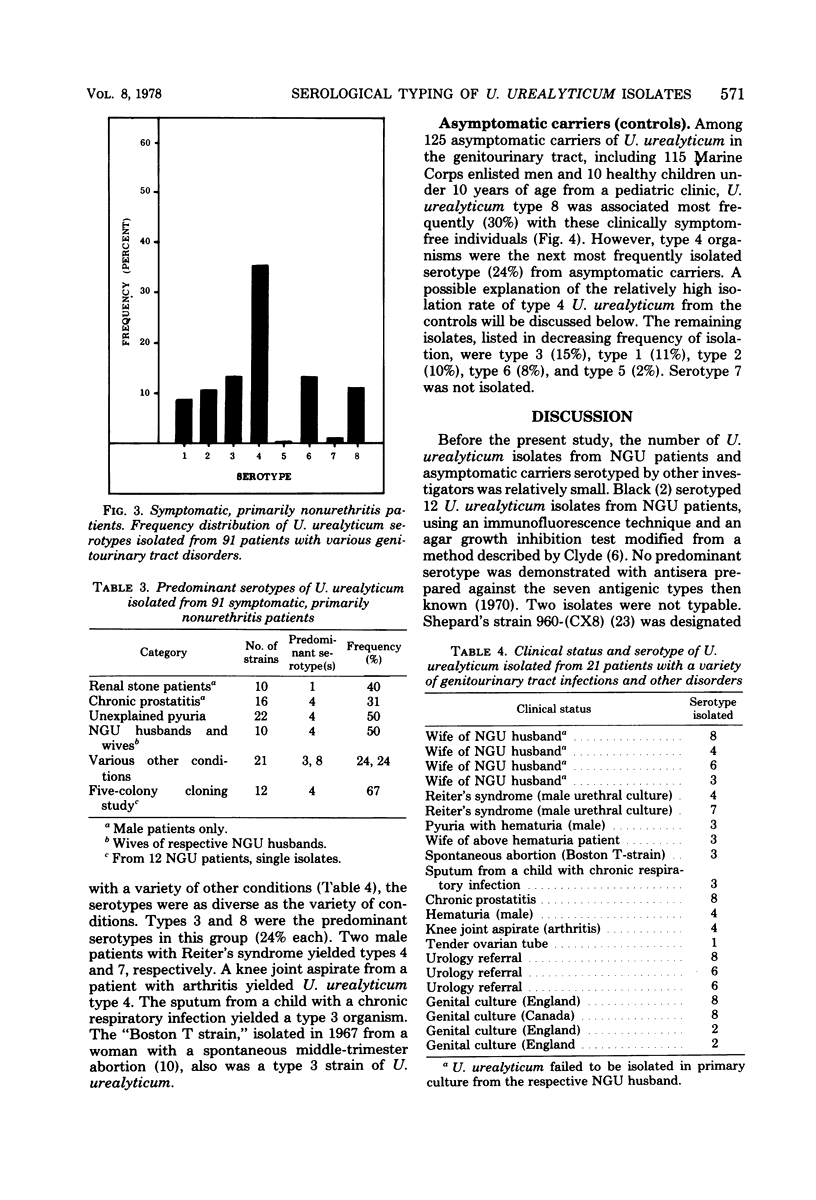
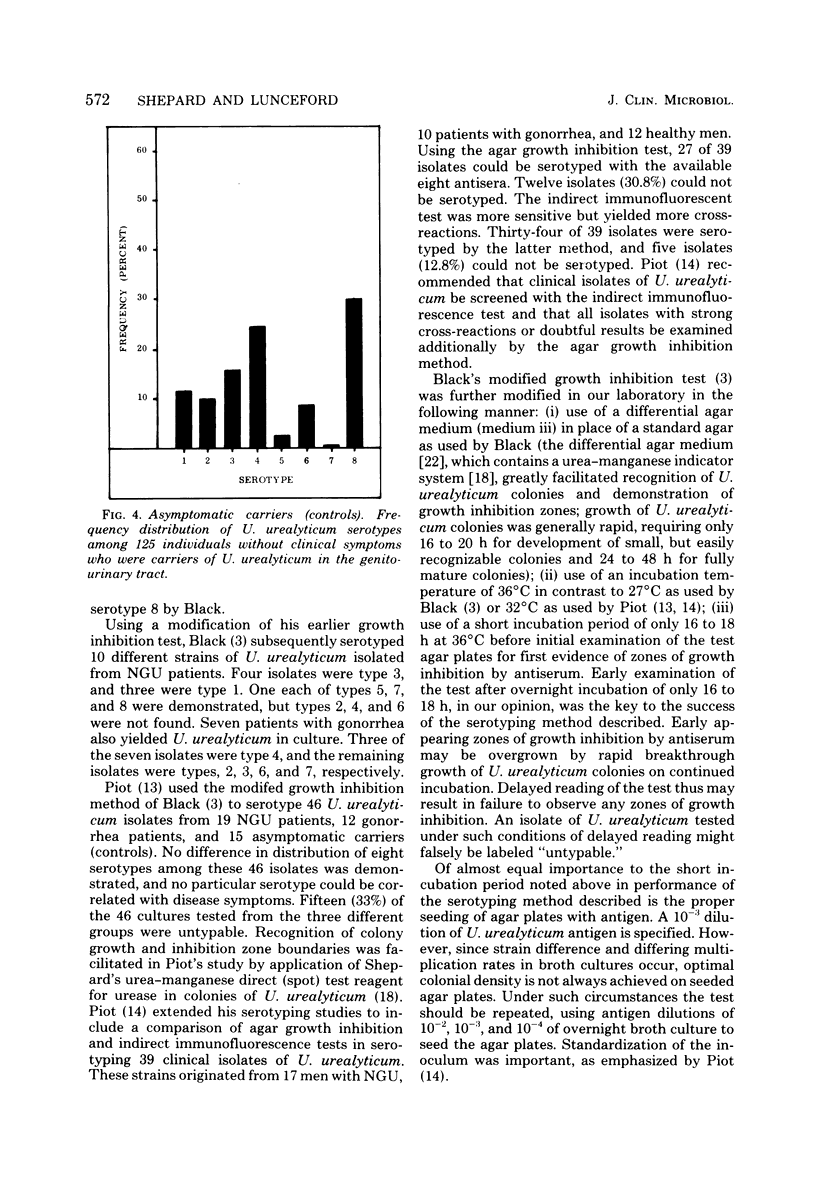
An agar growth inhibition method for serotyping Ureaplasma urealyticum is described, and the results of applying this method to serotyping 338 strains of the organism are presented. The serotyped strains consisted of cloned isolates from male patients with primary and recurrent nongonococcal urethritis (NGU), isolates from symptomatic patients with other genitourinary tract infections and disorders, and isolates from asymptomatic carriers of U. urealyticum in the genitourinary tract (controls). Among 122 male patients with NGU, serotype 4 was associated most frequently (52%) with this disease at Camp Lejeune, N.C. Seventeen percent of the isolates were type 2. The remaining isolates consisted of types 1, 3, 6, and 8 and accounted for 6 to 9% each of the serotypes isolated from the NGU group. Types 5 and 7 were not isolated. Among 91 symptomatic patients with other genitourinary tract infections and disorders, U. urealyticum type 4 also was associated most frequently (37%) with these disorders. The remaining isolates, represented by types 1, 2, 3, 6, 7, and 8, accounted for 9 to 15% each of the types isolated from this group. Type 5 was not isolated. Among 125 symptomfree carriers of U. urealyticum in the genitourinary tract, type 8 was recovered most frequently (30%), whereas type 4 was isolated next most frequently (24%). The remaining isolates consisted of types 1, 2, 3, 5, and 6 and accounted for 2 to 15% each in this asymptomatic control group. Type 7 was not isolated. Of the present eight serotypes of U. urealyticum studied in this investigation, type 4 was associated most frequently with disease (NGU) and certain other disorders of the genitourinary tract at Camp Lejeune. A previously unknown association of U. urealyticum with frequently abacteriuric, unexplained pyuria (with or without urethral pruritis and dysuria) is reported, suggesting the existence of asymptomatic Ureaplasma urethritis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black F. T., Krogsgaard-Jensen A. Application of indirect immunofluorescence, indirect haemagglutination and polyacrylamide-gel electrophoresis to human T-mycoplasmas. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):345–353. doi: 10.1111/j.1699-0463.1974.tb02336.x. [DOI] [PubMed] [Google Scholar]
- Black F. T. Modifications of the growth inhibition test and its application to human T-mycoplasmas. Appl Microbiol. 1973 Apr;25(4):528–533. doi: 10.1128/am.25.4.528-533.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLYDE W. A., Jr MYCOPLASMA SPECIES IDENTIFICATION BASED UPON GROWTH INHIBITION BY SPECIFIC ANTISERA. J Immunol. 1964 Jun;92:958–965. [PubMed] [Google Scholar]
- Ford D. K., MacDonald J. Influence of urea on the growth of T-strain mycoplasmas. J Bacteriol. 1967 May;93(5):1509–1512. doi: 10.1128/jb.93.5.1509-1512.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford D. K. Relationships between mycoplasma and the etiology of nongonococcal urethritis and Reiter's syndrome. Ann N Y Acad Sci. 1967 Jul 28;143(1):501–504. doi: 10.1111/j.1749-6632.1967.tb27694.x. [DOI] [PubMed] [Google Scholar]
- Kundsin R. B., Driscoll S. G., Ming P. L. Strain of mycoplasma associated with human reproductive failure. Science. 1967 Sep 29;157(3796):1573–1574. doi: 10.1126/science.157.3796.1573. [DOI] [PubMed] [Google Scholar]
- Lin J. S., Kass E. H. Immune inactivation of T-strain mycoplasmas. J Infect Dis. 1970 Jul-Aug;122(1):93–95. doi: 10.1093/infdis/122.1-2.93. [DOI] [PubMed] [Google Scholar]
- Lin J. S., Kendrick M. I., Kass E. H. Serologic typing of human genital T-mycoplasmas by a complement-dependent mycoplasmacidal test. J Infect Dis. 1972 Dec;126(6):658–663. doi: 10.1093/infdis/126.6.658. [DOI] [PubMed] [Google Scholar]
- Piot P. Comparison of growth inhibition and immunofluorescence tests in serotyping clinical isolates of Ureaplasma urealyticum. Br J Vener Dis. 1977 Jun;53(3):186–189. doi: 10.1136/sti.53.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piot P. Distribution of eight serotypes of Ureaplasma urealyticum in cases of non-gonococcal urethritis and of gonorrhoea, and in healthy persons. Br J Vener Dis. 1976 Aug;52(4):266–268. doi: 10.1136/sti.52.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell R. H., Taylor-Robinson D., Wong D., Chanock R. M. Color test for the measurement of antibody to T-strain mycoplasmas. J Bacteriol. 1966 Jul;92(1):6–12. doi: 10.1128/jb.92.1.6-12.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD M. C., LUNCEFORD C. D. EFFECT OF PH ON HUMAN MYCOPLASMA STRAINS. J Bacteriol. 1965 Feb;89:265–270. doi: 10.1128/jb.89.2.265-270.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard M. C. Differential methods for identification of T-mycoplasmas based on demonstration of urease. J Infect Dis. 1973 Mar;127(Suppl):S22–S25. doi: 10.1093/infdis/127.supplement_1.s22. [DOI] [PubMed] [Google Scholar]
- Shepard M. C., Howard D. R. Identification of "T" mycoplasmas in primary agar cultures by means of a direct test for urease. Ann N Y Acad Sci. 1970 Oct 30;174(2):809–819. doi: 10.1111/j.1749-6632.1970.tb45598.x. [DOI] [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Differential agar medium (A7) for identification of Ureaplasma urealyticum (human T mycoplasmas) in primary cultures of clinical material. J Clin Microbiol. 1976 Jun;3(6):613–625. doi: 10.1128/jcm.3.6.613-625.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Occurrence of urease in T strains of Mycoplasma. J Bacteriol. 1967 May;93(5):1513–1520. doi: 10.1128/jb.93.5.1513-1520.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Urease color test medium U-9 for the detection and identification of "T" mycoplasms in clinical material. Appl Microbiol. 1970 Oct;20(4):539–543. doi: 10.1128/am.20.4.539-543.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Csonka G. W., Prentice M. J. Human intra-urethral inoculation of ureplasmas. Q J Med. 1977 Jul;46(183):309–326. [PubMed] [Google Scholar]
- Wong J. L., Hines P. A., Brasher M. D., Rogers G. T., Smith R. F., Schachter J. The etiology of nongonococcal urethritis in men attending a venereal disease clinic. Sex Transm Dis. 1977 Jan-Mar;4(1):4–8. doi: 10.1097/00007435-197701000-00002. [DOI] [PubMed] [Google Scholar]
- al-Aubaidi J. M., Fabricant J. Characterization and classification of bovine mycoplasma. Cornell Vet. 1971 Jul;61(3):490–518. [PubMed] [Google Scholar]