Abstract
Senders and receivers influence dynamic characteristics of the signals used for mate attraction over different time scales. On a moment-to-moment basis, interactions among senders competing for a mate influence dynamic characteristics, whereas the preferences of receivers of the opposite gender exert an influence over evolutionary time. We observed and recorded the calling patterns of the bird-voiced treefrog Hyla avivoca, to assess how the dynamic characters of calls vary during interactions among groups of males in a chorus. This question was also addressed using playback experiments with males. Playback experiments with females showed how changes in dynamic call properties are likely to affect male mating success. Frogs calling in pairs, groups, or in response to playbacks produced longer calls than did isolated males. During call overlap, males often increased the duration of the silent interval (gaps) between the pulses of their calls so that the pulses of the calls of two neighbors interdigitated. This change resulted in increased variability of pulse rate, a traditionally static acoustic property; however, males also produced high proportions of non-overlapped calls in which variability in pulse rate was low and had species-typical values. Females preferred long calls to short and average-duration calls, and non-overlapped calls to overlapped calls. Given a choice between pairs of overlapped calls, females preferred pairs in which the proportion of overlap was low and pairs in which the pulses of such calls interdigitated completely. The observed patterns of vocal competition thus reflect the preferences of conspecific females, which have influenced the evolution of the calling behavior of H. avivoca.
Keywords: acoustic communication, male-male competition, call overlap, pulse interdigitation, mate choice, Hyla avivoca.
Introduction
The evolution of communication systems is complicated because senders and receivers are sources of mutual selection (Andersson, 1994; Kamo et al., 2002; Vehrencamp, 2000). In a reproductive context, senders typically compete with others to attract members of the opposite gender (Bradbury & Vehrencamp, 1998; Gerhardt and Huber, 2002; Wells and Schwartz, 2006) and alter the properties of their signals during interactions in ways that directly or indirectly increase their chances of mating (Greenfield, 1994; Gerhardt and Huber, 2002). The indirect effects of such interactions include the expulsion of other males from a resource required by females or from the immediate area of a signaler, which may increase the chances of the signaler being detected by females (see examples in Bee and Gerhardt, 2001; Bourne et al, 2001; Marshall et al, 2003; Narins et al, 2003). Direct effects, which are the focus of this study, are alterations occurring during vocal interactions that increase the attractiveness of the signal to females (e.g. Klump and Gerhardt, 1987; Gerhardt, et al, 1996; Hill, 1998; Schwartz et al, 2002).
Vocal communication in the bird-voiced treefrog Hyla avivoca, serves as an excellent system to study patterns of call modification and the behavioral advantages of such changes. As in other anurans, males engage in vocal and physical interactions within the chorus by varying the temporal properties of their pulsed calls (Fig. 1; sound file available on electronic version) allowing us to identify the call properties that result in changes in the vocal responses and timing of male advertisement calls. Additionally, gravid females readily respond to playbacks of synthetic calls allowing us to test hypotheses about the effects of female choice on advertisement call modifications and male competition.
Figure 1.
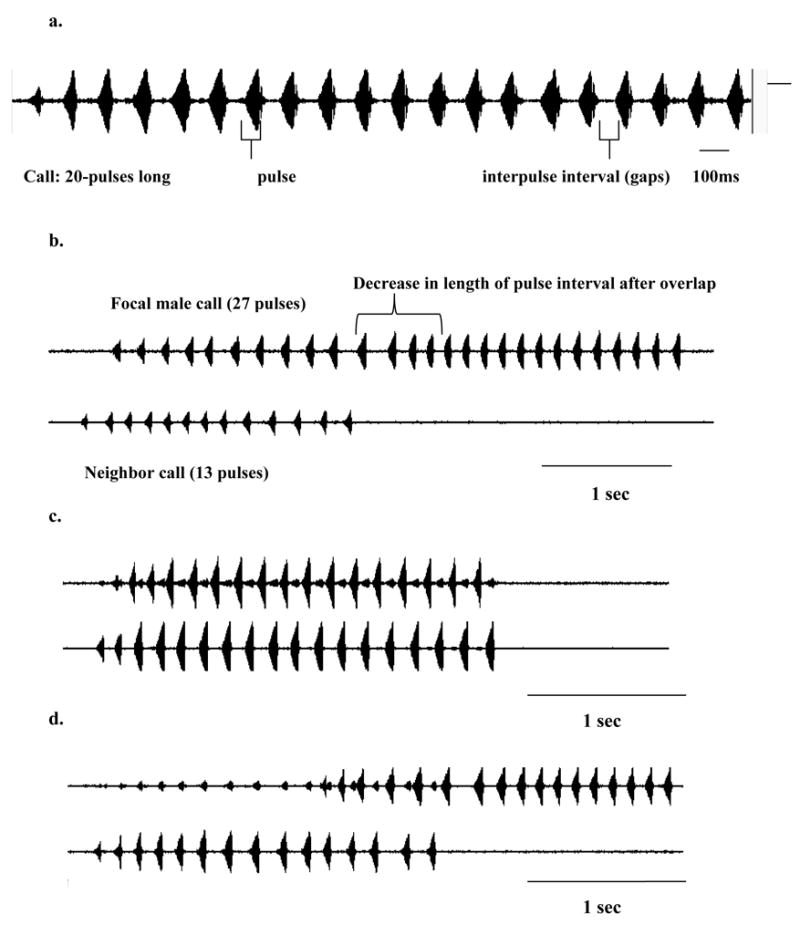
a. Oscillogram of a call of H. avivoca in isolation. b. Oscillograms of two overlapped calls showing an example of an extended duration call by the following male. Notice how interpulse interval returns to normal rate after neighbor stops calling. c. Call matching. d. Call interruption. Calls recorded at 22°C.
We focused on modifications in advertisement calls such as changes in call duration and call rate, and the patterns of call overlap and alternation that arise in the presence of neighbors (e.g., Wells and Taigen, 1986; Schwartz et al, 2002). We then tested how such changes are likely to affect a male’s chances of attracting a female. We will show that males calling in isolation had shorter calls and lower call rates compared to males calling in groups and that females preferred longer calls produced at higher rates.
A common phenomenon in calling frogs is that call overlap, which is often discriminated against by females, becomes unavoidable when calling from a chorus (Schwartz, 1987). What is unique among North American species is that males of H. avivoca interdigitate pulses within overlapped calls. Pulse interdigitation occurs when males overlapping their calls time the production of such calls so that the pulses within the calls completely alternate (sound file available on electronic version). In H. avivoca, this requires an increase in length of the intervals between pulses and consequently a significant decrease in pulse rate. Such fine-scale timing of pulses does not occur in either of the gray treefrogs (Hyla versicolor and H. chrysoscelis), which are closely related to H. avivoca.
Interdigitation of call notes has been reported in the Kuvangu running frog Kassina kuvangensis (Grafe, 2003) and in the small-headed and hourglass treefrogs, Hyla microcephala and H. ebraccata (Schwartz, 1987; 1993). In these species, however, both the patterns and time scale of interdigitation differ from the phenomenon we found in H. avivoca. In H. avivoca, pairs of males alternate the smallest acoustic unit of the advertisement call, the pulse, and they do so by increasing the interpulse silent interval in the range of 10–40 ms. In H. microcephala and H. ebraccata, pairs of males alternate pulsed introductory and secondary notes but do not alternate the pulses that make up these two kinds of notes (Schwartz 1987, 1993). The magnitude of changes in the inter-note intervals is of the order of 50 ms or more (Schwartz 1987, his Figure 1A; pers. comm.). Male K. kuvangensis alternate pulsed calls within overlapped call groups, and the silent intervals between these calls are all greater than 100 ms (Grafe 2003),
A fine-scale change in the length of pulse interval (=pulse rate) within an advertisement call is unusual and potentially poses a dilemma. Pulse rate in anuran signals is usually a static property (sensu Gerhardt, 1991) that shows little within-bout variation and is often essential for species recognition (Gerhardt and Huber, 2002). We will show, however, that pulse rate still qualifies as a static property in non-overlapped calls of H. avivoca and that females prefer overlapping calls with pulse interdigitation to overlapping calls lacking this timing pattern. Thus, signals with lower-than-average pulse rates are still treated as conspecific calls.
Materials and Methods
STUDY SYSTEM
The bird-voiced treefrog, Hyla avivoca, is a small treefrog found in forested floodplains and swamps near large rivers and in bald cypress-tupelo swamp areas in the southeastern United States. Within this habitat, bird-voiced treefrogs breed from April to August in permanent or semi-permanent swamps, where males call at various heights from overhanging vegetation or tree trunks directly above water (Dundee and Rossman, 1989). Males usually form small, dispersed choruses (sometimes of a few hundred) that spread through a vast area within the shoreline of a lake or swamp with an average male-male distance of about 2.4 (+/− 0.9) meters (Martinez-Rivera, unpublished data). We observed and recorded calling males and collected gravid females from five sites throughout the species range in Louisiana, Mississippi and Tennessee (Appendix 1). The sites in Louisiana and Mississippi are oxbow lakes formed from large rivers, which are the typical habitat for the species. The Tennessee population occurs along Reelfoot Lake, a ‘recent’ lake formed from intense geological activity in 1811–1812 (Kelson, 1996).
MALE CALLING BEHAVIOR
Recordings of natural male calling
We recorded isolated males and groups of interacting males for three consecutive breeding seasons (June to August 2002 and April to July 2003 and 2004) using a TasCam DAP1 Digital Audio Tape Recorder and Audio-Technica ATR-55 Line Cardioid Condenser Microphones. The sound pressure level (dB SPL re 20 μPa, fast root-mean-square [RMS]) was measured at 50, 100, and 150 cm using a CEL-254 digital impulse sound level meter (Bedford, UK). The SPL of the calls of nearest neighbors was measured for a sub-sample of males (N= 30) by placing the microphone of the SPL meter directly above a randomly selected focal male in the chorus and reading the average sound output when many the other frogs were calling. We also measured the physical distance of these calling males to the focal male. These measurements allowed us to estimate the average SPL of close neighbors in order to set appropriate intensity levels in the playbacks used for males. Deep-body temperature was taken using Schultheis quick-reading thermometers.
Recording sessions included bouts of at least 20 calls for each individual. We recorded males under three different calling regimes: (1) Solitary recordings – calling males that had no calling neighbor within a 5-metre radius; the overall chorus noise was 68 dB SPL or less at the location of the frog. (2) Pair recordings – pairs of males calling (about 2 m apart from each other) in choruses of low to moderate density (up to 30 males in a chorus). We focused on pairs of calling males that were each other’s closest neighbors, recorded each onto a separate channel of a two-channel stereo recorder, and haphazardly chose one of the two as our focal male for analysis (see below). (3) Groups recordings – males calling from high-density choruses that had over 20 calling males, where the median inter-male distance was less than 1 m between closest neighbors. We haphazardly chose one male from a group as a focal male and recorded its calls directly onto one channel of the stereo recorder; another microphone recorded the calls of the adjacent males (usually two) onto the other channel. After each recording, we placed the SPL meter directly on top of the focal male to measure the SPL of the calls of the nearest neighbors to assess their relative intensities.
Playback experiments with males
Recorded calls were digitized and analyzed using Raven 1.2.1 software (Cornell Laboratories 2003–2005) to obtain the values of an average call (Fig. 1; Table 1). We used SoundEdit 2.0.7 Software (Shockwave Macromedia 1990–1996) on an iBook (Apple Computer Inc., Cupertino, CA, USA) to generate a standard call that consisted of a sequence of synthetic pulses. Calls recorded from males calling at different temperatures were used to create the stimuli used at those temperatures. For example, to create a 23-pulse call at 16° C, we created a 2.4 kHz tone of 80 msec and inserted a silent gap of 120 msec. We then shaped the envelope of the pulse to approximate that of a typical pulse of an advertisement call of H. avivoca. The pulse and silent gap were then repeatedly copied and pasted to create a 23-pulse call. After the 23-pulse call was created, we inserted a 10 sec silent interval. All of our synthetic stimuli were created using this method, and the temporal components of the calls were adjusted to correlate with the parameters of the specific body temperature of the frogs being tested (Table 2). We created additional stimuli by varying the values of pulse duration, interpulse intervals, number of pulses and intervals between calls. Each synthetic call was played back from one of as many as four output channels; phase (timing) relationships of different synthetic calls and their relative intensities were also varied depending on the design of a particular experiment.
Table 1.
Mean values (SD) of the gross-temporal call parameters of Hyla avivoca under three different calling densities
| Mean values for temporal parameters (standard deviation) | ||||||
|---|---|---|---|---|---|---|
| Type of aggregation | Call duration in seconds | Call interval in seconds | Calls per minute | Pulse number | Pulse duration (msec) | Pulse interval (msec) |
| Solitary (n=26) | 2.27 (0.21) | 8.3(1.32) | 4.89 (0.91) | 17.9 (1.6) | 52.3 (0.21) | 62.63 (2.67) |
| Pair (n=15) | 2.98 (0.33)* | 6.8(2.51)* | 5.73 (1.1)* | 23.6 (2.53)* | 51.8(0.36) | 74.65 (6.34)* |
| Group (n=8) | 2.93 (0.41)* | 6.9 (3.42)* | 6.01 (0.7)* | 23 (3.14)* | 52.1 (0.32) | 73.29 (5.23)* |
Denotes a significance of P < 0.05 for a One-factor Repeated Measures ANOVA.
Table 2.
Temporal structure of the standard synthetic advertisement call created for playback experiments on male and female H. avivoca.
| Temperature | Pulse number | Call duration | Pulse duration | Pulse interval | Call interval | Calls per minute |
|---|---|---|---|---|---|---|
| 16° C | 23 | 4.7 sec | 70 msec | 140 msec | 10 sec | 4 |
| 20° C | 23 | 3.6 sec | 60 msec | 100 msec | 8.1 sec | 5 |
| 24° C | 23 | 3.1 sec | 48 msec | 90 msec | 6.8 sec | 6 |
We broadcast synthetic calls using a playback system consisting of a MacIntosh iBook computer connected to a custom-built 2-dB step attenuator that equalized the sound output, which was then amplified using a Virtual Reality Sound Laboratories 200watt VR3 car amplifier if needed. The attenuator-amplifier system allowed us to adjust the playback levels broadcasted from a 4″ × 10″ midrange horn speaker (Parts Express W-46-02-104) mounted on a Velbon 5000 tripod. We adjusted the SPL for the speaker output away from the experimental males prior to each test using a CEL-254 SPL meter. For most tests, we set the fast RMS level of the sound source at 80 dB SPL at a distance of 2 m, which simulates the intensity of the calls of a neighbor at that distance. Playing back a stimulus to a focal male at any higher intensity elicited aggressive responses from the focal male or from neighboring males. The aggressive responses of males are discussed elsewhere (Martínez-Rivera in preparation).
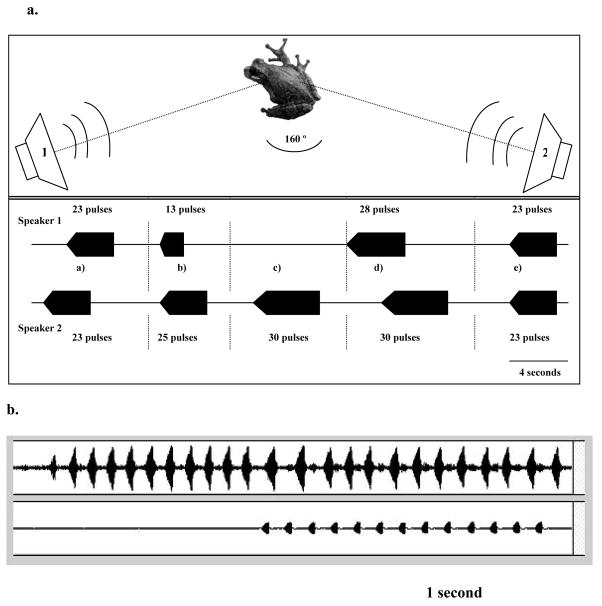
We performed four different playback tests on solitary males and used 18 different males for each of the first three tests: (1) Standard-call test. We presented males with a 20-call stimulus of the standard call (4.6 min. total duration; Table 2) at 80 dB SPL. (2) Longer-than-average call test. We broadcast a playback that contained sequences of five longer-than-average calls presented in random order (27, 30, 30, 27, 30 pulses) at 80 dB SPL (7 min total duration). (3) Distant-neighbor test. We used a 20-call stimulus of the standard 23-pulse call at 74 dB SPL at 2m, which simulates a male calling at a distance of about 3.4 meters (5 min total duration). (4) Multiple-male test. For this test, we presented 10 males with a pair of stimuli emitted from a two-speaker playback system (7 min total duration) using a stereo audio file that contained a simulated interaction between two males (Fig. 2). The values of call duration and the timing relationship of the two stimuli (see below) were based on data collected over the first two years of study. The arrangement of speakers (120° of angular separations) mimicked the typical positions of males calling under low-density conditions (Martinez-Rivera unpublished). One speaker was placed at 1.3 m from the focal male and the other speaker at 0.8 m; both speakers were pointed at the focal male and formed a roughly 160° angle with the focal male (Fig. 2). No male was used twice on the same test.
Figure 2.
a. Top panel shows a diagram of the playback setting used for the multi-neighbor male test. Bottom panel shows the stimulus used in our tests: a) Call alternation, b) complete call overlap, c) long calls, d) call jamming, and e) endurance rivalry. Overlapped calls have complete pulse interdigitation not shown in diagram. b. Oscillogram of a male interdigitating an overlapped call with a playback. A clear call (top panel) is interrupted on the 11th pulse by a 13 pulse long playback (bottom panel). As a result, the focal male extends the length of the interpulse interval of its call avoiding interference from the playback and produces a longer call of 24 pulses. Note how the interpulse interval increases as the call progresses, lowering pulse rate. The first four intervals, (before call overlap) are about 60 ms long, the last five (during call overlap) are about 110 ms. Playback is about 2 s in duration.
PLAYBACK EXPERIMENTS WITH FEMALES
Playback set-up
We broadcast synthetic calls generated with commercial software (Canary and SoundEdit) on a portable computer (iBook) using a playback system similar to the one described above for male playback experiments. We used Analog-Digital Systems 200 speakers (Boston, MA, USA) instead of horn speakers. The sound pressure level of each stimulus was adjusted to 86 dB SPL (fast RMS) midway between the speakers, which were separated by two meters and placed at opposite ends of the arena. Amplectant pairs were collected and placed in individual containers for at least two hours prior to being tested in the field. At this time, the gravid females were separated from their amplectant males; some females were tested on the night of capture, while others were tested on the following night. The latter females were held in their individual containers inside a cooler with ice and salty water to maintain a constant temperature of about 4° C, which inhibits oviposition and allows for testing of females on following nights. Females were acclimatized to the appropriate ambient temperature prior to testing.
All females were tested in the field at least 800 m away from the nearest frog chorus using a portable testing arena that consisted of six wooden frames measuring 1 m long by 0.50 m high covered with black cloth to minimize wind and light exposure to females. The arena measured 1 m × 2m × 0.5m with an open top and was placed on a flat surface (i.e. porous cement floor). A small holding container made out of acoustically transparent hardware cloth placed up-side down was used as a release box to retain each female at the release point midway between the speakers (unless otherwise stated). After broadcasting five calls from each speaker, we removed the release box with a pulley system and left the female in the arena free to make a choice between stimuli for up to 10 minutes. A response was recorded as positive when a female approached to within 10 cm of a speaker and showed the appropriate phonotactic behavior (Rheinlaender et al, 1979).
Females were tested only once per stimulus pair, and there was a time-out of 5 minutes between tests. The alternative stimuli were switched between speakers on every test to eliminate the chance of a side bias in the portable arena or from an outside source, (i.e. light, grade, ambient noise, etc.) which might make females approach or avoid a particular side preferentially. We recalibrated the sound pressure level of the speakers every time we alternated the direction of the source of the stimuli or changed the stimuli. Most females were released at the site of capture within two days of being collected.
We used synthetic signals that were generated as described above to test the selectivity of female phonotaxis with respect to different calling patterns. Given a choice between a typical pre-recorded call of 23 pulses and a synthetic call of the same length, thirteen females chose the natural call and twelve the synthetic call.
We conducted all playbacks at ambient temperatures ranging from 20° C to 26° C and used stimuli with values of call properties that were close (± 2 ° C) to those produced by males at the test temperature.
Female playback experiments
(1) Effects of call duration
We gave females a choice between the standard 23-pulse call and alternatives of various duration. For convenience, we grouped our stimuli as: (a) shorter-than-average calls of 10, 12 and 18 pulses; (b) calls that were plus/minus one standard deviation of the 23-pulse average call (a 21 and 24-pulse call), and (c) longer-than-average calls of 25, 27 and 30 pulses. We kept call rate (calls per minute) constant so that the alternative stimuli always alternated. The temporal parameters of stimuli fell within species-typical ranges of variation (Table 2).
(2) Effects of call overlap
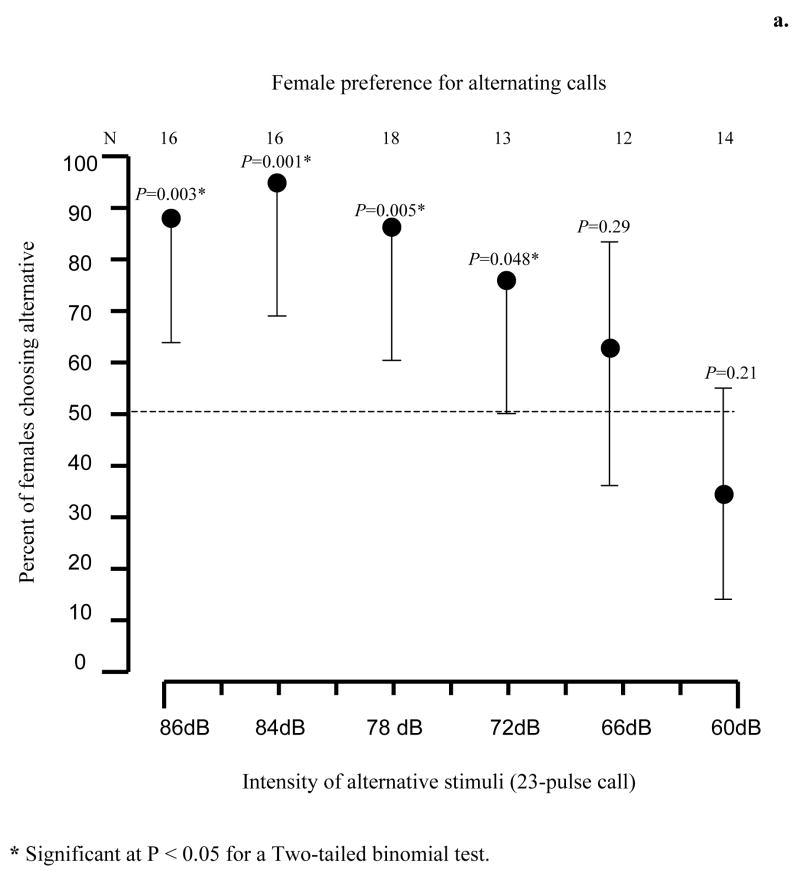
We gave females a choice between the standard 23-pulse call and a stimulus where every other call had a simulated overlap. The simulated overlapped call used for our stimulus was created by inserting a pulse of 50% of the relative amplitude of the previous pulse and a 5 msec silence gap to each pulse interval to obtain a simulated overlapped call consisting of a slightly longer call with 46 pulses; 23 pulses at full amplitude, each alternated by a pulse of 50% amplitude (Fig. 5). This stimulus thus simulated the pattern of pulse interdigitation typically observed in the overlapped calls of close neighbors where pulses are 180° out of phase (see Results). The speaker with the alternative stimulus broadcast a loop consisting of a 23-pulse call followed by two sets overlapping calls. We then performed five more tests in which we kept the intensity of the stimulus with the overlapped calls constant (86 dB) and varied the intensity of the 23-pulse call (84 dB, 78 dB, 72 dB, 66 dB, and 60 dB). The SPL (fast RMS) of the overlapped-call alternative was set to 86 dB SPL using a non-overlapped call prior to each test. This resulted in a slightly higher (about 87 dB) overall SPL for the overlapped calls in that stimulus, and the SPL of higher-amplitude overlapping call was 86 dB and that of the lower-amplitude call about 80 dB.
Figure 5.
a. Recognition and preference of call overlap. Error bars are 95% credible intervals; a single error bar denotes a significant (p < 0.05) preference in a two-tailed binomial test. b. Oscillograms of the stimuli used on this test. Top panel shows two simulated 23-pulsed call in overlap, bottom panel shows the 23-pulse stimulus. The overlapped call broadcast at an intensity of 86 dB @ 1m in all tests, the intensity of the single call in each test was broadcast at one of the following intensities: 84 dB, 78 dB, 72 dB, 66 dB, or 60 dB @ 1m.
(3) Effects of timing during call overlap
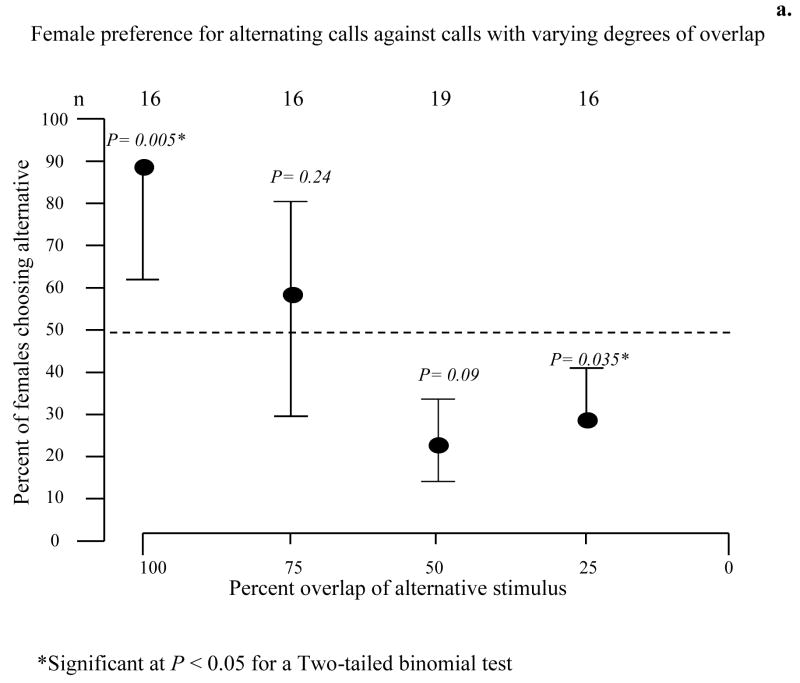
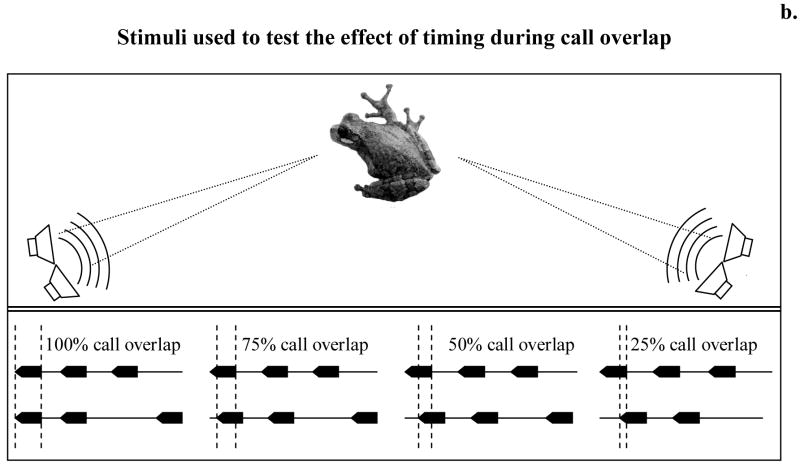
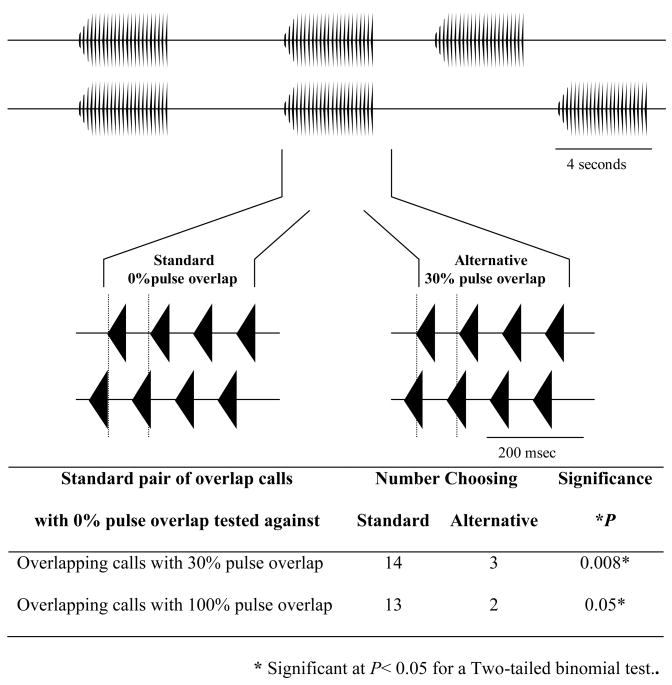
A. Gross temporal pattern-We used a four-speaker playback system to simulate two pairs of interacting males. The speakers in each pair were placed adjacent to each other and each pair was then placed in a separate corner of the arena to form a 120° angle of separation with reference to the female release point (Fig. 6B). This spatial arrangement is an approximation of those commonly observed in groups of calling males. We then varied the timing of calls from the two pairs of speakers. In one test, calls from one pair of speakers always alternated and calls from the other pair overlapped completely in two out of every three repetitions. Call rate was adjusted to avoid call overlap between the two sets of speakers. In three additional tests, calls from one pair of speakers always overlapped completely in two out of every three calls and was considered the standard stimulus-pair. In the alternative stimulus-pairs, two out of three calls also overlapped, but the amount of overlap for each alternative pair was 75%, 50%, or 25% respectively (Fig. 6B). B. Fine-scale temporal pattern. Females had a choice between a standard stimulus, in which the overlapping calls were timed so that pulse interdigitation was complete (0% pulse overlap) and two alternatives, one in which the alternative stimulus had no pulse interdigitation (100% pulse overlap) and a second alternative with less pulse interdigitation (30% pulse overlap) (Fig. 7).
Figure 6.
a. Female preference for overlapped calls with different degrees of call overlap. Error bars are 95% credible intervals; a single error bar denotes a significant (p < 0.05) preference in a two-tailed binomial test. b. Top panel - Cartoon of the four-speaker playback system used. Bottom panel - Schematic representation of the oscillograms used in our playback. Calls from one pair of speakers always alternated, calls from the other pair overlapped in two out of every three repetitions; the amount of call overlap varied according to the test (100%, 75%, 50%, or 25% overlap). Call rate not drawn to scale, was such as to avoid call overlap between the two sets of speakers.
Figure 7.
Representation of the stimuli used to test the effects of fine-scale temporal patterns during call overlap. In both stimuli two out of every three calls overlapped. The calls of the standard stimulus were timed so that complete pulse interdigitation occurred (0% pulse overlap; bottom left), the alternative stimuli had overlapping calls timed so that either 30% (bottom right) or 100% pulse overlap (not shown) occurred.
DATA ANALYSIS
Recording sessions and male playback experiments
We digitized and analyzed at least 20 calls per male using Raven 1.2.1 software (Cornell Laboratories 2003–2005); all recordings were made at 22.4 – 27.6 °C. We measured call length, pulse rate, call rate, call duty-cycle, and the duration of call overlap, counted the number of pulses per call and counted the number of overlapping calls. A call was considered overlapped if at least three pulses were completely overlapped by a neighbor. We determined the duration of call overlap of individual i by individual j by subtracting the starting time of individual j from the ending time of individual i (Brush and Narins, 1989) and analyzed treatment effects using a Friedman test for related samples. A One Factor Analysis of Variance for repeated measures with a multi-comparison significance level at 95% was used to analyze the male playback tests because we measured call parameters of the same male before, during and after treatment. We used Stat View SE+Graphics software for our analyses (Abacus Concepts, 1988).
Female playback experiments
The results of the female-preference test were analyzed in terms of preference functions. We computed 95%-exact confidence limits (binomial distribution) on the proportion of females choosing one of the alternatives. If the results of a two-tailed binomial test were significant (P<0.05), we show only the lower or upper confidence limit. The Animal Care and Use Committee of the University of Missouri approved our experimental procedures.
Results
MALE CALLING BEHAVIOR AND PLAYBACK EXPERIMENTS
Calling Patterns during Natural Interactions
Summary statistics for the measurements of the temporal properties of calls are given in Table 1. Because call rate between pairs of interacting males and groups of interacting males were not significantly different, (F1, 25= 0.036, P= 0.855, Table 1) we combined the two categories into a new category (interacting males) for the rest of our analyses. Mean call rate among interacting males was significantly higher than that of males calling in isolation (F1, 25= 9.821, P=0.044, Table 1). Interacting males increased pulse number (F1, 26= 9.96, P< 0.001) and produced calls at a higher rate (F1, 26= 12.667, P= 0.0015, Table 1) when compared to males calling in isolation.
Call overlap
Males calling in pairs overlapped 3.9 (+/− 1.3) out of every 10 calls on average, whereas males calling in groups overlapped 6.5 (+/− 0.9) calls out of every 10 on average (F1, 15= 15.4, P=0.0057). However, increased call overlap in groups of males was due to the increased number of calling males in the aggregation and not to changes in the calling patterns of individual males in the group. Call overlap occurred in three ways: 1. Simultaneous initiation of calls - Males began to call at the same time, but one male then produced a longer call (Fig. 1b). 2. Call matching - Both males produced simultaneous calls of similar lengths (duration and pulse number), causing the calls to overlap by 100% (Fig. 1c). 3. Extended duration calls by following male - The following male added pulses to match or exceed the number of pulses of the leading male, and then the leading male stopped abruptly, producing an unusually short call (Fig. 1b and 1d). This kind of interaction was most common in groups of calling males and could be characterized as a call interruption. The average pulse number of matched calls was significantly lower than the average pulse number of the alternating calls and extended calls (see below) in the same interacting pair [call matching = 17.5 (+/−2.4); alternating calls = 22.6 (+/− 3.1); extended calls = 23.6 +/−3.6) n = 15 calls, Friedman test; χ2= 15.63, df = 2; P< 0.001]. The calls that were stopped abruptly by an overlapping male (n = 27 calls) had 7.8 +/−3.2 pulses on average and were not tested for statistical differences but were clearly shorter than calls produced in other interactions.
Pulse interdigitation
Males often increased the silent interval between the pulses during call overlap such that the pulses of the calls of close neighbors interdigitated even when call overlap occurred during less than 10% of a call (i.e. 3 pulses). Furthermore, pulse interdigitation occurred at a scale of 10 milliseconds (Fig. 1) and resulted in a within-call reduction in pulse rate. Pulse interdigitation occurred in 62% of call overlaps when neighbors were about 3.5 meters apart (neighbor SPL of at least 80 dB) and in 90% of overlaps when neighbors call 2m apart or closer (neighbor SPL of 86 dB or more). Males were able to interdigitate pulses with more than one neighbor and with two speakers during the same call when these two alternated with each other but overlapped with the call produced by the focal male.
Male Response to Playback Experiments
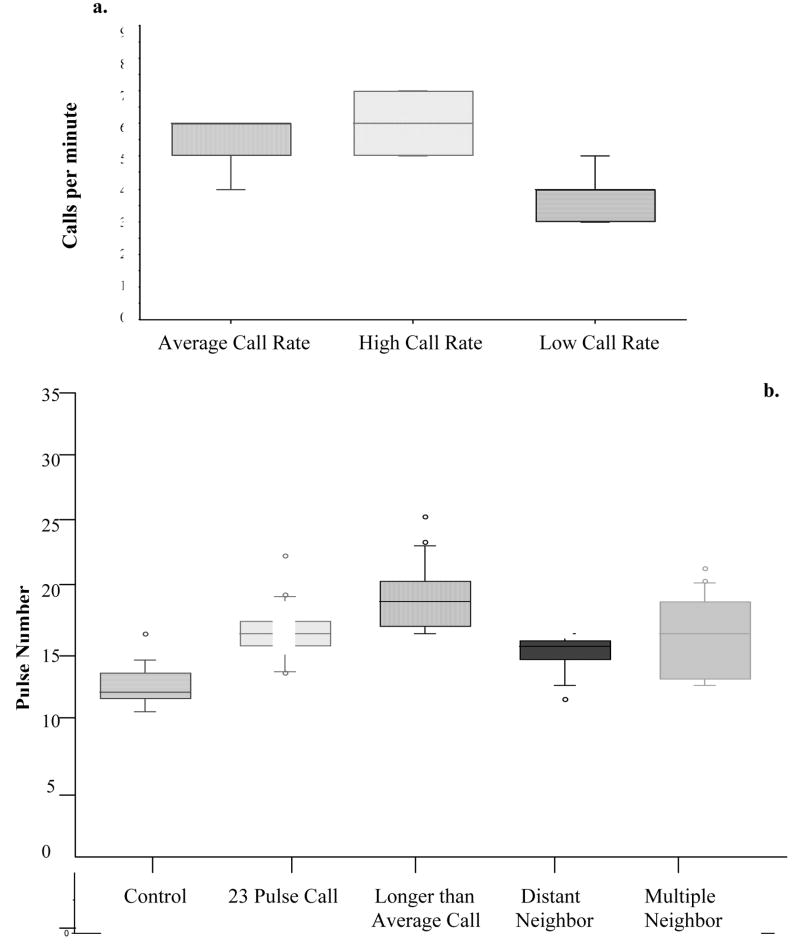
All males responded to our playbacks by engaging in at least one of the following behaviors: increased call production, increased number of pulses (call duration), increased call overlap, and pulse interdigitation with increased pulse intervals during call overlap (see Fig. 2b for an example). We compared the results of our male responses to its base-line calling behavior and observed the behavior of calling males after the stimuli ended. Our data are summarized in Figure 3 and Table 3. Males increased call length and call rate and engaged in call overlap with playbacks of calls of average length and playbacks of long calls. In the third test (distant neighbor), males increased call production but not necessarily call length. In the fourth test (multiple-male test), males responded to multiple speakers by increasing interpulse intervals and alternating pulses with both speakers during call overlap (Fig. 2b). However, after initially increasing call rate during the first minutes of being stimulated by a playback, males typically dropped their call rate to pre-stimulus levels within about 6.3 min of playback (Friedman test, Χ2 = 27.25, P= 0.001; Fig. 3).
Figure 3.
a. Box plot showing the average number of calls produced in a minute of continuous calling before playback stimulation. The second box the highest number of continuous calls produced during a minute of playback stimulation and the third shows the lowest number of continuous calls produced during a minute of playback stimulation. The variation between treatments is significant (Friedman test, X2 = 27.25, P = 0.001). b. Average pulse number per call given by frogs under different playback regimes. All males increase call length in response to playbacks. Bars indicate standard errors.
Table 3.
Mean response (SD) of calling males to the playback used in our different tests
| Tests | Variation in temporal structures of calls | Call production | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Call length in seconds | Average number of pulses | Average calls per minute | |||||||
| Before | During | After | Before | During | After | Before | During | After | |
| Average call | 2.32 (0.27) | 2.93 (0.18)* | 2.87 (0.76)* | 18.9 (1.7) | 22.2 (1.5)* | 23.2 (2.7)* | 4.8 (0.7) | 5.9 (1)* | 5.6 (0.8)* |
|
| |||||||||
| Longer call | 2.34 (0.25) | 3.06 (0.28)* | 2.98 (1.26)* | 17.8 (2.1) | 25.7 (2.6)* | 24.8 (1.9)* | 4.9 (0.6) | 6.2 (1.2)* | 5.4 (1.1) |
|
| |||||||||
| Distant neighbor | 2.43 (0.39) | 2.93 (0.29)* | 3.02 (1.44)* | 18.6 (0.6) | 21.5 (1.6)* | 23.7 (1.2)* | 4.8 (0.6) | 5.4 (1.3)* | 4.4 (0.9) |
|
| |||||||||
| Multiple Male | 2.08 (0.67) | 3.02 (0.27)* | 2.94 (0.93)* | 18.2 (1.3) | 23 (3.1)* | 23.2 (1.9)* | 4.8 (0.5) | 5.8 (0.3)* | 5.6 (1.2)* |
Denotes a significance of P < 0.05 for a One-factor Repeated Measures ANOVA for the differences observed on the measured temporal parameters between different times during the tests rather than between tests.
FEMALE PREFERENCES
(1) Effects of call duration
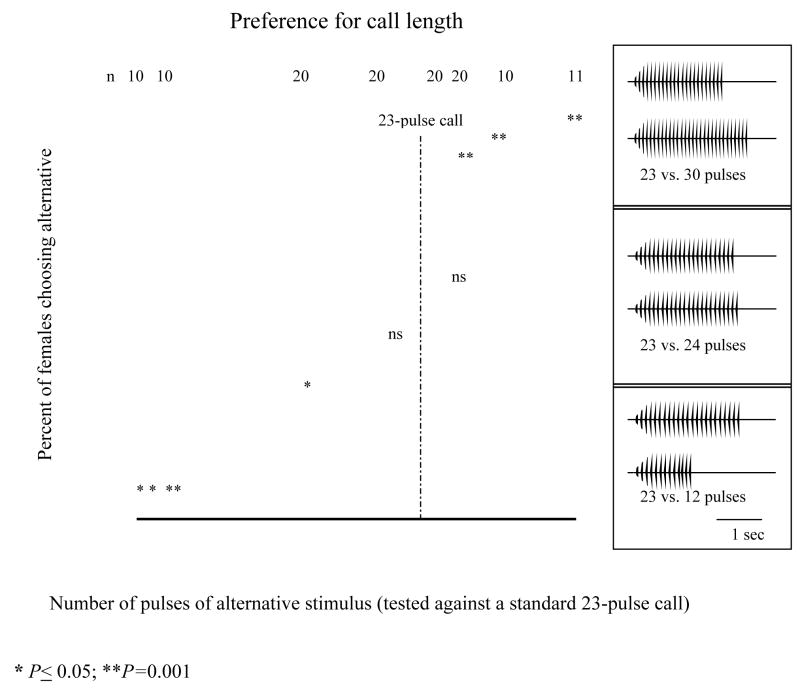
All females preferred the standard 23-pulse call to calls of shorter length (P< 0.001 vis-à-vis 10- and 12-pulse calls, and P=0.037 vis-à-vis 18-pulse calls). There was no preference in tests of the average 23-pulse call against 21-pulse calls (P=0.12) and 24-pulse calls (P=0.16). Females preferred longer alternatives with 25 or more pulses per call to the standard 23-pulse call (Fig. 4).
Figure 4.
Proportions of females choosing calls of varying length against the standard 23-pulse call. Error bars are 95% credible intervals; a single bar denotes a significant (p < 0.05) preference in a two-tailed binomial test.
(2) Effects of call overlap
Females strongly preferred the non-overlapped standard call even when its SPL was as much as 14 dB less than that of the stimulus in which every other call was overlapped (Fig. 5). This preference was abolished when the SPL of the standard call was reduced in intensity by 20 dB or more relative to the alternative with overlapped calls.
(3) Effects of timing during call overlap
A. Gross temporal patterns- females preferred a pair of alternating calls to a pair of overlapped calls in the four-speaker trials (P=0.005; Fig. 6) and preferred calls with a partial overlap of 25% to a pair of alternative calls. However, females showed no preference when the degree of overlap was 75% (P=0.024) or 50% (P=0.09) (Fig. 6). B. Fine-scale temporal pattern- females showed a significant preference for overlapped calls with interdigitated pulses over a set of overlapped calls in which pulses completely (P=0.05) or partially overlapped (P=0.008) (Fig. 7).
Discussion
The high levels of noise in a chorus limit the effectiveness of the signals produced by senders (Gerhardt and Klump, 1988; Wollerman and Wiley, 2001). Thus, it is in the best evolutionary interest of senders to maintain strategies that increase their chances of being detected and chosen by a female (Narins, 1992; Greenfield, 1994; Hill, 1998). Conversely, it is in the best interest of receivers to detect and orient towards the signaler that produces the best (clear) signal (reviewed in Wiley 2006). Receivers (females) that are selective towards advertisement calls would reduce the chances of costly mismatings (H. versicolor and H. chrysoscelis Gerhardt, 2005a; Marshall et al., 2006; Hyla cinerea and H. gratiosa; Höbel and Gerhardt, 2003) and reduce the probability of mating with lower-quality conspecific males (Spea bombifrons and S. multiplicata Pfennig and Simovich, 2002; reviewed in Pfennig, 1998; H. versicolor Welch et al., 1998). Moreover, noise reduces the effectiveness of sound localization (Schwartz and Gerhardt, 1989) and might increase possible costs associated with mate assessment, since females would require more time to locate a calling mate (reviewed in Gerhardt and Huber, 2002).
Dynamic properties of acoustic signals of insects and anurans (Gerhardt, 1991) are especially variable within calling bouts and can be related to on-going social interactions between rival males (Greenfield 1994; Gerhardt and Huber, 2002). In this study, we have described how different calling situations alter patterns of advertisement calling in the bird-voiced treefrog. We have also shown how several of these changes affect the relative attractiveness of signals to females. We now discuss relationships between calling patterns and female preferences and the implications for their co-evolution.
Increased call duration during male-male interactions
Males calling in groups or in response to playbacks produced longer calls by adding more pulses to their calls. The response of female H. avivoca to orient towards longer calls suggest an open-ended preference within the biological maxima of the calling behavior observed in the populations studied, a preference pattern that is typical for dynamic call properties (Gerhardt and Huber, 2002). As stated above, the production of longer calls in chorusing situations should benefit the male by increasing his detectability to females in noisy choruses (Schwartz et al., 2002; but see Passmore and Telford, 1981). However, females preferred long to short calls in the relatively quiet testing situations we used for our playback experiments. Thus, males would be expected to increase mating success by producing longer calls whenever they are calling near other males even if call detectability remains high. The fact that males increased duration and call rate only when stimulated vocally and reverted to producing fewer calls of normal duration after such a challenge, also suggests that the production of long calls is probably energetically more costly than producing short advertisement calls (see Schwartz et al., 1995) and could be reliable indicators of male reproductive fitness. Thus, the production of long calls and female preferences for such signals may have parallels with the gray treefrog (H. versicolor) system in which call duration may be an honest indicator of genetic as well as physical fitness (Welch et al., 1998). In order to test this hypothesis, breeding experiments with H. avivoca, that compare the fitness and attractiveness of the offspring of long and short callers would be needed.
Call Overlap: a conflict of calling strategies
Males of H. avivoca increased call production and call length in the presence of calling neighbors and during playback experiments. This change invariably resulted in increased call overlap. Even though pairs of calling males overlapped calls often, they still produced clear calls 58% of the time. Males calling in dense groups overlapped calls with more than one neighbor, and produced clear calls only about 35% of the time. Such overlap would be expected to counter-balance the attractive effect of increasing call duration because females preferred clear calls even when their intensity was as much as 14 dB lower than a set of calls that overlapped only 50% of the time. The results are generally consistent with other studies of chorus-breeding frogs showing that females discriminate against overlapped calls (Passmore and Telford, 1981; Schwartz 1987, Gerhardt and Klump, 1988; Wollerman and Wiley, 2001). However, one exceptional result was a preference for a pair of calls with 25% overlap rather than a pair of alternating calls. By broadcasting calls from adjacent speaker in our experiment, we might have created a stimulus that was perceived by females as a single longer-than-average call, which female anurans usually prefer (Gerhardt and Huber, 2002.
Pulse interdigitation: a partial solution?
We have shown in this study that many of the calls of close neighbors inevitably overlapped during male-male interactions and that male H. avivoca actively interdigitated the pulses of overlapped calls even in calls with partial overlap (Fig. 1). Perhaps the most significant of our results is the demonstration that females preferred overlapped signals with interdigitated pulses to overlapped signals where pulses partially overlapped (Fig. 7). This result could be considered unexpected in that the pulse rate, which is usually a species-specific, relatively invariant property, was lowered within each overlapped call in order to achieve pulse interdigitation. Furthermore, if the overlapped signals were perceived as a summed signal, then the perceived pulse rate would be significantly higher (i.e. double) than that of a non-overlapped call.
Interdigitation of call notes has been reported in other anuran species, but these patterns are not strictly comparable. In K. kuvangensis call groups are repeated in short succession followed by a period of silence; when males call synchronously they alternate the calls within the overlapped call groups (Grafe, 2003). Note alternation in H. microcephala can involve both the introductory component (a buzz-like pulsed note) and the shorter secondary notes (clicks) of two neighbors (Schwartz & Wells, 1985). The introductory note contains more of the species-identifying properties of the call and can attract females in the absence of secondary notes (Schwartz, 1987). Males add secondary click-notes in increasing numbers as male-male interactions in the chorus intensify (Schwartz & Wells, 1985; reviewed in Schwartz, 1993).
The effects of note interdigitation on female choice have been tested in H. microcephala. As in H. avivoca, alternating (interdigitating) notes were preferred to out-of-phase overlapping calls (Schwartz 1987; 1993). Even though males of H. versicolor do not show pulse-interval adjustments, a recent experimental study found that, unlike H. avivoca, pulse interdigitation of overlapping calls was less attractive to females than partial pulse overlap (Schwartz and Marshall, 2006). This species difference probably reflects the fact that two overlapping calls of H. versicolor with pulse interdigitation would result in a signal with a pulse timing that resembles that of the call of H. chrysoscelis, a genetically incompatible species that often breeds at the same time and place as H. versicolor. By comparison, the pulse rate of an interdigitated pair of calls of H. avivoca is significantly different from that of any other sympatric species.
Interdigitation of pulses: an exceptional pattern?
Interdigitation resulted in increased duration of silent gaps between pulses and hence sharp drops in pulse rate. Males are also able to return to the normal, faster pulse rate within that same call once an overlapping call stops (Fig. 1). Such within-call variation in pulse rate is an exception to the pattern observed in many other anurans. The within-male coefficient of variation (CV) in pulse rate is typically 4% or less (review in Gerhardt and Huber, 2002), and in H. versicolor, a close relative of H. avivoca the mean was 1.5% (range: 0.2 – 5.0%; Gerhardt, 1991). By contrast, the mean within-bout coefficient of variation for pulse rate in Hyla avivoca during call overlap, is about 9% (range 3.8 – 10.9 %) (n=30 males; this study). Because they responded to pairs of interdigitating calls in which the pulse rate of the calls of both signals were lower than average or in which the pulse rate of the composite signal was higher than average, females of H. avivoca may not be relying on pulse rate per se for mate recognition. Indeed, females of H. versicolor are selective for signals with a minimum pulse duration and maximum silent interval rather than the species typical pulse rate (Schul and Bush, 2002). Preliminary results of experiments suggest that females of H. avivoca use similar criteria (Martinez-Rivera and Gerhardt, unpubl. data).
Even if females of H. avivoca rely on pulse rate for species identification, there would almost certainly be sufficient numbers of clear calls (35% in groups and 58% between pairs of males) from which the unaltered rate could be assessed. Moreover, the mean CV for the pulse rate of calls produced by solitary males is about 2% (range 1.3 –3.4 %, n = 32), well within the usual range of variation for static properties (Gerhardt and Huber, 2002). Finally, the elevated mean CV for pulse rate in overlapped calls was still considerably lower than the mean CVs for classically dynamic properties such as call rate (mean CV 18.3%, range 12–25%, n = 32) and pulse number (mean CV – 25.3%, range 13–31%, n = 32) in males of H. avivoca calling in isolation. Indeed, a more biologically relevant perspective concerning the static-dynamic continuum emphasizes that within each species there are acoustic properties of relatively low and high within-bout variability rather than attempting to define an arbitrary and universal cutoff value of the mean CV for categorizing a property as static or dynamic (Gerhardt and Huber, 2002; see also Shaw and Herilihy, 2000).
Future Studies
Pairs of vocally interacting males showed four common patterns: synchronizing calls, matching call duration, increasing duration beyond the end of an overlap, and call interruptions. More research is need to evaluate the conditions in which each pattern is more likely to occur, and female-preference tests need to be conducted to assess which, if any, of these patterns females may favor. In such studies and in further investigations of pulse interdigitation, the angular separation of speakers should also be varied. Schwartz and Gerhardt (1995) and Marshall et al. (2006) show that when the angular separation of speakers was reduced, females of H. versicolor often failed to choose an alternative that was preferred when the angular separation was 90° or more. Thus, angular separation is likely to affect the choices of H. avivoca in tests in which a longer (i.e. more attractive) call is overlapped by a shorter call. For example, two overlapping calls of different length might be treated as a single longer call when presented from abutting or closely spaced speakers (or calling males) but not when there is greater angular separation.
In general, the calling patterns of H. avivoca, as in other frogs and insects become increasingly complex as group size increases. Uncovering complex patterns of interaction is a difficult challenge and will almost certainly require some new approaches and analytical techniques such as communication-network theory (McGregor and Peake 2000). In this approach, any member (signaler or receiver) of the network (i.e. chorus) can gain information from an interaction among other senders and influence its future behavior accordingly (McGregor and Dabelsteen 1996). For example, Siamese fighting fish (Betta splendens) exposed to a fighting event used the information about who won the fight to gauge the abilities of the opponent in future encounters and modified their behavior in the initial stages of agonistic interactions to avoid costly fights with the previous winner (Oliveira et al., 1998). Likewise, female black-capped chickadees eavesdropped on male-male vocal interactions and used this information to choose males with which they engaged in extra pair copulations (Mennill et al., 2002). Males and females of H. avivoca might also gain information by attending to vocal interactions and would be expected to alter their responses to a rival depending on his prior behavior. For example, females were attracted to a pair of speakers broadcasting a simulated interaction of only advertisement calls and not to a pair of speaker broadcasting a simulated interaction that contained both advertisement and aggressive calls (Martinez-Rivera, in prep.). Furthermore, females were not reliably attracted to playbacks of aggressive calls even when there was no other stimulus (Martinez-Rivera, unpubl. data). Hence, females probably avoid a male (or groups of calling males) engaged in aggressive behavior.
Acknowledgments
We thank Adam Boyette, Drew Dittmer and Zapic Martínez for assistance in collecting data and testing frogs in the field. Bob Jones, Kristy Wharton and Ray Semlitsch facilitated locality data for the Mississippi, Louisiana and Tennessee populations respectively. Mario and Crystal Sánchez, and Kristy Wharton kindly provided housing (and moral support) in the field; George K. and S. Adams made the long hours in the field feel less harsh. Bob Jones and Jerome Ford kindly granted permission to carry out our research in MS and LA respectively. Gerlinde Höbel and JJ Schwartz, along with two anonymous reviewers greatly improved this manuscript. This work was supported by a National Science Foundation – (Doctoral Dissertation Improvement Grant to Martínez-Rivera and IBN-0091993 to Gerhardt), the Public Health Service (DHHS R01 DC05760 to Gerhardt) and the City of Mayagüez Department of Education, Mayagüez, Puerto Rico (to Martínez-Rivera).
References
- Andersson M. Sexual Selection. Princeton Univ. Press; Princeton, NJ: 1994. [Google Scholar]
- Bee MA, Gerhardt HC. Habituation as a mechanism of reduced aggression between neighboring territorial male bullfrogs, Rana catesbeiana. J Comp Psych. 2001;115:68–82. doi: 10.1037/0735-7036.115.1.68. [DOI] [PubMed] [Google Scholar]
- Bourne GR, Collins AC, Hoder AM, McCarthy CL. Vocal communication and reproductive behavior of the frog Colostethus bebeei in Guyana. J Herpetol. 2001;35:272–81. [Google Scholar]
- Bradbury J, Vehrencamp S. Principles of animal communication. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- Brush JS, Narins PM. Chorus dynamics of a Neotropical amphibian assemblage: comparison of computer simulation and natural behavior. Anim Behav. 1989;37:33–44. [Google Scholar]
- Dundee HA, Rossman DA. The Amphibians and Reptiles of Louisiana. Paperback. Louisiana State University Press; 1989. pp. 87–89. 1996. [Google Scholar]
- Gerhardt HC. Female mate choice in treefrogs: static and dynamic acoustic criteria. Anim Behav. 1991;42:615–35. [Google Scholar]
- Gerhardt HC. Acoustic communication in two groups of closely related treefrogs. In: Slater PJB, Rosenblatt JS, Snowdon CT, Roper TJ, editors. Advances in the Study of Behavior. New York: Academic Press; 2001. pp. 99–167. [Google Scholar]
- Gerhardt HC. Advertisement-call preferences in diploid-tetraploid treefrogs (Hyla chrysoscelis and Hyla versicolor): implications for mate choice and the evolution of communication systems. Evolution. 2005a;59:395–408. [PubMed] [Google Scholar]
- Gerhardt HC. Acoustic spectral preferences in two cryptic species of grey treefrogs: implications for mate choice and sensory mechanisms. Anim Behav. 2005b;70:39–49. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic Communication in Insects and Frogs: Common Problems and Diverse Solutions. Chicago: University of Chicago Press; 2002. [Google Scholar]
- Gerhardt HC, Klump GM. Masking of acoustic signals by the chorus background noise in the green tree frog: a limitation on mate choice. Anim Behav. 1988;36:1247–49. [Google Scholar]
- Gerhardt HC, Dyson ML, Tanner SD. Dynamic properties of the advertisement calls of gray treefrogs: patterns of variability and female choice. Behav Ecol. 1996;7:7–18. [Google Scholar]
- Grafe TU. Synchronized interdigitated calling in the Kuvangu running frog, Kassina kuvangensis. Anim Behav. 2003;66:127–36. [Google Scholar]
- Greenfield MD. Synchronous and alternating choruses in insects and anurans: Common mechanisms and diverse functions. Am Zool. 1994;34:605–15. [Google Scholar]
- Hill PSM. Environmental and social influences on calling effort in the prairie mole cricket (Gryllotalpa major) Behav Ecol. 1998;9(1):101–108. [Google Scholar]
- Höbel G, Gerhardt CH. reproductive character displacement in the acoustic Communication system of green tree frogs (Hyla cinerea) Evolution. 2003;57:894–90. doi: 10.1111/j.0014-3820.2003.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Kamo M, Ghirlanda S, Enquist M. The evolution of signal form: effects of learned versus inherited recognition. Proc R Soc Lond B. 2002;269(1502):1765–71. doi: 10.1098/rspb.2002.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelson KI, Simpson GD, VanArsdale RB, Haraden CC, Lettis WR. Multiple late Holocene earthquakes along the Reelfoot fault, central New Madrid seismic zone. J Geophys Res. 1996;101(B3):6151–7. [Google Scholar]
- Klump GM, Gerhardt HC. Use of non-arbitrary acoustic criteria in mate choice by female gray tree frogs. Nature (London) 1987;326:286–288. [Google Scholar]
- Marshall VT, Humfeld SC, Bee MA. Plasticity of aggressive signaling and its evolution in male spring peepers Pseudacris crucifer. Anim Behav. 2003;65:1223–34. [Google Scholar]
- Marshall VT, Schwartz JJ, Gerhardt HC. Effects of heterospecific call overlap on the phonotactic behaviour of grey treefrogs. Anim Behav. 2006;72 (2):449–459. [Google Scholar]
- McGregor PK, Dabelsteen T. Communication networks. In: Kroodsma DE, Miller EH, editors. Ecology and evolution of acoustic communication in birds. Cornell Univ. Press; 1996. [Google Scholar]
- McGregor PK, Peake TM. Communication networks: social environments for receiving and signalling behaviour. Acta Ethologica. 2000;2:71–81. [Google Scholar]
- Mennill DJ, Ratcliffe LM, Boag PT. Female eavesdropping on male song contest in songbirds. Science. 2002;296:873. doi: 10.1126/science.296.5569.873. [DOI] [PubMed] [Google Scholar]
- Narins PM. Evolution of anuran chorus behavior: Neural and Behavioral constrain. Am Nat. 1992;139:S90–S104. [Google Scholar]
- Narins PM, Hödl W, Grabul DS. Bimodal signal requisite for agonistic behavior in a dart-poison frog, Epipedobates femoralis. PNAS. 2003;100:577–80. doi: 10.1073/pnas.0237165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RF, McGregor PK, Latruffe C. Know thine enemy: fighting fish gather information from observing conspecific interactions. Proc R Soc Lond B. 1998;265:1045–1049. [Google Scholar]
- Passmore NI, Telford SR. The effect of chorus organization on mate localization in the painted reed frog (Hyperolius marmoratus) Behav Ecol Sociobiol. 1981;9:291–293. [Google Scholar]
- Pfennig K. The evolution of mate choice and the potential for conflicts between species and mate-quality recognition. Proc R Soc Lond B. 1998;265:1743–1748. [Google Scholar]
- Pfennig K, Simovich MA. Differential selection to avoid hybridization in two toad species. Evolution. 2002;56(9):1840–1848. doi: 10.1111/j.0014-3820.2002.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Rheinlaender J, Gerhardt HC, Yager D, Capranica RR. Accuracy of phonotaxis in the green treefrog (Hyla cinerea) J Com Physiol. 1979;133:247–55. [Google Scholar]
- Schul J, Bush SL. Non-parallel coevolution of sender and receiver in the acoustic communication system of treefrogs. Proc R Soc Lond B. 2002;269:1847–1852. doi: 10.1098/rspb.2002.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JJ. The function of call alternation in anuran amphibians: a test of three hypotheses. Evolution. 1987;41:461–471. doi: 10.1111/j.1558-5646.1987.tb05818.x. [DOI] [PubMed] [Google Scholar]
- Schwartz JJ. Male calling behavior, female discrimination and acoustic interference in the Neotropical treefrog Hyla microcephala under realistic acoustic conditions. Behav Ecol Sociobiol. 1993;32:401–414. [Google Scholar]
- Schwartz JJ, Gerhardt HC. Directionality of the auditory system and call pattern recognition during acoustic interference in the gray treefrog, Hyla versicolor. Auditory Neurosci. 1995;1:195–206. [Google Scholar]
- Schwartz JJ, Wells KD. Intra-and interespecific vocal behavior of the neotropical treefrog Hyla microcephala. Copeia. 1985;1985:27–38. [Google Scholar]
- Schwartz JJ, Marshall VT. Forms of Call Overlap and Their Impact on Advertisement Call Attractiveness to Females of the Gray Treefrog, Hyla versicolor. Bioacoustics. 2006;16:39–56. [Google Scholar]
- Schwartz JJ, Ressel S, Bevier C. Carbohydrate and calling: Depletion of muscle glycogen and the chorusing dynamics of the Neotropical frog Hyla microcephala. Behav Ecol Sociobiol. 1995;37:125–35. [Google Scholar]
- Schwartz JJ, Buchanan BW, Gerhardt HC. Acoustic interactions among male gray treefrogs, Hyla versicolor, in a chorus setting. Behav Ecol Sociobiol. 2002;53:9–19. [Google Scholar]
- Shaw KL, Herhlihy D. Acoustic preference functions and song variability in the Hawaiian cricket Laupaula cerasina. Proc Royal Soc London B. 2000;267:577–584. doi: 10.1098/rspb.2000.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehrencamp SL. Handicap index and conventional signal elements of bird songs. In: Espmark Y, Amundsen T, Rosenquist G, editors. Animal signals: signaling and signal design in animal communication. Tapir Academic Press; Trodheim, Norway: 2000. pp. 277–300. [Google Scholar]
- Welch AM, Semlitsch RD, Gerhardt HC. Call duration as an indicator of genetic quality in male gray treefrogs. Science. 1998;280:1928–30. doi: 10.1126/science.280.5371.1928. [DOI] [PubMed] [Google Scholar]
- Wells KD, Schwartz JJ. The behavioral ecology of anuran communication. In: Narins PM, Feng AS, Fay RR, Popper AN, editors. Hearing and sound communication in amphibians. Vol. 28. Springer; Berlin Heidelberg New York: 2006. pp. 44–86. [Google Scholar]
- Wells KD, Taigen TL. The effect of social interactions on calling energetics in the gray treefrog (Hyla versicolor) Behav Ecol Sociobiol. 1986;19:9–18. [Google Scholar]
- Wiley RH. Signal detection and animal communication. Advances in the Study of Behavior. 2006;36:217–47. [Google Scholar]
- Wollerman L, Wiley RH. Background noise from a natural chorus alters female discrimination of male calls in a Neotropical frog. Anim Behav. 2001;63:15–22. [Google Scholar]